ABSTRACT
Objective
The purpose of the study is to quantitatively assess shear-wave elastography (SWE) value in American College of Radiology Thyroid Imaging Reporting and Data Systems (ACR TI-RADS) 4.
Materials and methods
One hundred and fifty-two ACR TI-RADS 4 thyroid nodules undergoing SWE were included in the study. The mean (EMean), minimum (EMin) and maximum (EMax) of SWE elasticity were measured.
Results
The areas under the receiver operating characteristic (ROC) curves for SWE EMean, EMin and EMax in detecting benign and malignant nodules were 0.95, 0.83 and 0.84, respectively. Cut-off value of EMean ≤ 23.30 kPa is able to downgrade the lesion category to ACR TI-RADS 3 and cut-off value of EMean ≥ 52.14 kPa is able to upgrade the lesion category to ACR TI-RADS 5.
Conclusions
The EMean of SWE will probably identify nodules that have a high potential for benignity in ACR TI-RADS 4. It may help identify and select benign nodules while reducing unnecessary biopsy of benign thyroid nodules.
Keywords: Shear-wave elastography, thyroid nodule, American College of Radiology Thyroid Imaging Reporting and Data Systems, fine needle aspiration biopsy
INTRODUCTION
Thyroid nodules are common findings in the general population and have increased in recent years ( 1 - 3 ). Most thyroid nodules are actually asymptomatic and benign, and only about 5% of thyroid nodules are malignant ( 4 - 8 ). Ultrasound is the most accurate way to image thyroid nodules because malignant ultrasound features are associated with a higher malignancy risk ( 9 ). But no ultrasound feature has both high sensitivity and high specificity ( 10 ). According to the guidelines, fine needle aspiration biopsy (FNAB) is the best way to identify benign and malignant tumors ( 11 - 13 ). But FNAB also has some limitations, such as being invasive and time consuming. Elastography, a new imaging technique, is a non-invasive procedure that is simple and convenient, the least but not the last, its capability of stiffness quantification as additional diagnostic metric, was added to the diagnostic list to evaluate thyroid nodules ( 14 ).
Several Thyroid Imaging Reporting and Data Systems (TI-RADS) have been developed for malignancy risk stratification that incorporate ultrasound features to categorize thyroid nodules, such as French TI-RADS, American Thyroid Association (ATA) guideline, Korean TI-RADS and American College of Radiology Thyroid Imaging Reporting and Data Systems (ACR TI-RADS) ( 15 - 19 ). The ACR TI-RADS developed a predictive model that assigned different risk scores to each suspicious ultrasound feature and determined the TI-RADS category of nodules by allocating points to more suspicious features and summing the total score ( 20 - 23 ). ACR TI-RADS 3 assessment assesses lesions with a malignancy of less than 5% and ACR TI-RADS 4 assessment for lesions with a malignant risk, ranging from 5% to 20% ( 24 ).
Ultrasound strain elastography measures the deformation of tissue responsiveness and display and derive its stiffness. The disadvantage of this kind ultrasound elastography is that diagnostic accuracy and quality of elastography may be affected by internal compression (e.g., depth and distance of carotid artery pulsation, depth of nodule to skin, tracheal motion) and external compression ( 25 ). However, shear wave elastography (SWE) estimates the shear wave velocity of the tissue by acoustic radiation force of the ultrasonic push pulses and directly reflects the tissue stiffness with Young’s modulus ( 26 ). Qualitative and quantitative assessments of tissue stiffness can be obtained ( 27 , 28 ). However, the nodule stiffness measurement can be biased by the pre-load due to the weight of the transducer and the operator’s hand over-compressing superficial and thyroid tissue. Fortunately, this can be avoided by using a thick gel layer. SWE is the most reproducible and least operator-dependent technique among other elastographic techniques ( 29 ).
MATERIALS AND METHODS
Patient selection
The patient cohort was retrospectively collected from patients assessed from January 2017 to May 2018. Informed consent was obtained from all patients. Most patients were selected for FNAB based on ACR TI-RADS, or because they were at high risk based on history, such as a family history of thyroid cancer or a history of radiation exposure. A very small part of patients were strongly recommended FNAB by themselves.
