Abstract
Analysis of volatile organic compounds (VOCs) in exhaled breath has shown great potential for disease detection including lung cancer, infectious respiratory diseases, and chronic obstructive pulmonary disease. Although many breath sample collection and analytical methods have been developed for breath analysis, analysis of metabolic VOCs in exhaled breath is still a challenge for clinical application. Many carbonyl compounds in exhaled breath are related to metabolic processes of diseases. This work reports a method of ultra-high performance liquid chromatography coupled with high resolution mass spectrometry (UHPLC-MS) for analysis of a broad range of carbonyl metabolites in exhaled breath. Carbonyl compounds in exhaled breath were captured by a fabricated silicon microreactor with a micropillar array coated with 2-(aminooxy)ethyl-N,N,N-trimethylammonium (ATM) triflate. A total of six subgroups consisting of saturated aldehydes and ketones, hydroxy-aldehydes, and hydroxy-ketones, unsaturated 2-alkenals, and 4-hydroxy-2-alkenals were identified in exhaled breath. The combination of a silicon microreactor for selective capture of carbonyl compounds with UHPLC-MS analysis may provide a quantitative method for analysis of carbonyls to identify disease markers in exhaled breath.
Graphical Abstract
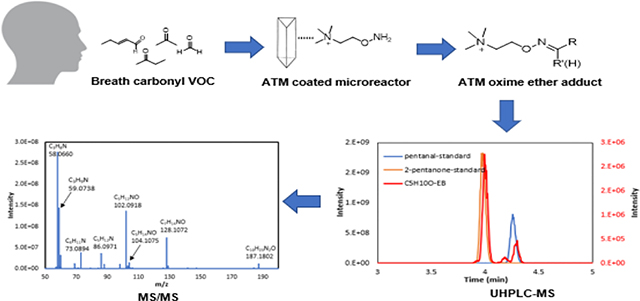
Breath analysis has received considerable interest because of its applications in noninvasive diagnosis of diseases.1–5 Carbonyl compounds have been investigated because elevated levels of these compounds in exhaled breath are considered as potential biomarkers for many types of diseases.6–10 Some carbonyl compounds, such as 2-butanone, hydroxy-acetaldehyde, 3-hydroxy-2-butanone, 2-pentanal, 2-hexanal, 4-hydroxy-2-hexenal (4-HHE), and 4-hydroxy-2-nonenal (4-HNE), are reported to have elevated levels in exhaled breath of lung cancer patients.8–12 Malondialdehyde (MDA) and 4-HNE in breath have been identified as biomarkers of asthma, chronic obstructive pulmonary disease (COPD), cardiovascular disease, and bronchiectasis induced by oxidative stress and inflammation.13–15 MDA and 4-HNE were also reported in several biological matrices including exhaled breath condensate,16 tissue,17 urine,18 serum,19 and bronchoalveolar lavage fluid.19 An elevated level of acrolein (propenal) has been found in the brain of Alzheimer’s disease subjects.20 Therefore, the measurement of carbonyl compounds in biofluids is of great interest for disease detection and the study of disease etiology.
Among all the developed methods for analysis of volatile organic compounds (VOCs) in exhaled breath, gas chromatography-mass spectrometry (GC-MS) is one of the most widely employed techniques.21–23 It allows separation and identification of VOCs from the breath matrix. However, GC-MS requires sample pre-treatment (pre-concentration and moisture removal), which leads to losses of analytes and contamination issues. GC-MS is also not suitable for real-time measurement.24 Other methods have been used for real-time measurements without sample processing, such as selected ion flow tube-mass spectrometry (SIFT-MS),25 proton transfer reaction-mass spectrometry (PTR-MS),26 ion mobility spectrometry (IMS)27 as well as electronic nose or gas sensor arrays.28 Although these techniques can be applied for real-time analysis and show good sensitivity, none have the chromatographic separation or spectral libraries for comparing results when compound identification is involved.
To focus on analysis of carbonyl compounds in exhaled breath for clinical applications, this work reports a new approach that combines a carbonyl trapping agent along with a microfabricated silicon microreactor to capture carbonyl compounds via oximation reactions with analysis using ultra high-performance liquid chromatography-mass spectrometry (UHPLC-MS) for quantitative measurements. Previously, we developed 2-(aminooxy)ethyl-N,N,N-trimethylammonium salts (ATM) to capture volatile aldehydes and ketones using a silicon-based microreactor followed by sample analysis using high resolution Fourier transform-ion-cyclotron resonance (FT-ICR) mass spectrometry (MS) without separation capacity.29, 30 By developing the present analytical platform technology, a broad range of carbonyl compounds including two subgroups of unsaturated 2-alkenals, and four subgroups of saturated ketones and aldehydes have been separated and identified in exhaled breath samples and their concentration ranges were determined. The subgroups of hydroxy-aldehydes and hydroxy-ketones are for the first time identified and measured. Although unsaturated 2-alkenals, 4-hydroxy-2-alkenals and 4-hydroxy-2,6-alkadienals have been reported in exhaled breath condensates, there have been no concentration ranges reported for these compounds and no unsaturated aldehydes with less than 6 carbon atoms have been previously reported.31 The present method enables detection of unsaturated aldehydes from C3 to C10 and unsaturated hydroxy-aldehydes from C3 to C9 in exhaled breath. A quantitative method for analysis of carbonyl compounds in exhaled breath can be used to identify disease biomarkers. Furthermore, the detection of these carbonyl compounds also provides evidence for metabolic and mechanistic studies of production of a large number of carbonyl compounds in exhaled breath from the oxidation of unsaturated fatty acids.32
EXPERIMENTAL SECTION
Material and methods
All reagents and solvents, including saturated aldehydes, ketones, hydroxy-ketones, hydroxy-aldehydes (except for 2-hydroxybutanal and 2-hydroxyheptanal, see supporting information for synthesis33, 34), acrolein (>99.5%), crotonaldehyde, 2-pentenal, 2-hexenal, 2-heptenal (>99.5%, mixture of cis and trans) were purchased from Sigma-Aldrich. 4-HHE and 4-HNE were purchased from Cayman. Water, acetonitrile, methanol, and formic acid of LC-MS grade were purchased from Fisher Scientific. Tedlar bags with a volume of 1 liter were also purchased from Sigma-Aldrich. 2-(Aminooxy)ethyl-N,N,N-trimethylammonium (ATM) triflate was synthesized from ATM iodide35 using a counterion exchange protocol.36 Eight ATM-carbonyl adduct standards were synthesized and purified according to the procedure described in Supporting Information (SI) for preparing calibration curves in Figure S1. Other adduct standards were prepared by mixing ATM with single carbonyl compounds with a molar ratio of 1.2:1 to ensure complete reaction of carbonyl compounds to obtain the retention times and MS2 data of UHPLC-MS.
Silicon microreactors
The silicon microreactors were fabricated from 4-inch silicon wafers using standard microelectromechanical systems (MEMS) fabrication techniques. Details of the microreactor design and fabrication and characterization have been published elsewhere.29, 30 The microreactor has thousands of equilateral triangular micropillars in an area of 21 mm in length and 7 mm in width and the surface of the micropillars were oxidized by wet (a mixture of H2O and O2) thermal oxidation. The triangular micropillars have a 50 μm lateral length and 400 μm in height, and the distance between the two closest pillars are 10 μm. Figure 1 (a) shows the size comparison of the microreactor with a dime coin, and (b) the SEM micrograph of the micropillars. The inlet and outlet of the microreactor were fitted with 350 μm O.D. and 250 μm I.D. deactivated fused silica tubes using a silica-based bonding agent. The total empty volume of the microreactor is about 30 μL. The surface of the channels and micropillars were functionalized with ATM by infusing ATM in methanol solution (1.5 μmol, 30 μL) into the microreactor from one connection port followed by evaporation of the solvent under vacuum oven at 50 °C. Figure 1 (c) shows the schematic illustration of ATM cations adsorbed on the surfaces of micropillars for oximation with ketones and aldehydes.
