Abstract
Leydig cell tumors of the ovary account for less than 0.1% of all ovarian tumors (1). We present two cases in which patients had markedly elevated serum testosterone levels and frank hirsutism. Both cases revealed right ovarian Leydig cell tumors upon oophorectomy with post-surgical resolution of hypertestosteronemia. While rare and difficult to diagnose, androgen secreting tumors should be suspected in women with hyperandrogenism and hirsutism, especially in the postmenopausal population. (www.actabiomedica.it)
Keywords: Leydig cell tumors, ovary, testosterone
Introduction
Hirsutism in women is most commonly attributed to polycystic ovarian syndrome or idiopathic cause. Less commonly, it is caused by congenital adrenal hyperplasia, Cushing syndrome, or ovarian or adrenal androgen secreting tumors. While ovarian and androgen secreting tumors affect only 0.001 to 0.003% of patients with hirsutism, ovarian steroid cell neoplasms cause hirsutism and virilization in 75% of cases (5, 8). Virilizing tumors make up less than 5% of ovarian neoplasms; of these, leydig cell tumors, which originate in ovarian stroma, account for less than 0.1% of all ovarian tumors and occur more commonly after menopause. Their unregulated testosterone secretion results in hyperandrogenism and virilization (1, 7). We present two cases in which patients with markedly elevated testosterone levels and frank hirsutism were found to have right ovarian Leydig cell tumors.
Case 1
A 56-year-old G3P2 female presented to endocrine and gynecology-oncology clinics with worsening hirsutism, acne, and hair thinning since the onset of menopause three years prior. She had a history of Type 2 diabetes mellitus, hyperlipidemia, hypertension, NASH cirrhosis, and possible PCOS. Her gynecologic history revealed menarche at age 13. She had two vaginal deliveries at full terms following clomid induction. On physical exam, she was overweight (body mass index 29 kg/m2) with temporal balding and midline hair thinning. She had coarse hair along her back and proximal aspects of her arms and legs with Ferriman-Galleway score of 36. Her labs revealed DHEA-sulfate 163 ng/mL (normal range 100-1600 ng/mL in postmenopausal females), androstenedione 169 ng/dL (normal <10-93 ng/dL in postmenopausal females), total testosterone 295 ng/dL (normal 2-45 ng/dL), and free testosterone 31.7 pg/mL (normal 0.1-6.4 pg/mL). Gonadotropins were in the menopausal range (follicle-stimulating hormone (FSH), 13.1 mIU/mL; luteinizing hormone (LH), 17.6 mIU/mL). Estradiol level was mildly elevated for the post-menopausal range at 29 pg/mL (normal <21 pg/mL).
Computed tomography scan (CT) of her abdomen and pelvis was notable for hepatosplenomegaly but no obvious adrenal hyperplasia or adrenal tumors. Transvaginal ultrasound showed a right ovary measuring 33 x 36 x 27 mm with a volume of 16.8 cc and left ovary measuring 32 mm x 40 mm x 25 with volume 16.8 cc. Both ovaries appeared prominent for postmenopausal status with increased vascularity, however no discrete tumors or free fluid were noted. Subsequent MRI revealed unremarkable ovaries measuring 2.7 cm on the left and 3.6 cm on the right with a subtle suggestion of signal shading in the right ovary but no discrete masses.
Figure 1.
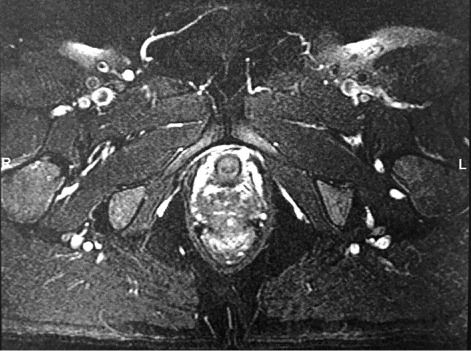
MRI Abdomen, Case 1: unremarkable ovaries, 2.7 cm on L and 3.6 cm on R. Signal shaesding in R ovary, no discrete masses
Figure 2.
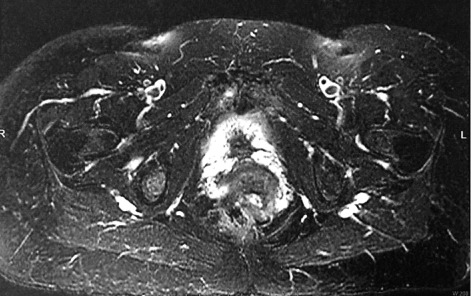
MRI Abdomen, Case 2: abnormally thickened endometrium with mild heterogenous enhancement. Myomatous uterus. Enhancing right adnexal mass
The patient underwent an exploratory laparotomy and bilateral salpingo-oophorectomy given high suspicion of a virilizing tumor. Intraoperatively, she was found to have an enlarged clitoris approximately 1.5 cm x 0.7 cm and enlarged ovaries, approximately 3 cm bilaterally. Microscopically the right ovary revealed fibrinoid necrosis of blood vessels, intracytoplasmic inclusions, and lipochrome pigment, most consistent with a Leydig cell tumor. No definitive Reinke crystals, which although pathognomonic are found in only 50% of tumors given irregular distribution in interstitial cells, were identified (6). Left ovary was normal. Pelvic washing was negative for malignant cells.
