Abstract
Microbial cell factories (MCFs) are typical and widely used platforms in biomanufacturing for designing and constructing synthesis pathways of target compounds in microorganisms. In MCFs, transporter engineering is especially significant for improving the biomanufacturing efficiency and capacity through enhancing substrate absorption, promoting intracellular mass transfer of intermediate metabolites, and improving transmembrane export of target products. This review discusses the current methods and strategies of mining and characterizing suitable transporters and presents the cases of transporter engineering in the production of various chemicals in MCFs.
1. Introduction
Biomanufacturing uses renewable biomass to produce bioenergy, biomaterials, natural products, and bulk chemicals, which has important significance for carbon emission reduction and sustainable development [1, 2]. Microbial cell factories (MCFs), as the core of biomanufacturing, are generally manipulated via metabolic engineering and synthetic biology techniques for producing diverse compounds. At present, many strategies have been used to improve the efficiency and capacity of MCFs, including enhancing pathway flux [3], inhibiting competitive pathways [4], cofactor engineering [5], and enzyme engineering [6]. In particular, since MCFs are regarded as “production workshops,” the mass transfer efficiency among the “production units (cells or organelles)” was usually insufficient, especially for eukaryotes, which extremely limits the further improvement of MCFs.
To solve this problem, transporter engineering was provided as an alternative strategy that can enhance substrate absorption, promote the intracellular mass transfer of intermediate metabolites, and improve the transmembrane export of target products. Generally, several strategies have been used for mass transfer intensification in MCFs. Subcellular compartmentalization strategy have been adopted to strengthen metabolic mass transfer by introducing a series of reactions into one compartment/organelle, which improved local concentrations in space for increasing product concentration [7–20]. Membrane engineering was also an effective strategy in the export of hydrophobic products by modifying the cell membrane structure genetically [21–23] or adding exogenous reagents such as cyclodextrins [24, 25], dodecane [26], and olive oil [27], which significantly released intracellular storage space and released product inhibition. Mining, expressing, and remolding transporters of target compounds (i.e., transporter engineering) is the most direct way to import or export a specific substrate. Transporter engineering has received more and more attention due to its specificity, efficiency, and simplicity.
Herein, we summarized the current methods and strategies for mining and characterizing suitable transporters and introduced cases for improving the manufacturing efficiency of MCFs through transporter engineering. The understanding of cellular transport process and the application of transporter engineering would provide novel insights into the construction of MCFs for the biomanufacturing process.
2. Mining and Characterizing Suitable Transporters
At present, the reported information about transporters is still inadequate, which causes tremendous challenge for engineering molecular transport in MCFs. Therefore, it has become exceptionally crucial for mining and characterizing more specific transporters for the target compounds (Table 1).
Table 1.
Transporter applications in microbial cell factories.
| Transporter | Species | Compound | Function | Reference |
|---|---|---|---|---|
| Substrates | ||||
| Gal2p | S. cerevisiae | Xylose | Improved the transport rate and accelerated utilization of xylose. | [40] |
| AraT | S. cerevisiae | L-arabinose | Transported L-arabinose with high specificity and high affinity. | [36] |
| XylE | P. putida | Xylose | Broadened metabolic capacity towards new substrates. | [43] |
| GatA | S. cerevisiae | D-galacturonic acid | Achieved coutilization of D-galUA and D-glucose. | [44] |
| Lac12 | S. cerevisiae | Lactose | Increased uptake of the lactose. | [66–68] |
| Intermediate metabolites | ||||
| ShiA | E. coli | 3-Dehydroshikimate | Enhanced reuptake of intermediate metabolite from extracellular to cytoplasm. | [45] |
| FadL | E. coli | Palmitate | Achieved reuptake of excreted intermediate metabolite. | [69] |
| Δpxa1 | S. cerevisiae | Fatty acyl-CoA | Increased production of fatty acyl-CoA in the cytoplasm. | [46] |
| NtJAT1, NtMATE2 | S. cerevisiae | Tropine | Alleviated vacuolar intermediate metabolite transport limitations. | [47] |
| Target products | ||||
| AcrE, MdtE, MdtC | E. coli | Medium-chain fatty acid | Increased extracellular MCFA concentration by 59.7%, 43.2%, and 83.1%. | [55] |