All examinations were performed by the same operator with 5 years of ultrasound experience. Patients with ACR-TIRADS 4 nodules who underwent FNAB or surgery after routine ultrasound and SWE were included in the study. Exclusion criteria were as follows: 1. Nodule with rim calcification or macrocalcification; 2. Nodule with cystic ingredient; 3. Nodule with depth > 3 cm; 4. Thyrodititis. SWE was performed after gray-scale ultrasound and prior to the FNAB.
Ultrasound examination
Patient was in the supine position with slightly extension of the neck on the examination table. After application of coupling agent, both conventional ultrasound imaging and SWE were performed with Mindray Resona 7 ultrasound machine equipped with high frequency (5.6 ∼ 10 MHz) linear array transducer before FNAB. Conventional ultrasound scanning protocols included both transverse and longitudinal imaging of thyroid nodules. Two thyroid radiologist with 7 years of clinical experience evaluated category according to ACR TI-RADS who were blinded to each other. Two radiologists agreed on the form of the discussion if there was disagreement between them. SWE images of longitudinally oriented thyroid nodules were obtained from the same two sonographers who underwent elastography training for more than 5 years. The transducer was placing lightly on the patient’s neck and it was necessary to make sure that the neck was not under pressure. Patients were asked to keep a breath for a while to minimize the effect of breathing when the sonographer collected standard images. The qualified images were as follows: 1. The color signal frame was almost completely filled with color and also the color was stable. 2. There was no obvious compression artifact. Analyzer was blind to the cytological result when placed region of interest (ROI) over the entire nodule. Choosing the highest stiffness region of each nodule, each ROI was recorded by the mean (EMean), min (EMin) and max (EMax) stiffness values of SWE.
ACR TI-RADS
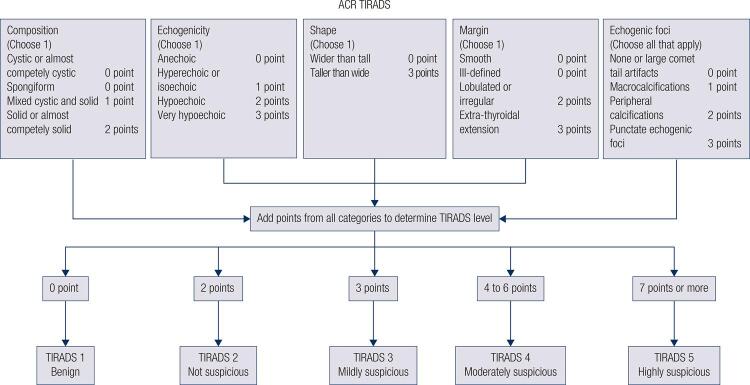
According to five features categories in the ACR-TIRADS Lexicon (shape, margin, composition, echogenicity, echogenic foci), the examiner assigned a malignancy risk that matched the five risk stratification levels (benign, not suspicious, mildly suspicious, moderately suspicious or highly suspicious) ( Figure 1 ). We elaborate on the most misunderstanding ultrasound findings about the five groups. A spongiform nodule which is included in composition must be composed predominantly (> 50%) of small cystic spaces. The feature of echogenicity uses the adjacent thyroid tissue as the basis for comparison. Except for very hypoechoic nodules, in which the echogenicity refers to a nodule’s reflectivity relative to the strap muscles. Taller-than-wide was categorized as when the anteroposterior diameter of a nodule was longer than its transverse diameter on a transverse or longitudinal plane for nodule shape. Microcalcification was defined as calcification with a diameter less than 1 mm, which was visualized as tiny and punctiform. Intra- and inter-observer reproducibility of ACR-TIRADS categories was assessed.
Figure 1. Schematic diagram of ACR TI-RADS was showing in the figure.
Final diagnosis
The final diagnosis was determined by histopathology or follow-up of each thyroid nodule. FNAB result was categorized as six grades. Bethesda category II to III was defined as benign and Bethesda category IV to VI was defined as malignant. For benign thyroid nodules, the final diagnosis was confirmed by surgery, repeated FNAB at least two benign outcomes, or one-time benign result on FNAB, and no change size on follow-up ultrasound more than 12-month. For malignant thyroid nodules, the final diagnosis was confirmed by surgery or a malignant result on FNAB at once.
