Abstract
Background:
Per- and polyfluoroalkyl substances (PFAS), endocrine disrupting chemicals with worldwide exposure, cause changes in mammary gland development in rodents. A few human studies report delay in pubertal events with increasing perfluorooctanoic acid (PFOA) exposure, but to our knowledge none have examined reproductive hormone levels at thelarche.
Methods:
In a cohort of Greater Cincinnati (GC) and San Francisco Bay Area (SFBA) girls recruited at 6–8 years of age, clinical examinations were conducted annually or semiannually with sequential Tanner staging. PFAS concentrations were measured in the first serum sample of 704 girls. In 304 GC girls, estradiol (), estrone (), testosterone (T), and dihydroepiandrosterone sulfate (DHEAS) were measured in serum at four time points around puberty. Relationships between PFAS and age at thelarche, pubarche, and menarche were analyzed using survival and structural equation models. The association between PFAS and reproductive hormones was assessed using linear regression models.
Results:
Median PFOA serum concentrations in GC (, ) and the SFBA (, ) were higher than in the U.S. population. In multivariable Cox proportional hazard models [adjusted for race, body mass index (BMI)], increasing serum log-transformed PFOA was associated with a delay in pubarche [; 95% CI: 0.70, 0.99] and menarche (; 95% CI: 0.01, 0.25). Structural equation models indicated a triangular relationship between PFOA, BMI percentile, and the age at the pubertal milestone. Increased PFOA had a statistically significant direct effect of delay on all three milestones, as did BMI. Perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDeA), and 2-(-methyl-perfluorooctane sulfonamido) acetic acid (Me-PFOSA-AcOH) also were associated with later thelarche, and Me-PFOSA-AcOH also with later pubarche. PFOA was inversely associated with DHEAS (), (), and T () concentrations at 6 months prior to puberty.
Conclusions:
PFAS may delay pubertal onset through the intervening effects on BMI and reproductive hormones. The decreases in DHEAS and associated with PFOA represent biological biomarkers of effect consistent with the delay in onset of puberty. https://doi.org/10.1289/EHP11811
Introduction
Early menarche is associated with increased breast cancer risk.1,2 Over time, the trend toward the decreasing age of initial breast development (thelarche) has led to conjecture and concern that earlier breast development also may lead to increased breast cancer risk3,4 and that environmental chemicals have contributed to this accelerated maturation.5 To address these concerns and further study breast cancer etiology, the Breast Cancer and the Environment Research Program (BCERP) was established to foster transdisciplinary research collaborations integrated across biologic, epidemiologic, and community outreach projects.5,6 Within the BCERP, a puberty cohort study was designed to explore potential factors associated with earlier breast development and other pubertal milestones, with a primary focus on exposure to endocrine disrupting chemicals (EDCs) prior to breast development.
PFAS are per- and polyfluoroalkyl substances that are man-made and were first introduced into the environment after World War II. They are used to make water- and stain-resistant materials used in carpeting, upholstery, clothing, and food packaging, as well as in the production of semiconductors. Given that these compounds do not break down once in the environment because of their strong carbon–fluorine bonds, they are not metabolized but, rather, bioaccumulate.7 PFAS have a long half-life in humans, ranging from 2.3 to 3.4 y, and have been detected in the serum of people in most industrialized countries.8–10 In the first report of PFAS in a representative sample of the U.S. population in 1999–2000 [National Health and Nutrition Examination Survey (NHANES)], the median serum concentration of perfluorooctanoic acid (PFOA) in children 12–19 years of age was 11,12 and in 2005–2006 was , but levels have decreased substantially to in 2015–2016. In the 2013–2014 exam cycle PFAS measurements were first made in children 6–11 years of age, and a median concentration of was found.12
Girls in the female BCERP puberty cohort study at the Greater Cincinnati (GC) and San Francisco Bay Area (SFBA) sites have been exposed to PFAS from sources beyond those in consumer products. Girls living in GC encountered exposure from an industrial plant in Hocking, West Virginia, that released PFOA into the Ohio River, which then traveled to communities downstream.13 In addition, hazardous waste sites along the Ohio River and other industrial facilities contributed to the amount in river water, which is used by the Cincinnati Water Works and the Northern Kentucky Water Department as a source of drinking water.13 During the 1990s, water departments serving southwestern Ohio used granular activated carbon (GAC) filtration to remove PFOA and other PFAS chemicals from drinking water. The water departments serving northern Kentucky did not use GAC filtration at that time.13 The SFBA, which includes the Silicon Valley area, has been home to many semiconductor companies14 that use PFAS in production.15,16 Girls in the BCERP puberty cohort from both the GC and SFBA have elevated PFAS serum concentrations17 measured at 6–8 years of age.
The reproductive health effects of perfluoroalkyl chemicals, including PFOA, have been studied extensively in animal models and in some study populations.18–20 However, we have found only one other previous study of PFAS exposure and thelarche and pubarche, but breast development staging was by self-assessment.21 One large study reported a delay in menarche with PFOA and perfluorooctanesulfonic acid (PFOS) exposure22 and another reported delay with maternal PFOA exposure.23 Two other studies reported no association with menarche.21,24 Limitations of previous studies of sex hormone measurements during puberty and PFAS exposure include being cross-sectional for pubertal staging and not linking the timing of reproductive hormone measurements to the time of thelarche.25–28
The primary objective of this study was to examine the longitudinal relationship between PFAS serum concentrations measured in young girls to determine whether PFAS exposure is associated with the age at three pubertal milestones: thelarche, pubarche, and menarche. To identify subclinical outcomes of the PFAS exposures, we also examined the relationship between serum reproductive hormone concentrations around the time of thelarche with PFAS exposure. Given that we have previously reported that elevated body mass index (BMI) accelerates the age at thelarche,29 and BMI is a covariate in these analyses, we also sought to determine whether BMI independently affects the levels of reproductive hormones.
Methods
Source Population
The BCERP puberty cohort study has been conducted at three sites: a) Mount Sinai School of Medicine, b) Kaiser Permanente Northern California, and c) Cincinnati Children’s Hospital/University of Cincinnati.30 Girls were recruited at 6–8 years of age in 2004–2007 and followed semiannually or annually to observe onset and progression of pubertal maturation. The study reported here included only GC and SFBA girls who had PFAS serum measurements (, 85.5% of 823 originally enrolled at these two sites; Table S1). The Mt. Sinai site did not obtain blood samples from the girls until relatively late in the study, and therefore PFAS measurements were not made in their serum (Figure S1).
The 379 participants at the GC site were recruited through public and parochial schools in the GC metropolitan area (; 85% of participants) as well as through the Breast Cancer Registry of Greater Cincinnati (; 15% of participants). Recruitment through the registry targeted those with a first- or second-degree family member with breast cancer. Girls eligible for recruitment at the Kaiser Permanente site (SFBA) were those who had been enrolled in the Kaiser Permanente system at SFBA clinics at birth and were enrolled at the time of study recruitment; 444 were enrolled (Table S1).
Previous Data Collection
Overall cohort study aims have been published previously.30 The studies were approved by the institutional review boards of Cincinnati Children’s Hospital Medical Center and Kaiser Permanente, and informed consent was obtained from parents or guardians and assent from the children. Informed written consent was obtained from each girl’s parent or guardian.
Data collection methods were consistent at the two sites and consisted of a yearly questionnaire and regular in-person clinical examinations and biospecimen collection. At both sites, baseline and annual follow-up questionnaires were administered to the girls’ parents or guardians. These included items about the health histories of the girl and her parents, such as whether the girl had been breastfed and the mother’s age at menarche. The girls were seen annually for clinical study visits (physical examinations including pubertal staging) in SFBA and semiannually (within wk of 6 months after the prior visit) in GC until 2010 and then annually until 2015.
Pubertal Maturation Assessment
Trained and certified female staff members performed standardized anthropometric measurements according to a common protocol, with two or more height and weight measurements using calibrated stadiometers and scales, as detailed previously.31 In addition, a limited number of female staff were trained and certified in assessment of pubertal maturation,32 using the criteria established by Marshall and Tanner for breast maturation (with palpation) and pubic hair stages,33 with photographs that demonstrated the maturation stages, published by van Wieringen.34 As a quality assurance measure, periodically a master trainer visited each site and conducted dual measurements, with interrater agreement of 87% () representing substantial agreement.30 Age of menarche, the first menstrual cycle, was determined by responses of each girl’s mother or guardian to interview questions (822/946 of girls at the three sites) or self-report of the study participant (124/946) regarding the girl’s first menstrual cycle regardless of its cyclicity.35 Given that parent/guardian information provided during the first 5 y of the study, was available on most of the girls, and usually closer in time to the menarche event, it was prioritized over self-report. Further details on pubertal milestone staging have been published previously.30,35
The first examination where Tanner breast stage was observed and breast tissue was palpated was termed the visit, and prior exams at Tanner breast stage 1 were TB1 visits. Pubarche, pubic hair maturation, as defined by the presence or absence of hair in the pubic area was determined by staff using accessory light sources, and the first examination where Tanner pubic hair stage was observed was termed the first visit. Girls with inconsistent staging of thelarche or pubarche at subsequent visits were considered to be at the earlier stage until continually at the higher sexual maturation stage.
Pubertal Milestone Outcomes
The primary outcomes of three ages at pubertal milestones—thelarche, pubarche, and menarche—were measured in months from birth. Each of these milestones was based on reaching Tanner sexual maturation stage or by self-report menarche in response to questions during an interview.
We also have previously reported our method for calculating estimated ages at thelarche and pubarche for all members of the cohort.29 With this method, we first defined a maturation interval (given that the event of first appearance of breast budding or first appearance of pubic hair is not observed) and then used accelerated failure time models to identify the age of the median probability of thelarche or pubarche across the interval. This method allowed us to estimate ages at thelarche and pubarche for girls who reached these milestones prior to the first study visit or who had missing visits or inconsistent staging across visits.
Covariates
Height and weight were measured at each study visit by trained female staff members. BMI was calculated as weight divided by height squared in kilograms per meter squared and then related to age- and sex-specific percentiles [BMI percentile (BMI%)] and -scores (BMIz) using the Centers for Disease Control and Prevention (CDC) growth charts.36 Some analyses were performed on substrata defined by girls at either below or at the median BMI% or above the median BMI% for girls in the analysis. Race/ethnicity and maternal age of menarche were obtained from the baseline and annual questionnaires. Race/ethnicity of the girl was self-reported and coded hierarchically as Black, non-Black Hispanic (Hispanic), non-Hispanic white, or Asian; participants could belong to only one race/ethnicity category. (We were unable to disaggregate race and ethnicity because of the design of the questionnaire item.) Maternal age of menarche was categorized as , but , or years of age.
