Abstract
Background and aim of the work:
Liquid levothyroxine (LT4) given at breakfast normalizes TSH in hypothyroid patients. However, a few studies are available on circulating free thyroxine (FT4) concentrations after liquid vs solid LT4 preparations.
Methods:
During an “ad interim” analysis on serum FT4 after 200 mcg liquid LT4 consumption while fasting in thyroidectomized thyroid cancer patients, we found that seven subjects fortuitously took liquid LT4 at breakfast. As established in the original protocol, serum FT4 was measured both at baseline as well as at 3 and 4 hours after solid or liquid LT4 consumption. We compared serum profile of FT4 in these subjects with those obtained in other subjects participating in the same study who took liquid LT4 (n. 7 subjects) or solid LT4 (n. 7 subjects) while fasting. The percentage increase of circulating FT4 was calculated at the above reported peak-times over the baseline values.
Results:
Circulating FT4 increased of about 40% in each group of subjects at both the 3rd and the 4th hour with no difference between these two time points in either group. The maximum FT4 % increase, irrespective of the time point, was 44.62 ± 3.05 (Mean ± SE), 44.84 ± 5.43, and 43.83 ± 1.30 after fasting solid, fasting liquid, and breakfast liquid LT4 consumption, respectively, with no differences among the three groups.
Conclusions:
Circulating FT4 obtained after 3 and 4 hours from the ingestion of 200 mcg liquid LT4 is not influenced by meal and is comparable with that observed after solid LT4 preparations ingested while fasting. (www.actabiomedica.it)
Keywords: thyroxine, absorption, liquid, FT4, breakfast, meal
Introduction
Levothyroxine (LT4) represents the most effective drug used for the treatment of hypothyroidism (1-3). It is also used in TSH-suppressive doses in patients with nontoxic uninodular or multinodular goiter and, after thyroidectomy, in those with differentiated thyroid cancer (4-6).
It represents one of the most commonly administered drugs in the world, and its use has been increasing in the last several years (7,8).
Approximately 60 to 90% of a LT4 dose is absorbed within 3 hours of ingestion with most of the absorption taking place in the jejunum and ileum (9,10). An empty stomach represents a very important condition for LT4 absorption with pre-breakfast fasting being associated with the highest intestinal absorption of the hormone (11). Also, a correct gastric acidity is reported to maximize LT4 absorption (12).
Therefore, LT4 is usually taken with water in the morning since its intestinal absorption has been shown to be altered by coffee (13) with fasting being maintained for approximately 30-60 minutes in order to have the drug correctly absorbed (14). The usual LT4 preparation is represented by tablets. However novel formulations, such as liquid form or soft-gel capsule are now available. The advent of these new LT4 preparations has prompt researchers to verify their impact on TSH control in hypothyroid patients and on the compliance of these patients with respect to the drug consumption. Liquid LT4 preparations taken while fasting has been shown to result in optimal TSH control (15,16). However, due to the fact that liquid LT4 does not need gastric dissolution and enters directly into the small bowel where it is absorbed (17-19), interest has been devoted to the possibility that the interference operated by meal on LT4 absorption may be less important with this LT4 formulation as compared with the classic tablet LT4 preparation. Recently, TSH serum concentrations within the normal range have been reported after six weeks of treatment with liquid LT4 taken at breakfast (20). These data are undoubtedly promising for the general considerations about LT4 treatment. However, little is known on the effects of meal on serum concentration of FT4 after liquid LT4 consumption.
Taking advantage of a group of patients who fortuitously took liquid LT4 at breakfast, during a study designed to determine serum FT4 after LT4 administration, we had the opportunity to evaluate circulating concentrations of free thyroxine after liquid LT4 consumption in this condition and to compare them with those obtained at the same time-points in patients who took, while fasting, liquid or solid LT4.
Methods
Data presented here are derived from an “ad interim” analysis of a study, which is currently performed in our institution on serum FT4 levels after LT4 administration in patients thyroidectomized for thyroid cancer. According to the original protocol, participants were evaluated after being randomly assigned to receive one oral dose of 200 mcg solid (Tirosint compresse 100 mcg®, 2 tablets) or liquid (Tirosint fiala monouso 100 mcg®, 2 vials) LT4 (IBSA Farmaceutici Italia srl, Lodi, Italy) soonafter thyroidectomy and before radioiodine ablation of thyroid remnant. Participants were requested to fast for one hour after LT4 ingestion. Serum FT4 was evaluated at the 3rd and 4th hour following LT4 intake.
