Abstract
Vibrio mimicus caused a seafood-associated outbreak in Florida, USA, in which 4 of 6 case-patients were hospitalized; 1 required intensive care for severe diarrhea. Strains were ctx-negative but carried genes for other virulence determinants (hemolysin, proteases, and types I–IV and VI secretion systems). Cholera toxin–negative bacterial strains can cause cholera-like disease.
Keywords: Vibrio mimicus, seafood, diarrhea, food safety, bacteria, enteric infections, Florida, United States
Vibrio mimicus, named because of its close metabolic and genetic similarity to V. cholerae, is recognized globally as a cause of foodborne and waterborne diarrheal disease (1–4). Limited data indicate that V. mimicus incidence is lower than that reported for V. parahaemolyticus and non-O1/non-O139 V. cholerae but comparable to that of V. fluvialis (3,4). Although some V. mimicus strains produce cholera toxin (CTX) or a cholera-like toxin or have genes from the ctx complex, most do not (1,5). Nonetheless, V. mimicus can cause severe, cholera-like illness; the hospitalization rate among case-patients reported in 2014 (the most recent year for which data are available) to the Centers for Disease Control and Prevention is 57% (3). We report a seafood-associated outbreak caused by V. mimicus in Florida, USA, in which 4 of 6 patients required hospitalization.
The Study
In June 2019, the Florida Department of Health in Alachua County (DOH-Alachua; Gainesville, FL, USA) received reports of multiple cases of diarrheal illnesses associated with eating at a local seafood restaurant. Six case-patients were subsequently identified who met the case definition of having eaten seafood at the implicated restaurant within a 2-day time window and who experienced acute onset of diarrhea within 96 hours of the reported meal or had a clinical diagnosis of vibriosis. Median case-patient age was 35.5 years; median incubation period was 24 (range 7–32) hours. All 6 case-patients had diarrhea; other signs/symptoms were vomiting (6 case-patients), headache (3 case-patients), and nausea (3 case-patients). Four patients were hospitalized, 3 at the University of Florida Heath Science Center (UFHealth; Gainesville). Among the UFHealth patients, 1 was admitted to the intensive care unit because of a diarrheal purge of 7–8 L. That patient was 39 years of age, had a history of hypertension and type 2 diabetes mellitus, and fully recovered after treatment with volume repletion and 4 days of doxycycline and ceftriaxone.
DOH-Alachua determined that the foods most commonly consumed by case-patients were steamed blue crab (5 case-patients), steamed snow crab (5 case-patients), and steamed shrimp (4 case-patients). Only 1 case-patient reported eating oysters. A joint environmental health assessment by DOH-Alachua, the Florida DOH regional environmental epidemiologist, and the Florida Department of Business and Professional Regulation documented multiple food safety violations (i.e., substantive overall sanitation issues, thawing frozen shrimp overnight at room temperature, returning cooked crabs to crates that previously held live crabs), and a lack of required state-approved employee education.
Fecal samples from the patients hospitalized at UFHealth were initially screened by using a culture-independent diagnostic PCR technique (BioFire FilmArray GI Panel; BioFire Diagnostics, https://www.biofiredx.com), which indicated possible V. cholerae and non-cholerae Vibrio spp. In follow-up studies at the University of Florida Emerging Pathogens Institute, fecal samples were plated on thiosulfate citrate bile-salts sucrose agar after enrichment in alkaline peptone water (pH 8.5) (Appendix). Green colonies, reflecting the lack of sucrose fermentation, characteristic of V. mimicus, grew from 2 of 3 samples. We confirmed that the isolates were V. mimicus by using convergent PCR primers (6); we designated the strains as D461B_US_2019 and E3_US_2019. Isolates were susceptible to doxycycline, azithromycin, ciprofloxacin, and ceftriaxone (Appendix Table 1).