Statistical analyses
All statistical analyses were conducted with IBM SPSS Statistics for Windows 22.0 version (Chicago) and Graph Pad Prism 6. Intra- and inter-observer reproducibility were tested in all cases using Kappa values. Comparing the velocities of benign and malignant lesions was used by the Mann-Whitney U test. The difference of measurement data was detected by Kolmogorov-Smirnov test. “rule out” and “rule in” elastography value was assessed by estimating the area under the receiver operating characteristic (ROC) curve. A sensitivity of at least 98% was considered a requirement to downgrade a lesion (“rule-out criterion”). A specificity of at least 98% was considered a requirement to upgrade a lesion (“rule-in criterion”). P value less than 0.05 considered statistically significant.
RESULTS
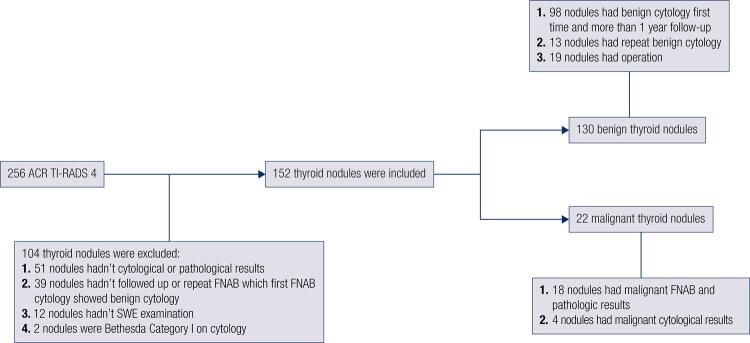
Two hundred and fifty-six lesions were included in the initial analysis from January 2017 to May 2018 with an ACR TI-RADS score of 4. One hundred and four lesions were eliminated from this analysis for some certain reasons. Definitive diagnosis was obtained via biopsy or surgical excision for 152 thyroid lesions form 152 patients. The mean age ± SD of the 152 patients (112 men and 40 women) were 45.2 ± 12.4 years (range, 19-74 years). Result revealed 22 malignant nodules and 130 benign nodules (including 37 benign nodules comfirmed by radiological follow-up and the remaining comfirmed by pathological results). The specific flow chart of thyroid lesion was described in figure 2 . The total thyroid cancer detection rate was 14.5% in our subjects. There were no significant differences in age and nodule diameter between malignant and benign groups ( Table 1 ). The Kappa values of intra- and inter-observer reproducibility of ACR-TIRADS categories were 0.81 and 0.77.
Figure 2. Two hundred and fifty-six ACR TI-RADS 4 lesions were evaluated in the initial analysis. One hundred and four lesions were excluded for some reasons as shown in figure. The remaining 152 lesions were used for the final analysis.
Table 1. Patient demographics.
| Characteristic | Value | P value | |
|---|---|---|---|
|
| |||
| Benign nodule | Malignant nodule | ||
| Number, n (%) | 130 (85.5%) | 22 (14.5%) | < 0.05 |
| Age, year (mean ± SD) | 45.88 ± 12.47 | 41.36 ± 11.95 | > 0.05 |
| Gender, n (%) | |||
| Female | 30 | 10 | < 0.05 |
| Male | 100 | 12 | < 0.05 |
| Longest diameter (cm) | 1.08 | 1.12 | > 0.05 |
| SWE, Emin (kPa) | 9.80 | 19.67 | < 0.05 |
| SWE, Emean (kPa) | 24.15 | 47.37 | < 0.05 |
| SWE, Emax (kPa) | 57.86 | 92.07 | < 0.05 |
SWE: shave wave elastography.
The area under the ROC curve value of EMean, EMin and EMax were 0.95 (95% CI 0.91-1.00), 0.83 (95% CI 0.73-0.93), 0.84 (95% CI 0.78-0.91) respectively ( Figure 3 ). EMean yielded the highest area under the ROC curve value, which was used for the diagnosis of nodules. Cut-off value of EMean ≤ 23.30 kPa was able to downgrade the lesion category (“rule-out criterion”) to ACR TI-RADS 3 with a sensitivity > 98%, Cut-off value of EMean ≥ 52.14 kPa was able to upgrade the lesion category (“rule-in criterion”) to ACR TI-RADS 5 with a specificity > 98% ( Figure 4 ).
