ABSTRACT
Superficial angiomyxoma is an extremely rare subcutaneously placed myxoid soft tissue neoplasm. There are few case reports with fine needle aspiration cytological and histopathological findings available for this tumor because of its rarity. Here, we describe a case of superficial angiomyxoma in a 24-year-old girl who had a solitary left ear pinna mass without a Carney's complex at the time of presentation or at the end of two years of follow-up next to the surgical removal of the tumor. The clinical, cytomorphological, and histological findings, together with the immunohistochemical markers, in a case of superficial angiomyxoma are described in this rare case report for the first time in the English literature.
Keywords: Superficial angiomyxoma, Ear pinna mass, Fine needle aspiration cytology, Squash smear cytology
INTRODUCTION
Angiomyxomas are a subclass of myxoid mesenchymal tumors that are relatively uncommon and are distinguished by their propensity for numerous local recurrences and lack of metastatic potential. Aggressive, superficial, and angiomyofibroblastoma angiomyxomas are the three categories that are recognized. The initial description of superficial angiomyxoma was made in 1988 by Allen et al. [1]. A superficial angiomyxoma is an uncommon, benign, solitary, cutaneous myxoid soft tissue tumor that affects adults and adolescents. It typically appears as papules, nodules, or polypoid lesions on the head, neck, and trunk. After inadequate excision, they tend to recur locally [2]. In the current case study, we describe the clinical, morphological, and immunohistochemical characteristics of a solitary superficial angiomyxoma that developed in the ear pinna without showing signs of Carney's complex and that remained free of recurrence for two years after the tumor was surgically removed.
CASE PRESENTATION
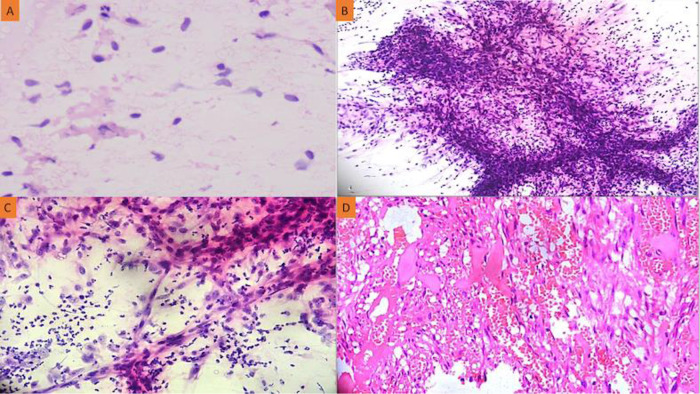
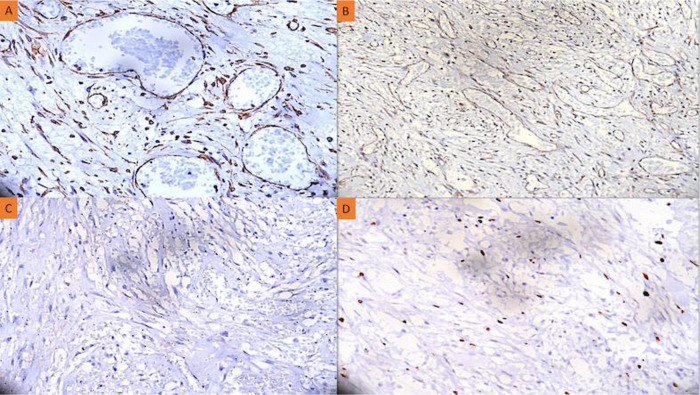
Here, we report a case of a 24-year-old girl who complained of a red, globular lesion on her left ear pinna over a period of two years (Figure 1). It did not cause any pain as it grew to its current size gradually. There was no prior history of injury or further skin lesions. The patient was instructed to undergo fine-needle aspiration cytology (FNAC). The cytology smears were moderately cellular and revealed isolated plump spindle-shaped and stellate cells on a fibrillary myxoid background. These spindle cells have elongated, bland nuclei and a moderate amount of cytoplasm (Figure 2A). There were no mitotic figures or necrosis discernible in aspirate smears. The following differentials were considered: (1) mesenchymal neoplasm with myxoid differentiation; and (2) adnexal tumor with myxoid differentiation based on FNAC smear findings. A squash smear cytology was requested by the operating surgeon because