Abstract
Due to increasing shortage of public healthcare resources in many countries around the globe, the use of simple, rapid and inexpensive laboratory parameters may be seen as a valuable aid for preliminary and cost-effective risk stratification of patients. Anisocytosis, conventionally measured by hematologic analyzers as the red blood cell distribution width (RDW), is an index of the heterogeneity of erythrocytes volumes. Several lines of evidence now attest that increased RDW values are commonplace in patients with many disorders, especially in those with the most prevalent conditions such as cardiovascular disease, diabetes, cancer and infections. Although the nature of this association remains to be definitely disclosed, what is strongly emerging from the recent scientific research is that the RDW should now be regarded as a “non” innocent bystander, wherein anisocytosis may be an active player in the pathogenesis of many pathologies. Therefore, major attention should be placed on this inexpensive but clinically meaningful parameter. The recent finding that dynamic changes of RDW are strongly predictors of mortality also suggests that continuous monitoring of anisocytosis may be an ancillary useful tool for establishing the effectiveness of managed care, as well as for deciding whether or not the overall clinical status is improving. (www.actabiomedica.it)
Keywords: red blood cell distribution width, RDW, mortality, outcome
Introduction
Laboratory diagnostics in the third millennium is now integral to the clinical decision making. Although we would all agree that it remains challenging to establish the real extent to which laboratory information really impacts managed care, it is now undeniable that the introduction of innovative biomarkers in certain diagnostic arenas have generated a paradigm shift in our understanding of pathophysiology, but have also revolutionized diagnosis, prognostication and therapeutic monitoring of many pathologies (1). Among these, myocardial infarction is a good example, wherein the routine use of high-sensitivity troponin immunoassays not only has allowed to reach a more efficient and timely diagnosis, but has also modified the actual definition of the disease, wherein the vast majority of cases of unstable angina can now be reclassified as non-ST elevation myocardial infarction (NSTEMI) (2). In a world with limited resources, however, broadening diagnostic panels with many innovative biomarkers is increasingly challenging, inasmuch as the final decision as to whether introducing or not a new laboratory test is dependent upon a good compromise between the real clinical usefulness and the overall expenditure (i.e., cost of the test, instrumentation and personnel) (3). Since establishing the exact breakeven between clinical efficacy and costs is seldom difficult, and many tests are hence waiting in line due to shortage of public healthcare funding in many laboratories, the rediscovery of some easy and inexpensive laboratory parameters should be regarded as an intriguing opportunity. Therefore, in this narrative review we make a paradigmatic example on how the red blood cell distribution width (RDW), a simple and frequently overlooked laboratory test, can often provide clinically meaningful information that may appear even greater than that generated by other, more expensive and analytically challenging biomarkers.
Anisocytosis
The red blood cells (RBCs), also known as erythrocytes, are non-nuclear cellular elements, the main function of which is transporting haemoglobin-bound oxygen from the lungs to the peripheral tissues. A typical human RBC has a diameter comprised between 6-8 µm and a thickness of 2 µm, which give it a typical biconcaval shape. The mature RBC has a total volume of approximately 90 fL, which can however vary from 60 to 150 fL in the presence of a discrete number of metabolic abnormalities influencing RBC production, survival and turnover (4). The modern generation of hematologic analyzers is capable to perform the complete blood cell count (CBC), but can also provide a number of additional parameters relative to the different blood cell populations, which may be helpful for identifying various genetic or acquired abnormalities of blood corpuscular elements. As regards RBC, and beside the measurement of the total RBC count, hematocrit and haemoglobin concentration, the most informative parameters that are automatically provided by all modern hematologic analyzers include the mean corpuscular volume (MCV), the mean corpuscular hemoglobin (MCH), the mean corpuscular hemoglobin concentration (MCHC) and the RDW. Traditionally, these indices are widely used for the differential diagnosis of anemia, but lesser interest is usually attributed to their potential clinical usefulness outside the boundaries of primary hematologic disorders (4).