Subjects were excluded from the study if they were affected by gastrointestinal disease, including coeliac disease, cardiovascular disease, renal failure, or if they were taking drugs known to interfere with LT4 absorption, including proton pump inhibitors or other gastric anti-acid drugs, anti-epileptic drugs, oral anti resorptive agent for osteoporosis, estrogens, amiodarone, lithium, cholestyramine or ezetimibe; oral calcium intake was allowed if it did not exceed 1g per day and was taken at night. Similarly, oral iron intake was allowed at night.
The protocol was approved by the local ethics committee and conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Conference on Harmonisation. All the participants provided prior written informed consent.
Subjects were studied while on daily treatment with solid LT4 (Tirosint ®) at a mean dose of 1,9 mcg/kg per day.
Patients were submitted to blood withdrawal at 0800 after an overnight fast. Two basal samples were collected at a 15-minute interval. Then LT4 was ingested according to the different formulations and study conditions, with subsequent blood withdrawal at further time points as above described. Upon collection, sample were sent to the Laboratory for blinded FT4 evaluation.
During an “ad interim” analysis of data from this protocol, we found that seven subjects fortuitously took liquid LT4 at breakfast which was purchased at the near vending machine and which consisted, on average, of 6 cookies corresponding to 31.2 gr (132 kcal; fat, 3 gr; carbohydrate, 24 gr; protein, 2.4 gr; fibers 1.2 gr; salt 0.3 gr) + 1 cup of expresso coffee with 5 gr sucrose or 1 cup of cappuccino (1 small cup of expresso coffee + 50 ml skimmed milk) with 5 gr sucrose.
For this reason, these subjects dropped out from the original study. However, their serum FT4 levels were already measured at the third and fourth hour after liquid LT4 intake, as established by the protocol. This gave us the opportunity to compare post-LT4 serum FT4 levels in this group of subjects (named “Liquid at Breakfast”, LB) with those measured in patients who took solid or liquid LT4 in fasting conditions. To this purpose, we randomly selected seven patients from those who took liquid LT4 while fasting (named “Liquid”, L) and seven from those who took solid LT4 while fasting (named “Solid”, S).
Serum FT4 was measured by chemiluminescence (DIX800 Beckmann) with a sensitivity of 0.25 ng/dl. The intra- and inter-assay coefficients of variation with this method were less than 5% and 9%, respectively.
The increase of FT4 with respect to basal values was calculated as percentage for each time point on the mean value of the two basal levels of FT4.
Statistical analysis
Demographic characteristics of the subjects were compared by ANOVA or chi-square test, as appropriate.
Comparison between 3- and 4-hour FT4 % increase within each treatment group was done by the Wilcoxon signed rank test. Comparison of the 3-hour and 4-hour FT4 % increase as well as of the maximum FT4 % increase among the three groups of treatment was done by the test of Kruskal-Wallis.
All values are expressed as mean ± SE unless other-wise specified. Significance was set at p<0.05. All statistical calculation were made by SPSS 22 software.
Results
The characteristics of patients are reported in Table 1. There were no differences in age, sex body mass index (BMI) or basal FT4 circulating concentrations among the three groups of subjects. Two subjects in the L group and in the S group had anti-thyroid peroxidase antibodies positivity; anti- thyroglobulin antibodies positivity was found in one subject of the LB group.
Table 1.
Demographic characteristics of the three groups of treatment
| S | L | LB | P* | |
|---|---|---|---|---|
| Age (years) (mean ± SD) | 49.57 ± 9.36 | 50.85 ± 8.93 | 53.42±10.39 | NS |
| Sex (female) n, (%) | 4 (57) | 5 (71) | 5 (71) | NS |
| BMI (Kg/m2) (mean ± SD) | 26.2 ± 4.7 | 27.5 ± 3.8 | 26.9 ± 5.3 | NS |
| Basal FT4 (ng/dL) (mean ± SD) | 0.92 ± 0.22 | 0.88 ± 0.10 | 0.91 ± 0.15 | NS |
S, solid in fasting conditions; L, liquid in fasting conditions; LB, liquid at breakfast; FT4, free thyroxine;
*, ANOVA or Chi-square as appropriate; SD, standard deviation
No differences were demonstrated in either treatment group in the comparison of FT4 % increase between the 3rd and 4th hour after LT4 ingestion, as reported in Table 2, although a slight, not significant, decrease was noted in L group.