Figure 1.
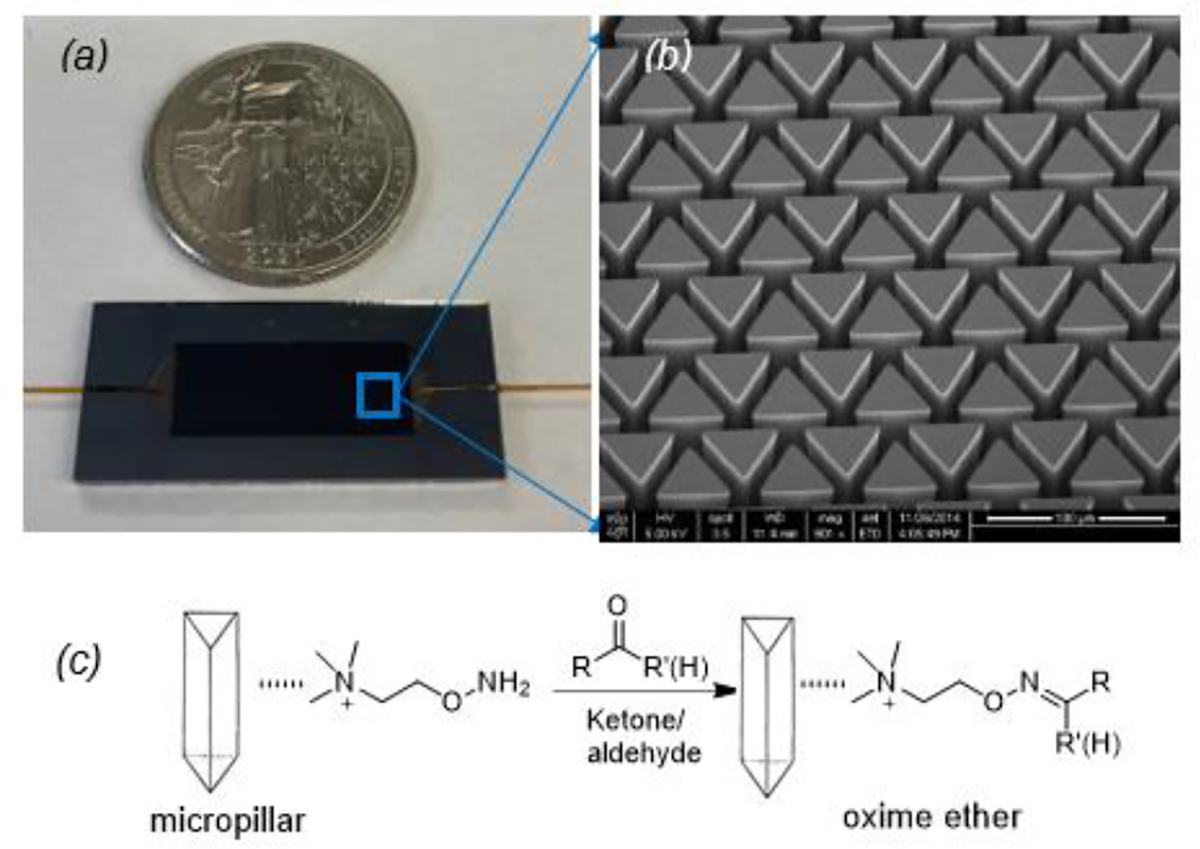
(a) Optical micrograph of a fabricated microreactor compared with a dime coin; (b) an SEM picture of the micropillar array; and (c) a schematic of ATM coated on micropillars and reaction with ketones and aldehydes to form corresponding oxime ether adducts.
Collection of exhaled breath samples
After approval by the Internal Review Board at the University of Louisville and obtained written informed consent, exhaled breath (EB) samples were collected from 20 healthy subjects using Tedlar bags. The Tedlar bags were cleaned with high purity nitrogen before each use to reduce background contamination. A 1L Tedlar bag was connected with a Teflon tube as mouthpiece. Subjects directly blew through the mouthpiece to fill the 1L Tedlar bag in one exhaled breath. A mixture of tidal and alveolar breath was collected. After collection, the gaseous breath in the Tedlar bag was evacuated through the microreactor at a flow rate of 7 mL/min. The setup for the evacuation to capture carbonyl VOCs requires a vacuum pump to pull gaseous breath samples from the Tedlar bag through the ATM-coated micropillar array in the microreactor.29, 30
UHPLC-MS analysis of carbonyl adducts
After the Tedlar bag was completely evacuated, ATM-carbonyl adducts in the microreactor were eluted with 200 μL of methanol from the microreactors. To the eluted sample 5 × 10−9 mol of ATM-acetone-d6 adduct was added as an internal reference (IR). Then the sample was diluted ten times with water for analysis. A Thermo Scientific ultra-high performance liquid chromatography-mass spectrometry (UHPLC-MS) system equipped with an automatic sampler, a Vanquish UHPLC and a Q Exactive Focus Orbitrap Mass Spectrometer (MS) was used for analysis. The UHPLC had an ACQUITY BEH phenyl column (2.1 mm × 100 mm, 1.7 μm, Waters, MA, USA) for separation of ATM-carbonyl adducts. The liquid flow rate through the column was set to 0.2 mL/min. The column temperature was stabilized at 30 °C. The autosampler tray temperature was set at 8 °C. 5 μL of the sample was injected into the column. The mobile phase A was 0.1% formic acid in water and mobile phase B was acetonitrile. The instrument was operated in positive electrospray ionization (ESI) mode with a spray voltage of 3.5 kV. Nitrogen was used as sheath, auxiliary, and sweep gas at flow rates of 49, 12, and 2 (arbitrary units), respectively. Chromatographic separation conditions were set via a gradient elution program: 0–1 min, 0%−10% B; 1–3.5 min, 10%−35% B; 3.5–9 min, 35–50% B; 9.0–9.1 min, 50%−0% B; 9.1–11 min, 0% B. The total program runtime was 11 min. Data acquisition and processing were carried out using Thermo Scientific Xcalibur version 4.4. For MS/MS analyses, parallel reaction monitoring (PRM) method was used by MS.
Method for quantitative analysis and validation
In order to obtain calibration curves, limit of detection (LOD), limit of quantification (LOQ), accuracy, intraday and interday precision for the UHPLC-MS method, different concentrations of eight synthesized ATM adducts in water including ATM-butanal, nonanal, acetone, 2-butanone, hydroxyacetaldehye, hydroxy-2-heptanal, pentenal and 4-HHE were prepared (Figure S1). The coefficients of determination (R2) were between 0.9918 to 0.9997 as shown in Table S1. LOD and LOQ were calculated from the calibration curves using a linear regression model. LOD = 3.3*(Sy/S) and LOQ = 10*(Sy/S), where Sy is the standard deviation of the response and S is the slope of the calibration curve. Sy is acquired by multiplying the square root of sample numbers with a standard error obtained from regression analysis. The precision results were expressed as relative standard deviations (RSD). RSD values ranged from 0.84% to 7.58% for intraday (n=3) and interday (n=3) tests, while accuracy ranged from 96.1% to 103.9%, indicating good study variation (Table S1).