Laboratory Analyses
Serum samples were drawn at each study visit in the early morning and stored at . The variance in time of blood collection was typically under 1 h. Missing PFAS and hormone measurements were due to a participant refusing to have her blood drawn or insufficient blood serum for measurement. PFAS in serum was measured by the CDC Environmental Laboratory using previously defined methods.37,38 PFAS measurements from each girl’s first serum sample were used for this analysis. Further details on PFAS assessment have been published previously.38,39 PFAS measured included PFOA, perfluorononanoic acid (PFNA), PFOS, perfluorohexane sulfonate (PFHxS), perfluorodecanoic acid (PFDeA), 2-(-methyl-perfluorooctane sulfonamido) acetic acid (Me-PFOSA-AcOH), 2-(-ethyl-perfluorooctane sulfonamido) acetic acid (Et-PFOSA-AcOH), and perfluorooctane sulfonaminde (PFOSA).
Only 304 girls from the GC site had serum samples for hormone analyses (Table S1). The other GC girls did not have an available serum sample before thelarche. Reproductive and adrenal hormones were measured in serum samples11 obtained at four time points around thelarche: 6 months prior to thelarche, at thelarche, 6 months after thelarche, and 12 months after thelarche. To be included in this analysis, a girl was required to have at least one of four hormone measurements from at least one of four time points. Many girls did not have hormone measurements at all four time points because serum aliquots had also been used for earlier serum biomarker measurements in multiple assays.40,41 The exact number for each assay ranged from 96 to 250. The four hormones analyzed were estradiol (), estrone (), dihydroepiandrosterone sulfate (DHEAS), and testosterone (T). LabCorp, certified by the CDC, performed the hormone assay. We used high-performance liquid chromatography–tandem mass spectrometry in analyses of the sex steroids, which allowed us a greater (10 times) sensitivity to examine hormonal concentrations even before the appearance of secondary sexual characteristics.42 The lower limit of quantification for DHEAS was , for , and for . The interassay precision (percentage coefficient of variation) for low, medium, and high control serum specimens was 4.9%, 4.6%, and 4.7% for ; 4.4%, 3.5%, and 3.3% for ; and 6.5%, 8.4%, and 7.3% for DHEAS.42 Further details on the hormone assays have been previously described.42
Statistical Analyses
All statistical analyses were performed using SAS (version 9.2; SAS Institute, Inc.). Missing data were set to “missing,” given that no imputations were performed to accommodate missing data. Summary statistic values for the characteristics of the GC girls were compared with those of the SFBA girls using Fisher exact test for categorical variables and Kruskal–Wallis for continuous variables. Following usual practice, PFAS levels below the limit of detection (LOD) were imputed using the value of the LOD divided by the square root of 2.43 The LOD specific to each batch and PFAS or hormone was used for imputation. This is a common method used for estimating analyte concentrations less than the LOD when they are not highly skewed and of the measurements are .43 The strength of the correlation of each PFAS with others was determined by calculating Pearson correlation coefficients.
Kaplan–Meier estimates of cumulative probability, stratified by PFOA serum concentration and BMI%, were used to visually examine the survival (time to pubertal milestone). For the longitudinal statistical analyses, PFAS serum concentrations were examined both as a log-transformed continuous variable and also categorized into quartiles for categorical analyses. We first performed analyses with either age at thelarche, at pubarche, or at menarche as the dependent variable, testing various independent variables: PFAS serum concentrations, BMIz at the clinical visit prior to the first observation of thelarche, pubarche, or menarche; race/ethnicity (four levels: Black, Hispanic, Asian, non-Hispanic white, and other); mother’s age at menarche (, but , or y); history of being breastfed (yes/no); parental education (four levels ranging from graduate to master’s degree or higher); and study site (GC/SFBA). Missing data were set to “missing”; no data imputations were performed except for the PFAS values , as described above.
We then conducted longitudinal analyses to determine the relative risk of advancing from thelarche or pubarche stages 1 to 2, or the risk of attaining menarche, in relation to PFAS serum concentrations and confounders. Each PFAS and the arithmetic sum of all PFAS were tested in separate models. These multivariable analyses were conducted using Cox proportional hazard models (PROC PHREG) and incorporating all of the covariates noted in the above paragraph to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for age at pubertal development ( or ) or menarche. Girls who had not reached thelarche, pubarche, or menarche at the end of observation (end of study or withdrawal) contributed person-time but not a pubertal event. Girls who either dropped out before the end of the study or did not reach a pubertal event during the study period were right-censored for the Cox models. For the Cox models, when the proportional hazards assumption failed, a time-dependent interaction between the age at the pubertal milestone in months and the exposure was added to each model to overcome this failure. The results of the final Cox models are expressed in HRs with Wald’s 95% CIs. Median ages at TB2, TP2, and menarche were predicted from the baseline survivor function of the adjusted Cox models.
Using the ages in months at thelarche and pubarche, logistic regression was used to estimate the odds of entering pubarche prior to, or at the same times as, thelarche. The results were expressed as odds ratios (ORs) with 95% CIs. We also used structural equation models (SEMs) to investigate the correct direction of the relationship between PFOA, BMI%, and the pubertal milestones. The SEM is a statistical method using the maximum likelihood estimator to model multiple related regression relationships. In these models, variables may be both dependent and independent, based on their relationships with other variables. Variables included in the SEMs were in the same format as the logistic regression models: PFOA (log-transformed); race/ethnicity, BMI%, Site (GC or SFBA). Separate models were constructed for the three outcome variables, namely, age at thelarche, pubarche, and menarche. Semiparametric SEMs used regression- and survival-mediated analysis to determine this relationship. Monte Carlo integration was used to stabilize the models.
We also performed a series of analyses on the girls from GC with at least one of four hormone (DHEAS, , , T) measurements from at least one of four time periods (6 months prior to thelarche, thelarche, 6 months after thelarche, 12 months after thelarche). Earlier in the program when the focus of analyses was on breast development, samples for hormone measurements were selected relative to the time of thelarche. Multiple variable linear regression models, adjusted for race and BMIz, were used to determine the relationship among PFOA and the hormones at the four time periods relative to thelarche.
All data preparation and analyses except SEM were conducted using SAS (version 9.2; SAS Institute, Inc.). All SEM analyses were performed using Mplus (version 5.2; Muthèn & Muthèn). Model assumptions for normal distribution of data for linear regression analyses were verified. As a result, the PFAS and hormone concentrations were log-transformed to overcome nonnormality. The log-transformed values were found to be normal in distribution and were used in all statistical models except descriptive statistics. A -value of was used to determine statistical significance.
Results
Participant Characteristics and PFAS Distribution
Girls with serum measurements of PFAS were equally represented by both sites. Of the 839 girls from these two sites, 704 had serum measurements of PFAS, or 93% (353/379) of the girls from GC and 79% (351/444) of the girls from the SFBA. Girls in the original cohort at these two sites but not eligible for these analyses (), did not have serum PFAS measurements and were not different by race, BMI at baseline, or age at menarche, but they were younger at thelarche and pubarche when compared with girls who were included (Table S2). Cohort members included in these analyses were distributed across four racial groups, although most of the Asian and Hispanic girls were from the SFBA site (Table 1). Slightly over half the girls were non-Hispanic white (51.7%) and 27.58% were Black, 6.53% Asian, and 13.92% Hispanic.
Table 1.
Characteristics of the girls in the puberty cohort with PFAS serum measurements, 2004–2014, by study site.
| Characteristic | Both sites combined () | Greater Cincinnati Area () | San Francisco Bay Area () | -Valuea |
|---|---|---|---|---|
| Median BMI% at baseline | 66.39 | 64.89 | 68.82 | 0.28 |
| Mean age of pubertal stage [months ( mean]b | ||||
| Thelarche | ||||
| Pubarche | 0.27 | |||
| Menarche | 0.65 | |||
| Pubertal stage attained by end of follow-up [ (%)] | ||||
| Thelarche | 655 (93.04) | 331 (93.75) | 324 (92.31) | 0.72 |
| Pubarche | 633 (89.91) | 316 (89.49) | 317 (90.31) | 0.52 |
| Menarche | 575 (81.67) | 263 (74.50) | 312 (88.89) | |
| Median BMI% closest to pubertal stagec | ||||
| Thelarche | 65.74 | 65.67 | 66.28 | 0.52 |
| Pubarche | 67.26 | 69.05 | 64.72 | 0.70 |
| Menarche | 67.28 | 68.11 | 65.83 | 0.20 |
| Race/ethnicity [ (%)] | ||||
| White | 364 (51.70) | 215 (61.08) | 149 (42.45) | |
| Asian | 46 (6.53) | 5 (1.42) | 41 (11.68) | |
| Hispanic | 98 (13.92) | 14 (3.98) | 84 (23.93) | |
| Black | 196 (27.84) | 119 (33.52) | 77 (21.94) | |
| Maternal age of menarche (y) [ (%)] | 0.22 | |||
| 153 (21.73) | 71 (20.17) | 82 (23.36) | ||
| Between 12 and | 404 (57.39) | 214 (60.51) | 190 (54.13) | |
| 147 (20.88) | 68 (19.32) | 79 (22.51) | ||
| Parent/caregiver education [ (%)] | ||||
| Grade school, some high school, high school grad or GED | 98 (13.92) | 33 (9.35) | 65 (18.52) | |
| Some college/technical/trade/vocational school or an associate’s degree | 226 (32.10) | 116 (32.86) | 110 (31.34) | |
| Bachelor’s degree | 204 (28.97) | 103 (29.18) | 101 (28.77) | |
| Master’s degree or higher | 127 (18.03) | 83 (23.51) | 44 (12.54) | |
| Missing | 49 (6.96) | 18 (5.10) | 31 (8.83) | |
Note: Significance declared at -value . BMI, body mass index; BMI%, BMI percentile; CDC, Centers for Disease Control and Prevention; GED, General Education Development; PFAS, per- and polyfluoroalkyl substances; y, years.
Test of significance of difference in values for Greater Cincinnati Area compared with San Francisco Bay Area; Fisher exact test for categorical variables and Kruskal–Wallis for continuous variables.
Of those who were not right-censored.
BMI% from CDC 2000 Female BMI-for-age table36; height and weight at closest visit prior to pubertal event.
Of the girls included in these analyses, the mean age at thelarche was 9.3 y (111.6 months) and at pubarche was 9.9 y (118.6 months) (Table 1). The age at thelarche was significantly younger in GC girls than in SFBA girls (107.1 vs. 116.2 months, ). The average age of menarche was 12.3 y, with 81.7% achieving menarche by the end of study follow-up. At enrollment, most girls (70.6%) had a BMI below the 85th percentile for their age,36 with a BMI% median value of 66.4. A higher percentage of GC girls had a BMI% greater than the 85th percentile than the SFBA girls at all the pubertal events. For those with a BMI percentile, the median BMI% value was 94.9. The mother’s age at menarche, which has previously been reported to be associated with the daughter’s age at pubertal milestones, was y in 19% of the GC girls and 23% of the SFBA girls.