The RDW is a calculated erythrocyte parameter, expressing the heterogeneity of RBC volumes in the blood sample (5). This definition is not ancillary, wherein the morphology of the erythrocytes is not always identical in circulating blood and in the blood tube. This may depend upon the nature of the additive used for inhibiting blood coagulation (i.e., different ethylenediaminetetraacetic acid [EDTA] salts may be used), the time between sample collection and analysis (the EDTA has a typical “swelling” effect on RBC, which is magnified 2-3 hours after blood sampling) (6), the procedure used for drawing blood and the potential problems emerging from this activity (i.e., spurious hemolysis, RBC agglutination, blood contamination with infusion fluids) (7). An additional source of problems is the current lack of harmonization of this measure, wherein the analytic techniques and the algorithms used for calculating both the MCV and its distribution may widely differ using different laboratory instrumentation (8). Irrespective of these preanalytical and analytical issues, the RDW may be finally expressed as the standard deviation (SD) of RBC volumes (i.e., RDW-SD; conventional reference range: 39-46 fL), or else can be calculated as the RDW-SD divided by the MCV and then multiplied for 100 (i.e., RDW-CV; conventional reference range: 11.6-14.6%) (5).
Anisocytosis in health and disease
In a rather pragmatic perspective, the RDW reliably reflects the degree of anisocytosis, and can hence be considered an efficient marker of perturbation of erythrocyte biology. Previous evidence has been brought that birth season (9), ageing (10) and eventually endurance physical exercise (11) should be considered physiological determinants of the RDW value. On the other hand, the leading paraphysiological and pathological conditions that may impact RBC volume and size distribution include genetic disorders (i.e., quantitative and/or qualitative abnormalities of hemoglobin production) (12), as well as many acquired metabolic disorders such as iron, folate or vitamin B deficiencies (12), blood transfusions (12), erythrocyte fragmentation (13), oxidative stress (14), inflammation (15), dyslipidemia (16) and glycosylation of cell surface proteins (17). It is hence little surprising that a various degree of anisocytosis may be present in association with many human pathologies, especially those in which the derangement of biological pathways also impacts RBC turnover. To date, the number of highly prevalent human disorders in which the RDW value may be frequently outside its conventional reference range is rather broad, and encompasses cardiovascular disease (including atrial fibrillation and heart failure), venous thromboembolism, trauma, cancer, diabetes, infections, severe allergic reactions, impaired renal function, liver dysfunction and thyroid disorders, among others (5). In most of these conditions the RDW value not only has a meaningful diagnostic utility (alone or in combination with other biomarkers, clinical signs and symptoms), but was also found to have a reliable prognostic role (i.e., in terms of cardiovascular and overall mortality) along with potentially useful therapeutic implications (5). More specifically, recent evidence attests that variations of RDW in patients undergoing therapy with hypocholesterolemic agents may provide valuable information for establishing the effectiveness of the treatment (18). It is also noteworthy that an increased RDW value significantly and independently predicts both cardiovascular and overall morality in the general population (19,20). In a recent study totalling more than 4,414 elderly subjects, the RDW was also found to be a significant predictor of frailty (21). Measuring anisocytosis would hence appear as an additional tool for deciding whether or not a certain patient may need a more accurate clinical monitoring, tailored preventive measures and/or a more aggressive management.
Interesting evidence is also emerging about the dynamic changes of RDW. Some recent studies showed that variations of anisocytosis during hospitalization (i.e., the so-called “delta RDW”) are independent predictors of outcome in patients with heart failure (22), but can also predict in-hospital mortality in the general inpatient population (23), thus underpinning the fact that longitudinal monitoring of anisocytosis may be a valuable aid for deciding the effectiveness of a certain therapy and tailoring treatments.
The “chicken and the egg” paradox
Given the fact that the clinical significance of measuring RDW seems now clearly affirmed in clinical practice, some important questions remain unanswered. Should the variation of RDW be considered an epiphenomenon (i.e., a consequence) of an underlying human disorder? Should the variation of RDW be considered an “accompanying abnormality” in many human disorders? Should the variation of RDW be considered a direct (or indirect) cause of human pathology? (24) Unfortunately, no definitive answers can be given to these questions at this point in time, but the possible scenario is that all these hypotheses may actually coexist.