RESULTS AND DISCUSSION
Characterization of carbonyl compound capture efficiencies by the microreactor
Since low molecular weight carbonyl compounds are ubiquitous in ambient air, deuterated ketones and aldehydes were used to characterize carbonyl capture efficiencies by the microreactors. The deuterated compounds were spiked into a 1L Tedlar bag filled with high purity synthetic air and flowed through the microreactors by applying a vacuum.29, 30 The capture efficiencies of deuterated saturated ketones (2-butanone-1,1,1,3,3-d5, and 2-pentanone-1,1,1,3,3-d5) and saturated aldehydes (propionaldehyde-2,2-d2, and butyraldehyde-2,2-d2) were determined. Figure S2 shows the relationship between the capture efficiency and the flow rate of spiked air samples flowing from the Tedlar bag through the microreactor. The capture efficiency decreases with an increase in the flow rate. Also, as the molecular weight of the carbonyl compounds increased, the capture efficiency decreased. The flow rate of the breath samples through the chips were fixed at 7 mL/min in order to achieve above 90% capture efficiencies of volatile carbonyl compounds.
Saturated aldehydes, ketones, hydroxy-aldehydes and hydroxy-ketones in exhaled breath
Our previous work has shown that the silicon microreactor technology for breath analysis can be used for detection of lung cancer by observing unique carbonyl VOC biomarkers.8, 9 However, the FT-ICR MS system used for breath analysis did not have the ability to separate constitutionally isomeric compounds. To enable the separation and identification of isomeric ketones and aldehydes, the UHPLC-MS system was used for analysis of captured carbonyl adducts from breath samples. Exhaled breath samples were collected from twenty subjects using ATM-coated microreactors according to the procedure described in the experimental section. Table 1 lists the study subject information. Fifteen subjects were never-smokers, and the other 5 subjects were current smokers. The effect of smoking on VOCs in exhaled breath is not considered in this study. An important advantage of analysis of ATM-carbonyl adducts from exhaled breath with UHPLC-MS is that there is no need for any further sample preparation and no thermal desorption (as in GC- MS), therefore no concern over thermal degradation. All ATM adducts are positively charged, thus there are no concerns over ionization efficiency. Extracted ion chromatograms were plotted with an isolation window of ±4 ppm. Figure 2 shows the chromatograms of ATM adducts of C5 to C8 saturated ketones and aldehydes in exhaled breath samples in comparison with in- house prepared ATM adducts as standards. The chromatograms showed excellent retention time matches between the breath-derived adducts and the prepared standards. As can be seen, the retention time increases with increasing the number of carbons of the alkyl chain from the same subgroups of aldehydes and ketones, which is important information for identification of other compounds whose adduct standards may not be easily accessible. E, Z isomers were found for the ATM-carbonyl adducts that had more than 6 carbons. Ketones generally eluted before the corresponding isomeric aldehydes.
Table 1.
Study subject information
| All subjects (N=20) | Male (N=10) | Female (N=10) | |
|---|---|---|---|
| Age (years) | 26–75 | 34–59 | 26–75 |
| White race | 16 | 8 | 8 |
| Height (cm) | 150–198 | 173–198 | 150–173 |
| Weight (kg) | 58–118 | 68–118 | 58–102 |
| Current smoker | 5 | 1 | 4 |
Figure 2.
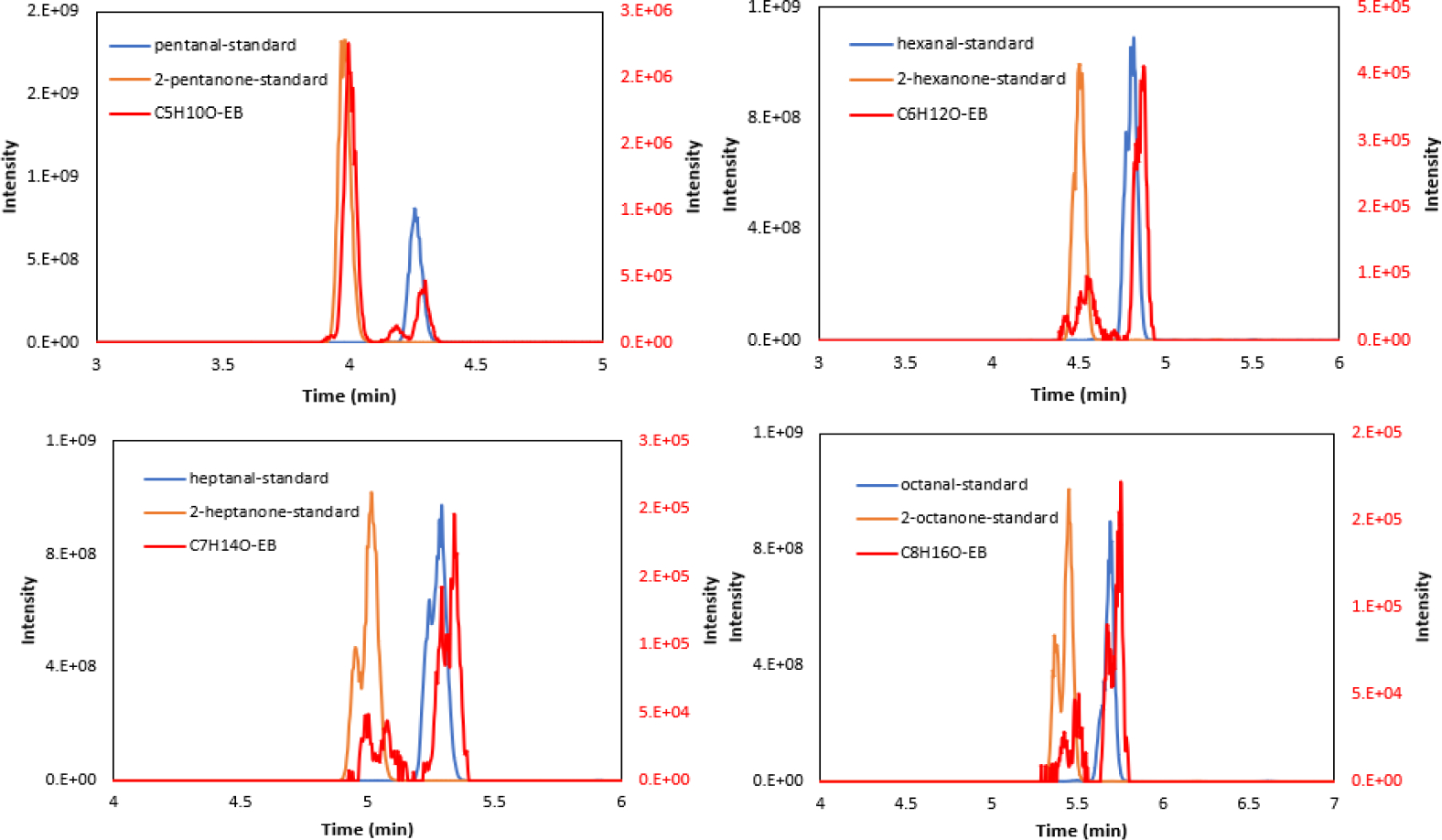
Extracted chromatograms for saturated ketones and aldehydes obtained from ATM-adduct standards of C5 to C8 carbonyls (blue and orange, y-axis on the left) and exhaled breath (EB) samples (red, y-axis on the right) by UHPLC-MS.
