Abstract
The pharyngeal arches form the cornerstone of the complex anatomy of the face and neck. These embryologic structures are the foundation of face and neck development, and anomalous growth can result in craniofacial abnormalities. Surgeons who manage head and neck pathology and pathoanatomy will invariably encounter conditions associated with aberrant pharyngeal arch anatomy, and a thorough understanding of the normal and pathological development of these important structures is paramount to accurate diagnosis and treatment.
This manuscript is the first of a three-part educational series that addressed the pharyngeal/branchial arch embryology, development, nomenclature, and normal anatomy (Part I), pathologic anomalies of ear and neck derived from abnormal development of the arches (Part II), and different types of orofacial clefts, including Tessier clefts (Part III).
Key Words: Craniofacial development, Face and neck anatomy, Pharyngeal arches, Pharyngeal arches derivatives
EMBRYOLOGY, MIGRATION, AND DEVELOPMENT
Development of the pharyngeal arches occurs during the third and fourth weeks of embryonic gestation. The arches are a succession of bulges on the lateral surface of the embryonic head which contain layers of different embryonic cell types that develop and give rise to the oropharyngeal apparatus.1,2 These paired structures are composed of the following embryonic germ layers: endoderm (internal layer); ectoderm (external layer); mesoderm; and neural crest (filling located between the external and internal embryonic cell layers).1–4 Throughout embryogenesis, each layer will differentiate and form different oropharyngeal structures consisting of pharyngeal epithelia, cartilage, muscles, vessels, and nerves.1,2,5 In humans, a total 6 pharyngeal arches form; however, arch 5 involutes during the embryo development process and does not give rise to an adult structure. Adjacent to the arches are 4 pharyngeal pouches and 4 pharyngeal clefts/grooves. All these structures will be growing following a cephalon-caudal direction and will give rise to different portions of the ear, face, and neck.6–8
Structures formed by the pharyngeal arches result from the contribution of the primary germ layers.8
Ectoderm – covers each pharyngeal arch externally and depressions of ectoderm form the pharyngeal clefts/grooves. This germ layer is also responsible for the formation of the keratinized epidermis that covers the face and throat, and oral epithelium and sensory neurons of the epibranchial ganglia.3
Endoderm – covers the inside of each pharyngeal arch. Depressions of this internal layer form the pharyngeal pouches, which are rich in actin fibers. Endoderm and ectoderm layers physically touch to form a closed pharyngeal membrane, and portions of this layer contact with ectoderm to form the pharyngeal membrane.3
Mesoderm – is the core of the pharyngeal arches and develops into the skeletal musculature of the head and neck. Endothelial cells for vascular structures are also derived from this germ layer.3,9,10
‘Neural crest – Surrounds the mesoderm core and contributes to the formation of smooth muscle structures, bones, cartilages, tendons, and nerves.3,9 Germ layers distribution within the pharyngeal apparatus are illustrated in Figure 1.
FIGURE 1.
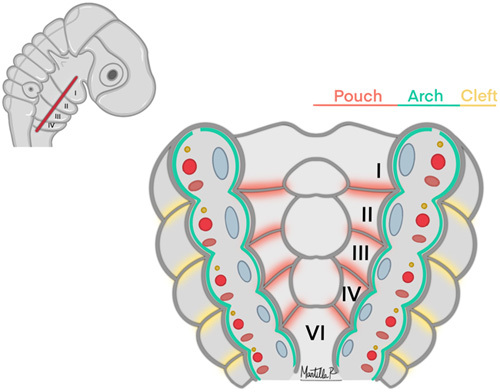
Germ layers distribution within the pharyngeal apparatus.