| FATP1 | S. cerevisiae | Fatty alcohol | Enabled an increased cell fitness for fatty alcohol production. | [56] |
| FATP1 | S. cerevisiae | 1-Alkenes | Improved the extracellular and total 1-alkene production. | [57] |
| MacA, TolC, MacB | E. coli | 6-Deoxyerythronolide B | Increased the 6dEB titers. | [60] |
| Orf14, Orf3 | Burkholderia | Epothilones | Raised the ratio of extracellular to intracellular accumulation from 9.3 : 1 to 13.7 : 1. | [62] |
| TolC, AcrB | E. coli | amorphadiene | Increased yield by 46%. | [59] |
| AcrA, TolC AcrB, | E. coli | Kaurene | Increased yield by 82%. | [59] |
| Snq2p | S. cerevisiae | β-Carotene | Improved β-carotene secretion level by 4.04-fold. | [64] |
| AcrAB | E. coli | Limonene | Reduced limonene toxicity. | [70] |
| Bfr1 | S. cerevisiae | Caffeine | Enhanced cellular resistance to caffeine. | [34] |
| AbPUP1, AbLP1 | S. cerevisiae | Littorine and hyoscyamine | Exported vacuolar littorine and hyoscyamine to the yeast cytosol. | [37] |
| AtDTX1 | E. coli | Reticuline | Achieved the secretion of high levels of reticuline. | [65] |
| MttA | A. niger | cis-aconitic acid | Secreted 9.8 g/L aconitic acid after 240 h of cultivation. | [71] |
| Spmae | S. cerevisiae | L-malic acid | Increased the accumulation. | [50] |
| AtABCG29 | S. cerevisiae | Coumaryl alcohol | Increased cellular tolerance to p-coumaryl alcohol. | [72] |
| PtPTP | Phaeodactylum tricornutum | Pyruvate | Enhanced biomass, lipid contents, and growth. | [73] |
| DCT1 | A. niger | Malic acid | Improved malic acid production by 36.8%. | [74] |
| MTT | Y. lipolytica | Itaconic acid | Enhanced itaconic acid titer by 10.5-folds. | [75] |
| RibM | B. subtilis | Riboflavin and roseoflavin | Increased the production of riboflavin and roseoflavin. | [76] |
| PP_1271 | P. putida | Propionic acid | Improved cellular tolerance to PA. | [48] |
| YbjE | Synechococcus sp | Lysine | Generated a large pool of lysine in the extracellular media. | [77] |
| Qdr3 | S. cerevisiae | Muconic acid | Increased cellular tolerance to glutaric, adipic, muconic, and glutaconic acid. | [78] |
| CexA | A. niger | Citric acid | Enhanced the secretion of citric acid. | [79] |
| M2 | E. coli | Proton | Increased acid tolerance. | [80] |
| SerE | C. glutamicum | L-serine | Increased L-serine efflux. | [51] |
| Tpo2p | S. cerevisiae | cis,cis-muconic acid, protocatechuic acid | Improved the production of target compound. | [39] |
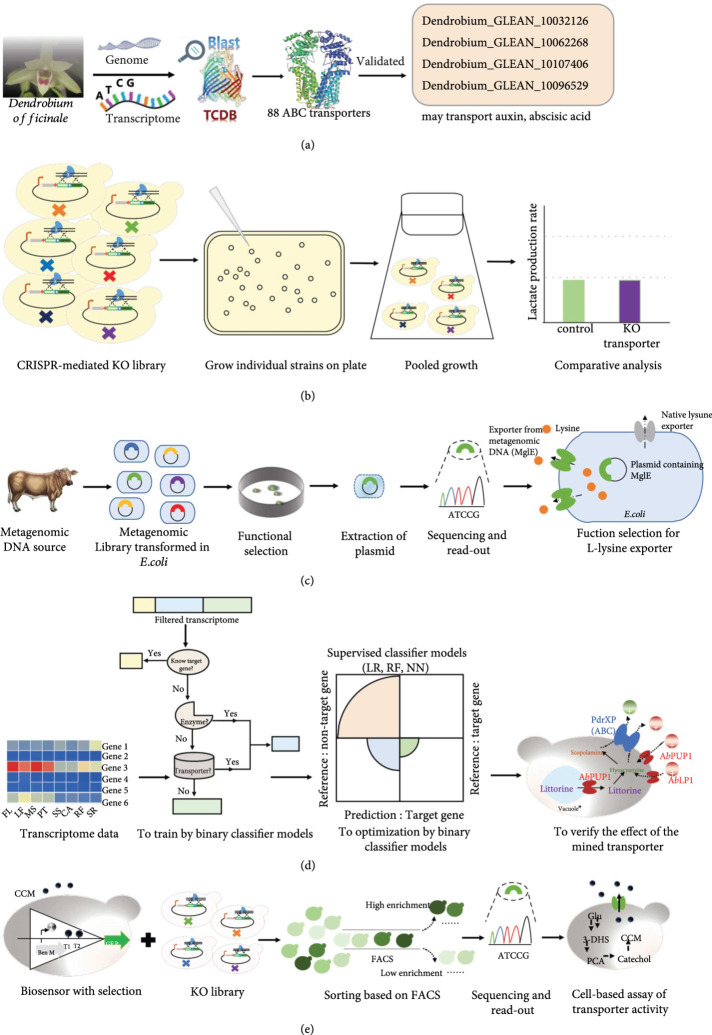
Bioinformatic tools are becoming popular due to their ability to analyze huge amounts of biological data [28]. AntiSMASH is a powerful tool for identifying gene clusters, which can annotate information about transporters [29]. Transporter classification database (TCDB; http://www.tcdb.org) is a common and freely accessible reference database [30]. Generally, the same transporter family can recognize similar substrate structures. Therefore, researchers have identified 88 ABC transporters from Dendrobium officinale via sequence alignment from TCDB [31], and four transporters were predicted for the transportation of abscisic acid and auxin by transcriptomic comparison [32] (Figure 1(a)).
Figure 1.
Methods for mining and characterizing suitable transporters: (a) predicting transporter via bioinformatics database; (b) screening natural transporters by establishing knock-out library; (c) identifying exporters by constructing and screening a metagenomic library; (d) mining transporters from the transcriptome by training, optimizing, and testing binary classifier models; and (e) high-throughput screening of transporters based on biosensors.
The function of natural transporters can be identified by gene knockout. However, the efficiency of this method was usually not obvious due to the redundancy of intracellular transporters and the complex network of their interactions. For instance, after knocking out the aqua (glycero) porin family and all known carboxylic acid transporters using CRISPR-Cas9 in Saccharomyces cerevisiae, the extracellular lactate production rate remained unchanged, indicating there existed some unknown transporters or mechanisms to export lactate (Figure 1(b)) [33]. Alternatively, constructing the mutation library of transporters was shown to be effective for high-throughput mining and characterizing the desired transporter. To export caffeine and relieve its toxicity to yeast, a mutation library of endogenous ABC-transporter brf1 was constructed, from which a mutant was screened out to increase caffeine resistance [34]. To find an efficient L-lysine export system, a metagenomic library of cow dung samples was constructed. After plating recombinants on high L-lysine concentration media, a novel lysine efflux transporter mglE was screened out, which improved the L-lysine tolerance of Escherichia coli by 40% and increased the L-lysine productivity of Corynebacterium glutamicum by 12% (Figure 1(c)) [35].