Neither strain carried genes encoding CTX (ctxA or ctxB) or other accessory genes linked to the CTX phage. However, both strains elicited strong hemolysis/hemolytic activity against sheep blood as measured by standard assays (Appendix Figure 1). Strains also exhibited protease activity, motility, and biofilm formation (Appendix Figures 2, 3). Whole-genome sequencing and bioinformatic analysis detected genes for a variety of possible virulence determinants, including hemolysin, various proteases, and types I–IV and VI secretion systems (Appendix Table 2). Strains carried both a heat stable and heat labile toxin, which showed >98% nucleotide and amino acid identity to similar genes in a V. mimicus strain (SCCF01) that was hypervirulent for freshwater catfish in China (7). In an earlier genomic study of 2 ctx-negative V. mimicus strains, Hasan et al. (1) reported the presence of genetic elements of the pre-CTX prophage (including ace and zot, implicated as contributors to diarrhea associated with CTX-negative V. cholerae strains [8]) and the VSP-II pathogenicity island, neither of which were present in our Florida V. mimicus strains. They also noted a gene cluster similar to that of the V. cholerae VPI-2 pathogenicity island; we found an identical gene cluster in our strains.
In a phylogenetic analysis based on the core genome (9) (Appendix), the 2 Florida strains clustered within a single, well-supported clade consistent with their high similarity, indicating a very recent common origin (Figure). Although the maximum-likelihood tree of available V. mimicus sequences shows several independent lineages of strains sampled worldwide with no apparent geographic structure, the closest lineages to the Florida strains were clinical isolates from the United Kingdom, as well as clinical and environmental strains from the United States, albeit with no strong bootstrap support.
Figure.
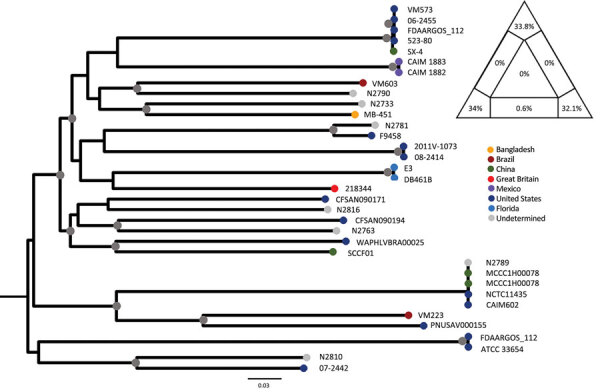
Maximum-likelihood tree of Vibrio mimicus strains. The tree was calculated by using 35 sequences; the best evolutionary model was selected by Bayesian information criterion. Nodes marked by a gray circle have bootstrap support (1,000 replicates) >90%. Scale bar represents the number of substitutions per site. The phylogenetic signal of the alignment was determined by likelihood mapping, as shown by the triangle in the top right corner. Likelihood mapping method is based on the analysis of maximum likelihoods for quartets of sequences randomly extracted from the alignment. There are only 3 possible fully resolved tree topologies deriving from 4 sequences (e.g., with 4 sequences named A,B,C, and D, sequence A can cluster with B and C with D; or A with C and D with B; or A with D and B with C); alternatively, if there is not enough information in the sequences, the result will be a star-like topology. The likelihoods of the 3 possible topologies are represented as 1 point in an equilateral triangle, in which each vertex represents 1 of the possible topologies. The triangle is partitioned in 7 regions: the region in the center represents completely unresolved quartets with star-like evolution topology; the 3 regions in the corners represent well-resolved topologies; the regions along the sides represent the situation in which it is difficult to decide between 2 of the 3 possible topologies. Percentages in the triangle reflect the number of quartets assigned to each region. A higher sum of the percentages in the triangle corners (completely resolved quartets) indicates a higher phylogenetic signal contained in the alignment. The alignment signal of >99% therefore shows a strong phylogenetic signal, which enables reliable calculation of the tree.