As a result of the neural tube closure, neural crest cells (NCC) in the neural plate will migrate from the central nervous system to different zones of the body and develop into a variety of vital structures.11 Cephalic NCC have the ability to create cartilage and ossification processes. These cells originate from the hindbrain’s rhombomeres (segments r1 to r7)3,9,11 and migrate via epithelial-to-mesenchymal transition to the pharyngeal arches, where they will surround and merge with the mesodermal layer to create definitive embryogenic structures.9,12 The first and second rhombomeres (r1 and r2) will migrate to the first pharyngeal arch. Additional to structures derived from this arch, r1 and r2 will also contribute to form the frontonasal process (which is not a derivate of the pharyngeal apparatus). The fourth rhombomere (r4) will migrate to the second pharyngeal arch, while the sixth and seventh rhombomeres (r6 and r7) will populate the third, fourth, and sixth arches. Third and fifth rhombomere cells (r3 and r5) do not form part of any specific arch, as these are not involved in cell migration13,14(Fig. 2). Although the neural crest plays an essential role in the patterning, distribution, and development of the oropharyngeal apparatus,14,15 recent studies have shown pharyngeal development can occur in the absence of NCC and that differentiation starts even before the neural crest migration. This highlights that, alongside the neural crest, mesoderm plays a paramount role in the patterning process.16–19
FIGURE 2.
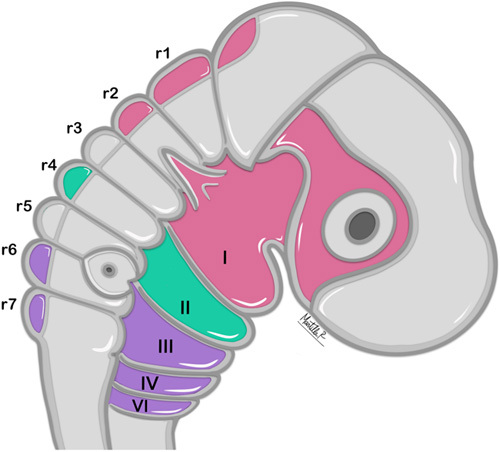
Rhombomere cell migration and derivatives within the pharyngeal apparatus.
On a molecular basis, the formation and development of each branchial arch will depend on the adequate expression of several genes. Hox genes, which are dependent of retinoic acid and key for an adequate growth and division along the anterior-posterior axis in vertebrates, serve to enable proper segmentation of arches 2 through 6.8,17,20,21 Dlx genes establish the pattern and polarity of pharyngeal arch 1. In addition, Dlx genes are paramount for the development of the upper and lower jaw.8,22
DERIVED STRUCTURES
Each pharyngeal arch is vital for the development of osseous, muscular, vascular, and nerve structures that will comprise the face, neck, and oropharyngeal apparatus. A detailed description of the derivatives of each arch, pouch, and cleft are given below, and a summary of each of these derivatives by arch number and germ layers are summarized in Supplemental Digital Contents 1 and 2, Supplemental Digital Content 1 and 2, http://links.lww.com/SCS/E980 and http://links.lww.com/SCS/E981, respectively. In addition, four cranial nerves will invade the pharyngeal arches and give rise to branches that will provide innervation to structures derived from each arch. These nerves include the trigeminal (CN V), facial (CN VII), glossopharyngeal (CN IX), and Vagus (CN X). A schematic illustration showing the osseous and muscle structures derived from each pharyngeal arch, along with the nerves associated with each arch are seen in Figure 3. Vascular derivatives of the pharyngeal arches are summarized in Figure 4.
FIGURE 3.
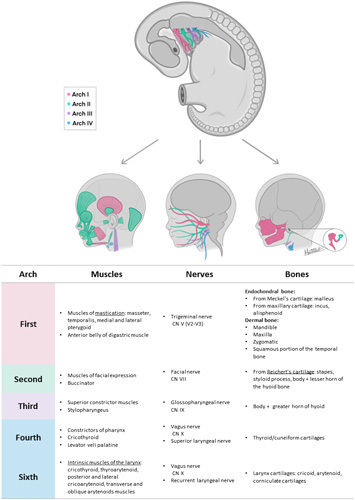
Osseous and muscle structures derived from each pharyngeal arch + schematic illustration of cranial nerves involved in each pharyngeal arch.
FIGURE 4.