As for the first question, the many human disorders that were found to be associated with increased RDW values may have a strong impact on effective production, release and survival of erythroid precursors (i.e., usually reticulocytes) from the bone marrow, as well as on RBC turnover in the circulation. Incidentally, effective erythropoiesis not only is influenced by the “mainstream hormone” erythropoietin, but also by a number of growth factors and cytokines such as stem cell factor (SCF), thrombopoietin (TPO), colony stimulating factor 2 (CSF2), interleukin (IL)-3 and IL-11 (25). The metabolism of many of these important biological mediators is variably perturbed in patients with severe pathologies such as cardiovascular disease, cancer, diabetes and infections. IL-6, another cytokine strongly up-regulated in many human disorders characterized by a strong inflammatory component such as infections, cardiovascular disease, cancer and diabetes (26), alters mitochondrial function in maturing erythroid cells, thus impairing hemoglobin production and erythroid maturation (27). Oxidative stress may also be a frequent determinant of RDW values in many disease conditions, wherein a higher oxidative stress may reduce erythrocyte survival, thus producing a higher degree anisocytosis due to enhanced rate of premature circulating erythrocytes (28). Several additional and disease-specific mechanisms (5) may be brought to justify the derangement of erythropoiesis and the possible development of anisocytosis in patients with the most prevalent human pathologies (i.e., cardiovascular disease, diabetes, cancer and infections) (29) (Fig. 1). Therefore, it is nothing but likely that anisocytosis may be a direct (or indirect) consequence of other underlying disorders. Notwithstanding this explanation, the main biological determinants of RDW, as those previously described in table 1, are frequently encountered in patients with multifaceted chronic disorders, as well as in those with specific organ injury (Table 2). Incidentally, the variable combination of these triggering conditions may be the concomitant cause of both anisocytosis and human pathology. Last but not least, due to the extraordinary oxygen-carrying capacity of the erythrocytes, it is not so illogical to conceive that a perturbation of RBC biology may also worsen, or even actively contribute to, tissue injury. Myocardial infarction may be seen as a paradigmatic example, wherein a significant and inverse association has been observed between RDW and oxygen saturation of blood, so implying that the higher the degree of anisocytosis, the lower the availability of hemoglobin-bound oxygen in peripheral tissues (30). In another elegant study, Patel et al demonstrated that increased RDW values were significantly and independently associated with reduced RBC deformability (31), which implies that a higher degree of anisocytosis would also impair the ability of RBCs to enter the microcirculation, ultimately decreasing the oxygenation of the target organs. Interestingly, it has also been speculated that anisocytosis due to a significant increases of the number of older RBC in the circulation may be associated with the inhibition of nitric oxide (NO) bioactivity, and may hence be responsible for lower flow-dependent dilatation of conduit arteries (14). All these mechanisms may substantially contribute to enhance the severity of ischemic injuries and reduce the chance of tissue recovery from ischemic and/or oxidative damages (Fig. 2).
Figure 1.
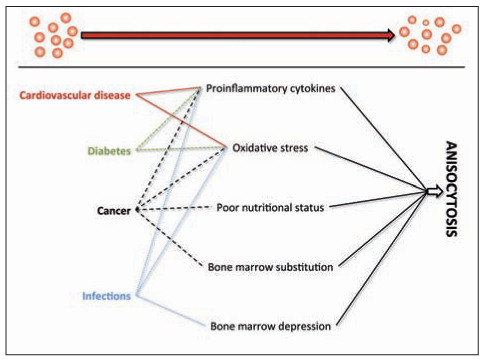
Putative mechanisms responsible for the derangement of erythropoiesis and the possible development of anisocytosis in patients with the most prevalent human disorders (29)
Table 1.
Main determinants of red blood cell distribution width
Physiological
Paraphysiological and pathological
|
Table 2.