of the diagnostic dilemma on cytomorphology. The squash material was not easily squashable, and smears revealed a cellular spindle cell neoplasm composed of minimally anisomorphic cells having oval to elongated nuclei with a moderate amount of cytoplasm on a myxoid background with numerous neutrophils (Figures 2B and C). There was no necrosis or mitotic figure visible. A provisional diagnosis of a benign spindle cell lesion was rendered based on squash smear cytology. A wide local excision of the left ear pinna tumor was done, and the specimen was sent for histopathological examination and immunohistochemistry. The hematoxylin and eosin (H-E) stained section underwent histopathological analysis, which revealed variable-sized congested blood vessels with scattered interspersed bland spindle and stellate-shaped cells proliferation within a scanty fibromyxoid stroma having scattered acute inflammatory cells infiltrate (Figure 2D). Nuclear hyperchromasia, mitosis, or necrosis were not discernible. The stromal tumor cells showed positive expression for CD34 and vimentin. There was no expression noted for S100, smooth muscle actin (SMA), muscle-specific antigen (MSA), or desmin. The Ki-67 proliferation index was ∼4% among the tumor cells (Figure 3A–D). A definitive diagnosis of superficial angiomyxoma was rendered based on clinical presentation, cytology, histomorphology, and immunohistochemistry. Carney's complex was ruled out because there were no pigmented skin lesions, nevus, schwannomas, or myxomas elsewhere. The tests for endocrine overactivity, such as levels of growth hormone, prolactin, insulin-like growth factor, cortisol, and the response of urinary glucocorticosteroids to dexamethasone administration, excluded a relationship with Carney's complex. Ultrasonography and echocardiography were also performed to identify the possibility of a breast myxoma, thyroid lesion, or cardiac myxoma. However, the findings ruled out a link with Carney's complex. The patient was kept in regular follow-up for two years, and there has been no recurrence noted to date.
Fig. 1.
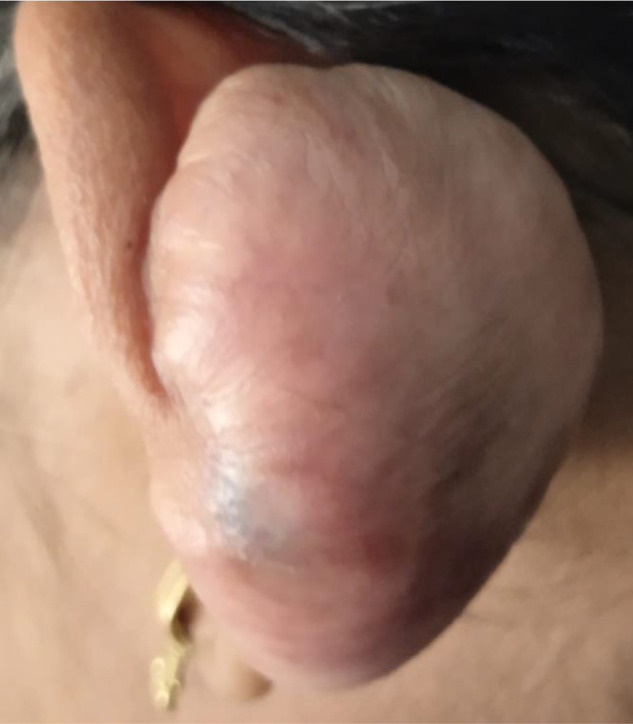
Clinical photograph of the patient displaying a tumor protruding from the ear pinna.
Fig. 2.
(A) A fine needle aspiration cytology smear shows bland spindle-shaped and stellate cells on a fibrillary myxoid background (Giemsa, x400); (B) Squash cytology showing cellular smears comprising of fragments of spindle-shaped tumor cells on a myxoid background (Giemsa, x100); (C) Scattered bland spindle cells displaying elongated nuclei and a moderate amount of cytoplasm with numerous neutrophils in the background (Giemsa, x400); (D) High-power view of the tumor displaying numerous congested vascular channels and interspersed spindle-shaped and stellate cells with minimal inflammatory cell infiltrate (HE, x400).