System biology and machine learning provided new approaches for mining transporter genes. For example, through analyzing transcriptomic data of Penicillium chrysogenum in D-glucose and L-arabinose restricted culture, respectively, the fungal transporter PcAraT specifically transporting L-arabinose instead of xylose and glucose was identified [36]. The transcriptomic data of Atropa belladonna were analyzed through binary classifier supervised learning models (logical regression, random forest, and feedforward neural network). The supervised classifier models based on tissue description showed greater efficacy in predicting transporters than traditional regression- and clustering-based methods. As a result, two identified transporters, AbPUP1 and AbLP1, were found to increase the production of target alkaloids in the engineered yeast (Figure 1(d)) [37].
Genetically encoded biosensors [38] are another powerful tool for high-throughput screening of strains producing or transporting target compounds. Researchers constructed a knockout library of 361 nonessential native transporters in S. cerevisiae via CRISPR/Cas9 followed by fluorescence-activated cell sorting (FACS) based on the biosensor of the target organic acid compounds cis, cis-muconic (CCM) and protocatechuic acids (PCA) [39]. As a result, Tpo2 was validated as an importer of CCM and PCA through Xenopus expression assays (Figure 1(e)) [39].
3. Enhancement of Substrate Absorption by Importer in MCFs
Low substrate uptake rates would hamper the productivity of MCFs, especially in engineered strains that use unnatural substrates (Table 1). Therefore, it is particularly important to improve substrate absorption and enhance transfer efficiency through targeted importer expression in MCFs.
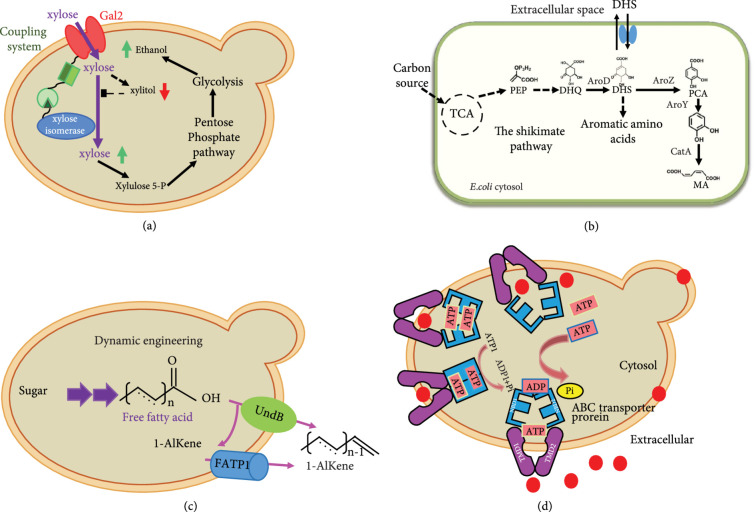
Lignocellulose-derived pentose sugars (mainly D-xylose and L-arabinose) are not natural substrates of baker’s yeast. The utilization of pentose can substantially improve bioresource utilization. An artificial complex consisting of endogenous sugar transporter Gal2 and heterologous xylose isomerase (XI) was constructed in S. cerevisiae, which significantly improved the substrate uptake rate and simultaneously reduced the production of byproduct xylitol (Figure 2(a)) [40]. The galactose transporter Gal2 and the low-activity hexose transporter Hxt9 could transport L-arabinose with low affinity () [41, 42]. Therefore, a high-affinity () and high-specificity L-arabinose transporter PcAraT from Penicillium chrysogenum was identified by characterizing sugar uptake kinetics in S. cerevisiae, which contributed to rapid and efficient conversion of L-arabinose [36]. In another study, the absorptivity of oligosaccharides and pentose was reinforced in Pseudomonas putida when the native ABC transporter complex PP1015~PP1018 was overexpressed [43]. D-galacturonic acid and D-glucose were coutilized by identifying and expressing a heterologous transporter GatA from Aspergillus niger, which realized the production of galactonic acid directly from industrial orange peel waste [44].
Figure 2.
Application of transporter engineering in the construction of MCFs. (a) The Gal2 transporter was coupled with xylose isomerase to improve the xylose transport rate; (b) schematic of the cis, cis-muconic acid biosynthetic pathway and the intermediate DHS transporter; (c) 1-alkene secretion by expressing the long-chain fatty acid transporter FATP1 from Homo sapiens; and (d) improvement of the export and production of β-carotene by overexpressing endogenous ABC transporters.
4. Promotion of the Intracellular Mass Transfer of Intermediate Metabolites
Strengthening the reuptake of intermediate metabolites or the mass transfer between cells and extracellular media or subcellular compartments could significantly improve the flux of metabolic pathways, which is essential for the manufacturing efficiency and capacity of the MCFs. In the cis, cis-muconic acid production strain, the crucial intermediate 3-dehydroshikimic acid (DHS) can diffuse to the outside of the cell along the concentration gradient, resulting in the draining of precursor. By expressing a membrane-bound transporter ShiA to import DHS into the cytosol, the production of cis, cis-muconic acid was significantly improved [45] (Figure 2(b)). Acyl-CoA degrades to acetyl-CoA through the peroxisomal β-oxidation pathway in S. cerevisiae, which limits the production of cytosolic acyl-CoA. Therefore, the fatty acyl-CoA peroxisomal transporter Pxa1 was knocked out to prevent oxidation, which improved the production of acyl-CoA-derived triacylglycerols [46]. Researchers introduced the tobacco-derived multidrug and toxic compound efflux proteins NtJAT1 and NtMATE2 with nicotine transport ability into yeast, which alleviated vacuolar intermediate metabolite transport limitations, eventually increasing the titers of the target alkaloids hyoscyamine and scopolamine by 74% and 18%, respectively [47].