Using the ESI positive mode, we were able to detect saturated subgroups of aldehydes (C1 to C12) and ketones (C3 to C8) (CxH2xO), hydroxy-aldehydes (C2 to C10) and hydroxy-ketones (C3 to C16) (CxH2xO2), unsaturated subgroups of 2-alkenals (C3 to C10) (CxH2x-2O), and hydroxy-2-alkenals (C3 to C9) (CxH2x-2O2). Table 2 shows the retention times, concentration ranges, median and mean of four subgroups of saturated carbonyl compounds detected in exhaled breath. It should be noted that the concentrations listed in Table 2 and 3 represent nanomoles of carbonyl compounds in one-liter gaseous breath samples. However, the calibration curves in Fig. S1 and the LOD and LOQ in Table S1 reflect aqueous preparations of purified ATM adduct standards, the latter of which would be free from any potential matrix effect. Volatile carbonyl compounds are often labile, with limited commercial availability and short shelf lives. Therefore, ATM-acetone-d6 was used as IR for quantitation of the detected carbonyl compounds. The quantification was done by multiplying ATM-adduct peak area to the IR peak rata ratio with the amount of added IR. This method is imperfect and can introduce error. Although the absolute intensity of the MS signal of ATM adducts results primarily from the presence of a cationic ammonium headgroup common to all ATM adducts, the compositional differences among carbonyl analytes will introduce some variation in the theoretical curves of MS signal versus concentration. A more accurate quantification should be obtained from calibration curves. We are gratified to see that with just one-liter of exhaled breath collected for each subject, the concentrations of recovered ATM-carbonyl adducts were above the LOD and LOQ in Table S1, validating the reliability of our UHPLC-MS method. Acetone had the highest mean and median concentrations of all carbonyl compounds. Breath acetone is affected by changes in dietary macronutrient composition, caloric restriction, exercise, pulmonary factors, smoking and other assorted factors that increase fat metabolism or inhibit acetone metabolism.37 The concentrations were lower for compounds with longer alkyl chains, which is probably related to their lower volatility and vapor pressure as well as their source abundance decreasing in exhaled breath.38
Table 2.
Concentrations of saturated carbonyl compounds detected in gaseous exhaled breath samples by UHPLC-MSa
| Saturated aldehydes (CxH2xO) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Carbon atoms | m/z | acc. (ppm) | RT (min) | Conc. range (nmol/L) | Conc. median (nmol/L) | Conc. mean (nmol/L) |
|
| ||||||
| 1 | 131.1177 | 1.144 | 1.38 | 0.544–10.054 | 4.740 | 4.484 |
|
| ||||||
| 2 | 145.1333 | 1.516 | 2.06 | 0.104–1.998 | 0.358 | 0.482 |
|
| ||||||
| 3 | 159.1489 | 1.634 | 3.10 | 0.065–0.313 | 0.170 | 0.165 |
|
| ||||||
| 4 | 173.1645 | 1.848 | 3.64 | 0.113–0.323 | 0.206 | 0.203 |
|
| ||||||
| 5 | 187.1802 | 1.710 | 4.23 | 0.050–0.564 | 0.086 | 0.116 |
|
| ||||||
| 6 | 201.1957 | 2.038 | 4.82 | 0.050–0.307 | 0.145 | 0.153 |
|
| ||||||
| 7 | 215.2114 | 1.998 | 5.28/5.33 | 0.019–0.149 | 0.054 | 0.059 |
|
| ||||||
| 8 | 229.2269 | 2.356 | 5.68/5.76 | 0.024–0.187 | 0.075 | 0.076 |
|
| ||||||
| 9 | 243.2426 | 1.932 | 6.12/6.21 | 0.365–2.245 | 0.871 | 0.919 |
|
| ||||||
| 10 | 257.2582 | 2.216 | 6.96/7.17 | 0.014–0.371 | 0.061 | 0.098 |
|
| ||||||
| 11 | 271.2740 | 1.585 | 8.25/8.45 | 0–0.092 | 0.005 | 0.013 |
|
| ||||||
| 12 | 285.2893 | 2.524 | 9.15/9.30 | 0–0.081 | 0.002 | 0.008 |
|
| ||||||
| Saturated ketones (CxH2xO) | ||||||
|
| ||||||
| Carbon atoms | m/z | acc. (ppm) | RT (min) | Conc. range (nmol/L) | Conc. median (nmol/L) | Conc. mean (nmol/L) |
|
| ||||||
| 3 | 159.1489 | 1.634 | 2.99 | 1.512–27.324 | 11.638 | 11.5 |
|
| ||||||
| 4 | 173.1645 | 1.848 | 3.45 | 0.414–3.447 | 1.868 | 1.955 |
|
| ||||||
| 5 | 187.1802 | 1.710 | 3.94 | 0.076–1.403 | 0.886 | 0.845 |
|
| ||||||
| 6 | 201.1957 | 2.038 | 4.47/4.50 | 0.001–0.043 | 0.023 | 0.024 |
|
| ||||||
| 7 | 215.2114 | 1.998 | 4.95/5.02 | 0.003–0.034 | 0.012 | 0.015 |
|
| ||||||
| 8 | 229.2269 | 2.356 | 5.37/5.45 | 0.002–0.016 | 0.009 | 0.009 |
|
| ||||||
| Saturated hydroxy-aldehydes (CxH2xO2) | ||||||
|
| ||||||
| Carbon atoms | m/z | Acc. (ppm) | RT (min) | Conc. range (nmol/L) | Conc. median (nmol/L) | Conc. mean (nmol/L) |
|
| ||||||
| 2 | 161.1281 | 1.986 | 1.29/1.36 | 0.143–0.619 | 0.370 | 0.364 |
|
| ||||||
| 3 | 175.1438 | 1.770 | 1.83/2.14 | 0.006–0.067 | 0.025 | 0.031 |
|
| ||||||
| 4 | 189.1594 | 2.009 | 3.07/3.13 | 0.004–0.045 | 0.018 | 0.019 |
|
| ||||||
| 5 | 203.1751 | 1.624 | 3.32/3.48 | 0.001–0.005 | 0.004 | 0.004 |
|
| ||||||
| 6 | 217.1906 | 2.302 | 3.56/3.84 | 0.002–0.011 | 0.007 | 0.007 |
|
| ||||||
| 7 | 231.2061 | 2.465 | 4.37/4.49 | 0.027–0.093 | 0.033 | 0.038 |
|
| ||||||
| 8 | 245.2218 | 2.365 | 5.00 | 0–0.001 | 0 | 0 |
|
| ||||||
| 9 | 259.2374 | 2.314 | 5.39 | 0–0.003 | 0.001 | 0.001 |
|
| ||||||
| 10 | 273.2529 | 2.818 | 5.75 | 0–0.006 | 0.001 | 0.001 |
| Saturated hydroxy-ketones (CxH2xO2) | ||||||
|
| ||||||
| Carbon atoms | m/z | acc. (ppm) | RT (min) | Conc. range (nmol/L) | Conc. median (nmol/L) | Conc. mean (nmol/L) |
|
| ||||||
| 3 | 175.1438 | 1.770 | 1.42 | 0.022–0.070 | 0.047 | 0.048 |
|
| ||||||
| 4 | 189.1594 | 2.009 | 2.16/2.33 | 0.001–0.312 | 0.035 | 0.059 |
|
| ||||||
| 5 | 203.1751 | 1.624 | 3.04/3.11 | 0.005–0.028 | 0.014 | 0.014 |
|
| ||||||
| 6 | 217.1906 | 2.302 | 3.19/3.27 | 0.004–0.026 | 0.012 | 0.013 |
|
| ||||||
| 7 | 231.2061 | 2.465 | 3.51/3.79 | 0–0.021 | 0.004 | 0.005 |
|
| ||||||
| 8 | 245.2218 | 2.365 | 4.20/4.41 | 0.001–0.014 | 0.006 | 0.007 |
|
| ||||||
| 9 | 259.2374 | 2.314 | 4.59/4.90 | 0.001–0.019 | 0.004 | 0.005 |
|
| ||||||
| 10 | 273.2529 | 2.818 | 4.95/5.11 | 0.004–0.109 | 0.012 | 0.018 |
|
| ||||||
| 11 | 287.2686 | 2.506 | 5.27 | 0.001–0.007 | 0.005 | 0.005 |
|
| ||||||
| 12 | 301.2841 | 2.987 | 5.56 | 0.002–0.035 | 0.021 | 0.022 |
|
| ||||||
| 13 | 315.2997 | 2.918 | 5.91 | 0–0.007 | 0.003 | 0.033 |
|
| ||||||
| 14 | 329.3155 | 2.399 | 6.55 | 0–0.007 | 0.003 | 0.002 |
|
| ||||||
| 15 | - | - | - | - | - | |
|
| ||||||
| 16 | 357.3465 | 2.910 | 10.32 | 0–0.012 | 0.002 | 0.003 |
Table 3.