The patient’s testosterone levels normalized following successful surgical treatment of her benign Leydig cell tumor. There was no evidence of recurrence or metastases on follow up imaging.
Table 1.
Hormonal status, Cases 1 and 2
| DHEA sulfate, ng/mL | Androstenedione, ng/dL | Total testosterone, ng/dL | Free testosterone, pg/mL | FSH, mIU/mL | LH, mIU/mL | Estradiol, pg/mL | |
|---|---|---|---|---|---|---|---|
| Case 1 | 163 | 169 | 295 | 31.7 | 13.1 | 17.6 | 29 |
| Case 2 | 1660 | 287 | 34.7 | 21.1 |
Normal ranges in post-menopausal females: DHEA 100-1600 ng/mL, androstenedione <10-93 ng/dL, total testosterone 2-45 ng/dL, free testosterone 0.1-6.4 pg/mL, FSH 25.8-134.8 mIU/mL, LH 14.2-52.3 IU/L, estradiol <21 pg/mL
Case 2
Similarly, a 66 year old post-menopausal female presented to obstetrics and gynecology clinic with central hirsutism for one and a half years. She had a history of hypertension and hyperlipidemia. Her gynecologic history revealed menarche at age 12; she had two vaginal deliveries at full term with onset of menopause at age 57. Laboratory workup revealed serum testosterone level 287 ng/dL; FSH and LH were in menopausal range at 34.7 mIU/mL and 21.1 mIU/mL, respectively, with elevated DHEAS at 1660 ng/mL. CT scan showed multiple uterine fibroids and a right adnexal mass measuring 2.2 x 1.8 cm with increased prominence in the right ovarian vessels. Subsequent PET/CT scan showed a thickened endometrium and increased uptake in the ovaries bilaterally, although the ovaries were not well visualized; pelvic ultrasound revealed a heterogeneously thickened endometrium and uterine fibroids. MRI of the abdomen revealed abnormally thickened endometrium with mild heterogeneous enhancement and a myomatous uterus, as well as an enhancing right adnexal mass.
The patient underwent exploratory laparotomy with total abdominal hysterectomy and bilateral salpingo-oophorectomy. Intraoperatively, she was noted to have an irregular fibroid uterus with normal appearing ovaries. The right ovary appeared lobulated with a tan-yellow capsular surface and measured 2 x 1 x 0.5 cm. Bisection revealed a 1.8 x 1.4 x 1.3 cm tan-brown mass consistent with a steroid cell/Leydig tumor on electron microscopy. Histologic sections of the mass showed a well-circumscribed tumor composed of aggregates of large, monomorphic polypoid cells in nests and lobules. The cells contained large, round, centrally placed nuclei with prominent nucleoli and an abundant amount of granular, eosinophilic cytoplasm without definite Reinke crystals. Mitotic figures were inconspicuous and necrosis was not identified, however occasional tumor cells in the omental biopsy contained round cytoplasmic inclusions with mini-crystalline structures consistent with immature Reinke crystals. Left ovary was normal. Pelvic wash was negative for malignant cells.
As expected, the patient’s testosterone levels normalized following surgery with no further evidence of disease on follow up imaging.
Discussion
Leydig cell tumors are rare testosterone producing ovarian tumors that result in hyperandrogenism and virilization in women. While Leydig cell tumors are typically benign with excellent prognosis, they provide a diagnostic challenge as they are typically < 4 cm in size and difficult to detect on multiple imaging modalities (1).
Our review of the medical literature reveals five cases of unilateral Leydig cells tumors with peripheral serum testosterone ranging from 28 to >1600 ng/dL, normalized following bilateral oophorectomy (1, 3, 4, 6, 9). We report two additional cases that further confirm the difficulty in diagnosing virilization tumors biochemically or with imaging studies. While clinical and biochemical cues can help guide management, unfortunately the mainstay for diagnosis remains surgical intervention.
It is thought that serum testosterone levels above 200 ng/dL point to androgen-secreting tumors more so than non-neoplastic lesions such as PCOS. Similarly, DHEAS levels above 600 mg/dL suggest adrenal neoplasms more so than ovarian etiology (7). In Leydig cell tumors, serum testosterone is expected to be elevated with normal or only mildly elevated DHEAS levels as seen in our first case. However this was not true in our second patient whose DHEAS level was markedly elevated at > 1600 mg/dL. It is important to note that the specificity of DHEAS in detecting adrenal origin of hyperandrogenism ranges widely from 85-98% (3). Hormone markers alone are therefore not sufficient to diagnose underlying cause of hirsutism and virilization.