Figure 3. The SWE value of mean (EMean), min (EMin) and max (EMax) with areas under the receiver operative characteristic (ROC) curve of 0.95, 0.83 and 0.84, respectively.
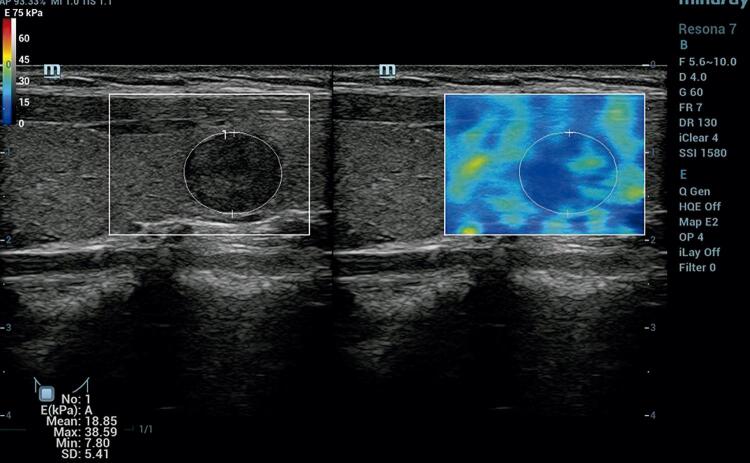
Figure 4. According to composition (solid), echogenicity (very hypoechoic), shape (wider than tall), margin (smooth) and echogenic foci (none), this nodule was ACR TI-RADS 4 with 5 points. The EMean of SWE was 18.85 kPa which was able to downgrade the lesion category to ACR TI-RADS 3. The pathology of this nodule was nodular hyperplasia.
DISCUSSION
Previous studies had focused on the overall study of thyroid nodules at all levels, while rare reports had discussed ACR TI-RADs 4 nodules individually ( 30 ). However, in clinical work, it was the ACR TI-RADS 4 nodule that made the examining physician more confused ( 31 ). In particular, it was more difficult to conduct a good assessment, and it was impossible to provide meaningful guidance to clinicians when there was no experience ( 32 ).
Routine detection thyroid nodules used by high-frequency ultrasound couldn’t detect the biophysical parameters closely related to the above-mentioned tumor tissues. The measurements of traditional ultrasound elastography technology were affected by probe pressure and the operator’s subjective experience, which led to unsatisfactory diagnostic specificity and sensitivity to benign and malignant thyroid nodules. SWE technology measured the speed of shear waves emitted by transducers in different tissues and evaluates nodules with Young’s modulus values ( 33 ). It was the only technique that could quantitatively determine the stiffiness value of nodules at present ( 34 ). SWE had achieved “sound and palpation” with strong objectivity, good repeatability and little influence by the operator.
Benign thyroid lesions were mainly consisted of follicular cells, which were filled with colloid components and were soft in texture, while the intratumoral cancer cells replacing the soft follicles and glial components in malignant thyroid lesions ( 35 - 37 ). The increased in the stiffiness of malignant thyroid lesion was due to the fact that the tissue mainly contains many interstitial fibrous tissues, blood vessels and concentrically arranged calcified bodies and other reasons. In addition, the increased in the stiffiness of papillary thyroid cancer was related to the formation of a large number of psammoma bodies within the nodule.