5. Improvement of the Transmembrane Export of Target Products in MCFs
Export of target products in MCFs has many benefits, including releasing intracellular space, eliminating product inhibition, and reducing potential product toxicity. Propionic acid is a valuable C3 platform chemical, but it is toxic to microorganisms. Propionic acid tolerance and production in P. putida were increased by overexpressing the major facilitator superfamily (MFS) transporter gene cluster PP_1271 [48]. However, the production did not fluctuate greatly after deleting the cluster PP_1271, which showed more than one transporter regulating propionic acid tolerance and confirmed the complexity of the transport mechanism. A C4-dicarboxylate transporter Spmae from Schizosaccharomyces pombe was found to export L-malic acid effectively [49]. Researchers found that Spmae can be modified by ubiquitin, which might result in significant degradation. By employing a deubiquitination strategy, the accumulation of L-malic acid was improved in S. cerevisiae [50]. In another case, to export L-serine from the cell, a novel exporter SerE was overexpressed, and the titer of L-serine reached 43.9 g/L in C. glutamicum combining with the strengthening of L-serine synthetic pathway, which further enhanced its industrial application [51].
The export of many fuel chemicals was conducive to their bioproduction, such as fatty acids [52], fatty alcohols [53, 54], and alkanes [52]. The coexpression of efflux transporters mdtE, acrE, and mdtC in combination with the deletion of the influx transporter cmr increased extracellular medium-chain fatty acids (C6-C10, MCFAs) titer and endowed host strains with more adaptability to harsh environments [55]. In order to alleviate growth inhibition and reduce extraction cost, the export of fatty alcohols was promoted by about fivefold after expressing human fatty acid transporter FATP1 in yeast [56]. FATP1 also facilitated the production and secretion of alkenes according to the similar hydrophobic properties between long-chain fatty acids and alkenes. As a result, more than 80% of alkene was exported, which immensely reduced the cost of downstream extraction and separation and further improved the economics of the process (Figure 2(c)) [57].
Polyketides are a large class of natural products with great therapeutic value. Resistance-nodulation-cell division (RND) family efflux pumps play major roles in the resistance of gram-negative bacteria to a wide range of compounds, such as polyketides [58]. An RND efflux pump typically consists of three different components, an inner membrane protein (e.g., MacB), an outer membrane protein (e.g., TolC), and a periplasmic membrane adapter protein (e.g., MacA), which are organized in a complex structure with a specific ratio [59]. The highest titer of 6-deoxygibberellin B (6dEB, erythromycin precursor) was achieved with the combination of MacA, MacB, and TolC in E. coli [60]. It is noteworthy that the improvement was significantly higher than those of expressing all single components of pumps alone. Therefore, the coordinative interaction between pump components is indeed important for transporter engineering. Generally, the expression of RND efflux pumps is often tightly controlled by the relevant regulatory proteins. For example, five transcriptional activators YdeO, MarA, RpoH, EvgA, and Fnr, which are responsible for activating the multidrug efflux pumps, were tested for improving polyketide production [60]. In the treatment of cancer, epothilone is a polyketide compound with a better curative effect and milder side effects than taxane [61]. The ratio of extracellular to intracellular accumulation of epothilone was boosted from 9.3 : 1 to 13.7 : 1 by applying two multidrug efflux pumps, Orf14 and Orf3 in Burkholderia, thereby promoting the forward biosynthesis of the heterologous polyketide compound epothilone [62].
Terpenoids are the largest family of secondary metabolites of plants, and they are widely distributed in archaea, bacteria, and eukaryotes [63]. The RND efflux pump was also efficient for the cellular exportation of the sesquiterpene amorphadiene and the diterpene kaurene [59]. Interestingly, the three components of tripartite efflux pumps played varied effect on different compounds. For amorphadiene production, the highest yield was achieved with the combination of TolC and AcrB; the three-component combination AcrA-TolC-AcrB achieved the highest yield of kaurene in E. coli [59]. The coordinative interaction between pump components was vital for transporter engineering. The extracellular production of hydrophobic β-carotene was enhanced by 4.04-fold through adopting an inducible GAL promoter to overexpress the endogenous plasma membrane ABC transporter Snq2p in S. cerevisiae [64] (Figure 2(d)). As an important intermediate compound in the alkaloid synthesis pathway, the yield of reticuline increased by 11-fold in E. coli by introducing the multidrug and toxic compound efflux family transporter AtDTX1 from Arabidopsis thaliana [65].
6. Conclusion and Perspectives
Transporter engineering has been documented to improve substrate absorption, promote the intracellular mass transfer of intermediate metabolites, and reinforce the transmembrane export of target products, which play a decisive role in the metabolism and mass transfer of the MCFs. However, reported information about transporters is insufficient, severely limiting the application of transporter engineering in MCFs. Therefore, it is extremely necessary to vigorously develop efficient methods and strategies for mining and characterizing transporters. The current methods for identifying specific transporters still have limitations, such as relatively low throughput, low efficiency, and labor intensive. It is necessary to develop high throughput, low-cost, and efficient methods for automated identification and characterization of transporters.
Transporters generally have a broad substrate spectrum, which increases the transport flux of target compounds through the synergistic effect of multiple transporters. The heterologous production of various classes of compounds could combine different types of pumps. However, selecting the proper type of pump is also vital for a specific heterologous product. In nature, the expression of some efflux pumps is often tightly controlled by the relevant regulatory proteins. Thus, tuning the expression of pump regulators may be an effective option for transporter engineering as well. As the transport mechanism becomes clear, the semirational or rational design based on the protein structure could further expand their substrate spectrum and improve the transport affinity and transport rate of target compounds. In addition, combining transporter engineering with other regulation strategies may further boost production and efflux of target compounds based on their synergism.
Acknowledgments
This work was financially supported by the National Basic Research Program of China (973 Program) (2018YFA0901800) and the National Natural Science Foundation of China (22138006, 21736002, and 22078020).
Contributor Information
Lei Qin, Email: qinleithu@tsinghua.edu.cn.
Chun Li, Email: lichun@tsinghua.edu.cn.
Data Availability
All data are available in the main text.