Concentrations of unsaturated carbonyl compounds detected in gaseous exhaled breath sample by UHPLC-MSa
| 2-alkenals (CxH2x-2O) | ||||||
|---|---|---|---|---|---|---|
| Carbon atoms | m/z | acc. (ppm) | RT (min) | Conc. range (nmol/L) | Conc. median (nmol/L) | Conc. mean (nmol/L) |
| 3 | 157.1333 | 1.527 | 3.07 | 0.003–0.032 | 0.009 | 0.011 |
| 4 | 171.1489 | 1.636 | 3.55/3.63 | 0.002–0.050 | 0.011 | 0.015 |
| 5 | 185.1646 | 1.566 | 4.04/4.16 | 0.007–0.048 | 0.025 | 0.025 |
| 6 | 199.1802 | 1.607 | 4.61/4.76 | 0–0.022 | 0 | 0.003 |
| 7 | 213.1956 | 2.439 | 5.24 | 0–0.004 | 0 | 0.001 |
| 8 | 227.2111 | 2.905 | 5.53/5.67 | 0–0.015 | 0.002 | 0.003 |
| 9 | 241.2269 | 2.280 | 5.96/6.21 | 0.002–0.041 | 0.008 | 0.012 |
| 10 | 255.2424 | 2.899 | 7.23/7.47 | 0–0.001 | 0 | 0 |
| hydroxy-2-alkenals (CxH2x-2O2) | ||||||
| Carbon atoms | m/z | acc. (ppm) | RT (min) | Conc. range (nmol/L) | Conc. median (nmol/L) | Conc. mean (nmol/L) |
| 3 | 173.1282 | 1.532 | 3.01 | 0.003–0.026 | 0.008 | 0.009 |
| 4 | 187.1438 | 1.502 | 3.11 | 0.001–0.011 | 0.005 | 0.006 |
| 5 | 201.1594 | 1.496 | 3.30 | 0.001–0.018 | 0.002 | 0.003 |
| 6 | 215.1749 | 1.988 | 3.45 | 0.028–0.095 | 0.036 | 0.038 |
| 7 | 229.1911 | 2.138 | 3.75 | 0–0.134 | 0.002 | 0.011 |
| 8 | 243.2062 | −0.218 | 4.25 | 0–0.007 | 0 | 0.001 |
| 9 | 257.2218 | 2.220 | 4.91 | 0–0.021 | 0.002 | 0.003 |
MS2 were obtained for m/z features in bold. acc stands for the mass accuracy calculated as the measurement error of the m/z found to the calculated m/z. RT=retention time. See Supporting Information for detailed chromatography and MS2 spectra.
Formaldehyde had the highest level of all detected aldehydes as shown in Table 2. For current cigarette smokers, acetaldehyde levels could be even higher than formaldehyde in exhaled breath because of higher levels of acetaldehyde in cigarette smoke.10 Furthermore, nonanal had the second highest median concentration followed by acetaldehyde, propanal and hexanal. Nonanal and hexanal are produced from peroxidation of unsaturated ω3, ω6 fatty acids.6, 32 Nonanal has been reported as the most abundant aldehyde among the C4 to C10 aldehydes in human breath.39
A review of published data on detected aldehydes indicates that in general the detected aldehyde concentrations in exhaled breath were less than 1 ppb.39 The data in Table 2, however, indicates that formaldehyde, acetaldehyde, propanal, pentanal, hexanal, and nonanal have mean concentrations higher than 1 ppb. The discrepancy may be attributable to the limitations of the methodology used in previous reports. The literature reveals that solid phase microextraction (SPME) with either physical adsorption or chemical reaction was used most often for extraction of aldehydes in exhaled breath and GC-MS was then used for analysis of these compounds.10,11,39,40 Furthermore, the literature on detected saturated ketones in breath, from acetone to nonanone, shows that only 2-butanone, 2-pentanone, and heptanone were above detection limits when using GC coupled to a triple quadrupole mass spectrometer for analysis.40 In contrast, the present method enabled the determination of ketones from acetone to octanone. Many published works indicate that diabetic subjects have much higher acetone levels than healthy controls,1–6 while lung cancer patients have higher levels of 2-butanone and 2-pentanone in their exhaled breath.29, 30, 41 Therefore, quantitative methods for analysis of these saturated ketones and aldehydes in exhaled breath can be expected to facilitate noninvasive detection of the corresponding diseases.
Saturated hydroxy-aldehydes from hydroxy-acetaldehyde (C2) to hydroxy-decanal (C10) and hydroxy-ketones from hydroxy-acetone (C3) to hydroxy-hexadecanone (C16) were detected and their concentration ranges were determined. Hydroxy-acetaldehyde had the highest level of all hydroxy-aldehydes, close to acetaldehyde in exhaled breath. Most of the hydroxy-aldehydes and hydroxy-ketones have concentrations below 1 ppb (or 0.041 nmol/L) except hydroxy-acetaldehyde, hydroxy-acetone and 3-hydroxy-2-butanone. The hydroxy-aldehydes were predicted as arising from peroxidation of unsaturated fatty acids, but no hydroxy-ketone subgroup was similarly predicated from the mechanistic study.32 To date, only hydroxy-acetaldehyde and 3-hydroxy-2-butanone have been reported in exhaled breath.8, 9, 12, 40–42 These two compounds were reported to have higher levels in the exhaled breath of lung cancer patients compared to that in healthy controls.8, 9, 12, 41, 42
Unsaturated aldehydes and hydroxy-aldehydes
Table 3 shows the retention times, concentration ranges, median and mean of 2-alkenal, and unsaturated hydroxy-alkenals detected in exhaled breath. 2-Alkenals from acrolein (C3) to decenal (C10) were detected. Unsaturated hydroxy-aldehydes from hydroxy-propenal or MDA (C3) to 4-hydroxy-2-nonenal (4-HNE) (C9) were also detected. 4-HHE had the highest level among the hydroxy-alkenal subgroup. In general, all 2-alkenals and hydroxy-alkenals have concentrations lower than 1 ppb except 2-pentenal and 4-HHE. 2-Alkenals from C9 to C14 and 4-hydroxy-2-alkenals from C6 to C16 have been reported in exhaled breath condensate samples.31 Both 2-alkenal and 4-hydroxy-2-alkenal subgroups were predicted in exhaled breath from peroxidation of unsaturated fatty acids.40 Acrolein was reported in exhaled breath condensate samples.43, 44 Elevated acrolein and MDA in exhaled breath are important indicators of oxidative stress and inflammation.14–16 Elevated MDA, 4-HHE and 4-HNE in exhaled breath condensates were reported as biomarkers of COPD and lung cancer.43, 44 Reviews of aldehydes in exhaled breath clearly indicate that these compounds are closely related to diseases.6, 7 Thus, quantitative analysis of unsaturated aldehydes in exhaled breath will be critical for developing a diagnostic tool for detection of these diseases.