We previously reported the PFAS concentrations measured in serum of these girls collected in 2004–200617 when they were 6–8 years of age, showing that GC girls had higher levels of exposure.17 Of the eight PFAS measured, six had detectable serum concentration in of the girls and are included in these analyses (Table 2). Two PFAS concentrations, Et-PFOSA-AcOH and PFOSA, had concentrations below the LOD in of the serum samples and are only included in descriptive statistics. Median concentration of PFOA significantly differed by study site ( in GC vs. in SFBA girls) and the range of values also differed ( to in GC girls vs. 2.4– in SFBA girls). PFOA serum concentrations exceeded the NHANES 2005–2006 95th percentile value for children 12–19 years of age in 38.6% of the GC site girls (136/352) and in 14% of the SFBA girls (50/351). (Measurements for younger children were not available from the NHANES 2005–2006 examinations.). Girls at the GC site also had significantly higher adjusted geometric mean concentrations of PFOA than SFBA girls (7.1 vs. , ).
Table 2.
PFAS serum measurements by site for the girls in the study, Greater Cincinnati and San Francisco Bay Area, 2004–2014.
| PFAS (ng/mL) | Median | Minimum | Maximum | LODa | Percentage (%) | |
|---|---|---|---|---|---|---|
| Both sites combined () | ||||||
| PFOA | 704 | 6.40 | 55.90 | 0.1 | 0.14 | |
| PFOS | 704 | 13.10 | 104.00 | 0.2 | 0.14 | |
| PFNA | 704 | 1.50 | 15.50 | 0.1 | 0.14 | |
| PFHxS | 704 | 3.50 | 192.00 | 0.1 | 0.14 | |
| PFDeA | 641 | 0.30 | 1.20 | 0.1 and 0.2 | 22.62 | |
| Me-PFOSA-AcOH | 704 | 0.70 | 14.65 | 0.2 | 4.4 | |
| Et-PFOSA-AcOH | 704 | 0.14 | 3.10 | 0.1 and 0.2 | 85.37 | |
| PFOSA | 704 | 0.07 | 1.60 | 0.05 and 0.1 | 85.51 | |
| Greater Cincinnati () | ||||||
| PFOA | 353 | 7.30 | 55.90 | 0.1 | 0.28 | |
| PFOS | 353 | 13.60 | 96.00 | 0.2 | 0.28 | |
| PFNA | 353 | 1.40 | 6.80 | 0.1 | 0.28 | |
| PFHxS | 353 | 5.20 | 185.00 | 0.1 | 0.28 | |
| PFDeA | 293 | 0.30 | 1.00 | 0.1 and 0.2 | 24.23 | |
| Me-PFOSA-AcOH | 353 | 0.70 | 8.11 | 0.2 | 5.10 | |
| Et-PFOSA-AcOH | 353 | 0.14 | 1.20 | 0.1 and 0.2 | 85.55 | |
| PFOSA | 353 | 0.07 | 0.70 | 0.05 and 0.1 | 81.02 | |
| San Francisco Bay Area () | ||||||
| PFOA | 351 | 5.80 | 2.4 | 18.20 | NA | 0 |
| PFOS | 351 | 12.50 | 3.8 | 104.00 | NA | 0 |
| PFNA | 351 | 1.60 | 0.6 | 15.50 | NA | 0 |
| PFHxS | 351 | 2.30 | 0.3 | 192.00 | NA | 0 |
| PFDeA | 348 | 0.30 | 1.20 | 0.2 | 21.26 | |
| Me-PFOSA-AcOH | 351 | 0.61 | 14.65 | 0.2 | 3.70 | |
| Et-PFOSA-AcOH | 351 | 0.14 | 3.10 | 0.2 | 85.19 | |
| PFOSA | 351 | 0.07 | 1.60 | 0.05 and 0.1 | 90.03 | |
Note: Summary statistics were determined by using the univariate procedure in SAS. CDC, Centers for Disease Control and Prevention; Et-PFOSA-AcOH, acetic acid; LOD, limit of detection reported by CDC lab; Me-PFOSA-AcOH, acetic acid; NA, not available; PFAS, per- and polyfluoroalkyl substances; PFDeA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perflurooctanesulfonic acid; PFOSA, perfluorooctane sulfonaminde.
Samples were sent to the CDC lab in several batches. LODs of the PFAS were batch specific.
GC girls in these analyses had higher levels of PFOA exposure than girls in SFBA, especially those living in the northern Kentucky area of GC.17 Median PFOA in the GC girls () was higher than the median of the SFBA girls () with levels in the GC girls ranging from to and levels ranging from to in SFBA girls. Serum concentrations of most of the PFAS were correlated with each other (Table S3).
Relationship between PFOA Serum Concentrations and Age at Thelarche
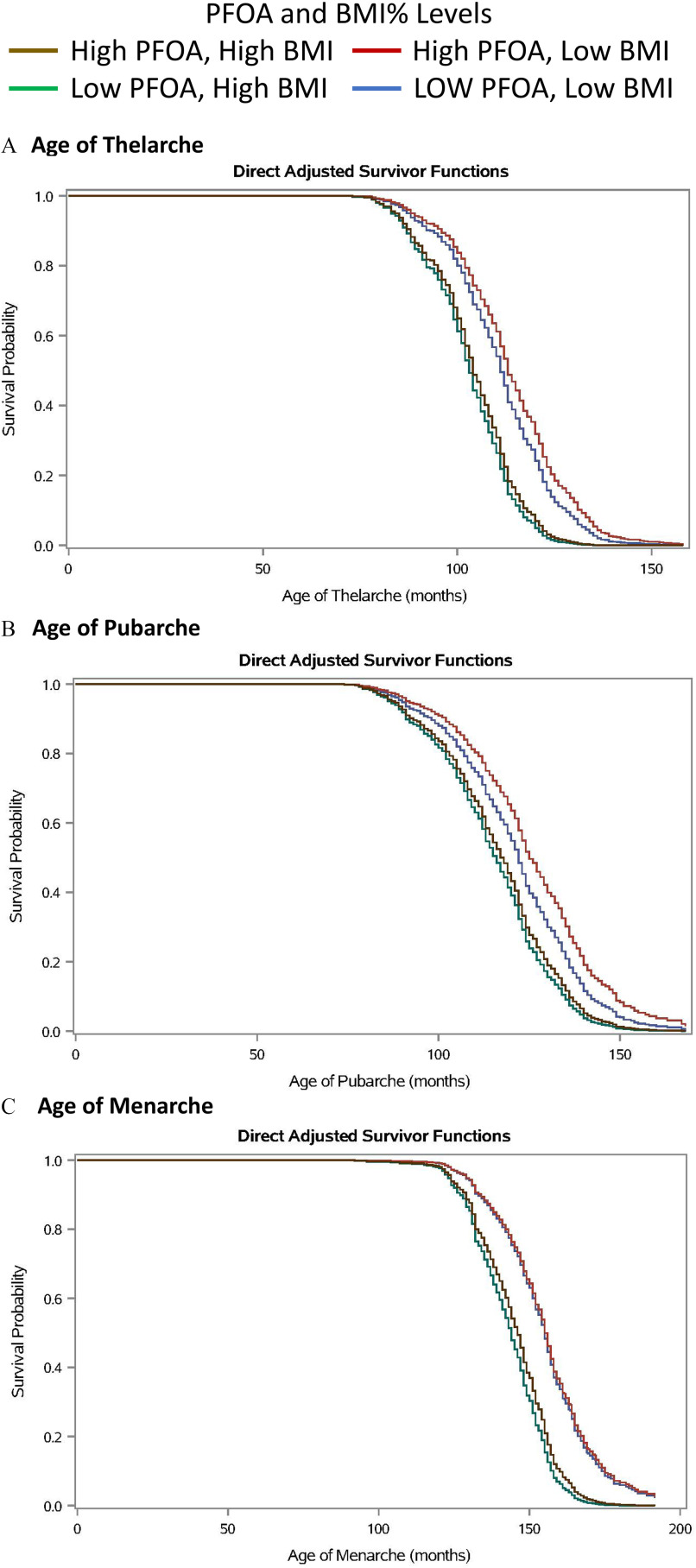
Visual examination of Kaplan–Meier curves stratified by the median BMI% and median PFOA of girls in the thelarche analysis revealed the group most likely to reach thelarche at an older age were those in the lower BMI% () and higher PFOA (serum concentrations ) groups (Figure 1A). Girls with higher BMI% () were more likely to reach thelarche at an earlier age, regardless of their PFOA serum concentration. When we performed Cox regression analyses, with adjustment for BMIz at the examination closest to thelarche and race, we found no associations between PFOA and age at thelarche regardless of whether PFOA was modeled as log-transformed continuous or categorical (Table 3). Having reached thelarche at an earlier age was associated with being Black (; 95% CI: 1.46, 2.14) and a higher BMIz (; 95% CI: 1.35, 1.56) and inversely related to being from the SFBA site (; 95% CI: 0.41, 0.59) in the analyses with log-transformed PFOA.
Figure 1.
Kaplan–Meier curve estimates of cumulative probability of achieving a pubertal milestone, stratified by PFOA serum concentration and BMI%. (Greater Cincinnati and San Francisco Bay Area girls, , 2004–2014.) Pubertal milestones: (A) age of thelarche, (B) age of pubarche, and (C) age of menarche. BMI strata for milestone A: BMI% strata, low , high ; PFOA strata, low , high . BMI strata for milestone B: BMI% strata, low , high ; PFOA strata, low , high . BMI strata for milestone C: BMI% strata, low , high ; PFOA strata, low , high . Note: BMI, body mass index; BMI%, BMI percentile; CDC, Centers for Disease Control and Prevention; PFOA, perfluorooctanoic acid.
Table 3.