References
- 1.Herwig C., Slouka C., Neubauer P., and Delvigne F., “Editorial: continuous biomanufacturing in microbial systems,” Frontiers in Bioengineering and Biotechnology, vol. 9, p. 665940, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otero-Muras I., and Carbonell P., “Automated engineering of synthetic metabolic pathways for efficient biomanufacturing,” Metabolic Engineering, vol. 63, pp. 61–80, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Lim H., Park J., and Woo H. M., “Overexpression of the key enzymes in the methylerythritol 4-phosphate pathway in Corynebacterium glutamicum for improving farnesyl diphosphate-derived terpene production,” Journal of Agricultural and Food Chemistry, vol. 68, no. 39, pp. 10780–10786, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y., Fan J., Wang C., Feng X., and Li C., “Enhancing oleanolic acid production in engineered _Saccharomyces cerevisiae_,” Bioresource Technology, vol. 257, pp. 339–343, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Li X., Chen J., Andersen J. M., Chu J., and Jensen P. R., “Cofactor engineering redirects secondary metabolism and enhances erythromycin production in Saccharopolyspora erythraea,” ACS Synthetic Biology, vol. 9, no. 3, pp. 655–670, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Fisher A. K., Freedman B. G., Bevan D. R., and Senger R. S., “A review of metabolic and enzymatic engineering strategies for designing and optimizing performance of microbial cell factories,” Computational and Structural Biotechnology Journal, vol. 11, no. 18, pp. 91–99, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu G.-S., Li T., Zhou W., Jiang M., Tao X.-Y., Liu M., Zhao M., Ren Y.-H., Gao B., Wang F.-Q., and Wei D.-Z., “The yeast peroxisome: a dynamic storage depot and subcellular factory for squalene overproduction,” Metabolic Engineering, vol. 57, pp. 151–161, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Deloache W. C., Russ Z. N., and Dueber J. E., “Towards repurposing the yeast peroxisome for compartmentalizing heterologous metabolic pathways,” Nature Communications, vol. 7, no. 1, p. 11152, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J. E., Jang I. S., Son S. H., Ko Y. J., Cho B. K., Kim S. C., and Lee J. Y., “Tailoring the _Saccharomyces cerevisiae_ endoplasmic reticulum for functional assembly of terpene synthesis pathway,” Metabolic Engineering, vol. 56, pp. 50–59, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Poirier Y., Erard N., and Petetot J. M., “Synthesis of polyhydroxyalkanoate in the peroxisome of Saccharomyces cerevisiae by using intermediates of fatty acid β-oxidation,” Applied and Environmental Microbiology, vol. 67, no. 11, pp. 5254–5260, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma T., Shi B., Ye Z., Li X., Liu M., Chen Y., Xia J., Nielsen J., Deng Z., and Liu T., “Lipid engineering combined with systematic metabolic engineering of _Saccharomyces cerevisiae_ for high-yield production of lycopene,” Metabolic Engineering, vol. 52, pp. 134–142, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Farhi M., Marhevka E., Masci T., Marcos E., Eyal Y., Ovadis M., Abeliovich H., and Vainstein A., “Harnessing yeast subcellular compartments for the production of plant terpenoids,” Metabolic Engineering, vol. 13, no. 5, pp. 474–481, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Zhang C., Li M., Zhao G. R., and Lu W., “Harnessing yeast peroxisomes and cytosol acetyl-CoA for sesquiterpene α-humulene production,” Journal of Agricultural and Food Chemistry, vol. 68, no. 5, pp. 1382–1389, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Guo Q., Shi T. Q., Peng Q. Q., Sun X. M., Ji X. J., and Huang H., “Harnessing Yarrowia lipolytica peroxisomes as a subcellular factory for α-humulene overproduction,” Journal of Agricultural and Food Chemistry, vol. 69, no. 46, pp. 13831–13837, 2021 [DOI] [PubMed] [Google Scholar]
- 15.Cao X., Yang S., Cao C., and Zhou Y. J., “Harnessing sub-organelle metabolism for biosynthesis of isoprenoids in yeast,” Synthetic and systems biotechnology, vol. 5, no. 3, pp. 179–186, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y., Wang D., Li R., Huang L., Dai Z., and Zhang X., “Engineering yeast subcellular compartments for increased production of the lipophilic natural products ginsenosides,” Metabolic Engineering, vol. 67, pp. 104–111, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Yu Y., Rasool A., Liu H., Lv B., Chang P., Song H., Wang Y., and Li C., “Engineering _Saccharomyces cerevisiae_ for high yield production of α-amyrin via synergistic remodeling of α-amyrin synthase and expanding the storage pool,” Metabolic Engineering, vol. 62, pp. 72–83, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Yee D. A., DeNicola A. B., Billingsley J. M., Creso J. G., Subrahmanyam V., and Tang Y., “Engineered mitochondrial production of monoterpenes in _Saccharomyces cerevisiae_,” Metabolic Engineering, vol. 55, pp. 76–84, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arendt P., Miettinen K., Pollier J., de Rycke R., Callewaert N., and Goossens A., “An endoplasmic reticulum-engineered yeast platform for overproduction of triterpenoids,” Metabolic Engineering, vol. 40, pp. 165–175, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Sadre R., Kuo P., Chen J., Yang Y., Banerjee A., Benning C., and Hamberger B., “Cytosolic lipid droplets as engineered organelles for production and accumulation of terpenoid biomaterials in leaves,” Nature Communications, vol. 10, no. 1, p. 853, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J.-L., Bai Q.-Y., Peng Y.