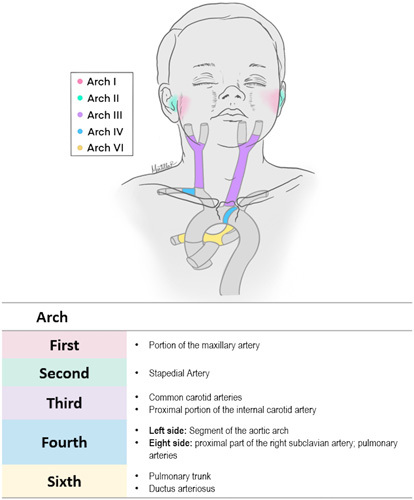
Vascular derivatives of each pharyngeal arch.
First Arch, Pouch, and Cleft
During the 5th week of embryo development, the first arch divides into two mandibular and two maxillary processes. The mandibular processes will merge forming the lower jaw, while the maxillary processes will extend and fuse with the primary palate to form the soft palate and a portion of the hard palate. Furthermore, this arch will give rise to additional osseous structures such as the malleus, incus, zygomatic bone, maxillary bone, and squamous portion of the temporal bone. Additional structures that arise from this arch are a small part of the maxillary artery, branches 2 and 3 of the trigeminal nerve (CN V2, V3) , and mastication muscles, including the masseter, temporalis, anterior belly of the digastric muscle, and both lateral and medial pterygoid muscles.8,23,24
The first pouch and cleft are paramount to the formation of the external and middle ear. Epithelium of the external auditory meatus derives from the ectoderm of the first pharyngeal cleft, while the endoderm composing the first pharyngeal pouch will give rise to the epithelium of the middle ear, tympanic cavity, and eustachian tube.3,25 Apposition of the external auditory meatus, tympanic cavity epithelia, and mesenchymal layer will form the tympanic membrane, which will play an essential role in translating the sound from waves to mechanical vibrations before transmitting it toward the inner ear.3,25 These first pouches and cleft will also be essential to coordinate the signaling and development of the remaining auditive structures, such as the outer and middle ear.26
Second Arch and Pouch
The second pharyngeal arch forms a wide array of derivates. This arch will contribute to completing the remaining ear structures with the formation of the stapes bone. The styloid process of the temporal bone and parts of the hyoid also come from this arch. Furthermore, facial muscles such as the occipitofrontalis, orbicularis oculi, corrugator supercilia, nasalis, procerus, depressor septi nasi, orbicularis oris, buccinator, depressor anguli oris, levator angulis ori, risorius, zygomaticus major and minor, levator labii superioris, alaeque nasi, depressor labii inferioris, mentalis, and platysma derived from the second arch mesoderm. Finally, this structure is also responsible for the formation of both the stapedial artery and the facial nerve (CN VII).8
The second pharyngeal pouch will contribute to the formation of the palatine tonsil epithelium. This pouch starts developing at week 8 of embryologic development, and tonsil-lymphoid infiltration will occur around the 7th month of gestation; however, adequate immune response will not occur immediately after birth. During the first year of life, the tonsils will increase its size due to intense lymphoid proliferation.3,27
Third Arch and Pouch
The third pharyngeal arch will form the inferior parts of the hyoid bone, the stylopharyngeus, and the superior constrictor muscle of the pharynx. The glossopharyngeal nerve (CN IX) also rise from this arch. Vascular structures derived from the second arch include the common carotid artery and the first part of the internal carotid.8
During the 6th week of embryogenesis, the third pharyngeal pouch will split into 2 wings. The ventral portion will form part of the thymus which will migrate downward to merge with its other portion in the anterior mediastinum, while the dorsal wing will create the inferior parathyroid glands, which will descend to their final position during week 7. This pouch is also in charge of creating the piriform fossa.28,29
Fourth Arch and Pouch
The fourth arch oversees the development of the thyroid and cuneiform cartilages, as well as the middle and inferior pharyngeal constrictor muscles and the cricothyroid levator veli palatine.8 The neural crest cells of this arch form the segment of the aortic arch artery located between the common carotid and the left subclavian artery. They will also give rise to the proximal portion of the right subclavian artery and the pulmonary arteries. As for nerve derivates, the superior laryngeal branch of the vagus nerve (CN X) will arise from this arch.8,28
The fourth pouch is responsible for the formation of the superior parathyroid glands and the ultimobranchial bodies. This latter structure will give rise to the parafollicular cells (C cells) of the thyroid gland, which are responsible for the secretion of calcitonin.30–32
Second, Third, and Fourth Clefts
These structures will organize and give rise to the cervical sinus. This sinus will obliterate around gestational week 7 due to the rapid overgrowth of the second pharyngeal arch; thus, no permanent derivatives arise from any of these clefts. Persistence of the cervical sinus will lead to embryogenic defects, which will be discussed in more detail in chapters 2 and 3 of this series.28,33
Sixth Arch
The 6th arch – in conjunction with the fourth arch, will contribute to the formation of the larynx cartilages by differentiating into the cricoid, arytenoid, and corniculate cartilages. It will also constitute the intrinsic muscles of the larynx such as the cricothyroid, thyroarytenoid, posterior and lateral cricoarytenoid, and transverse and oblique arytenoid muscles. This arch will also give rise to the ductus arteriosus and the pulmonary trunk.8,32
The sixth pouch will combine with the fourth pouch and contribute to the formation of the parafollicular C cells of the thyroid gland8,32.