Proportional hazards analysis to determine the effects of PFAS serum concentrations on time to pubertal event for girls in the puberty cohort study, 2004–2024.
| Thelarche | Pubarche | Menarche | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFAS (ng/mL)a | HR | 95% CI | -Value | HR | 95% CI | -Value | HR | 95% CI | -Value | |||
| PFOAa | 703 | 0.92 | 0.76, 1.10 | 0.36 | 700 | 0.83 | 0.70, 0.99 | 0.04 | 698 | 0.04b | 0.01, 0.25 | |
| PFOA categorical | ||||||||||||
| Serum PFOA | 182 | 1.00 | Ref | — | 181 | 1.00 | Ref | — | 181 | 1.00 | Ref | — |
| PFOA | 178 | 1.05 | 0.84, 1.30 | 0.68 | 178 | 0.98 | 0.79, 1.23 | 0.87 | 177 | 0.93 | 0.74, 1.17 | 0.56 |
| PFOA | 174 | 0.82 | 0.65, 1.02 | 0.07 | 172 | 0.79 | 0.63, 0.99 | 0.04 | 171 | 0.93 | 0.73, 1.17 | 0.53 |
| PFOA | 169 | 0.88 | 0.70, 1.12 | 0.29 | 169 | 0.78 | 0.61, 0.99 | 0.04 | 169 | 0.02b | 0.00, 0.20 | |
| PFOSa | 704 | 0.45b | 0.17, 1.23 | 0.12 | 701 | 0.90 | 0.79, 1.01 | 0.08 | 699 | 0.18b | 0.05, 0.63 | 0.01 |
| PFOS categorical | ||||||||||||
| Serum PFOS | 180 | 1.00 | Ref | — | 179 | 1.00 | Ref | — | 178 | — | Ref | — |
| PFOS | 177 | 0.95 | 0.76, 1.19 | 0.63 | 176 | 1.02 | 0.82, 1.29 | 0.84 | 177 | 0.96 | 0.76, 1.22 | 0.76 |
| PFOS | 172 | 0.88 | 0.70, 1.10 | 0.27 | 171 | 0.19b | 0.05, 0.78 | 0.02 | 169 | 0.75 | 0.59, 0.96 | 0.02 |
| PFOS | 175 | 0.79 | 0.63, 0.98 | 0.03 | 175 | 0.74 | 0.59, 0.92 | 0.01 | 175 | 0.09b | 0.01, 0.76 | 0.03 |
| PFNAa | 704 | 0.23b | 0.07, 0.79 | 0.02 | 701 | 0.89 | 0.76, 1.05 | 0.16 | 699 | 1.06 | 0.89, 1.27 | 0.50 |
| PFHxSa | 704 | 0.93 | 0.87, 1.01 | 0.07 | 701 | 0.98 | 0.91, 1.05 | 0.55 | 699 | 0.95 | 0.88, 1.03 | 0.23 |
| PFDeAa | 641 | 0.85 | 0.72, 1.00 | 0.05 | 638 | 0.90 | 0.76, 1.06 | 0.20 | 636 | 0.94 | 0.79, 1.11 | 0.47 |
| Me-PFOSA-AcOHa | 704 | 0.42b | 0.19, 0.93 | 0.03 | 701 | 0.88 | 0.80, 0.97 | 0.01 | 699 | 0.97 | 0.87, 1.08 | 0.61 |
| Sum of PFASa | 641 | 0.89 | 0.77, 1.02 | 0.89 | 638 | 0.87 | 0.76, 1.00 | 0.06 | 636 | 0.19b | 0.04, 0.84 | 0.03 |
Note: All models were adjusted for race, BMI at closest visit prior to pubertal event, and site. Models of menarche were also adjusted for mother’s age of menarche. Significance declared at -value . —, Not applicable; BMI, body mass index; CI, confidence interval; HR, hazard ratio; Me-PFOSA-AcOH, acetic acid; PFAS, per- and polyfluoroalkyl substances; PFDeA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perflurooctanesulfonic acid; Ref, reference.
All exposures were modeled as continuous and log transformed except when noted as categorical.
For models in which the proportional hazards assumption failed, a time-dependent covariate was included in the model. HRs reported in this table are prior to incorporating the time-dependent covariate. For example, with incorporating the covariate of the girl, the effect of log-transformed PFOA serum concentration on age at menarche was statistically significant at 11 ( 0.57; 95% CI: 0.43, 0.77) or 12 years of age ( 0.73; 95% CI: 0.59, 0.89).
Relationship between PFOA Serum Concentrations and Age at Pubarche
The Kaplan–Meier curves displayed a later age at pubarche with higher PFOA serum concentration among leaner girls (Figure 1B). The cut point for BMI% for girls in the pubarche analysis was the median value of the study population, 67.6% and those with a BMI% () were again likely to reach pubarche at an earlier age regardless of PFOA serum concentration. In the Cox regression analyses of all girls, we found a statistically significant inverse relationship between log-transformed serum concentration of PFOA and having reached pubarche, as well as for the two highest quartiles of PFOA serum concentrations (Table 3). An increase in 1 logged unit of PFOA in serum resulted in a 17% less risk of having reached pubarche (0.83; 95% CI: 0.70, 0.99). Effect estimates for BMIz at the study visit closest to pubarche (1.31; 95% CI: 1.22, 1.40) and being Black (1.97; 95% CI: 1.62, 2.40) were similar to those detected in the thelarche analyses. The site effect, however, was not present. (0.49; 95% CI: 0.41, 0.59). When girls were stratified by the median value of BMI% at the closest exam ( vs. ), higher PFOA was associated with having reached pubarche in the stratum with lower BMI% when PFOA characterized as either log-transformed () or categorical in the group with PFOA (). Those findings were not present in the group with higher BMI% (Table S4).
Logistic regression analyses were conducted to determine whether the serum concentration of PFOA altered the odds of entering pubarche prior to thelarche. We found a statistically significant lower odds of entering pubarche prior to thelarche with increasing levels of continuous PFOA (; 95% CI: 0.37, 0.95) (Table S5).
Relationship between PFOA Serum Concentrations and Age at Menarche
Visual examination of adjusted Kaplan–Meier curves stratified by median BMI% and median PFOA revealed a delay in age of menarche for girls with lower vs. higher BMI% regardless of PFOA serum concentration (Figure 1C). Consistent with the adjusted Kaplan–Meier curves, Cox regression analyses with both continuous PFOA and the fourth quartile of PFOA exposure revealed statistically significant inverse associations with age at menarche (Table 3). Given that the proportional hazard assumption failed for the continuous and categorical exposure models, a time-dependent interaction between the age at menarche in months and PFOA exposure was added to the model to overcome this failure. With this added interaction term, the effect of log-transformed PFOA serum concentration on age at menarche was statistically significant at 11 or 12 years of age (0.57; 95% CI: 0.43, 0.77; 0.73; 95% CI: 0.59, 0.89) but not at 13 or 14 years of age. An increase in 1 logged unit of PFOA in serum resulted in a 43% less risk of entering menarche for girls 11 years of age (0.57; 95% CI: 0.43, 0.77) (footnote b in Table 3). BMIz at the exam closest to menarche (1.51; 95% CI: 1.39, 1.64), mother’s age at y (1.37; 95% CI: 1.11, 1.68), and being Black (1.36; 95% CI: 1.09, 1.67) also were associated with an earlier age of menarche. When girls were stratified by BMI% at the closest exam ( percentile vs. percentile), there was no difference in the strength of the association with menarche in the two strata (Table S4).
Relationship between Other PFAS Serum Concentrations Age at Pubertal Milestones
In adjusted Cox regression models to examine the effect of other PFAS, PFNA, PFDeA, and Me-PFOSA-AcOH had a significant impact on the age of thelarche, with higher serum concentrations associated with later thelarche (Table 3). In categorical analyses, the highest category of PFOS exposure also was associated with an earlier age at thelarche (Table 3). Although PFOS when modeled as a continuous variable did not have a significant impact on age of any of the pubertal milestones, when modeled categorically, the two highest quartiles were associated with a lower HR (a lower likelihood of reaching pubarche) (Table 3). Higher levels of PFNA, PFDeA, and Me-PFOSA-AcOH also were associated with a delay in age at thelarche, and Me-PFOSA-AcOH was associated with a delay in age at pubarche (Table 3). PFOS had a statistical effect on age of menarche in both continuous and categorical analyses, with a higher level of PFOS being associated with a later age at menarche (Table 3). The sum of all PFAS serum concentrations was associated with a significantly lower HR for menarche, and it was borderline for pubarche (Table 3).
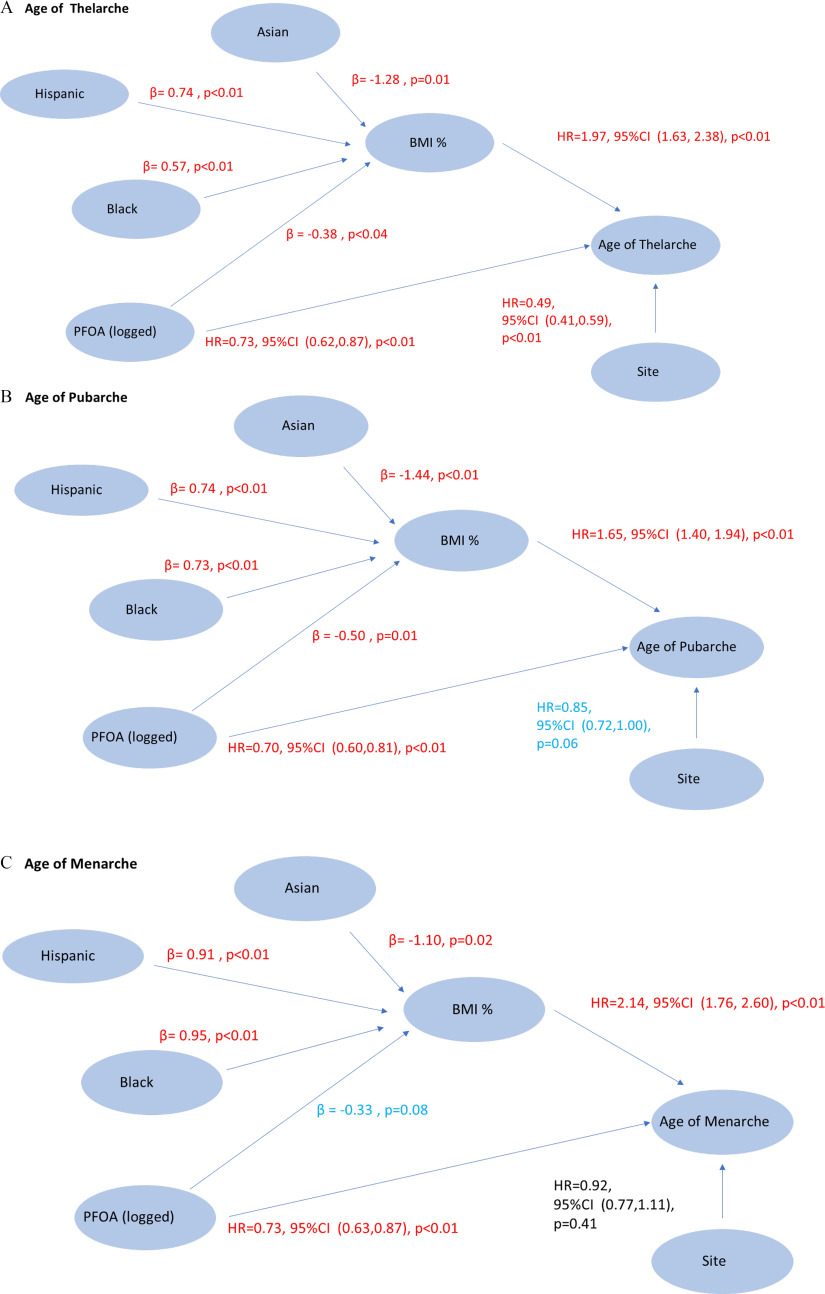
SEM Analyses
To further understand the correct direction of relationships among PFOA, BMI, and the pubertal milestones, we conducted SEMs for each pubertal milestone (thelarche, pubarche, and menarche). Final SEMs indicated a triangular relationship between PFOA, BMI%, and the age at the pubertal milestone. Higher log-transformed PFOA had a statistically significant direct effect of delaying the ages of the pubertal milestones: thelarche, 0.73 (95% CI: 0.62, 0.87); pubarche 0.70 (95% CI: 0.60, 0.81); and menarche, 0.73 (95% CI: 0.63, 0.87) (Figure 2). Higher BMI% was independently significantly related to earlier ages of thelarche, pubarche, and menarche. PFOA was a significant determinant of lower BMI% (thelarche and pubarche) or had a borderline effect (menarche). The SFBA site was associated with later age menarche compared with the GC site. Being either Hispanic or non-Hispanic black was associated with higher BMI% than being non-Hispanic white, and Asian girls had a lower BMI% than non-Hispanic white girls (Figure 2).