-Z., Fan J., Jin C. C., Cao Y. X., and Yuan Y. J., “High production of triterpenoids in Yarrowia lipolytica through manipulation of lipid components,” Biotechnology for Biofuels, vol. 13, no. 1, p. 13, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu T., Li S., Ye L., Zhao D., Fan F., Li Q., Zhang B., Bi C., and Zhang X., “Engineering an artificial membrane vesicle trafficking system (AMVTS) for the excretion of β-carotene in Escherichia coli,” ACS Synthetic Biology, vol. 8, no. 5, pp. 1037–1046, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Oh H. Y., Lee J. O., and Kim O. B., “Increase of organic solvent tolerance of Escherichia coli by the deletion of two regulator genes, fadR and marR,” Applied Microbiology and Biotechnology, vol. 96, no. 6, pp. 1619–1627, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ni J., Zhang G., Qin L., Li J., and Li C., “Simultaneously down-regulation of multiplex branch pathways using CRISPRi and fermentation optimization for enhancing β-amyrin production in _Saccharomyces cerevisiae_,” Synthetic and systems biotechnology, vol. 4, no. 2, pp. 79–85, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moses T., Pollier J., Almagro L., Buyst D., van Montagu M., Pedreño M. A., Martins J. C., Thevelein J. M., and Goossens A., “Combinatorial biosynthesis of sapogenins and saponins in Saccharomyces cerevisiaeusing a C-16α hydroxylase from Bupleurum falcatum,” Proceedings of the National Academy of Sciences of the United States of America, vol. 111, no. 4, pp. 1634–1639, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang H. J., Yoon S. H., Ryu H. K., Kim J. H., Wang C. L., Kim J. Y., Oh D. K., and Kim S. W., “Retinoid production using metabolically engineered Escherichia coli with a two-phase culture system,” Microbial Cell Factories, vol. 10, no. 1, pp. 1–12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun L., Kwak S., Jin Y. S., and Vitamin A., “Vitamin A production by engineeredSaccharomyces cerevisiae from Xylosevia two-phase in situ extraction,” ACS Synthetic Biology, vol. 8, no. 9, pp. 2131–2140, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Almeida O. G. G., and De Martinis E. C. P., “Bioinformatics tools to assess metagenomic data for applied microbiology,” Applied Microbiology and Biotechnology, vol. 103, no. 1, pp. 69–82, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Blin K., Shaw S., Steinke K., Villebro R., Ziemert N., Lee S. Y., Medema M. H., and Weber T., “antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline,” Nucleic Acids Research, vol. 47, no. W1, pp. W81–W87, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saier M. H., Reddy V. S., Moreno-Hagelsieb G., Hendargo K. J., Zhang Y., Iddamsetty V., Lam K. J. K., Tian N., Russum S., Wang J., and Medrano-Soto A., “The Transporter Classification Database (TCDB): 2021 update,” Nucleic Acids Research, vol. 49, no. D1, pp. D461–D467, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan B., Liu S. S., Chen J., and Guo S. X., “Identification and differential expression analysis of ABC transporter gene from medicinal plant Dendrobium officinale,” Acta Pharmaceutica Sinica, pp. 1177–1189, 2018
- 32.Yan L., Wang X., Liu H., Tian Y., Lian J., Yang R., Hao S., Wang X., Yang S., Li Q., Qi S., Kui L., Okpekum M., Ma X., Zhang J., Ding Z., Zhang G., Wang W., Dong Y., and Sheng J., “The genome of _Dendrobium officinale_ illuminates the biology of the important traditional Chinese orchid herb,” Molecular Plant, vol. 8, no. 6, pp. 922–934, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Mans R., Hassing E. J., Wijsman M., Giezekamp A., Pronk J. T., Daran J. M., and van Maris A. J. A., “A CRISPR/Cas9-based exploration into the elusive mechanism for lactate export in Saccharomyces cerevisiae,” FEMS Yeast Research, vol. 17, no. 8, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Wang M., Deng W. W., Zhang Z. Z., and Yu O., “Engineering an ABC transporter for enhancing resistance to caffeine in Saccharomyces cerevisiae,” Journal of Agricultural and Food Chemistry, vol. 64, no. 42, pp. 7973–7978, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Malla S., van der Helm E., Darbani B., Wieschalka S., Förster J., Borodina I., and Sommer M. O., “A novel efficient L-lysine exporter identified by functional metagenomics,” Frontiers in microbiology, p. 1260, 2020 [DOI] [PMC free article] [PubMed]
- 36.Bracher J. M., Verhoeven M. D., Wisselink H. W., Crimi B., Nijland J. G., Driessen A. J. M., Klaassen P., van Maris A. J. A., Daran J. M. G., and Pronk J. T., “The Penicillium chrysogenum transporter PcAraT enables high-affinity, glucose-insensitive l-arabinose transport in Saccharomyces cerevisiae,” Biotechnology for Biofuels, vol. 11, no. 1, p. 63, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srinivasan P., and Smolke C. D., “Engineering cellular metabolite transport for biosynthesis of computationally predicted tropane alkaloid derivatives in yeast,” Proceedings of the National Academy of Sciences, vol. 118, no. 25, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demeke Teklemariam A., Samaddar M., Alharbi M. G., al-Hindi R. R., and Bhunia A. K., “Biosensor and molecular-based methods for the detection of human coronaviruses: a review,” Molecular and Cellular Probes, vol. 54, p. 101662, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang G., Møller-Hansen I., Babaei M., D’Ambrosio V., Christensen H. B., Darbani B., Jensen M. K., and Borodina I., “Transportome-wide engineering of _Saccharomyces cerevisiae_,” Metabolic Engineering, vol. 64, pp. 52–63, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomik T., Wittig I., Choe J. Y., Boles E., and Oreb M., “An artificial transport metabolon facilitates improved substrate utilization in yeast,” Nature Chemical