CONCLUSION
The embryologic pharyngeal apparatus – comprised of several arches, pouches, and clefts – is a paramount assembly responsible for the development of numerous structures that play an essential role in the human body. The correct development of these structures is achieved by a series of complex embryologic and genetic mechanisms. In addition, an adequate formation and migration of all primordial germ layers is essential for the development of all the pharyngeal arches-derived structures. If normal development of the pharyngeal arches occurs, physical and functional abnormalities can occur.
Supplementary Material
Footnotes
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.jcraniofacialsurgery.com.
Contributor Information
Sara Toro-Tobon, Email: torotobonsara@gmail.com.
Monica Manrique, Email: mmanrique@childrensnational.org.
Juliana Paredes-Gutierrez, Email: julipagu@gmail.com.
Esperanza Mantilla-Rivas, Email: esmantilla4@gmail.com.
Haley Oh, Email: sulin.oh@hotmail.com.
Laiba Ahmad, Email: laibaa1@umbc.edu.
Albert K. Oh, Email: aoh@childrensnational.org.
Gary F. Rogers, Email: grogers@childrensnational.org.
REFERENCES
- 1. Graham A. Development of the pharyngeal arches. Am J Med Genet A 2003;119A:251–256 [DOI] [PubMed] [Google Scholar]
- 2. Graham A, Poopalasundaram S, Shone V, et al. A reappraisal and revision of the numbering of the pharyngeal arches. J Anat 2019;235:1019–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grevellec A, Tucker AS. The pharyngeal pouches and clefts: development, evolution, structure and derivatives. Semin Cell Dev Biol 2010;21:325–332 [DOI] [PubMed] [Google Scholar]
- 4. Graham A. The development and evolution of the pharyngeal arches. J Anat 2001;199(Pt1-2):133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. David NB, Saint-etienne L, Tsang M, et al. Requirement for endoderm and FGF3 in ventral head skeleton formation. Development 2002;129:4457–4468 [DOI] [PubMed] [Google Scholar]
- 6. Casale J, Giwa AO. Embryology, Branchial Arches. [Updated 2022 Aug 8]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538487/ [PubMed]
- 7. Benson MT, Dalen K, Mancuso AA, et al. Congenital anomalies of the branchial apparatus: embryology and pathologic anatomy. Radiographics 1992;12:943–960 [DOI] [PubMed] [Google Scholar]
- 8. Frisdal A, Trainor PA. Development and evolution of the pharyngeal apparatus. Wiley Interdiscip Rev Dev Biol 2014;3:403–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graham A, Richardson J. Developmental and evolutionary origins of the pharyngeal apparatus. Evodevo 2012;3:24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grenier J, Teillet M-A, Grifone R, et al. Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS ONE 2009;4:e4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kulesa PM, Bailey CM, Kasemeier-kulesa JC, et al. Cranial neural crest migration: new rules for an old road. Dev Biol 2010;344:543–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dupin E, Creuzet S, Le Douarin NM, et al. The contribution of the neural crest to the vertebrate body. Adv Exp Med Biol 2006;589:96–119 [DOI] [PubMed] [Google Scholar]
- 13. Namba K. Carotid-vertebrobasilar anastomoses with reference to their segmental property. Neurol Meidco-Chiqurgica 2017;57:267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Köntges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development 1996;122:3229–3242 [DOI] [PubMed] [Google Scholar]
- 15. Noden D. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol 1983;96:144–165 [DOI] [PubMed] [Google Scholar]