Relative contributions of the main determinants of anisocytosis to the pathogenesis of the most prevalent human disorders (29)
| Disease | Cardiovascular disease | Cancer | Diabetes | Infections |
|---|---|---|---|---|
| Ageing | ** | ** | * | - |
| Nutrition deficiencies | -/* | *** | -/* | - |
| Erythrocyte injury | -/* | * | ** | * |
| Oxidative stress | *** | ** | *** | *** |
| Inflammation | *** | * | -/* | *** |
| Dyslipidemia | *** | -/* | ** | - |
| Glycosylation of surface proteins | * | -/* | *** | - |
-/*, present or non-present; * possibly present; **, frequently present; ***, commonplace
Figure 2.
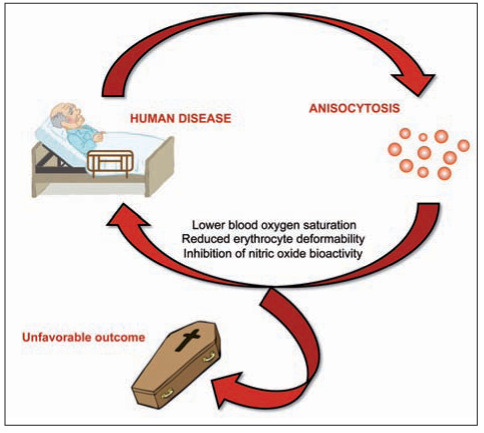
The vicious circle linking human pathology and anisocytosis
Conclusions
Due to an unprecedented economic crisis, which is still distressing the available resources of most health care systems around the globe (32), the use of simple, rapid and inexpensive laboratory parameters may be seen as a valuable aid for a preliminary and cost-effective risk stratification of patients in terms of probability of unfavourable clinical outcomes, including mortality (33,34). In the legendary Sherlock Holmes short stories, the British writer and physician Sir Arthur Conan Doyle wrote that “…once you eliminate the impossible, whatever remains, no matter how improbable, must be the truth” (35). Accordingly, the large and constantly increasing amount of biological and clinical evidence accumulated in recent years about the role of anisocytosis in health and disease (Fig. 3) would ultimately suggest that, no matter the many challenges remaining for explaining the multifaceted interplay between anisocytosis and human pathology, the role of RDW in modern science and medicine cannot be refuted. What is prepotently emerging from the current scientific research is that anisocytosis should now be regarded as a “non” innocent bystander, since it may be a crucial part of a vicious circle in which some human pathologies trigger anisocytosis, which in turn contributes to worsen the clinical outcome or else predispose the patient to the development of additional comorbidities and unfavourable outcomes (Fig. 2). We hence believe that major attention should be paid to this informative parameter in the initial clinical assessment, but continuous monitoring of anisocytosis may also generate mindful information for establishing the effectiveness of managed care, as well as for deciding whether or not the overall clinical status is improving. Importantly, the emerging evidence that patients with decreasing RDW values exhibit a much better prognosis than those with increasing anisocytosis (19) suggests that RDW-guided treatment may be regarded as an intriguing and cost-effective perspective to be investigated in future studies.
Figure 3.