Figure 2.
SEM of effects of PFOA and other factors on age at pubertal milestone in months (Greater Cincinnati and San Francisco Bay Area girls, , 2004–2014. Pubertal milestones: (A) age of thelarche, (B) age of pubarche, and (C) age of menarche. SEM analysis using Mplus: survival- and regression-mediated analysis; percentile (reference), percentile; Asian: , Hispanic, Black (reference), ; Hispanic: , Asian, Black (reference), ; Black: , Asian, Hispanic (reference), ; Site: (reference), ; serum concentration (logged). Note: BMI, body mass index; CI, confidence interval; GC, Greater Cincinnati; HR, hazard ratio; PFOA, perfluorooctanoic acid (logged); SEM, structural equation model; SFBA, San Francisco Bay Area.
Associations among Circulating Reproductive Hormones and PFAS
Multivariable regression analyses, controlling for race and BMIz, on the GC girls with hormone values () around the time of thelarche showed inverse relationships between , T, and DHEAS and serum PFOA concentration at 6 months prior to thelarche but no relationship with (Table 4). Significant associations observed with other PFAS included an inverse relationships between PFDeA and and the sum of PFAS and T at thelarche (Table 4). When girls were stratified by median BMI% during each time period, all significant relationships between PFOA and the hormones were seen only in the girls with a BMI% greater than the study population median (Table S6). PFOA was significantly inversely associated with , T, and DHEAS at 6 months prior to thelarche; with DHEAS at thelarche; and with T at 6 months after thelarche. Analyses of the associations between BMIz and the circulating reproductive hormones (without PFAS in the models) showed significant universal inverse associations between BMIz and at all four time periods (at 6 months prior to thelarche, at thelarche, at 6 months after thelarche, at 12 months after thelarche); for at 6 months prior to thelarche, at 6 months after thelarche, and at 12 months after thelarche; and for T at 6 months after thelarche and at 12 months after thelarche (Table 5). BMIz had no association with DHEAS.
Table 4.
Effect estimates from regression analyses to determine the association of PFAS serum concentrations with serum reproductive hormone concentrations measured at different time points around thelarche in the girls from Greater Cincinnati, 2004–2014.
| Six months before thelarche | Thelarche | Six months after thelarche | Twelve months after thelarche | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SE | -Value | SE | -Value | SE | -Value | SE | -Value | |||||||||
| PFOA | ||||||||||||||||
| Estradiol | 207 | 0.09 | 0.76 | 185 | 0.08 | 0.10 | 0.44 | 223 | 0.12 | 0.39 | 125 | 0.06 | 0.20 | 0.76 | ||
| Estrone | 208 | 0.06 | 0.04 | 186 | 0.07 | 0.12 | 222 | 0.08 | 0.07 | 125 | 0.12 | 0.77 | ||||
| Testosterone | 206 | 0.07 | 0.03 | 190 | 0.06 | 0.21 | 241 | 0.06 | 0.12 | 142 | 0.10 | 0.68 | ||||
| DHEAS | 221 | 0.08 | 197 | 0.09 | 0.26 | 250 | 0.08 | 0.37 | 141 | 0.11 | 0.91 | |||||
| PFOS | ||||||||||||||||
| Estradiol | 207 | 0.09 | 0.38 | 185 | 0.09 | 0.55 | 223 | 0.11 | 0.11 | 0.33 | 125 | 0.11 | 0.18 | 0.56 | ||
| Estrone | 208 | 0.06 | 0.07 | 186 | 0.06 | 0.06 | 222 | 0.08 | 0.77 | 125 | 0.00 | 0.11 | 0.99 | |||
| Testosterone | 206 | 0.07 | 0.18 | 190 | 0.06 | 0.11 | 241 | 0.06 | 0.14 | 142 | 0.05 | 0.66 | ||||
| DHEAS | 221 | 0.08 | 0.09 | 197 | 0.08 | 0.34 | 250 | 0.07 | 0.59 | 141 | 0.02 | 0.09 | 0.86 | |||
| PFNA | ||||||||||||||||
| Estradiol | 207 | 0.15 | 0.13 | 0.24 | 185 | 0.15 | 0.57 | 223 | 0.26 | 0.17 | 0.12 | 125 | 0.33 | 0.29 | 0.27 | |
| Estrone | 208 | 0.08 | 0.85 | 186 | 0.10 | 0.06 | 222 | 0.07 | 0.11 | 0.55 | 125 | 0.22 | 0.17 | 0.20 | ||
| Testosterone | 206 | 0.05 | 0.10 | 0.62 | 190 | 0.09 | 0.67 | 241 | 0.08 | 0.92 | 142 | 0.01 | 0.13 | 0.93 | ||
| DHEAS | 221 | 0.12 | 0.51 | 197 | 0.12 | 0.66 | 250 | 0.11 | 0.55 | 141 | 0.15 | 0.77 | ||||
| PFHxS | ||||||||||||||||
| Estradiol | 228 | 0.02 | 0.05 | 0.67 | 185 | 0.06 | 0.78 | 223 | 0.10 | 0.06 | 0.10 | 125 | 0.02 | 0.11 | 0.83 | |
| Estrone | 208 | 0.04 | 0.96 | 186 | 0.01 | 0.16 | 222 | 0.01 | 0.01 | 0.83 | 125 | 0.06 | 0.49 | |||
| Testosterone | 206 | 0.00 | 0.04 | 0.97 | 190 | 0.04 | 0.29 | 241 | 0.04 | 0.99 | 142 | 0.05 | 0.58 | |||
| DHEAS | 221 | 0.00 | 0.05 | 0.88 | 197 | 0.02 | 0.87 | 250 | 0.06 | 0.01 | 0.17 | 141 | 0.04 | 0.06 | 0.50 | |
| PFDeA | ||||||||||||||||
| Estradiol | 169 | 0.12 | 0.12 | 0.34 | 148 | 0.13 | 0.29 | 178 | 0.01 | 0.16 | 0.95 | 96 | 0.21 | 0.12 | ||
| Estrone | 170 | 0.00 | 0.09 | 0.97 | 148 | 0.09 | 0.03 | 177 | 0.11 | 0.49 | 96 | 0.13 | 0.67 | |||
| Testosterone | 170 | 0.10 | 0.08 | 0.25 | 149 | 0.08 | 0.42 | 192 | 0.09 | 0.40 | 111 | 0.03 | 0.12 | 0.78 | ||
| DHEAS | 179 | 0.12 | 0.60 | 156 | 0.11 | 0.58 | 199 | 0.11 | 0.24 | 109 | 0.13 | 0.62 | ||||
| Me-PFOSA-AcOH | ||||||||||||||||
| Estradiol | 207 | 0.07 | 0.14 | 185 | 0.07 | 0.43 | 223 | 0.15 | 0.08 | 0.05 | 125 | 0.11 | 0.95 | |||
| Estrone | 208 | 0.04 | 0.25 | 186 | 0.05 | 0.48 | 222 | 0.08 | 0.05 | 0.12 | 125 | 0.07 | 0.98 | |||
| Testosterone | 206 | 0.02 | 0.46 | 190 | 0.04 | 0.37 | 241 | 0.00 | 0.04 | 0.96 | 142 | 0.06 | 0.24 | |||
| DHEAS | 221 | 0.06 | 0.94 | 197 | 0.02 | 0.06 | 0.68 | 250 | 0.02 | 0.05 | 0.66 | 141 | 0.07 | 0.50 | ||
| Sum of PFAS | ||||||||||||||||
| Estradiol | 169 | 0.12 | 0.76 | 148 | 0.13 | 0.37 | 178 | 0.13 | 0.16 | 0.39 | 96 | 0.24 | 0.79 | |||
| Estrone | 170 | 0.08 | 0.13 | 148 | 0.08 | 0.08 | 177 | 0.11 | 0.67 | 96 | 0.14 | 0.59 | ||||
| Testosterone | 170 | 0.09 | 0.31 | 149 | 0.08 | 0.04 | 192 | 0.08 | 0.11 | 111 | 0.11 | 0.65 | ||||
| DHEAS | 179 | 0.11 | 0.10 | 156 | 0.11 | 0.42 | 199 | 0.10 | 0.67 | 109 | 0.04 | 0.14 | 0.79 | |||
Note: Models were controlled for race and BMIz. Six months prior to thelarche was defined as 9 months prior to months prior to thelarche. Thelarche was defined as 3 months prior to months after thelarche. Six months after thelarche was defined as 3 months after thelarche to months after thelarche. Twelve months after thelarche was defined as 9 months after thelarche to months after thelarche. Significant at -value . BMIz, body mass index -score based on 2000 CDC tables; CDC, Centers for Disease Control and Prevention; DHEAS, dihydroandrostendione sulfate; Me-PFOSA-AcOH, acetic acid; PFAS, per- and polyfluoroalkyl substances; PFDeA, perfluorodecanoic acid; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perflurooctanesulfonic acid; SE, standard error.
Table 5.
Effect estimates from regression analyses to determine the association of BMIz with hormone concentrations for the girls from Greater Cincinnati, 2004–2014.
| Six months before thelarche | Thelarche | Six months after thelarche | Twelve months after thelarche | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SE () | -Value | SE (β) | -Value | SE (β) | -Value | SE () | -Value | |||||||||
| Estradiol | 207 | 0.05 | 0.02 | 205 | 0.06 | 0.11 | 223 | 0.06 | 125 | 0.09 | ||||||
| Estrone | 208 | 0.03 | 186 | 0.04 | 224 | 0.04 | 125 | 0.06 | ||||||||
| Testosterone | 206 | 0.04 | 0.08 | 190 | 0.04 | 0.11 | 241 | 0.04 | 142 | 0.04 | 0.05 | |||||
| DHEAS | 221 | 0.05 | 0.28 | 197 | 0.05 | 0.27 | 250 | 0.04 | 0.36 | 141 | 0.02 | 0.05 | 0.68 | |||
Note: All models were controlled for race and PFOA. Six months prior to thelarche was defined as 9 months prior to months prior to thelarche. Thelarche defined as 3 months prior to months after thelarche. Six months after thelarche was defined as 3 months after thelarche to months after thelarche. Twelve months after thelarche was defined as 9 months after thelarche to months after thelarche. Significant at -value . BMIz, body mass index -score based on 2000 CDC tables; CDC, Centers for Disease Control and Prevention; DHEAS, dihydroandrostendione sulfate; SE, standard error.