Conclusions
V. mimicus is present either as a free-living microorganism or in biofilm in the aquatic/estuarine environment; infection is strongly associated with seafood consumption (4,10,11). For this outbreak, we could not identify a single source for the infection, given that case-patients had eaten a variety of seafoods and there were sufficient breakdowns in food safety practices within the kitchen to make cross-contamination of multiple foods highly likely. Although oysters have been implicated as a V. mimicus source, only 1 case-patient reported eating oysters, making oysters an unlikely primary source of infection. We also note the risk inherent in placing cooked seafood back into containers in which live seafood has been shipped, a practice linked with previous V. mimicus and other Vibrio spp. outbreaks (12).
Most hospitals and commercial laboratories have now moved to use of culture-independent diagnostic techniques, resulting in a strikingly increased number of cases of Vibrio-associated infections reported in the United States (13). The outbreak reported here highlights the utility of such techniques for providing a quick, preliminary diagnosis of a possible Vibrio infection as well as problems with such systems. Exact isolate speciation was not possible with the system used by UFHealth, and speciation subsequently required careful classical microbiological approaches (with enrichment procedures) to isolate and identify the organism, without which this outbreak caused by a less common Vibrio species would almost certainly have been missed.
V. mimicus evolved from a common V. cholerae ancestor with a prototypic sixth pandemic genomic backbone (1). Although most V. mimicus strains have not retained the ctx genes (the primary virulence factor responsible for the severe diarrhea seen in cholera), the 2 strains isolated in this outbreak carried a wide variety of potential virulence determinants, showing varying degrees of similarity with potential virulence factors reported for other V. mimicus strains (1,7). Common to many other Vibrio species (including V. cholerae), V. mimicus has a superintegron (1,14), which can serve as a capture system for acquiring DNA from the surrounding environment and may have contributed to the accumulation of virulence genes. We did not see evidence of distinct clinical and environmental lineages (associated with V. cholerae and V. parahaemolyticus), underscoring the idea that most V. mimicus strains, regardless of origin, have the potential for carrying genes capable of causing illness in humans.
Although incidence data for V. mimicus are limited, partially because of diagnostic difficulties, Vibrio case numbers are clearly increasing with rising surface water temperatures (13,15,16). Thus, diagnostic capabilities for V. mimicus and other Vibrio species need to be enhanced. There also needs to be recognition that strains that do not carry cholera toxin can cause cholera-like disease and that further work is needed to more clearly identify the pathogenic mechanisms by which such strains cause illness.
Supplemental information for study of seafood-associated outbreak of ctx-negative Vibrio mimicus causing cholera-like illness, Florida, USA.
Acknowledgments
Laboratory studies were funded through internal funding sources at the University of Florida and the University of Florida Emerging Pathogens Institute.
Biography
Dr. Alam is an investigator in the Cholera Laboratory at the University of Florida Emerging Pathogens Institute, where he oversees studies of V. cholerae and other Vibrio species, including recent studies in Haiti and the Democratic Republic of the Congo. Dr. Stern is a resident physician at Atrium Health/Wake Forest Baptist and Wake Forest University School of Medicine, in Wake Forest, North Carolina.
Footnotes
Suggested citation for this article: Alam MT, Stern SR, Frison D, Taylor K, Tagliamonte MS, Nazmus S, et al. Seafood-associated outbreak of ctx-negative Vibrio mimicus causing cholera-like illness, Florida, USA. Emerg Infect Dis. 2023 Oct [date cited]. https://doi.org/10.3201/eid2910.230486
These first authors contributed equally to this article.
These senior authors contributed equally to this article.