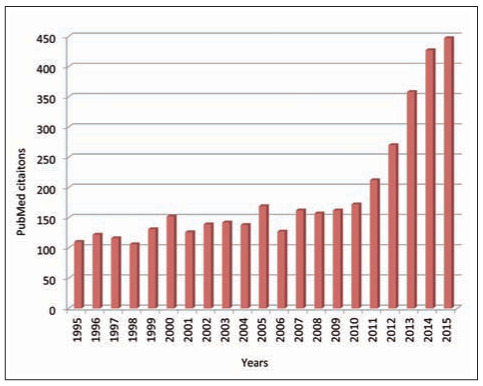
Number of PubMed citations in the past 20 years using the key word “red blood cell distribution width”
References
- Plebani M, Lippi G. Improving diagnosis and reducing diagnostic errors: the next frontier of laboratory medicine. Clin Chem Lab Med. 2016;54:1117–8. doi: 10.1515/cclm-2016-0217. [DOI] [PubMed] [Google Scholar]
- Braunwald E, Morrow DA. Unstable angina: is it time for a requiem? Circulation. 2013;127:2452–7. doi: 10.1161/CIRCULATIONAHA.113.001258. [DOI] [PubMed] [Google Scholar]
- Lippi G, Plebani M. Laboratory economics. Risk or opportunity? Clin Chem Lab Med. 2016 May 20 doi: 10.1515/cclm-2016-0313. doi: 10.1515/cclm-2016-0313. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lippi G, Cervellin G, Favaloro EJ, Plebani M. In vitro and in vivo hemolysis. An unresolved dispute in laboratory medicine. De Gruyter, Berlin, Germany. 2012 [Google Scholar]
- Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52:86–105. doi: 10.3109/10408363.2014.992064. [DOI] [PubMed] [Google Scholar]
- Banfi G, Salvagno GL, Lippi G. The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes. Clin Chem Lab Med. 2007;45:565–76. doi: 10.1515/CCLM.2007.110. [DOI] [PubMed] [Google Scholar]
- Lima-Oliveira G, Lippi G, Salvagno GL, Picheth G, Guidi GC. Laboratory Diagnostics and Quality of Blood Collection. J Med Biochem. 2015;34:288–94. doi: 10.2478/jomb-2014-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Pavesi F, Bardi M, Pipitone S. Lack of harmonization of red blood cell distribution width (RDW). Evaluation of four hematological analyzers. Clin Biochem. 2014;47:1100–3. doi: 10.1016/j.clinbiochem.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Lippi G, Salvagno GL, Montagnana M, Danese E, Guidi GC. Birth season predicts the values of red blood cell distribution width (RDW) in adulthood. Clin Chem Lab Med. 2016;54:667–71. doi: 10.1515/cclm-2015-0829. [DOI] [PubMed] [Google Scholar]
- Lippi G, Salvagno GL, Guidi GC. Red blood cell distribution width is significantly associated with aging and gender. Clin Chem Lab Med. 2014;52:e197–9. doi: 10.1515/cclm-2014-0353. [DOI] [PubMed] [Google Scholar]
- Lippi G, Salvagno GL, Danese E, Tarperi C, Guidi GC, Schena F. Variation of red blood cell distribution width and mean platelet volume after moderate endurance exercise. Adv Hematol. 2014;2014:192173. doi: 10.1155/2014/192173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttarello M. Laboratory diagnosis of anemia: are the old and new red cell parameters useful in classification and treatment, how? Int J Lab Hematol. 2016 May 16 doi: 10.1111/ijlh.12500. doi: 10.1111/ijlh.12500. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Bessman JD, Gilmer PR, Jr, Gardner FH. Improved classification of anemias by MCV and RDW. Am J Clin Pathol. 1983;80:322–6. doi: 10.1093/ajcp/80.3.322. [DOI] [PubMed] [Google Scholar]
- Owusu BY, Stapley R, Honavar J, Patel RP. Effects of erythrocyte aging on nitric oxide and nitrite metabolism. Antioxid Redox Signal. 2013;19:1198–208. doi: 10.1089/ars.2012.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628–32. doi: 10.5858/133.4.628. [DOI] [PubMed] [Google Scholar]
- Lippi G, Sanchis-Gomar F, Danese E, Montagnana M. Association of red blood cell distribution width with plasma lipids in a general population of unselected outpatients. Kardiol Pol. 2013;71:931–6. doi: 10.5603/KP.2013.0228. [DOI] [PubMed] [Google Scholar]