Structure identification of detected carbonyl subgroup compounds
Rasche et al. introduced fragmentation trees as a method of annotating tandem mass spectra to identify unknown small molecules.45 In the present work, tandem mass spectra were obtained to confirm the identity of the compounds based on both retention times and fragment spectra and were used for generating fragmentation trees for the compounds analyzed.
Heptanal is a possible lipid peroxidation product of both ω−6 and ω −7 fatty acids.6 Several groups have reported heptanal as a biomarker of lung cancer in exhaled breath.10, 11 Figure 3 shows the fragmentation pathway for the m/z feature identified as the ATM adduct of heptanal. To determine which peak registering an m/z of 215.2114 is the ATM adduct of heptanal as opposed to the ATM adduct of 2-heptanone, tandem MS spectra were acquired from breath samples for the m/z 215.2114 peaks and compared with the MS/MS spectra obtained from the corresponding standards. The collision energy of 20 eV was used to perform the tandem MS experiments. Figures 4(a) and 4(b) were obtained from prepared standards of ATM-heptanal and ATM-2-heptanone, respectively. Figures 4(c) and 4(d) were obtained from an exhaled breath sample at m/z of 215.2114 tentatively identified as heptanal and 2-heptanone. ATM-heptanal and ATM-2-heptanone showed very similar fragmentation ions except that fragment ion m/z 104.1070 only arises from the ATM-heptanal adduct. Activation of oxime ethers derived from aldehydes often results in nitrile formation through loss of the aldoxime hydrogen. This elimination reaction to form a nitrile cannot occur with oxime ethers derived from ketones (Scheme S1).46, 47 The same fragment m/z of 104.1070 is also seen for ATM-pentanal, but not for ATM-2-pentanone, which also cannot eliminate in this manner (Fig. S3). Figure S4 shows good matches of retention times and MS2 analysis of ATM-hydroxyacetaldehyde standard and ATM-hydroxyacetaldehyde in exhaled breath samples. Both 2-hydroxy-butanal and 3-hydroxy-2-butanone were detected in exhaled breath samples based on comparison with the retention times of their standards as shown Fig. S5. Further structural confirmation of these two compounds was done by MS2 analysis of ATM adducts of 2-hydroxy-butanal and 3-hydroxy-2-butanone standards and 3-hydroxy-2-butanone in breath samples as shown Fig. S6. The signal of ATM-2-hydroxybutanal in breath sample is too low to perform MS2. The fragment ion at m/z 104.1071 was also observed in both ATM-hydroxyaldehyde (Fig. S4) adducts and hydroxyketone adducts (Fig. S6). The presence of an alpha-hydroxy group in these cases can be expected to facilitate formation of this fragment via oxygen-assisted elimination of acetonitrile (Scheme S2).48 The retention times and MS2 analysis of ATM adducts of pentenal (Fig. S7) and 4-HHE (Fig. S8) likewise show good matches between the standards and breath samples. Most of the detected alkenals and hydroxy-alkenals in exhaled breath samples were confirmed by using their standards with retention times in Table 3 and MS2 analyses. These unsaturated aldehydes were produced from peroxidation of unsaturated fatty acids.6,7,32
Figure 3.
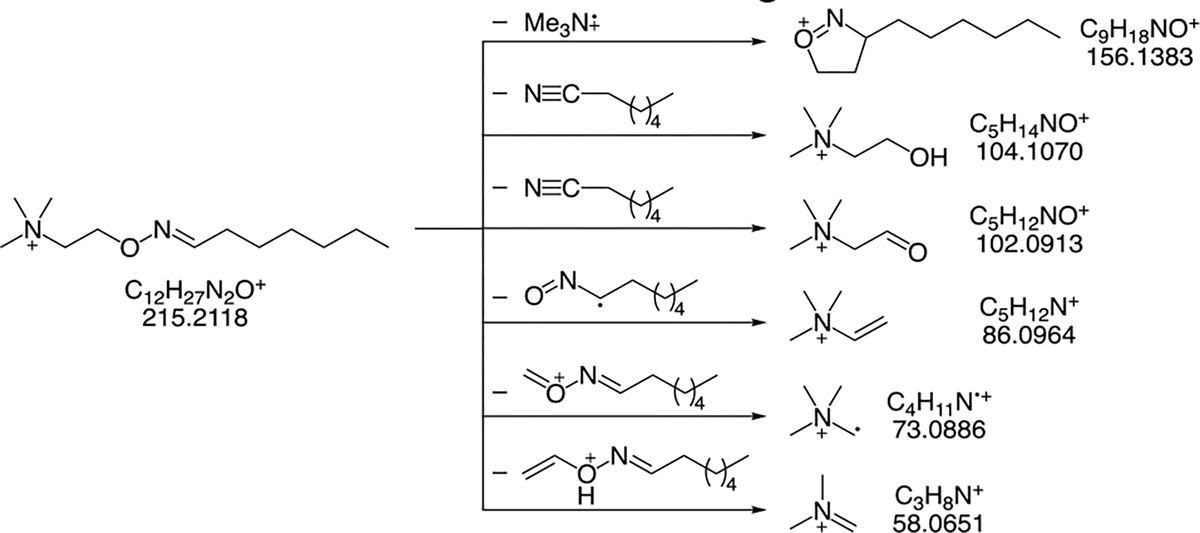
Fragmentation pathways obtained from the MS/MS spectrum of the m/z feature 215.2114, identified as the ATM adduct of heptanal. Exact masses depicted are theoretical values and are within ±0.0015 amu of the observed value for each fragment ion.
Figure 4.
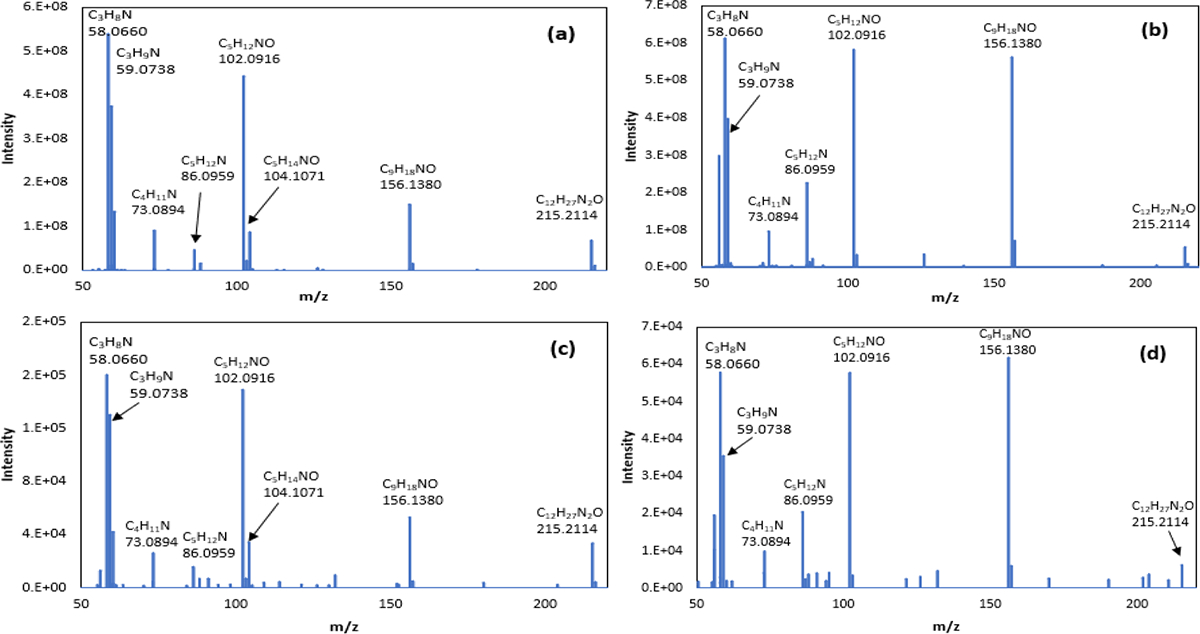
MS/MS spectra obtained from standards (a, b) and from exhaled breath (c, d) for the compounds identified as ATM-heptanal (RT = 5.33 min, Figure 2) and ATM-2-heptanone (RT = 5.02 min, Figure 2), where RT = retention time.