Biology, vol. 13, no. 11, pp. 1158–1163, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Kou S. C., Christensen M. S., and Cirillo V. P., “Galactose transport in Saccharomyces cerevisiaeII. Characteristics of galactose uptake and exchange in galactokinaseless cells,” Journal of Bacteriology, vol. 103, no. 3, pp. 671–678, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subtil T., and Boles E., “Improving L-arabinose utilization of pentose fermenting Saccharomyces cerevisiae cells by heterologous expression of L-arabinose transporting sugar transporters,” Biotechnology for Biofuels, vol. 4, no. 1, p. 38, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dvorak P., and De Lorenzo V., “Refactoring the upper sugar metabolism of _Pseudomonas putida_ for co- utilization of cellobiose, xylose, and glucose,” Metabolic Engineering, vol. 48, pp. 94–108, 2018 [DOI] [PubMed] [Google Scholar]
- 44.Protzko R. J., Latimer L. N., Martinho Z., de Reus E., Seibert T., Benz J. P., and Dueber J. E., “Engineering _Saccharomyces cerevisiae_ for co-utilization of d-galacturonic acid and d-glucose from citrus peel waste,” Nature Communications, vol. 9, no. 1, p. 5059, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H., Pereira B., Li Z., and Stephanopoulos G., “Engineering Escherichia colicoculture systems for the production of biochemical products,” Proceedings of the National Academy of Sciences of the United States of America, vol. 112, no. 27, pp. 8266–8271, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferreira R., Teixeira P. G., Gossing M., David F., Siewers V., and Nielsen J., “Metabolic engineering of _Saccharomyces cerevisiae_ for overproduction of triacylglycerols,” Metabolic engineering communications, vol. 6, pp. 22–27, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srinivasan P., and Smolke C. D., “Biosynthesis of medicinal tropane alkaloids in yeast,” Nature, vol. 585, no. 7826, pp. 614–619, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma C., Mu Q., Xue Y., Xue Y., Yu B., and Ma Y., “One major facilitator superfamily transporter is responsible for propionic acid tolerance in Pseudomonas putidaKT2440,” Microbial Biotechnology, vol. 14, no. 2, pp. 386–391, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zelle R. M., De Hulster E., Van Winden W. A., De Waard P., Dijkema C., Winkler A. A., Geertman J. M., Van Dijken J. P., Pronk J. T., and Van Maris A. J., “Malic acid production by Saccharomyces cerevisiae: engineering of pyruvate carboxylation, oxaloacetate reduction, and malate export,” Applied and Environmental Microbiology, vol. 74, no. 9, pp. 2766–2777, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X., Wang Y., Dong X., Hu G., and Liu L., “Engineering rTCA pathway and C4-dicarboxylate transporter for L-malic acid production,” Applied Microbiology and Biotechnology, vol. 101, no. 10, pp. 4041–4052, 2017 [DOI] [PubMed] [Google Scholar]
- 51.Zhang X., Gao Y., Chen Z., Xu G., Zhang X., Li H., Shi J., Koffas M. A. G., and Xu Z., “High-yield production of L-serine through a novel identified exporter combined with synthetic pathway in Corynebacterium glutamicum,” Microbial Cell Factories, vol. 19, no. 1, p. 115, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rutter C. D., Zhang S., and Rao C. V., “Engineering Yarrowia lipolytica for production of medium-chain fatty acids,” Applied Microbiology and Biotechnology, vol. 99, no. 17, pp. 7359–7368, 2015 [DOI] [PubMed] [Google Scholar]
- 53.Tang X., and Chen W. N., “Enhanced production of fatty alcohols by engineering the TAGs synthesis pathway in Saccharomyces cerevisiae,” Biotechnology and Bioengineering, vol. 112, no. 2, pp. 386–392, 2015 [DOI] [PubMed] [Google Scholar]
- 54.Youngquist J. T., Schumacher M. H., Rose J. P., Raines T. C., Politz M. C., Copeland M. F., and Pfleger B. F., “Production of medium chain length fatty alcohols from glucose in _Escherichia coli_,” Metabolic Engineering, vol. 20, pp. 177–186, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu J., Wang Z., Zhang X., Zhou P., Xia X., and Dong M., “Improving medium chain fatty acid production in _Escherichia coli_ by multiple transporter engineering,” Food Chemistry, vol. 272, pp. 628–634, 2019 [DOI] [PubMed] [Google Scholar]
- 56.Hu Y., Zhu Z., Nielsen J., and Siewers V., “Heterologous transporter expression for improved fatty alcohol secretion in yeast,” Metabolic Engineering, vol. 45, pp. 51–58, 2018 [DOI] [PubMed] [Google Scholar]
- 57.Zhou Y. J., Hu Y., Zhu Z., Siewers V., and Nielsen J., “Engineering 1-alkene biosynthesis and secretion by dynamic regulation in yeast,” ACS Synthetic Biology, vol. 7, no. 2, pp. 584–590, 2018 [DOI] [PubMed] [Google Scholar]
- 58.Puentes-Cala E., and Harder J., “An RND transporter in the monoterpene metabolism of Castellaniella defragrans,” Biodegradation, vol. 30, no. 1, pp. 1–12, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J. F., Xiong Z. Q., Li S. Y., and Wang Y., “Enhancing isoprenoid production through systematically assembling and modulating efflux pumps in Escherichia coli,” Applied Microbiology and Biotechnology, vol. 97, no. 18, pp. 8057–8067, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Yang J., Xiong Z. Q., Song S. J., Wang J. F., Lv H. J., and Wang Y., “Improving heterologous polyketide production in Escherichia coli by transporter engineering,” Applied Microbiology and Biotechnology, vol. 99, no. 20, pp. 8691–8700, 2015 [DOI] [PubMed] [Google Scholar]
- 61.Gerth K., Bedorf N., Höfle G., Irschik H., and Reichenbach H., “Epothilons A and B: antifungal and cytotoxic compounds from Sorangium cellulosum (myxobacteria). Production, physico-chemical and biological properties,” The Journal of Antibiotics, vol. 49, no. 6, pp. 560–563, 1996 [DOI] [PubMed] [Google Scholar]