Fig. 3.
(A) Immunohistochemistry showing positive expression for CD34 (anti-CD34 Ab, x400); (B) Positive expression of vimentin in the tumor cells (anti-Vimentin Ab, x400); (C) No expression was seen for S100 by the tumor cells (anti-S100 Ab, x400); (D) The Ki-67 proliferation index was ∼4% (anti-Ki67 Ab, x400).
DISCUSSION
A superficial angiomyxoma was initially described by Carney et al. in 1985 [3]. Myxomas, patchy pigmentation, endocrine overactivity, and schwannomas are all symptoms of Carney's complex, an autosomal dominant familial illness. Multiple superficial angiomyxomas, particularly those over the ear pinna, together with signs of endocrine overactivity, can be considered when diagnosing a Carney complex [4]. In the current case, the patient had a solitary subcutaneous lump of the left ear pinna without any other syndromic symptoms linked to Carney's complex. In the literature, only a few cases of solitary superficial angiomyxoma without the presence of Carney's complex have been documented, including those reported by Rosado et al. in the parotid, Kozo et al. in the inguinal area, and Mahendra et al. in the toe [5-7]. However, only a couple of cases of superficial angiomyxoma of the ear pinna have been described in the literature, and none have demonstrated the complete assimilation of clinical findings, cytomorphological features, histomorphology, and immunohistochemistry characteristics [8]. Males are more likely to develop superficial angiomyxoma in their third or fourth decade [9]. In order to distinguish it from aggressive angiomyxoma, the term superficial angiomyxoma was employed. Aggressive angiomyxomas differ from superficial angiomyxomas by appearing in deeper sites and as bigger nodules in the genital, pelvic, and perineal regions [10]. Patients with superficial angiomyxoma typically have asymptomatic pink-colored, fleshy lesions that tend to appear on the neck, head, and lower limbs. In this particular case, a solitary subcutaneous mass was observed on the ear pinna, an exceedingly rare location for such neoplasms.
A superficial angiomyxoma is a dermal-based lesion with a clearly defined border and subcutaneous involvement. The tumor is multilobulated and made up of a reticulin network, myxoid stroma, and cells that range in shape from stellate to spindle-shaped and are prominently associated with varied numbers of thin-walled blood vessels. A key diagnostic indicator for superficial angiomyxoma is its unusual histological appearance, with the presence of stromal inflammatory cells, chiefly neutrophils. Superficial angiomyxomas have positive expression of CD34 and vimentin in the stromal spindle cells, and the endothelial lining of vascular channels serves as a positive control. Histomorphology findings and results of immunohistochemistry support the diagnosis of superficial angiomyxoma in the index case.
For these myxoid lesions near the skin's surface, there are several possible diagnoses, such as aggressive angiomyxoma, angiomyolipoma, dermal nerve sheath myxoma, and low-grade myxofibrosarcoma [11] (Table 1). Superficial angiomyxoma differs from aggressive angiomyxoma by having well-defined borders, non-infiltrative patterns, dilated vascular channels, and being immunopositive for CD34 and vimentin [12,13]. Angiomyolipomas are primarily seen in the kidney and are extremely rare at a subcutaneous location. In cases of angiomyolipomas, the tumor mass is comprised of thick-walled blood vessels, smooth muscles, and fibroadipose tissue with co-expression of smooth muscle (SMA/MSA) and melanocytic markers (HMB-45/Melan A) [14-16]. The architecture of a nerve sheath myxoma is lobulated, with smaller individual nodules, benign spindle-shaped cells organized in fascicles and whorls like Pacinian corpuscles, and occasionally histiocytic cells are noted. Intralesional nerve bundles lying in myxoid stroma show diffuse positive expression of S100 and neuron-specific enolase [17-19]. The last entity, low-grade myxofibrosarcoma, can be distinguished from superficial angiomyxoma by having infiltrative margins, cellular atypia, brisk mitosis, and necrosis [20,21].