Table 2.
FT4 % increase at the 3rd and 4th hour after LT4 ingestion (mean ±SE)
| 3rd hour | 4th hour | P | |
|---|---|---|---|
| S | 42.08±3.33 | 42.56±2.90 | 0.86 |
| L | 43.67±5.78 | 40.47±4.03 | 0.24 |
| LB | 40.46±1.07 | 40.75±2.11 | 1.00 |
S, solid in fasting conditions; L, liquid in fasting conditions; LB, liquid at breakfast
The overall comparison of FT4 % increase within the 3rd hour and the 4th hour values among the three treatment groups did not show any statistical significance (Figure 1).
Figure 1.
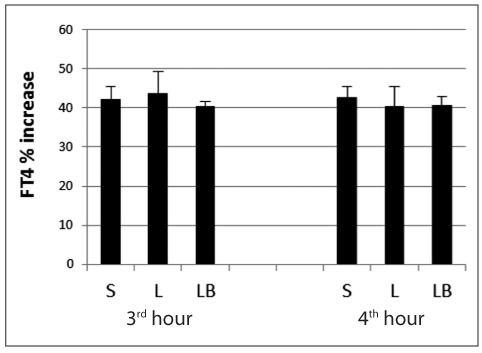
FT4 percentage (%) increase evaluated at the third and fourth hour after LT4 intake in the three groups of subjects.
S, solid; L, liquid; LB, liquid at breakfast.
Comparison of the maximum FT4 % increase, irrespective of the time point is reported in Figure 2 with no difference among the three groups of treatment.
Figure 2.
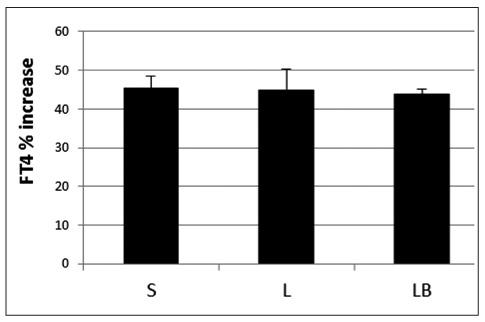
Maximum FT4 percentage (%) increase irrespective of the time-point evaluation calculated after LT4 intake in the three groups of subjects.
S, solid; L, liquid; LB, liquid at breakfast.
Discussion
In this study we found no difference in serum FT4 % increase among the three groups of treatment (i.e., fasting liquid LT4, at-breakfast liquid LT4, and fasting solid LT4).
Considering the liquid LT4 preparation, the suggestion coming from this study is that the increase in serum FT4 after liquid LT4 intake is not influenced by meal.
The blunting effect of meal, (i.e, breakfast), and even some beverages such as coffee, on LT4 absorption is well known (11-14,21) and represents an important characteristic of LT4 treatment which may compromise the compliance of patients with respect to their therapy. For this reason, the current guidelines for the treatment of hypothyroidism by a Task Force of the American Thyroid Association recommend that for optimal and consistent absorption, LT4 should, if possible, be taken at least 30 minutes before breakfast (or at bedtime, at least three hours after the evening meal) (2).
The advent of new LT4 preparations prompted researchers to challenge their potential advantage on the traditional tablet formulation.
In 2012, Yue et al demonstrated that the solution formulation of LT4 is associated with a similar rate and overall extent of exposure/bioavailability as the soft gel capsule and the tablets formulations (22). More recently, the liquid form of LT4 has been demonstrated to be more effective than LT4 tablet in controlling TSH levels in hypothyroid patients without malabsorption, gastric disorders, or drug interference (15).
One of the most interesting point is whether the absorption of LT4 administered through these new formulations is impaired by food intake as observed for the tablet preparation.
A recent randomized placebo-controlled study demonstrated no statistically significant differences in basal serum TSH, FT4, or FT3 levels whether LT4 was taken at breakfast or 30 minutes before, in a fasting state, suggesting that a liquid LT4 formulation can be ingested directly at breakfast (20). However, it is important to note that, notwithstanding the extreme interest of this kind of studies, the long inter-observation time of these study design cannot ascertain the compliance of patients about the correct intake of the drug.