Discussion
We report consistent evidence that exposure to PFAS is associated with later age at onset of pubarche and menarche in girls from two areas of the United States with a history of exposure to PFAS. In both Cox regression analyses and in SEMs, we identified statistically significant inverse associations between serum PFOA and age at pubarche and menarche. Girls with higher serum PFOA concentrations also were less likely to enter pubarche before thelarche, possibly reflecting their lower DHEAS serum concentrations. Analyses using SEMs also detected a significant inverse association between serum PFOA and age at thelarche. We also detected consistent delay in the age at pubertal milestones with serum concentrations of other PFAS, with no evidence of an earlier age at the milestone. Therefore, in contrast to the original hypothesis, we found that the PFAS class of EDCs were associated with later rather than earlier breast development. In girls with serum measurements of reproductive hormones, the later pubertal development findings were accompanied by inverse associations between serum PFOA and serum , DHEAS, and possibly T around the time of thelarche (and in most girls, also pubarche, given that these milestones are usually within 6 months of each other). Given that is produced in adipose tissue, an inverse association between PFOA and in circulating serum may align with our earlier published finding that BMI was lower in girls with greater PFOA exposure.44 Based on findings from exposure studies of other populations in these areas,13,45,46 we infer that their mothers were also exposed to PFAS in drinking water and the girls were exposed in utero, although we do not have any direct information about maternal exposure levels of the girls in our cohort. We conclude that PFAS may delay pubertal onset through the intervening effects on BMI and reproductive hormones. The decreases in DHEAS and associated with PFOA represent biological biomarkers of effect consistent with the delay in onset of puberty.
The novelty of our study is that longitudinal assessment of thelarche was performed by trained clinicians in dedicated clinical examinations. We have identified only one other study that examined the association of PFAS exposure with age at thelarche and pubarche. In the Danish National Birth Cohort, among girls with maternal gestational measurements of PFAS, pubertal staging information was obtained by questionnaire self-report, collected biannually from 11 years of age until full maturation21; median maternal PFOA exposure was . Late age at enrollment was one of the limitations of that study, especially for thelarche and pubarche, given that 85% of the girls were at and 54% were at at enrollment. Ernst reported that the only statistically significant findings in girls were delays in entry into Tanner stage 2 breast development with PFOA exposure (Ernst et al.21, Table 4) and Tanner stage 4 and 5 pubic hair appearance. None of the other ages at pubertal stages were associated with PFOA exposure, nor were any of the pubertal staging relationships with other PFAS significant. The direction of effect (delay) was, in general, consistent with our study findings regarding relationships of PFAS with both thelarche and pubarche, and we also found a similar significant delay in thelarche with PFNA exposure.
Several other studies have reported on investigations of the age at menarche and PFAS exposure. No significant associations were found in the Danish birth cohort study,21 nor in a recent study of girls in the longitudinal Avon cohort of British girls,24 with both studies using maternal gestational exposure measurements. An earlier report from the Avon cohort noted nonsignificant decreased odds of earlier age at menarche.47 Median values of PFOA and PFOS exposure in the Danish cohort were similar to that in our population ( and ) but were lower in the Avon cohort ( and ). In a different Danish population study of 254 girls, Kristensen23 reported that those with mothers with a gestational higher level of PFOA exposure (range: 4.4–) had an estimated 5.3 months later age at menarche, similar to findings that we report. No statistically significant differences were reported for reproductive hormone levels by PFAS exposure. The very large C-8 study of persons in the Mid-Ohio Valley of the United States also reported a delay in menarche of 130 d (4.3 months) and 138 d (4.5 months) in 2,931 girls with the highest concentration quartiles of PFOA and PFOS (geometric means of 9.8 and , respectively).22
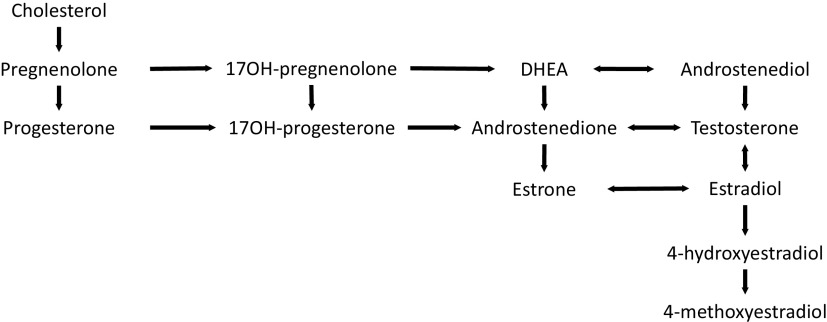
Classification of environmental chemicals as endocrine disruptors is often based on their ability to bind to specific receptors and the nuclear receptor superfamily, which are the most abundant classes of transcriptional receptors.48 EDCs bind to estrogen receptors, androgen receptors, or thyroid hormone receptors, all having roles in regulating development, metabolism, and reproduction. Small amounts of androgens (primarily androstenedione and T) are produced by females in the ovaries, adrenal glands, and fat cells and are essential for producing estrogens. Androgen production from cholesterol and then dehydroepiandrosterone (DHEA) occurs in the ovaries after stimulation from luteinizing hormone and is regulated by the hypothalamus. In women, T is produced in the ovaries and adrenal glands from androstenedione; T is the source of . Androstenedione is also converted into (Figure 3). In girls, estrogen is important for linear growth, breast, and uterine development, menstrual cycles, mood and cognition, and bone density. Timing of puberty—specifically earlier age of peak height velocity and menarche—are associated with risk for breast cancer. In adult life, estrogen is important for all of the health parameters noted for girls, as well as for bone and cardiovascular health. A recently published paper noted the relationship between pubertal parameters with breast cancer are likely mediated via hormone changes in puberty; for example, age at menarche was associated with the estrogen:androgen ratio.49 EDCs can also impact the hormonal milieu.
Figure 3.
Estrogen biosynthesis pathway (circulating hormones and at tissue level). Note: DHEA, dehydroepiandrosterone; OH, hydroxy.
De novo synthesis of cholesterol takes place in the liver. Metabolomic and transcriptomic studies of PFAS in humans have noted perturbations in the lipid metabolism-related pathways, starting with fatty acid catabolism and mitochondrial oxidation of fatty acids and extending to the biosynthesis of cholesterol,50 including specifically promoting cholesterol biosynthesis in the normal human liver cell L-02. However, findings of human studies of PFAS and cholesterol are opposite of those in animal studies. The finding that PFAS exposure promotes biosynthesis of cholesterol aligns with the reported positive association between relatively high PFOA exposure, , and cholesterol in the C-8 cohort51 and also higher total and low-density lipoprotein cholesterol in children and adolescents.52 However, PFOS was inversely associated with essential fatty acid and triglyceride serum levels in pregnant mothers with relatively low serum concentration ().53 Moreover, PFOS and other exposures have been shown to decrease the mitochondrial of fatty acids,54 a finding that could partially explain the decrease BMI with PFOA exposure that we have noted in the girls in the cohort and which disappeared around the time of menarche.44 Martinsson55 found no effect of early pregnancy maternal exposure on weight at 4 years of age, although PFAS serum concentrations were relatively low.
Cholesterol is the substance from which other hormones are synthesized, and it is the precursor of many hormones. It is converted to pregnenolone, the “mother hormone.” Pregnenolone is oxidized to produce progesterone, and two carbon chains are then cleaved to form androstendione, from which is derived. T is formed by reduction of the 17-keto group of androstendione, and then is formed from T.56
Perturbations by PFAS in either of two pathways ( formation from androstenedione, or formation from T through androstenediol) could result endocrine disruption. PFAS also could interfere with steroidogenesis, impacting the production of cholesterol or pregnenolone, or it could interact with nuclear hormone receptors. Many rodent studies in maternal serum have noted that PFAS affects lipid metabolism, including the biosynthesis of cholesterol. PFOS has decreased steroidogenic enzyme gene (CYP17, CYP19a, CYP19b) expression of genes involved in sex hormone synthesis.57
The analyses of circulating reproductive hormone levels strongly suggest a mechanism for the observed delay in pubarche given that a significant inverse association existed between PFOA and PFOS and DHEAS at 6 months prior to thelarche, as well as for PFOA at all three time points in girls with BMI % less than the study population median value. No relationship was found between PFOA and , and we found no association between PFOA and age at thelarche. Independent of PFAS exposure, we report that increased BMI is consistently associated with decreased reproductive hormone levels around the time of thelarche, as noted in our earlier report.58 Previous studies also have consistently reported associations, almost always inverse, between PFAS and reproductive hormones in girls although there is substantial variation in the age at the serum samples and the strength of associations between various PFAS analytes and the hormone concentrations.
One study of Japanese mothers and children (maternal serum median ) reported inverse associations with progesterone and prolactin.59 Previous studies of reproductive hormone levels in girls have almost exclusively been cross-sectional and not linked to observation of either thelarche or pubarche. The level of PFAS exposure in these populations was quite diverse. In a cross-sectional study of children in the Mid-Ohio Valley, girls 6–9 years of age who were exposed to substantial amounts of PFOA (), no significant associations were detected with PFOA serum concentrations, but PFOS concentrations were significantly inversely associated with serum levels of T and insulin-like growth factor-1 (IGF-1), and PFNA with IGF-1.25 However, because they were unable to differentiate children by Tanner stages, they could not associate measurements with thelarche. A second cross-sectional study in Taiwan of 330 females, mostly 18–30 years of age, reported that PFOA exposure was associated with lower sex hormone binding globulin (SHBG), PFOS with lower T, and perfluoroundecanoic acid (PFUA) with lower follicle stimulating hormone.27 Another cross-sectional study of Taiwanese children in an asthma cohort, 13–15 years of age, reported that in females, perfluorododecanoic acid (PFDoA) was inversely associated with T levels, but no other associations between PFAS and reproductive hormones were detected.28 However, a prospective study of pregnant women in Avon, UK, reported that relatively low PFAS exposure in utero (mother’s median PFOA serum ) was positively associated with serum T levels in daughters at 15 years of age.26
In the Danish Odense Child Cohort, with 373 maternal–child pairs from Denmark, a 2-fold increase in prenatal exposure to perfluorodecanoic acid (PFDA) predisposed infants to significantly lower serum concentration of DHEA, a steroid hormone precursor.60 DHEA is one of the most abundant circulating steroid hormones in humans and represents a large reservoir of precursors for intracellular production of androgens and estrogens in nonreproductive tissues,61 which could include breast tissue. Fetal exposure to PFDA, at the time of mini-puberty (at about 4 months of age) has been noted to predispose infants to lower levels of androstenedione and DHEA, but only in female infants.60 A very recently published study of pubertal milestones in 200 boys and girls of a Cincinnati birth cohort used self-reported pubertal stage assessment and self-report of time of menses initiation in girls at 12 years and measurements of PFAS in serum at age 12. In girls, they found that adolescent PFAS serum concentrations were associated with later pubic hair growth, later breast maturation, later age at menarche, and lower concentrations of estradiol. No patterns of pubic hair appearance or hormone concentrations were associated with PFAS in males.66
Genetic variants that influence the expression of genes responsible for encoding enzymes in the cholesterol metabolism and steroid synthesis pathway certainly can influence the production of hormones. The difference in reported findings across studies suggest possible variation in genetic susceptibility. A future study of germline genetic variants of genes such as of CYP17, CYP19, HSD3B1, and HSD173B1 in these girls would enhance the understanding of mechanisms of reproductive hormone variations, allowing separate estimation of the effect of inherited variability and the effect of environmental exposure on gene expression. Incorporating genetic variants into future metabolomic and transcriptomic analyses would enhance the understanding of the effect of environmental PFAS exposure and lead to better clinical care through environmental precision health.