CONCLUSIONS
A MEMS-fabricated silicon microreactor was used to derivatize carbonyl compounds in exhaled breath and UHPLC-MS was used to analyze and identify the adducts. A very broad range of carbonyl compounds including saturated aldehydes, ketones, hydroxy-aldehydes, hydroxy-ketones, and unsaturated aldehydes, and hydroxy-aldehydes were detected. The concentration ranges for these carbonyl sub-types were determined. ATM-derivatized ketone and aldehyde isomers from exhaled breath were separated by UHPLC. Retention times and MS2 spectra of compounds detected in breath samples were compared with standards for compound identification. The MS2 spectra and fragmentation trees were shown to provide valuable complementary information. This approach of combining microreactor technology for capture of carbonyl compounds from exhaled breath and UHPLC-MS for adduct analyses could be a powerful method for quantitative analysis of carbonyl compounds in exhaled breath. The more accurate quantifications might improve breath analysis for detection of lung cancer, COPD, or infectious respiratory diseases.
Supplementary Material
ACKNOWLEDGMENT
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R21CA229057 and the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number U18TR003787. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors gratefully acknowledge Dr. Bradley Hart at Thermo-Fisher Scientific for the generous support of using the Thermo-Fisher UHPLC-MS system for developing the analytical method.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
ATM adduct calibration curves, microreactor flow rate effect, fragmentation scheme, UHPLC chromatogram, and MS2 spectra for standards and exhaled breath samples. The Supporting Information is available free of charge on the ACS Publications website.
REFERENCES
- (1).Amor RE; Nakhleh MK; Barash O; Haick H Breath analysis of cancer in the present and the future. Eur Respir Rev 2019, 28 (152). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Marzorati D; Mainardi L; Sedda G; Gasparri R; Spaggiari L; Cerveri P A review of exhaled breath: a key role in lung cancer diagnosis. J Breath Res 2019, 13 (3). [DOI] [PubMed] [Google Scholar]
- (3).Zhang J; Tian YH; Luo ZW; Qian C; Li WW; Duan YX Breath volatile organic compound analysis: an emerging method for gastric cancer detection. J Breath Res 2021, 15 (4). [DOI] [PubMed] [Google Scholar]
- (4).Hua QL; Zhu YZ; Liu H Detection of volatile organic compounds in exhaled breath to screen lung cancer: a systematic review. Future Oncol 2018, 14 (16), 1647–1662. [DOI] [PubMed] [Google Scholar]
- (5).Jia ZN; Patra A; Kutty VK; Venkatesan T Critical Review of Volatile Organic Compound Analysis in Breath and In Vitro Cell Culture for Detection of Lung Cancer. Metabolites 2019, 9 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Sutaria SR; Gori SS; Morris JD; Xie ZZ; Fu XA; Nantz MH Lipid Peroxidation Produces a Diverse Mixture of Saturated and Unsaturated Aldehydes in Exhaled Breath That Can Serve as Biomarkers of Lung Cancer-A Review. Metabolites 2022, 12 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Floss MA; Fink T; Maurer F; Volk T; Kreuer S; Muller-Wirtz LM Exhaled Aldehydes as Biomarkers for Lung Diseases: A Narrative Review. Molecules 2022, 27 (16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Li MX; Yang DK; Brock G; Knipp RJ; Bousamra M; Nantz MH; Fu XA Breath carbonyl compounds as biomarkers of lung cancer. Lung Cancer 2015, 90 (1), 92–97. [DOI] [PubMed] [Google Scholar]
- (9).Fu XA; Li MX; Knipp RJ; Nantz MH; Bousamra M Noninvasive detection of lung cancer using exhaled breath. Cancer Med-Us 2014, 3 (1), 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Fuchs P; Loeseken C; Schubert JK; Miekisch W Breath gas aldehydes as biomarkers of lung cancer. Int J Cancer 2010, 126 (11), 2663–2670. [DOI] [PubMed] [Google Scholar]
- (11).Poli D; Goldoni M; Corradi M; Acampa O; Carbognani P; Internullo E; Casalini A; Mutti A Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME-GC/MS. J Chromatogr B 2010, 878 (27), 2643–2651. [DOI] [PubMed] [Google Scholar]
- (12).Song G; Qin T; Liu H; Xu GB; Pan YY; Xiong FX; Gu KS; Sun GP; Chen ZD Quantitative breath analysis of volatile organic compounds of lung cancer patients. Lung Cancer 2010, 67 (2), 227–231. [DOI] [PubMed] [Google Scholar]
- (13).Khoubnasabjafari M; Ansarin K; Jouyban A Critical Review of Malondialdehyde Analysis in Biological Samples. Curr Pharm Anal 2016, 12 (1), 4–17. [Google Scholar]
- (14).Antus B; Harnasi G; Drozdovszky O; Barta I Monitoring oxidative stress during chronic obstructive pulmonary disease exacerbations using malondialdehyde. Respirology 2014, 19, 74–79. [DOI] [PubMed] [Google Scholar]
- (15).Casimirri E; Stendardo M; Bonci M; Andreoli R; Bottazzi B; Leone R; Schito M; Vaccari A; Papi A; Contoli M; et al. Biomarkers of oxidative-stress and inflammation in exhaled breath condensate from hospital cleaners. Biomarkers 2016, 21 (2), 115–122. [DOI] [PubMed] [Google Scholar]
- (16).Kartavenka K; Panuwet P; Greenwald R; Ehret KM; D’Souza PE; Barr DB; Ryan PB Quantification of malondialdehyde in exhaled breath condensate using pseudo two-dimensional ultra-performance liquid chromatography coupled with single quadrupole mass spectrometry. J Chromatogr B 2019, 1105, 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Ilhan N; Halifeoglu I; Ozercan HI; Ilhan N Tissue malondialdehyde and adenosine triphosphatase level after experimental liver ischaemia-reperfusion damage. Cell Biochem Funct 2001, 19 (3), 207–212. [DOI] [PubMed] [Google Scholar]
- (18).Stalikas CD; Konidari CN Analysis of malondialdehyde in biological matrices by capillary gas chromatography with electron-capture detection and mass spectrometry. Anal Biochem 2001, 290 (1), 108–115. [DOI] [PubMed] [Google Scholar]
- (19).Ozaras R; Tahan V; Turkmen S; Talay F; Besirli K; Aydin S; Uzun H; Cetinkaya A Changes in malondialdehyde levels in bronchoalveolar fluid and serum by the treatment of asthma with inhaled steroid and beta2-agonist. Respirology 2000, 5 (3), 289–292, [DOI] [PubMed] [Google Scholar]
- (20).Lovell MA; Xie CS; Markesbery WR Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging 2001, 22 (2), 187–194. [DOI] [PubMed] [Google Scholar]