- Symeonidis A, Athanassiou G, Psiroyannis A, Kyriazopoulou V, Kapatais-Zoumbos K, Missirlis Y, et al. Impairment of erythrocyte viscoelasticity is correlated with levels of glycosylated haemoglobin in diabetic patients. Clin Lab Haematol. 2001;23:103–9. doi: 10.1046/j.1365-2257.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- Lippi G. Red blood cell distribution width and mean platelet volume: Surrogate markers for, or treatment targets in, dyslipidemia? Clin Biochem. 2015;48:555–6. doi: 10.1016/j.clinbiochem.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. 2009;169:515–23. doi: 10.1001/archinternmed.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker S, Stevens J, Howell MD. Red cell distribution width and mortality in newly hospitalized patients. Am J Med. 2012;125:283–91. doi: 10.1016/j.amjmed.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Aarts S, Patel KV, Garcia ME, et al. Co-Presence of Multimorbidity and Disability with Frailty: An Examination of Heterogeneity in the Frail Older Population. J Frailty Aging. 2015;4:131–8. doi: 10.14283/jfa.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlestein JB, Lappe DL, Anderson JL, et al. Both initial red cell distribution width (RDW) and change in RDW during heart failure hospitalization are associated with length of hospital stay and 30-day outcomes. Int J Lab Hematol. 2016;38:328–37. doi: 10.1111/ijlh.12490. [DOI] [PubMed] [Google Scholar]
- Shteinshnaider M, Barchel D, Almoznino-Sarafian D, et al. Prognostic significance of changes in red cell distribution width in an internal medicine ward. Eur J Intern Med. 2015;26:616–22. doi: 10.1016/j.ejim.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Lippi G, Cervellin G, Sanchis-Gomar F. Red blood cell distribution width and cardiovascular disorders. Does it really matter which comes first, the chicken or the egg? Int J Cardiol. 2016;206:129–30. doi: 10.1016/j.ijcard.2016.01.122. [DOI] [PubMed] [Google Scholar]
- Dzierzak E, Philipsen S. Erythropoiesis: development and differentiation. Cold Spring Harb Perspect Med. 2013;3:a011601. doi: 10.1101/cshperspect.a011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisman EZ, Tenenbaum A. The ubiquitous interleukin-6: a time for reappraisal. Cardiovasc Diabetol. 2010;9:62. doi: 10.1186/1475-2840-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCranor BJ, Kim MJ, Cruz NM, et al. Interleukin-6 directly impairs the erythroid development of human TF-1 erythroleukemic cells. Blood Cells Mol Dis. 2014;52:126–33. doi: 10.1016/j.bcmd.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S. Red cell distribution width, inflammatory markers and cardiorespiratory fitness: results from the National Health and Nutrition Examination Survey. Indian Heart J. 2012;64:380–7. doi: 10.1016/j.ihj.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Plebani M. Biomarker research and leading causes of death worldwide: a rather feeble relationship. Clin Chem Lab Med. 2013;51:1691–3. doi: 10.1515/cclm-2013-0210. [DOI] [PubMed] [Google Scholar]
- Tomkiewicz-Pajak L, Plazak W, Kolcz J, et al. Iron deficiency and hematological changes in adult patients after Fontan operation. J Cardiol. 2014;64:384–9. doi: 10.1016/j.jjcc.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Patel KV, Mohanty JG, Kanapuru B, Hesdorffer C, Ershler WB, Rifkind JM. Association of the red cell distribution width with red blood cell deformability. Adv Exp Med Biol. 2013;765:211–6. doi: 10.1007/978-1-4614-4989-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano R. Health, medical care, and economic crisis. N Engl J Med. 2009;360:749–51. doi: 10.1056/NEJMp0809122. [DOI] [PubMed] [Google Scholar]
- Lippi G, Mattiuzzi C. The biomarker paradigm: between diagnostic efficiency and clinical efficacy. Pol Arch Med Wewn. 2015;125:282–8. doi: 10.20452/pamw.2788. [DOI] [PubMed] [Google Scholar]
- Lippi G, Mattiuzzi C, Cervellin G. No correlation between health care expenditure and mortality in the European Union. Eur J Intern Med. 2016 Mar 14 doi: 10.1016/j.ejim.2016.02.025. doi: 10.1016/j.ejim.2016.02.025. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Conan Doyle A. The Case-Book of Sherlock Holmes. London, UK: John Murray Publisher; 1927. [Google Scholar]