Our study used a different approach to evaluate the feasibility of at-breakfast liquid LT4 treatment, looking at the rise in serum FT4 after both at-breakfast and fasting liquid LT4 ingestion as well as fasting LT4 tablets and comparing these FT4 increments among these three different conditions of LT4 ingestion. With respect to liquid LT4 formulations, our results, demonstrating the lack of differences in serum FT4 percentage increase after liquid LT4 taken either in fasting condition or at breakfast, give further evidence that liquid LT4 taken at breakfast has no disadvantage in comparison with liquid LT4 taken in fasting conditions.
An interesting point of this study is that the FT4 increases after at-breakfast- or fasting liquid LT4 intake were comparable to those detected after solid LT4 taken in fasting conditions. This is an important observation since LT4 treatment is traditionally considered as carried out by tablet LT4 preparation. Our data are in line with the reports demonstrating a comparable bioequivalence of different LT4 preparations including liquid and solid formulations (17,22). However, in our study we included in the comparison the other way of ingestion which is represented by at-breakfast liquid LT4 and found no difference in FT4 increase among these three conditions.
Therefore, the results of the present study give substantial support to the concept that liquid LT4 taken at breakfast may represent a valuable alternative to other type of LT4 treatment.
A point of strength of the present study is that subjects were all totally thyroidectomized before participating in the study. This avoided any effects of endogenous hormone secretion on the results.
We had no groups of patients who took solid LT4 at breakfast. This could be considered as a limitation of the study. However, this condition was not planned in the original protocol from which these results were drawn. Beside that, since our thyroid cancer patients needed to be daily treated in order to decrease their circulating thyrotropin, it would have been probably questionable to ethically accept even a single challenge with solid LT4 at breakfast, since it is known that this would have result in a reduced LT4 absorption and hence a reduction of the suppressive action on thyrotropin.
One limitation of this study is represented by the low number of cases; however the findings of our “ad interim” analysis we show in these results did not represent the outcome the original study was planned for.
The fact that we analyzed FT4 increase as percentages over the baseline values rather than increases in the absolute values could also represent a limitation; however, it has to be pointed out that the values of the percentages of FT4 increase were higher, in each time points, than the % coefficient of variations in FT4 assay, indicating a good and reliable detection of the true increase in FT4 serum concentrations.
The response of serum FT4 following liquid LT4 ingestion has been shown to peak about 30 min earlier than that observed with solid LT4 which occurs between the second and the third hour after LT4 consumption (17,22), although variations in t-max between 1 hour and 6 hours have been reported for both solid and liquid LT4. Since our goal was to compare solid vs liquid formulations, the patients were sampled at the 3rd and the 4th hour after LT4 ingestion, because pharmacokinetic studies have shown that at those time-points circulating concentrations of thyroxine, still significantly high vs baseline, are comparable between the two formulations (22). For this reason we believe that our data may provide a real comparison between solid and liquid LT4 formulations.
Altogether, these data are in line with previous experiences from our group from more prolonged observations of FT4 increases after LT4 intake (unpublished data).
In conclusion, our study demonstrate that FT4 percentage increase observed after LT4 ingestion is comparable among liquid preparation ingested while fasting, liquid preparation ingested at breakfast, and solid preparation ingested while fasting. These data represent a new tool to suggest that LT4 treatment may be carried out by liquid LT4 ingested at breakfast.