SWE like other technologies had a certain false positive and negative rate. Overlapping of lesion information was because when some benign nodules develop chronic fibrosis and coarse calcification during growth, the stiffiness increased accordingly and also EMax value increased, resulting in false positives; Szczepanek-Parulska and cols. also reported that benign lesions with microcalcifications were stiffer than benign lesions without calcifications, complicating the use of maximal SWE stiffness to discriminate benign and malignant lesions ( 38 ). On the other hand, malignant nodules decrease stiffness due to necrosis, liquefaction, and hemorrhage and also EMax values decreased, resulting in false negatives. It was difficult to distinguish with benign nodules in certain situation. EMean yielded the highest area under the ROC curve value, which was the best indicator used for the diagnosis of nodules. Cut-off value of EMean ≤ 23.30 kPa was able to downgrade the lesion category (“rule-out criterion”) with a sensitivity > 98%, Cut-off value of EMean ≥ 52.14 kPa was able to upgrade the lesion category (“rule-in criterion”) with a specificity > 98%. The easily accessible and reliable SWE will complement the ACR TI-RADS 4, support clinical decision making and reduce necessary thyroid biopsy.
There were some limitations in this study. First, there was a retrospective and single study, a selection bias was inevitable. Second, stiffness value measurements only achieved by longitudinal images. We favored of longitudinal orientation due to the lower incidence of images affected by carotid pulsation as well as lack of tracheal compression on thyroid. Third, operator-dependent factors (lack of contact of probe with neck, blurring caused by probe motion and so on) of SWE were not excluded in this study.
Our study support the recommendation that ACR-TIRADS 4 combined with SWE could be used in clinical diagnosis as a noninvasive diagnostic approach, which may lead to a great improvement in accurate diagnosis, and the number of unnecessary FANB could be reduced for benign thyroid nodules.
Acknowledgement
The research was supported by the National Natural Science Foundation of China (No. 81420108018, 81527803), China Ultrasound Physician Technology Star Program (No.KJXX2018003), National Key R&D Program of China (No.2018YFC0115900) and Zhejiang Science and Technology Project (No.2019C03077).
REFERENCES
- 1.Cao J, Huang YQ, Jiao-Sun, Lan XB, Ge MH. Clinicopathological and prognostic significance of SHP2 and Hook1 expression in patients with thyroid carcinoma. Hum Pathol. 2018 ;81:105-12. [DOI] [PubMed]
- 2.Janus D, Wojcik M, Taczanowska A, Soltysiak P, Wedrychowicz A, Roztoczynska D, et al. Follow-up of parenchymal changes in the thyroid gland with diffuse autoimmune thyroiditis in children prior to the development of papillary thyroid carcinoma. J Endocrinol Invest. 2019 ;42(3):261-70. [DOI] [PMC free article] [PubMed]
- 3.Murugan AK, Munirajan AK, Alzahrani AS. Long noncoding RNAs: emerging players in thyroid cancer pathogenesis. Endocr Relat Cancer. 2018 ;25(2):R59-82. [DOI] [PubMed]
- 4.Na KJ, Choi H. Immune landscape of papillary thyroid cancer and immunotherapeutic implications. Endocr Relat Cancer. 2018 ;25(5):523-31. [DOI] [PubMed]
- 5.Tori M, Shimo T. Long-term efficacy of lenvatinib for recurrent papillary thyroid carcinoma after multimodal treatment and management of complications: a case report. BMC Cancer. 2018 ;18(1):698. [DOI] [PMC free article] [PubMed]