Table 1.
Differential diagnosis of superficial angiomyxoma.
| Differential diagnosis | Age and gender predisposition | Site | Gross findings | Histomorphological findings | Immunohistochemistry |
|---|---|---|---|---|---|
| Aggressive angiomyxoma[12,13] | Adults F>>M | Lower genital tract, perineum, and pelvic | Subcutis/subfascial, large lesions, mostly (>10cm), usually solitary | Infiltrative locally aggressive spindle cell neoplasm having myxoid stroma with interspersed thick-walled blood vessels, hyalinization of vascular walls, stroma at periphery entraps fat and smooth muscle bundles. | Desmin+ SMA+ MSA+ |
| Angiomyolipoma[14-16] | Fourth to seventh decade F>M | Kidney Liver | Unilateral and unifocal, multiple or bilateral tumors suggest association with tuberous sclerosis | Comprising of admixture of thick-walled blood vessels, smooth muscles and adipose tissue, interspersed vascular component is present in the form of dysmorphic thick-walled blood vessels with absence of elastic lamina. | HMB45+ Melan A+ SMA+ MSA+ |
| Dermal nerve sheath myxoma[17-19] | Young and middle age adults F>M | Extremetes and head/neck | Primarily solitary dermal based neoplasm, flesh colored to transparent nodule, rubbery to firm consistency, usually < 3cm | Well demarcated lobulated dermal neoplasm with fibrous tissue septation, myxoid stroma, spindle-shaped tumor cells with mild nuclear atypia. | S100+ Neuron specific enolase + |
| Low grade myxofibrosarcoma[20,21] | Sixth to eighth decade, F<M | Extremities, trunk and retroperitoneum | Subfascial, variable size slowly growing malignant neoplasms, Size <10cm | Subfascial neoplasm with infiltrative margins, myxoid and fibrous stroma, variable cytological atypia and characteristics curvilinear blood vessels, hemorrhage and necrosis can be present. | Vimentin+ SMA+/- MSA+/- |
A positive prognosis is often expected for superficial angiomyxomas because they do not invade deeper structures and remain only superficial. According to Allen et al. and Calonje et al., the primary treatment for superficial angiomyxoma is complete wide local excision with follow-up because these tumors are known for having a high potential for local recurrence if the resection is marginal or incomplete [1,2]. According to Rodriguez et al.'s study, when there is inadequate resection, the local recurrence of the tumor is predicted to be between 30%-40% [5]. The most recent follow-up, in the current case study, has not shown tumor recurrence.
CONCLUSION
A differential diagnosis of superficial angiomyxoma should be kept in mind if there is the presence of myxoid stroma in the background of aspiration smears, together with cells of bland spindle morphology and the presence of acute inflammatory cells. Complete assimilation of clinical findings, cytomorphological features, histopathology, and immunohistochemistry is mandatory for the definitive diagnosis of such rare tumors at unusual locations. The mainstay of treatment for this extremely rare neoplasm is a wide local excision because a recurrence can result from an incomplete excision.
Informed consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Funding
None.
Conflict of interest
None.