- (21).Zhou JM; Huang ZA; Kumar U; Chen DDY Review of recent developments in determining volatile organic compounds in exhaled breath as biomarkers for lung cancer diagnosis. Anal Chim Acta 2017, 996, 1–9. [DOI] [PubMed] [Google Scholar]
- (22).Dent AG; Sutedja TG; Zimmerman PV Exhaled breath analysis for lung cancer. J Thorac Dis 2013, 5, S540–S550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Kim KH; Jahan SA; Kabir E A review of breath analysis for diagnosis of human health. Trac-Trend Anal Chem 2012, 33, 1–8. [Google Scholar]
- (24).Ibrahim B; Basanta M; Cadden P; Singh D; Douce D; Woodcock A; Fowler SJ Non-invasive phenotyping using exhaled volatile organic compounds in asthma. Thorax 2011, 66 (9), 804–809. [DOI] [PubMed] [Google Scholar]
- (25).Spanel P; Smith D Selected ion flow tube mass spectrometry for on-line trace gas analysis in biology and medicine. Eur J Mass Spectrom 2007, 13 (1), 77–82. [DOI] [PubMed] [Google Scholar]
- (26).Amann A; Poupart G; Telser S; Ledochowski M; Schmid A; Mechtcheriakov S Applications of breath gas analysis in medicine. Int J Mass Spectrom 2004, 239 (2–3), 227–233. [Google Scholar]
- (27).Ruzsanyi VB, Analysis JI of human breath using IMS. Int. J. Ion Mobility Spectrom 2005, (1), 4. [Google Scholar]
- (28).Xie ZZ; Raju MVR; Stewart AC; Nantz MH; Fu XA Imparting sensitivity and selectivity to a gold nanoparticle chemiresistor through thiol monolayer functionalization for sensing acetone. Rsc Adv 2018, 8 (62), 35618–35624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Fu XA; Li MX; Biswas S; Nantz MH; Higashi RM A novel microreactor approach for analysis of ketones and aldehydes in breath. Analyst 2011, 136 (22), 4662–4666. [DOI] [PubMed] [Google Scholar]
- (30).Li M; Biswas S; Nantz MH; Higashi RM; Fu XA Preconcentration and analysis of trace volatile carbonyl compounds. Analytical chemistry 2012, 84 (3), 1288–1293, WorldCat.org. [DOI] [PubMed] [Google Scholar]
- (31).Garcia-Gomez D; Sinues PML; Barrios-Collado C; Vidal-de-Miguel G; Gaugg M; Zenobi R Identification of 2-Alkenals, 4-Hydroxy-2-alkenals, and 4-Hydroxy-2,6-alkadienals in Exhaled Breath Condensate by UHPLC-HRMS and in Breath by Real-Time HRMS. Analytical Chemistry 2015, 87 (5), 3087–3093. [DOI] [PubMed] [Google Scholar]
- (32).Ratcliffe N; Wieczorek T; Drabinska N; Gould O; Osborne A; Costello BL A mechanistic study and review of volatile products from peroxidation of unsaturated fatty acids: an aid to understanding the origins of volatile organic compounds from the human body. J Breath Res 2020, 14 (3). [DOI] [PubMed] [Google Scholar]
- (33).Bohman B; Flematti GR; Unelius CR Practical one-pot stereospecific preparation of vicinal and 1,3-diols. Tetrahedron Lett 2017, 58 (1), 75–77. [Google Scholar]
- (34).Keck GE; Palani A; Mchardy SF Total Synthesis of (+)-Carbonolide-B. J Org Chem 1994, 59 (11), 3113–3122. [Google Scholar]
- (35).Biswas S; Huang X; Badger WR; Nantz MH Nucleophilic cationization reagents. Tetrahedron Lett 2010, 51 (13), 1727–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Pereira R; Wolstenhulme J; Sandford G; Claridge TDW; Gouverneur V; Cvengros J Synthesis and characterization of a novel N-F reagent derived from the ethano-Troger’s base: (1)J(FN) coupling constants as a signature for the N-F bond. Chem Commun 2016, 52 (8), 1606–1609. [DOI] [PubMed] [Google Scholar]
- (37).Anderson JC Measuring Breath Acetone for Monitoring Fat Loss: Review. Obesity 2015, 23 (12), 2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Gaugg MT; Bruderer T; Nowak N; Eiffert L; Martinez-Lozano Sinues P; Kohler M; Zenobi R Mass-Spectrometric Detection of Omega-Oxidation Products of Aliphatic Fatty Acids in Exhaled Breath. Anal Chem 2017, 89 (19), 10329–10334, [DOI] [PubMed] [Google Scholar]
- (39).McCartney MM; Thompson CJ; Klein LR; Ngo JH; Seibel JD; Fabia F; Simms LA; Borras E; Young BS; Lara J; et al. Breath carbonyl levels in a human population of seven hundred participants. J Breath Res 2020, 14 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Lomonaco T; Romani A; Ghimenti S; Biagini D; Bellagambi FG; Onor M; Salvo P; Fuoco R; Di Francesco F Determination of carbonyl compounds in exhaled breath by on-sorbent derivatization coupled with thermal desorption and gas chromatography-tandem mass spectrometry. J Breath Res 2018, 12 (4). [DOI] [PubMed] [Google Scholar]
- (41).Wang PY; Huang Q; Meng SS; Mu T; Liu Z; He MQ; Li QY; Zhao S; Wang SD; Qiu MT Identification of lung cancer breath biomarkers based on perioperative breathomics testing: A prospective observational study. Eclinicalmedicine 2022, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Bajtarevic A; Ager C; Pienz M; Klieber M; Schwarz K; Ligor M; Ligor T; Filipiak W; Denz H; Fiegl M; et al. Noninvasive detection of lung cancer by analysis of exhaled breath. Bmc Cancer 2009, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Corradi M; Pignatti P; Manini P; Andreoli R; Goldoni M; Poppa M; Moscato G; Balbi B; Mutti A Comparison between exhaled and sputum oxidative stress biomarkers in chronic airway inflammation. Eur Respir J 2004, 24 (6), 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Andreoli R; Manini P; Corradi M; Mutti A; Niessen WMA Determination of patterns of biologically relevant aldehydes in exhaled breath condensate of healthy subjects by liquid chromatography/atmospheric chemical ionization tandem mass spectrometry. Rapid Commun Mass Sp 2003, 17 (7), 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Rasche F; Svatos A; Maddula RK; Bottcher C; Bocker S Computing fragmentation trees from tandem mass spectrometry data. Anal Chem 2011, 83 (4), 1243–1251, [DOI] [PubMed] [Google Scholar]
- (46).Asano Y; Kato Y Z-phenylacetaldoxime degradation by a novel aldoxime dehydratase from Bacillus sp. strain OxB-1. Fems Microbiol Lett 1998, 158 (2), 185–190. [Google Scholar]
- (47).Betke T; Higuchi J; Rommelmann P; Oike K; Nomura T; Kato Y; Asano Y; Groger H Biocatalytic Synthesis of Nitriles through Dehydration of Aldoximes: The Substrate Scope of Aldoxime Dehydratases. Chembiochem 2018, 19 (8), 768–779. [DOI] [PubMed] [Google Scholar]
- (48).Laulhe S; Bogdanov B; Johannes LM; Gutierrez O; Harrison JG; Tantillo DJ; Zhang X; Nantz MH Fragmentation of oxime and silyl oxime ether odd-electron positive ions by the McLafferty rearrangement: new insights on structural factors that promote alpha,beta fragmentation. J Mass Spectrom 2012,47, 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