- 6.Cabanillas ME, Ferrarotto R, Garden AS, Ahmed S, Busaidy NL, Dadu R, et al. Neoadjuvant BRAF- and Immune-Directed Therapy for Anaplastic Thyroid Carcinoma. Thyroid. 2018 ;28(7):945-51. [DOI] [PMC free article] [PubMed]
- 7.Amendoeira I, Maia T, Sobrinho-Simoes M. Non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): impact on the reclassification of thyroid nodules. Endocr Relat Cancer. 2018 ;25(4):R247-58. [DOI] [PubMed]
- 8.Corver WE, Demmers J, Oosting J, Sahraeian S, Boot A, Ruano D, et al. ROS-induced near-homozygous genomes in thyroid cancer. Endocr Relat Cancer. 2018 ;25(1):83-97. [DOI] [PubMed]
- 9.Werner TA, Forster CM, Dizdar L, Verde PE, Raba K, Schott M, et al. CXCR4/CXCR7/CXCL12-Axis in Follicular Thyroid Carcinoma. J Cancer. 2018 ;9(6):929-40. [DOI] [PMC free article] [PubMed]
- 10.Horn-Ross PL, Lichtensztajn DY, Clarke CA, Dosiou C, Oakley-Girvan I, Reynolds P, et al. Continued rapid increase in thyroid cancer incidence in california: trends by patient, tumor, and neighborhood characteristics. Cancer Epidemiol Biomarkers Prev. 2014 ;23(6):1067-79. [DOI] [PMC free article] [PubMed]
- 11.Nabhan F, Porter K, Lupo MA, Randolph GW, Patel KN, Kloos RT. Heterogeneity in Positive Predictive Value of RAS Mutations in Cytologically Indeterminate Thyroid Nodules. Thyroid. 2018 ;28(6):729-38. [DOI] [PubMed]
- 12.Tam S, Amit M, Boonsripitayanon M, Busaidy NL, Cabanillas ME, Waguespack SG, et al. Effect of Tumor Size and Minimal Extrathyroidal Extension in Patients with Differentiated Thyroid Cancer. Thyroid. 2018 ;28(8):982-90. [DOI] [PubMed]
- 13.Abi-Raad R, Prasad M, Baldassari R, Schofield K, Callender GG, Chhieng D, et al. The Value of Negative Diagnosis in Thyroid Fine-Needle Aspiration: a Retrospective Study with Histologic Follow-Up. Endocr Pathol. 2018 ;29(3):269-75. [DOI] [PubMed]
- 14.Wang HW, Shi HN, Cheng J, Xie F, Luo YK, Tang J. Real-time shear wave elastography (SWE) assessment of short- and long-term treatment outcome in Budd-Chiari syndrome: A pilot study. PLoS One. 2018 ;13(5):e0197550. [DOI] [PMC free article] [PubMed]
- 15.Sawka AM, Carty SE, Haugen BR, Hennessey JV, Kopp PA, Pearce EN, et al. American Thyroid Association Guidelines and Statements: Past, Present, and Future. Thyroid. 2018 ;28(6):692-706. [DOI] [PubMed]
- 16.Shangguan R, Hu YP, Huang J, Yang SJ, Ye L, Lin RX, et al. Association Between BRAF(V600E) Mutation and the American College of Radiology Thyroid Imaging, Reporting and Data System in Solitary Papillary Thyroid Carcinoma. Acad Radiol. 2019 ;26(2):154-60. [DOI] [PubMed]
- 17.Middleton WD, Teefey SA, Reading CC, Langer JE, Beland MD, Szabunio MM, et al. Comparison of Performance Characteristics of American College of Radiology TI-RADS, Korean Society of Thyroid Radiology TIRADS, and American Thyroid Association Guidelines. AJR Am J Roentgenol. 2018 ;210(5):1148-54. [DOI] [PubMed]
- 18.Hoang JK, Middleton WD, Farjat AE, Teefey SA, Abinanti N, Boschini FJ, et al. Interobserver Variability of Sonographic Features Used in the American College of Radiology Thyroid Imaging Reporting and Data System. AJR Am J Roentgenol. 2018 ;211(1):162-7. [DOI] [PubMed]
- 19.Russ G. Risk stratification of thyroid nodules on ultrasonography with the French TI-RADS: description and reflections. Ultrasonography. 2016 ;35(1):25-38. [DOI] [PMC free article] [PubMed]
- 20.Robenshtok E, Nachalon Y, Benbassat C, Hirsch D, Shimon I, Grossman A, et al. Disease Severity at Presentation in Patients with Disease-Related Mortality from Differentiated Thyroid Cancer: Implications for the 2015 ATA Guidelines. Thyroid. 2017;27(9):1171-6. [DOI] [PubMed]