Strengths
The many strengths of the design of the present study include the large number of girls in the cohort, a rigorous protocol for height and weight measurements, and the measurements of PFAS at the CDC laboratory, internationally recognized for high quality analyses. Pubertal maturation was staged at each clinical visit and included both observation and palpation. Statistical analyses were conducted using Cox proportional hazards models that account for the amount of time under observation. [Note: At an International Society of Environmental Epidemiology (ISEE) meeting in 2009, we initially reported earlier breast development with PFOA exposure in a cross-sectional analysis done at the second year of follow-up, but when only 29% if the girls at the two sites had reached thelarche.62 When we conducted the first longitudinal analysis in 2011, we then reported an older age at thelarche with PFOA exposure at the ISEE meeting that year.63]
Limitations
Given that both participation in this cohort study and retention was voluntary, it is possible that healthier girls were included in our analysis, which may have led us to underestimate the effects of PFAS. The GC subcohort had few Asian or Hispanic girls. We did not have serum reproductive hormone measurements on all girls. Only girls at the GC site had serial serum samples banked for future hormone analyses, and some girls did not return for visits or refused phlebotomy. Girls without hormone measurements, many who dropped out before reaching the age of pubertal milestones, did not contribute data to the hormone analyses at different time points around thelarche (Tables 3 and 4). The girls who dropped out of the study were more likely to have reached thelarche and therefore the findings of our study may be more representative of girls who would have been later in achieving pubarche or menarche. Furthermore, those GC girls with hormone measurements did not differ in race or BMI at entry from the girls without hormone and PFAS measurements. Statistical analyses with multiple comparisons may result in random findings at a nominal significance level of 0.05, inferring a 5% chance of incorrectly rejecting the null hypothesis.64 We did not elect to adjust for multiple comparisons because to do so would have increased the probability of false negative findings, and the effect estimates and -values we do report can be used in the inference of the importance of the findings. Very likely, simultaneous exposure to other chemical compounds can influence the level of reproductive hormones.
Future Studies
Future multivariable exposure mixture analyses of the effect on reproductive hormones will determine whether the reproductive hormones with significant findings in the PFAS alone analysis remain significant in the mixture analysis. In addition, using our banked DNA samples for detecting susceptibility genetic variants and conducting metabolomic analyses will further contribute to our understanding of the effects of PFAS exposure. Future long-term follow-up of these girls, including their reproductive hormone levels as adults in both the follicular and luteal phases of the menstrual cycle and their pregnancy outcomes, coupled with pubertal findings could provide additional insight into the effects of PFAS, as a disrupted endocrine environment early in life can affect reproductive health in adulthood.65
Supplementary Material
Acknowledgments
This publication was made possible by the Breast Cancer and the Environment Research Program (U01ES012770, U01ES019453 to F.M.B., U01ES012801 to L.H.K., U01ES019435 to L.H.K., U01ES019457) and by the National Institute of Environmental Health Sciences (NIEHS R01ES029133 to S.M.P. and T32-ES010957), by the National Center for Research Resources UL1RR024131, and CSTA-UL1RR026314). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or NCI, the National Institutes of Health, the Centers for Disease Control and Prevention, or the California Department of Public Health.
References
- 1.Bernstein L. 2002. Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Biol Neoplasia 7(1):3–15, PMID: , 10.1023/a:1015714305420. [DOI] [PubMed] [Google Scholar]
- 2.Golub MS, Collman GW, Foster PMD, Kimmel CA, Rajpert-De Meyts E, Reiter EO, et al. 2008. Public health implications of altered puberty timing. Pediatrics 121(suppl 3):S218–S230, PMID: , 10.1542/peds.2007-1813G. [DOI] [PubMed] [Google Scholar]
- 3.Bodicoat DH, Schoemaker MJ, Jones ME, McFadden E, Griffin J, Ashworth A, et al. 2014. Timing of pubertal stages and breast cancer risk: the Breakthrough Generations Study. Breast Cancer Res 16(1):R18, PMID: , 10.1186/bcr3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biro FM, Deardorff J. 2013. Identifying opportunities for cancer prevention during preadolescence and adolescence: puberty as a window of susceptibility. J Adolesc Health 52(suppl 5):S15–S20, PMID: , 10.1016/j.jadohealth.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiatt RA, Haslam SZ, Osuch J, Breast Cancer and the Environment Research Centers. 2009. The Breast Cancer and the Environment Research Centers: transdisciplinary research on the role of the environment in breast cancer etiology. Environ Health Perspect 117(12):1814–1822, PMID: , 10.1289/ehp.0800120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terry MB, Michels KB, Brody JG, Byrne C, Chen S, Jerry DJ, et al. 2019. Environmental exposures during windows of susceptibility for breast cancer: a framework for prevention research. Breast Cancer Res 21(1):96, PMID: , 10.1186/s13058-019-1168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ATSDR (Agency for Toxic Substances and Disease Registry). 2021. Toxicological profile for Perfluoroalkyls. CAS# 335-67-1, 1763-23-1, 355-46-4, 375-95-1. Atlanta, GA: ATSDR. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=1117&tid=237 [accessed 8 September 2023]. [PubMed] [Google Scholar]
- 8.Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, et al. 2004. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol 38(17):4489–4495, PMID: , 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- 9.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. 2007. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99(2):366–394, PMID: , 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 10.Fromme H, Tittlemier SA, Völkel W, Wilhelm M, Twardella D. 2009. Perfluorinated compounds—exposure assessment for the general population in Western countries. Int J Hyg Environ Health 212(3):239–270, PMID: , 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. 2007. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect 115(11):1596–1602, PMID: , 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC (Centers for Disease Control and Prevention). 2022. Biomonitoring Data Tables for Environmental Chemicals. Updated March 24, 2022. https://www.cdc.gov/exposurereport/index.html. [accessed 5 November 2022].
- 13.Herrick RL, Buckholz J, Biro FM, Calafat AM, Ye X, Xie C, et al. 2017. Polyfluoroalkyl substance exposure in the Mid-Ohio River Valley, 1991–2012. Environ Pollut 228:50–60, PMID: , 10.1016/j.envpol.2017.04.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbo A. 2020. 19 Bay Area semiconductor companies making tech innovation possible. Built In SF. 22 September 2020. https://www.builtinsf.com/2020/02/06/bay-area-semiconductor-companies [accessed 29 July 2021].
- 15.Glüge J, Scheringer M, Cousins IT, DeWitt JC, Goldenman G, Herzke D, et al. 2020. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ Sci Process Impacts 22(12):2345–2373, PMID: , 10.1039/d0em00291g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demircan E. 2020. Fluorinated chemicals are essential to semiconductor manufacturing and innovation. Semi. 15 December 2020. https://semi.org/en/blogs/semi-news/fluorinated-chemicals-are-essential-to-semiconductor-manufacturing-and-innovation [accessed 8 September 2023].
- 17.Pinney SM, Biro FM, Windham GC, Herrick RL, Yaghjyan L, Calafat AM, et al. 2014. Serum biomarkers of polyfluoroalkyl compound exposure in young girls in Greater Cincinnati and the San Francisco Bay Area, USA. Environ Pollut 184:327–334, PMID: , 10.1016/j.envpol.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson LM, Angrish M, Shirke AV, Radke EG, Schulz B, Kraft A, et al. 2022. Systematic evidence map for over one hundred and fifty per- and polyfluoroalkyl substances (PFAS). Environ Health Perspect 130(5):056001, PMID: , 10.1289/EHP10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29(2):131–147, PMID: , 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blake BE, Fenton SE. 2020. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: a review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology 443:152565, PMID: , 10.1016/j.tox.2020.152565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst A, Brix N, Lauridsen LLB, Olsen J, Parner ET, Liew Z, et al. 2019. Exposure to perfluoroalkyl substances during fetal life and pubertal development in boys and girls from the Danish National Birth Cohort. Environ Health Perspect 127(1):017004, PMID: , 10.1289/EHP3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Espinosa MJ, Fletcher T, Armstrong B, Genser B, Dhatariya K, Mondal D, et al. 2011. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ Sci Technol 45(19):8160–8166, PMID: , 10.1021/es1038694. [DOI] [PubMed] [Google Scholar]
- 23.Kristensen SL, Ramlau-Hansen CH, Ernst E, Olsen SF, Bonde JP, Vested A, et al. 2013. Long-term effects of prenatal exposure to perfluoroalkyl substances on female reproduction. Hum Reprod 28(12):3337–3348, PMID: , 10.1093/humrep/det382. [DOI] [PubMed] [Google Scholar]
- 24.Marks KJ, Howards PP, Smarr MM, Flanders WD, Northstone K, Daniel JH, et al. 2021. Prenatal exposure to mixtures of persistent endocrine disrupting chemicals and early menarche in a population-based cohort of British girls. Environ Pollut 276:116705, PMID: , 10.1016/j.envpol.2021.116705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Espinosa MJ, Mondal D, Armstrong BG, Eskenazi B, Fletcher T. 2016. Perfluoroalkyl substances, sex hormones, and insulin-like growth factor-1 at 6–9 years of age: a cross-sectional analysis within the C8 Health Project. Environ Health Perspect 124(8):1269–1275, PMID: , 10.1289/ehp.1509869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maisonet M, Calafat AM, Marcus M, Jaakkola JJ, Lashen H. 2015. Prenatal exposure to perfluoroalkyl acids and serum testosterone concentrations at 15 years of age in female ALSPAC study participants. Environ Health Perspect 123(12):1325–1330, PMID: , 10.1289/ehp.1408847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai MS, Lin CY, Lin CC, Chen MH, Hsu SHJ, Chien KL, et al. 2015. Association between perfluoroalkyl substances and reproductive hormones in adolescents and young adults. Int J Hyg Environ Health 218(5):437–443, PMID: , 10.1016/j.ijheh.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Hu LW, Qian ZM, Chang JJ, King C, Paul G, et al. 2016. Association of perfluoroalkyl substances exposure with reproductive hormone levels in adolescents: by sex status. Environ Int 94:189–195, PMID: , 10.1016/j.envint.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Biro FM, Greenspan LC, Galvez MP, Pinney SM, Teitelbaum S, Windham GC, et al. 2013. Onset of breast development in a longitudinal cohort. Pediatrics 132(6):1019–1027, PMID: , 10.1542/peds.2012-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biro FM, Galvez MP, Greenspan LC, Succop PA, Vangeepuram N, Pinney SM, et al. 2010. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics 126(3):e583–e590, PMID: , 10.1542/peds.2009-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cattran AM, Kalkwarf HJ, Pinney SM, Huang B, Biro FM. 2015. Bone density and timing of puberty in a longitudinal study of girls. J Pediatr Adolesc Gynecol 28(3):170–172, PMID: , 10.1016/j.jpag.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorn LD, Dahl RE, Woodward HR, Biro F. 2006. Defining the boundaries of early adolescence: a user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Appl Dev Sci 10(1):30–56, 10.1207/s1532480xads1001_3. [DOI] [Google Scholar]
- 33.Marshall WA, Tanner JM. 1969. Variations in pattern of pubertal changes in girls. Arch Dis Child 44(235):291–303, PMID: , 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Wieringen JC, Roede MJ, Wit JM. 1985. Growth diagrams for patient care [in Dutch]. Tijdschr Kindergeneeskd 53(4):147–152, PMID: . [PubMed] [Google Scholar]
- 35.Biro FM, Pajak A, Wolff MS, Pinney SM, Windham GC, Galvez MP, et al. 2018. Age of menarche in a longitudinal US cohort. J Pediatr Adolesc Gynecol 31(4):339–345, PMID: , 10.1016/j.jpag.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CDC. 2000. 2000 CDC Growth Charts. Girls BMI for age, Data table for BMI for age charts. https://www.cdc.gov/growthcharts/cdc_charts.htm [accessed 8 September 2023].