References
- Biondi B, Wartofsky L. Treatment with thyroid hormone. Endocrine Reviews. 2014;35:433–512. doi: 10.1210/er.2013-1083. [DOI] [PubMed] [Google Scholar]
- Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the Treatment of Hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670–751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga WM. Paradigm shifts in thyroid hormone replacement therapies for hypothyroidism. Nat Rev Endocrinology. 2014;10:164–74. doi: 10.1038/nrendo.2013.258. [DOI] [PubMed] [Google Scholar]
- Fiore E, Rago T, Provenzale MA, et al. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27914 patients. Endocr Relat Cancer. 2010;17:231–9. doi: 10.1677/ERC-09-0251. [DOI] [PubMed] [Google Scholar]
- Hegedus L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev. 2003;24:102–32. doi: 10.1210/er.2002-0016. [DOI] [PubMed] [Google Scholar]
- Haugen BR, Alexander EK, Bible KC, et al. 2015. American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira C, Knudsen N, Ovesen L, et al. Doubling in the use of thyroid hormone replacement therapy in Denmark: association to iodization of salt? Eur J Epidemiol. 2011;26:629–35. doi: 10.1007/s10654-011-9590-5. [DOI] [PubMed] [Google Scholar]
- IMS Institute for Healthcare Informatics. The use of medicines in the United States: review of 2011. April 2012. IMS Health website. http://www.imshealth.com/ims/Global/Content/Insights/IMS%20Institute%20for%20Healthcare% 20Informatics/IHII_Medicines. [Google Scholar]
- Benvenga S, Bartolone L, Squadrito S, Lo Giudice F, Trimarchi F. Delayed intestinal absorption of levothyroxine. Thyroid. 1995;5:249–53. doi: 10.1089/thy.1995.5.249. [DOI] [PubMed] [Google Scholar]
- Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414–19. doi: 10.1089/thy.2011.0526. [DOI] [PubMed] [Google Scholar]
- Bach-Huynh TG, Nayak B, Loh J, Soldin S, Jonklaas J. Timing of levothyroxine administration affects serum thyrotropin concentration. J Clin Endocrinol Metab. 2009;94:3905–12. doi: 10.1210/jc.2009-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787–95. doi: 10.1056/NEJMoa043903. [DOI] [PubMed] [Google Scholar]
- Benvenga S, Bartolone L, Pappalardo MA, et al. Altered intestinal absorption of L-thyroxine caused by coffee. Thyroid. 2008;18:293–301. doi: 10.1089/thy.2007.0222. [DOI] [PubMed] [Google Scholar]
- Vita R, Saraceno G, Trimarchi F, Benvenga S. A novel formulation of L-thyroxine (L-T4) reduces the problem of L-T4 malabsorption by coffee observed with traditional tablet formulations. Endocrine. 2013;43:154–60. doi: 10.1007/s12020-012-9772-2. [DOI] [PubMed] [Google Scholar]
- Fallahi P, Ferrari SM, Antonelli A. Oral L-thyroxine liquid versus tablet in patients with hypothyroidism without malabsorption: a prospective study. Endocrine. 2016;52:597–601. doi: 10.1007/s12020-015-0836-y. [DOI] [PubMed] [Google Scholar]
- Cassio A, Monti S, Rizzello A, et al. Comparison between liquid and tablet formulations of levothyroxine in the initial treatment of congenital hypothyroidism. J Pediatr. 2013;162:1264–9. doi: 10.1016/j.jpeds.2012.11.070. [DOI] [PubMed] [Google Scholar]
- Walter-Sack I, Clanget C, Ding R, et al. Assessment of levothyroxine sodium bioavailability: recommendations for an improved methodology based on the pooled analysis of eight identically designed trials with 396 drug exposures. Clin Pharmacokinet. 2004;43:1037–53. doi: 10.2165/00003088-200443140-00006. [DOI] [PubMed] [Google Scholar]
- Koytchev R, Lauschner R. Bioequivalence study of levothyroxine tablets compared to reference tablets and an oral solution. Arzneimittelforschung. 2004;54:680–4. doi: 10.1055/s-0031-1297021. [DOI] [PubMed] [Google Scholar]
- Yannovits N, Zintzaras E, Pouli A, et al. A bioequivalence study of levothyroxine tablets versus an oral levothyroxine solution in healthy volunteers. Eur J Drug Metab Pharmacokinet. 2006;31:73–8. doi: 10.1007/BF03191122. [DOI] [PubMed] [Google Scholar]
- Cappelli C, Pirola I, Daffini L, et al. A Double-Blind Placebo-Controlled Trial of Liquid Thyroxine Ingested at Breakfast: Results of the TICO Study. Thyroid. 2016;26:197–202. doi: 10.1089/thy.2015.0422. [DOI] [PubMed] [Google Scholar]
- Benvenga S. When thyroid hormone replacement is ineffective? Curr Opin Endocrinol Diabetes Obes. 2013;20:467–77. doi: 10.1097/MED.0000000000000003. [DOI] [PubMed] [Google Scholar]
- Yue CS, Scarsi C, Ducharme MP. Pharmacokinetics and potential advantages of a new oral solution of levothyroxine vs. other available dosage forms. Arzneimittelforschung. 2012;62:631–6. doi: 10.1055/s-0032-1329951. [DOI] [PubMed] [Google Scholar]