- 16. Veitch E, Begbie J, Schilling TF, et al. Pharyngeal arch patterning in the absence of neural crest. Curr Biol 1999;9:1481–1484 [DOI] [PubMed] [Google Scholar]
- 17. Gavalas A, Trainor P, Ariza-mcnaughton L, et al. Synergy between hoxa1 and hoxb1 : the relationship between arch patterning and the generation of cranial neural crest. Development 2001;128:3017–3027 [DOI] [PubMed] [Google Scholar]
- 18. Crump JG, Swartz ME, Kimmel CB. An integrin-dependent role of pouch endoderm in hyoid cartilage development. PLoS Biol 2004;2:E244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Couly G, Creuzet S, Bennaceur S, et al. Interactions between hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development 2002;129:1061–1073 [DOI] [PubMed] [Google Scholar]
- 20. Mark M, Ghyselinck NB, Chambon P. Retinoic acid signalling in the development of branchial arches. Curr Opin Genet Dev 2004;14:591–598 [DOI] [PubMed] [Google Scholar]
- 21. Trainor PA, Krumlauf R. Hox genes, neural crest cells and branchial arch patterning. Curr Opin Cell Biol 2001;13:698–705 [DOI] [PubMed] [Google Scholar]
- 22. Depew MJ, Simpson CA, Morasso M, et al. Reassessing the Dlx Code: the genetic regulation of branchial arch skeletal pattern and development. J Anat 2005;207:501–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heuzé Y, Kawasaki K, Schwarz T, et al. Developmental and evolutionary significance of the zygomatic bone. Anat Rec 2016;299:1616–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Som PM, Naidich TP. Illustrated review of the embryology and development of the facial region, part 2: late development of the fetal face and changes in the face from the newborn to adulthood. ANJR Am J Neuroradiol 2014;35:10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mallo M. Formation of the middle ear: recent progress on the developmental and molecular mechanisms. Dev Biol 2001;231:410–419 [DOI] [PubMed] [Google Scholar]
- 26. Mayer TE, Brueckmann H, Siegert R, et al. High-resolution CT of the temporal bone in dysplasia of the auricle and external auditory canal. ANJR Am J Neuroradiol 1997;18:53–65 [PMC free article] [PubMed] [Google Scholar]
- 27. Isaacson G, Parikh T. Developmental anatomy of the tonsil and its implications for intracapsular tonsillectomy. Int J Pediatr Otorhinolaryngol 2008;72:89–96 [DOI] [PubMed] [Google Scholar]
- 28. Johnson J, Moonis G, Green GE, et al. Syndromes of the first and second branchial arches, part 1: embryology and characteristic defects. ANJR Am J Neuroradiol 2011;32:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosen RD, Bordoni B. Embryology, parathyroid. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK554580/ Published 2022. Accessed December 16, 2022. [PubMed]
- 30. Allen E, Fingeret A. Anatomy, head and neck, thyroid. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK470452/ . Published 2022. Accessed December 16, 2022. [PubMed]
- 31. Mérida-Velasco JA, García-García JD, Espín-Ferra J, et al. Origin of the ultimobranchial body and its colonizing cells in human embryos. Acta Anat 1989;136:325–330 [DOI] [PubMed] [Google Scholar]
- 32. Miles B, Srinivasan VN. Embryology, pharyngeal pouch. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK557724/ . Published 2022. Accessed December 16, 2022. [PubMed]
- 33. Adams A, Mankad K, Offiah C, et al. Branchial Cleft anomalies: a pictorial review of embryological development and spectrum of imaging findings. Insights Imaging 2016;7:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]