- 62.Liu C., Yu F., Liu Q., Bian X., Hu S., Yang H., Yin Y., Li Y., Shen Y., Xia L., Tu Q., and Zhang Y., “Yield improvement of epothilones in Burkholderia strain DSM7029 via transporter engineering,” FEMS Microbiology Letters, vol. 365, no. 9, 2018 [DOI] [PubMed] [Google Scholar]
- 63.Rohdich F., Bacher A., and Eisenreich W., “Isoprenoid biosynthetic pathways as anti-infective drug targets,” Biochemical Society Transactions, vol. 33, no. 4, pp. 785–791, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Bu X., Lin J. Y., Cheng J., Yang D., Duan C. Q., Koffas M., and Yan G. L., “Engineering endogenous ABC transporter with improving ATP supply and membrane flexibility enhances the secretion of β-carotene in Saccharomyces cerevisiae,” Biotechnology for Biofuels, vol. 13, no. 1, p. 168, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamada Y., Urui M., Oki H., Inoue K., Matsui H., Ikeda Y., Nakagawa A., Sato F., Minami H., and Shitan N., “Transport engineering for improving the production and secretion of valuable alkaloids in _Escherichia coli_,” Metabolic engineering communications, vol. 13, article e00184, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu J. J., Kwak S., Pathanibul P., Lee J. W., Yu S., Yun E. J., Lim H., Kim K. H., and Jin Y. S., “Biosynthesis of a functional human milk oligosaccharide, -fucosyllactose, andl-fucose using engineeredSaccharomyces cerevisiae,” ACS Synthetic Biology, vol. 7, no. 11, pp. 2529–2536, 2018 [DOI] [PubMed] [Google Scholar]
- 67.Yu S., Liu J. J., Yun E. J., Kwak S., Kim K. H., and Jin Y. S., “Production of a human milk oligosaccharide -fucosyllactose by metabolically engineered Saccharomyces cerevisiae,” Microbial Cell Factories, vol. 17, no. 1, p. 101, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hollands K., Baron C. M., Gibson K. J., Kelly K. J., Krasley E. A., Laffend L. A., Lauchli R. M., Maggio-Hall L. A., Nelson M. J., Prasad J. C., Ren Y., Rice B. A., Rice G. H., and Rothman S. C., “Engineering two species of yeast as cell factories for -fucosyllactose,” Metabolic Engineering, vol. 52, pp. 232–242, 2019 [DOI] [PubMed] [Google Scholar]
- 69.Kim J., Yoo H. W., Kim M., Kim E. J., Sung C., Lee P. G., Park B. G., and Kim B. G., “Rewiring FadR regulon for the selective production of ω-hydroxy palmitic acid from glucose in _Escherichia coli_,” Metabolic Engineering, vol. 47, pp. 414–422, 2018 [DOI] [PubMed] [Google Scholar]
- 70.Dunlop M. J., Dossani Z. Y., Szmidt H. L., Chu H. C., Lee T. S., Keasling J. D., Hadi M. Z., and Mukhopadhyay A., “Engineering microbial biofuel tolerance and export using efflux pumps,” Molecular Systems Biology, vol. 7, no. 1, p. 487, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steiger M. G., Punt P. J., Ram A. F. J., Mattanovich D., and Sauer M., “Characterizing MttA as a mitochondrial _cis_ -aconitic acid transporter by metabolic engineering,” Metabolic Engineering, vol. 35, pp. 95–104, 2016 [DOI] [PubMed] [Google Scholar]
- 72.Alejandro S., Lee Y., Tohge T., Sudre D., Osorio S., Park J., Bovet L., Lee Y., Geldner N., Fernie A. R., and Martinoia E., “AtABCG29 is a monolignol transporter involved in lignin biosynthesis,” Current Biology, vol. 22, no. 13, pp. 1207–1212, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Seo S., Kim J., Lee J. W., Nam O., Chang K. S., and Jin E. S., “Enhanced pyruvate metabolism in plastids by overexpression of putative plastidial pyruvate transporter in Phaeodactylum tricornutum,” Biotechnology for Biofuels, vol. 13, no. 1, p. 120, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cao W., Yan L., Li M., Liu X., Xu Y., Xie Z., and Liu H., “Identification and engineering a C4-dicarboxylate transporter for improvement of malic acid production in Aspergillus niger,” Applied Microbiology and Biotechnology, vol. 104, no. 22, pp. 9773–9783, 2020 [DOI] [PubMed] [Google Scholar]
- 75.Zhao C., Cui Z., Zhao X., Zhang J., Zhang L., Tian Y., Qi Q., and Liu J., “Enhanced itaconic acid production in Yarrowia lipolytica via heterologous expression of a mitochondrial transporter MTT,” Applied Microbiology and Biotechnology, vol. 103, no. 5, pp. 2181–2192, 2019 [DOI] [PubMed] [Google Scholar]
- 76.Hemberger S., Pedrolli D. B., Stolz J., Vogl C., Lehmann M., and Mack M., “RibM from Streptomyces davawensis is a riboflavin roseoflavin transporter and may be useful for the optimization of riboflavin production strains,” BMC Biotechnology, vol. 11, no. 1, pp. 1–10, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Korosh T. C., Markley A. L., Clark R. L., McGinley L. L., McMahon K. D., and Pfleger B. F., “Engineering photosynthetic production of L-lysine,” Metabolic Engineering, vol. 44, pp. 273–283, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pereira R., Wei Y., Mohamed E., Radi M., Malina C., Herrgård M. J., Feist A. M., Nielsen J., and Chen Y., “Adaptive laboratory evolution of tolerance to dicarboxylic acids in _Saccharomyces cerevisiae_,” Metabolic Engineering, vol. 56, pp. 130–141, 2019 [DOI] [PubMed] [Google Scholar]
- 79.Steiger M. G., Rassinger A., Mattanovich D., and Sauer M., “Engineering of the citrate exporter protein enables high citric acid production in _Aspergillus niger_,” Metabolic Engineering, vol. 52, pp. 224–231, 2019 [DOI] [PubMed] [Google Scholar]
- 80.Shin J., Jin Y. S., Park Y. C., Park J. B., Lee Y. O., Kim S. K., and Kweon D. H., “Enhancing acid tolerance of _Escherichia coli_ via viroporin-mediated export of protons and its application for efficient whole-cell biotransformation,” Metabolic Engineering, vol. 67, pp. 277–284, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the main text.