REFERENCES
- 1.Allen PW, Dymock RB, MacCormac LB. Superficial angiomyxomas with and without epithelial components. Report of 30 tumors in 28 patients. Am J Surg Pathol. 1988;12(7):519–530. doi: 10.1097/00000478-198807000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Calonje E, Guerin D, McCormick D, et al. Superficial angiomyxoma: clinicopathologic analysis of a series of distinctive but poorly recognized cutaneous tumors with tendency for recurrence. Am J Surg Pathol. 1999;23(8):910–917. doi: 10.1097/00000478-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Carney JA, Gordon H, Carpenter PC, et al. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64(4):270–283. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Ferreiro JA, Carney JA. Myxomas of the external ear and their significance. Am J Surg Pathol. 1994;18(3):274–280. doi: 10.1097/00000478-199403000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Rosado Rodríguez P, de Vicente JC, de Villalaín L, et al. Angiomixoma superficial de la región parotídea y revisión de la literatura. [Superficial angiomyxoma of the parotid region and review of the literature]. Acta Otorrinolaringol Esp. 2012;63(2):147–149. doi: 10.1016/j.otorri.2010.11.014. Spanish. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto K, Kondo A, Iwashita K, et al. A case of superficial angiomyxoma. Tokai J Exp Clin Med. 2006;31(2):53–55. [PubMed] [Google Scholar]
- 7.Kura MM, Jindal SR. Solitary superficial acral angiomyxoma: an infrequently reported soft tissue tumor. Indian J Dermatol. 2014;59(5):529. doi: 10.4103/0019-5154.139893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khadilkar UN, Khadilkar NP, Rao PS, et al. Superficial angiomyxoma of the external ear not associated with Carney's complex: a case report. Kathmandu Univ Med J (KUMJ) 2007;5(4):546–549. [PubMed] [Google Scholar]
- 9.Hwang YJ, Lee HW, Lee IS, et al. Superficial angiomyxoma of the posterior neck. Arch Craniofac Surg. 2021;22(1):62–65. doi: 10.7181/acfs.2020.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shukla S, Sehgal S, Prabhat P, et al. Congenital Presentation of a Solitary Superficial Angiomyxoma in the Parotid Region Masquerading as Parotid Tumor. Turk Patoloji Derg. 2018;34(3):262–264. doi: 10.5146/tjpath.2015.01358. English. [DOI] [PubMed] [Google Scholar]
- 11.Bembem K, Jaiswal A, Singh M, et al. Cyto-Histo Correlation of a Very Rare Tumor: Superficial Angiomyxoma. J Cytol. 2017;34(4):230–232. doi: 10.4103/0970-9371.216119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graadt van Roggen JF, Hogendoorn PC, Fletcher CD. Myxoid tumours of soft tissue. Histopathology. 1999;35(4):291–312. doi: 10.1046/j.1365-2559.1999.00835.x. [DOI] [PubMed] [Google Scholar]
- 13.Gorgulu G, Kole MÇ, Ayaz D, et al. Aggressive Angiomyxoma. A case series of eight years of experience. Ann Ital Chir . 2022;93:562–565. [PubMed] [Google Scholar]
- 14.Ammanagi AS, Dombale VD, Shindholimath VV. Cutaneous angiomyolipoma. Indian Dermatol Online J. 2012;3(1):40–41. doi: 10.4103/2229-5178.93501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh K, Pai RR, Kini H, et al. Cutaneous angiomyolipoma. Indian J Pathol Microbiol. 2009;52(2):242–243. doi: 10.4103/0377-4929.48932. [DOI] [PubMed] [Google Scholar]
- 16.Khaddam S, Gulati S. Spectrum of Presentations and Management Strategies in Renal Angiomyolipoma. J Kidney Cancer VHL. 2022;9(1):42–47. doi: 10.15586/jkcvhl.v9i1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagrani NS, Bhawan J. Histopathological Variants of Cutaneous Neurofibroma: A Compendious Review. Dermatopathology (Basel) 2022;10(1):1–19. doi: 10.3390/dermatopathology10010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakayama R, Mori T, Okita Y, et al. A multidisciplinary approach to soft-tissue sarcoma of the extremities. Expert Rev Anticancer Ther. 2020;20(10):893–900. doi: 10.1080/14737140.2020.1814150. [DOI] [PubMed] [Google Scholar]
- 19.Meng H, Jiang C. A Case of Dermal Nerve Sheath Myxoma: A Different Tumor from Cellular Neurothekeoma. Indian J Dermatol. 2021;66(4):416–419. doi: 10.4103/ijd.IJD_166_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamed M, Fisher C, Thway K. Low-grade fibromyxoid sarcoma: Clinical, morphologic and genetic features. Ann Diagn Pathol. 2017;28:60–67. doi: 10.1016/j.anndiagpath.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Desai S, Choudhury J, Varghese K, Kapoor T. Primary Osseous Low-grade Myxofibrosarcoma of Metatarsal Masquerading as Enchondroma: A Case Report. J Orthop Case Rep. 2022;12(5):35–39. doi: 10.13107/jocr.2022.v12.i05.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]