References
- 1.Hasan NA, Grim CJ, Haley BJ, Chun J, Alam M, Taviani E, et al. Comparative genomics of clinical and environmental Vibrio mimicus. Proc Natl Acad Sci U S A. 2010;107:21134–9. 10.1073/pnas.1013825107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chitov T, Kirikaew P, Yungyune P, Ruengprapan N, Sontikun K. An incidence of large foodborne outbreak associated with Vibrio mimicus. Eur J Clin Microbiol Infect Dis. 2009;28:421–4. 10.1007/s10096-008-0639-7 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Enteric Disease Surveillance: COVIS Annual Summary, 2014. [cited 2022 Jul 25]. https://www.cdc.gov/nationalsurveillance/pdfs/covis-annual-summary-2014-508c.pdf
- 4.Louisiana Office of Public Health. Vibrios [cited 2022 Jul 27]. https://ldh.la.gov/assets/oph/Center-PHCH/Center-CH/infectious-epi/Annuals/Vibrios_LaIDAnnual.pdf
- 5.Shinoda S, Nakagawa T, Shi L, Bi K, Kanoh Y, Tomochika K, et al. Distribution of virulence-associated genes in Vibrio mimicus isolates from clinical and environmental origins. Microbiol Immunol. 2004;48:547–51. 10.1111/j.1348-0421.2004.tb03551.x [DOI] [PubMed] [Google Scholar]
- 6.Kim H-J, Ryu J-O, Lee S-Y, Kim E-S, Kim H-Y. Multiplex PCR for detection of the Vibrio genus and five pathogenic Vibrio species with primer sets designed using comparative genomics. BMC Microbiol. 2015;15:239. 10.1186/s12866-015-0577-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Z, Wang E, Geng Y, Wang K, Chen D, Huang X, et al. Complete genome analysis of Vibrio mimicus strain SCCF01, a highly virulent isolate from the freshwater catfish. Virulence. 2020;11:23–31. 10.1080/21505594.2019.1702797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trucksis M, Galen JE, Michalski J, Fasano A, Kaper JB. Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette. Proc Natl Acad Sci U S A. 1993;90:5267–71. 10.1073/pnas.90.11.5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guardiola-Avila I, Sánchez-Busó L, Acedo-Félix E, Gomez-Gil B, Zúñiga-Cabrera M, González-Candelas F, et al. Core and accessory genome analysis of Vibrio mimicus. Microorganisms. 2021;9:191. 10.3390/microorganisms9010191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abioye OE, Osunla AC, Okoh AI. Molecular detection and distribution of six medically important Vibrio spp. in selected freshwater and brackish water resources in Eastern Cape Province, South Africa. Front Microbiol. 2021;12:617703. 10.3389/fmicb.2021.617703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Álvarez-Contreras AK, Quiñones-Ramírez EI, Vázquez-Salinas C. Prevalence, detection of virulence genes and antimicrobial susceptibility of pathogen Vibrio species isolated from different types of seafood samples at “La Nueva Viga” market in Mexico City. Antonie van Leeuwenhoek. 2021;114:1417–29. 10.1007/s10482-021-01591-x [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC). Notes from the field: Vibrio mimicus infection from consuming crayfish --- Spokane, Washington, June 2010. MMWR Morb Mortal Wkly Rep. 2010;59:1374. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. FoodNet Fast [cited 2022 Jul 25]. https://wwwn.cdc.gov/foodnetfast
- 14.Mazel D, Dychinco B, Webb VA, Davies J. A distinctive class of integron in the Vibrio cholerae genome. Science. 1998;280:605–8. 10.1126/science.280.5363.605 [DOI] [PubMed] [Google Scholar]
- 15.Trinanes J, Martinez-Urtaza J. Future scenarios of risk of Vibrio infections in a warming planet: a global mapping study. Lancet Planet Health. 2021;5:e426–35. 10.1016/S2542-5196(21)00169-8 [DOI] [PubMed] [Google Scholar]
- 16.Brehm TT, Berneking L, Sena Martins M, Dupke S, Jacob D, Drechsel O, et al. ; German Vibrio Study Group. Heatwave-associated Vibrio infections in Germany, 2018 and 2019. Euro Surveill. 2021;26:2002041. 10.2807/1560-7917.ES.2021.26.41.2002041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental information for study of seafood-associated outbreak of ctx-negative Vibrio mimicus causing cholera-like illness, Florida, USA.