- 37.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. 2011. Trends in exposure to polyfluoroalkyl chemicals in the US population: 1999–2008. Environ Sci Technol 45(19):8037–8045, PMID: , 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- 38.Kuklenyik Z, Needham LL, Calafat AM. 2005. Measurement of 18 perfluorinated organic acids and amides in human serum using on-line solid-phase extraction. Anal Chem 77(18):6085–6091, PMID: , 10.1021/ac050671l. [DOI] [PubMed] [Google Scholar]
- 39.Kato K, Basden BJ, Needham LL, Calafat AM. 2011. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 1218(15):2133–2137, PMID: , 10.1016/j.chroma.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 40.Windham GC, Pinney SM, Voss RW, Sjödin A, Biro FM, Greenspan LC, et al. 2015. Brominated flame retardants and other persistent organohalogenated compounds in relation to timing of puberty in a longitudinal study of girls. Environ Health Perspect 123(10):1046–1052, PMID: , 10.1289/ehp.1408778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Attfield KR, Pinney SM, Sjödin A, Voss RW, Greenspan LC, Biro FM, et al. 2019. Longitudinal study of age of menarche in association with childhood concentrations of persistent organic pollutants. Environ Res 176:108551, PMID: , 10.1016/j.envres.2019.108551. [DOI] [PubMed] [Google Scholar]
- 42.Biro FM, Pinney SM, Huang B, Baker ER, Walt Chandler D, Dorn LD. 2014. Hormone changes in peripubertal girls. J Clin Endocrinol Metab 99(10):3829–3835, PMID: , 10.1210/jc.2013-4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolff MS, Teitelbaum SL, Lioy PJ, Santella RM, Wang RY, Jones RL, et al. 2005. Exposures among pregnant women near the World Trade Center site on 11 September 2001. Environ Health Perspect 113(6):739–748, PMID: , 10.1289/ehp.7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinney SM, Windham GC, Xie C, Herrick RL, Calafat AM, McWhorter K, et al. 2019. Perfluorooctanoate and changes in anthropometric parameters with age in young girls in the Greater Cincinnati and San Francisco Bay Area. Int J Hyg Environ Health 222(7):1038–1046, PMID: , 10.1016/j.ijheh.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sánchez-Soberón F, Sutton R, Sedlak M, Yee D, Schuhmacher M, Park JS. 2020. Multi-box mass balance model of PFOA and PFOS in different regions of San Francisco Bay. Chemosphere 252:126454, PMID: , 10.1016/j.chemosphere.2020.126454. [DOI] [PubMed] [Google Scholar]
- 46.Sedlak M, Sutton R, Wong A, Lin D. 2018. Per and Polyfluoroalkyl Substances (PFASs) in San Francisco Bay: Synthesis and Strategy. RMP Contribution No. 867. Richmond, CA: San Francisco Estuary Institute. https://www.sfei.org/sites/default/files/biblio_files/PFASs%20Synthesis%20and%20Strategy.pdf [accessed 8 September 2023]. [Google Scholar]
- 47.Christensen KY, Maisonet M, Rubin C, Holmes A, Calafat AM, Kato K, et al. 2011. Exposure to polyfluoroalkyl chemicals during pregnancy is not associated with offspring age at menarche in a contemporary British cohort. Environ Int 37(1):129–135, PMID: , 10.1016/j.envint.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duarte J, Perrière G, Laudet V, Robinson-Rechavi M. 2002. NUREBASE: database of nuclear hormone receptors. Nucleic Acids Res 30(1):364–368, PMID: , 10.1093/nar/30.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biro FM, Huang B, Wasserman H, Gordon CM, Pinney SM. 2021. Pubertal growth, IGF-1, and windows of susceptibility: puberty and future breast cancer risk. J Adolesc Health 68(3):517–522, PMID: , 10.1016/j.jadohealth.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng S, Yan L, Zhang J, Wang Z, Tian M, Shen H. 2013. An integrated metabonomics and transcriptomics approach to understanding metabolic pathway disturbance induced by perfluorooctanoic acid. J Pharm Biomed Anal 86:56–64, PMID: , 10.1016/j.jpba.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 51.Steenland K, Fletcher T, Savitz DA. 2010. Epidemiologic evidence on the health effects of perfluorooactanoic acid (PFOA). Environ Health Perspect 118(8):1100–1108, PMID: , 10.1289/ehp.0901827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khalil N, Ebert JR, Honda M, Lee M, Nahhas RW, Koskela A, et al. 2018. Perfluoroalkyl substances, bone density, and cardio-metabolic risk factors in obese 8–12 year old children: a pilot study. Environ Res 160:314–321, PMID: , 10.1016/j.envres.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 53.Kishi R, Nakajima T, Goudarzi H, Kobayashi S, Sasaki S, Okada E, et al. 2015. The association of prenatal exposure to perfluorinated chemicals with maternal essential and long-chain polyunsaturated fatty acids during pregnancy and the birth weight of their offspring: the Hokkaido Study. Environ Health Perspect 123(10):1038–1045, PMID: , 10.1289/ehp.1408834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L, Li YY, Zeng HC, Li M, Wan YJ, Schluesener HJ, et al. 2011. Perfluorooctane sulfonate induces apoptosis in N9 microglial cell line. Int J Toxicol 30(2):207–215, PMID: , 10.1177/1091581810387832. [DOI] [PubMed] [Google Scholar]
- 55.Martinsson M, Nielsen C, Björk J, Rylander L, Malmqvist E, Lindh C, et al. 2020. Intrauterine exposure to perfluorinated compounds and overweight at age 4: a case–control study. PLoS One 15(3):e0230137, PMID: , 10.1371/journal.pone.0230137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berg JM, Tymoczko J, Stryer L. 2002. Important derivatives of cholesterol include bile salts and steroid hormones. In: Biochemistry. 5th ed. New York, NY: W.H. Freeman, 1–7. [Google Scholar]
- 57.Du G, Hu J, Huang H, Qin Y, Han X, Wu D, et al. 2013. Perfluorooctane sulfonate (PFOS) affects hormone receptor activity, steroidogenesis, and expression of endocrine-related genes in vitro and in vivo. Environ Toxicol Chem 32(2):353–360, PMID: , 10.1002/etc.2034. [DOI] [PubMed] [Google Scholar]
- 58.Fassler CS, Pinney SE, Xie C, Biro FM, Pinney SM. 2019. Complex relationships between perfluorooctanoate, body mass index, insulin resistance and serum lipids in young girls. Environ Res 176:108558, PMID: , 10.1016/j.envres.2019.108558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Itoh S, Araki A, Mitsui T, Miyashita C, Goudarzi H, Sasaki S, et al. 2016. Association of perfluoroalkyl substances exposure in utero with reproductive hormone levels in cord blood in the Hokkaido Study on Environment and Children’s Health. Environ Int 94:51–59, PMID: , 10.1016/j.envint.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 60.Jensen RC, Glintborg D, Gade Timmermann CA, Nielsen F, Kyhl HB, Frederiksen H, et al. 2020. Prenatal exposure to perfluorodecanoic acid is associated with lower circulating concentration of adrenal steroid metabolites during mini puberty in human female infants. The Odense Child Cohort. Environ Res 182:109101, PMID: , 10.1016/j.envres.2019.109101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Traish AM, Kang HP, Saad F, Guay AT. 2011. Dehydroepiandrosterone (DHEA)—a precursor steroid or an active hormone in human physiology. J Sex Med 8(11):2960–2982, PMID: , 10.1111/j.1743-6109.2011.02523.x. [DOI] [PubMed] [Google Scholar]
- 62.Pinney SM, Windham GC, Biro FM, Kushi LH, Yaghjyan L, Calafat A, et al. 2009. Perfluorooctanoic acid (PFOA) and pubertal maturation in young girls. [Poster.] Epidemiology 20(6):S80, 10.1097/01.ede.0000362949.30847.cb. [DOI] [Google Scholar]
- 63.Pinney SM, Windham GC, Biro FM, Kushi LH, Herrick RL, Yaghjyan L, et al. 2011. Age at puberty in relation to prepubertal measures of PFOA—new findings. Environ Health Perspect 2011(1):1, 10.1289/isee.2011.00776. [DOI] [Google Scholar]
- 64.Hubbard R. 2011. The widespread misinterpretation of p-values as error probabilities. J Appl Stat 38(11):2617–2626, 10.1080/02664763.2011.567245. [DOI] [Google Scholar]
- 65.Lagiou P, Samoli E, Okulicz W, Xu B, Lagiou A, Lipworth L, et al. 2011. Maternal and cord blood hormone levels in the United States and China and the intrauterine origin of breast cancer. Ann Oncol 22(5):1102–1108, PMID: , 10.1093/annonc/mdq565. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Calafat AM, Chen A, Lanphear BP, Jones NY, Cecil KM, et al. 2023. Associations of prenatal and postnatal exposure to perfluoroalkyl substances with pubertal development and reproductive hormones in females and males: The HOME study. Sci Total Environ 890:164353, PMID: , 10.1016/j.scitotenv.2023.164353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.