Imaging too can be nondiagnostic, often with no detection of ovarian change despite presence of a Leydig cell tumor. CT, MRI and transvaginal ultrasound did not reveal an obvious mass in our first patient. CT and MRI showed a right adnexal mass in our second patient, while transvaginal ultrasound was unremarkable despite being considered the most sensitive imaging tool for ovarian tumor detection. Diagnostic difficulty with imaging is likely due to the small size of Leydig cell tumors and the fact that they are isoechoic to the uterus on ultrasound and isodense to the uterus on CT (7).
Although five cases of bilateral tumors are reported in the literature, Leydig cell tumors are unilateral 95% of the time which raises the debate of selective ovarian venous sampling (5). In one case of suspected androgen secreting ovarian neoplasm in a peri-menopausal woman, intraoperative ovarian venous sampling showed more elevated levels in the right vein (1). In another menopausal patient with virilization and testosterone level 1330 ng/dL, venous sampling showed significantly higher total serum testosterone level in the left than in the right ovarian vein (9). While Leydig cell tumor localization was consistent with venous sampling in both cases, bilateral oophorectomy was performed regardless. Given the tendency toward bilateral resection despite venous findings, as well the inherent risk in ovarian vein catheterization and performer discordance, we agree against the general use of diagnostic catheterization, although perhaps it could play a beneficial role in women of reproductive age in which imaging is unrevealing (2).
In conclusion, we report two cases of Leydig cell tumors in post-menopausal women presenting with hirsutism. Although rare and difficult to diagnose biochemically or with imaging studies, androgen secreting tumors should be considered in the differential diagnosis in women with hyperandrogenism and hirsutism especially if postmenopausal. If virilization tumors are suspected, we recommend laparoscopic oophorectomy with or without total abdominal hysterectomy for both diagnostic and therapeutic purposes.
References
- Nardo LG, Ray DW, Laing I, Williams C, McVey RJ, Seif MW. Ovarian Leydig cell tumor in a peri-menopausal woman with severe hyperandrogenism and virilization. Gynecol Endocrinol. 2005;21(4):238–41. doi: 10.1080/09513590500369005. [DOI] [PubMed] [Google Scholar]
- Siekierska-Hellmann M, Sworczak K, Babińska A, Wojtylak S. Ovarian thecoma with androgenic manifestations in a postmenopausal woman. Gynecol Endocrinol. 2006;22(7):405–8. doi: 10.1080/09513590600842539. [DOI] [PubMed] [Google Scholar]
- Hofland M, Cosyns S, De Sutter P, Bourgain C, Velkeniers B. Leydig cell hyperplasia and Leydig cell tumour in postmenopausal women: report of two cases. Gynecol Endocrinol. 2013;29(3):213–5. doi: 10.3109/09513590.2012.705375. [DOI] [PubMed] [Google Scholar]
- Salim S, Shantha GP, Patel AD, et al. Virilizing ovarian steroid cell tumor in a 40 year old South Indian female: a case report. Cases J. 2009;2:7521. doi: 10.1186/1757-1626-2-7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz OA, Martinez PR, Guarch RT, Goñi MJ, Alcazar JL. Bilateral Leydig cell of the ovary: A rare cause of virilization in postmenopausal patient. Maturitas. 2007;57(2):214–6. doi: 10.1016/j.maturitas.2006.11.013. Epub 2007 Feb 7. [DOI] [PubMed] [Google Scholar]
- Yetkin DO, Demirsoy ET, Kadioglu P. Pure leydig cell tumour of the ovary in a post-menopausal patient with severe hyperandrogenism and erythrocytosis. Gynecol Endocrinol. 2011;27(4):237–40. doi: 10.3109/09513590.2010.490611. [DOI] [PubMed] [Google Scholar]
- Souto SB, Baptista PV, Braga DC, Carvalho D. Ovarian Leydig cell tumor in a post-menopausal patient with severe hyperandrogenism. Arq Bras Endocrinol Metabol. 2014;58(1):68–75. doi: 10.1590/0004-2730000002461. [DOI] [PubMed] [Google Scholar]
- Legro RS, Azziz R. Androgen excess disorders. Danforth’s Obstretrics and gynecology. 9th edition. Philadelphia: Lippincott Williams and Wilkins Publishers; 2003. pp. 663–85. [Google Scholar]
- Ozgun MT, Batukan C, Turkyilmaz C, Dolanbay M, Mavili E. Selective ovarian vein sampling can be crucial to localize a Leydig cell tumor: an unusual case in a postmenopausal woman. Maturitas. 2008;61(3):278–80. doi: 10.1016/j.maturitas.2008.09.003. [DOI] [PubMed] [Google Scholar]