- 21.Ha SM, Baek JH, Choi YJ, Chung SR, Sung TY, Kim TY, et al. Malignancy risk of initially benign thyroid nodules: validation with various Thyroid Imaging Reporting and Data System guidelines. Eur Radiol. 2019 ;29(1):133-40. [DOI] [PubMed]
- 22.Chaigneau E, Russ G, Royer B, Bigorgne C, Bienvenu-Perrard M, Rouxel A, et al. TIRADS score is of limited clinical value for risk stratification of indeterminate cytological results. Eur J Endocrinol. 2018 ;179(1):13-20. [DOI] [PubMed]
- 23.Weiss VL, Andreotti RF, Ely KA. Use of the thyroid imaging, reporting, and data system (TI-RADS) scoring system for the evaluation of subcentimeter thyroid nodules. Cancer Cytopathol. 2018 ;126(8):518-24. [DOI] [PubMed]
- 24.Griffin AS, Mitsky J, Rawal U, Bronner AJ, Tessler FN, Hoang JK. Improved Quality of Thyroid Ultrasound Reports After Implementation of the ACR Thyroid Imaging Reporting and Data System Nodule Lexicon and Risk Stratification System. J Am Coll Radiol. 2018 ;15(5):743-8. [DOI] [PubMed]
- 25.Kim MH, Luo S, Ko SH, Bae JS, Lim J, Lim DJ, et al. Thyroid nodule parameters influencing performance of ultrasound elastography using intrinsic compression. Ultrasound Med Biol. 2015 ;41(9):2333-9. [DOI] [PubMed]
- 26.Hu X, Liu Y, Qian L. Diagnostic potential of real-time elastography (RTE) and shear wave elastography (SWE) to differentiate benign and malignant thyroid nodules: A systematic review and meta-analysis. Medicine (Baltimore). 2017 ;96(43):e8282. [DOI] [PMC free article] [PubMed]
- 27.Kocaoglu C, Durmaz MS, Sivri M. Shear wave elastography evaluation of testes with non-communicating hydrocele in infants and toddlers: A preliminary study. J Pediatr Urol. 2018 ;14(5):445e1-445.e6. [DOI] [PubMed]
- 28.Chang N, Zhang X, Wan W, Zhang C, Zhang X. The Preciseness in Diagnosing Thyroid Malignant Nodules Using Shear-Wave Elastography. Med Sci Monit. 2018 ;24:671-7. [DOI] [PMC free article] [PubMed]
- 29.Dighe M, Hippe DS, Thiel J. Artifacts in Shear Wave Elastography Images of Thyroid Nodules. Ultrasound Med Biol. 2018 ;44(6):1170-6. [DOI] [PMC free article] [PubMed]
- 30.Hoang JK, Middleton WD, Farjat AE, Langer JE, Reading CC, Teefey SA, et al. Reduction in Thyroid Nodule Biopsies and Improved Accuracy with American College of Radiology Thyroid Imaging Reporting and Data System. Radiology. 2018 ;287(1):185-93. [DOI] [PubMed]
- 31.Grant EG, Tessler FN, Hoang JK, Langer JE, Beland MD, Berland LL, et al. Thyroid Ultrasound Reporting Lexicon: White Paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) Committee. J Am Coll Radiol. 2015 ;12(12 Pt A):1272-9. [DOI] [PubMed]
- 32.Richman DM, Benson CB, Doubilet PM, Peters HE, Huang SA, Asch E, et al. Thyroid Nodules in Pediatric Patients: Sonographic Characteristics and Likelihood of Cancer. Radiology. 2018 ;288(2):591-9. [DOI] [PMC free article] [PubMed]
- 33.Wang Q, Guo LH, Li XL, Zhao CK, Li MX, Wang L, et al. Differentiating the acute phase of gout from the intercritical phase with ultrasound and quantitative shear wave elastography. Eur Radiol. 2018 ;28(12):5316-27. [DOI] [PubMed]
- 34.Park J, Woo OH, Shin HS, Cho KR, Seo BK, Kang EY. Diagnostic performance and color overlay pattern in shear wave elastography (SWE) for palpable breast mass. Eur J Radiol. 2015 ;84(10):1943-8. [DOI] [PubMed]
- 35.Naoum GE, Morkos M, Kim B, Arafat W. Novel targeted therapies and immunotherapy for advanced thyroid cancers. Mol Cancer. 2018 ; 17(1):51. [DOI] [PMC free article] [PubMed]
- 36.de la Fouchardière C, Decaussin-Petrucci M, Berthiller J, Descotes F, Lopez J, Lifante JC, et al. Predictive factors of outcome in poorly differentiated thyroid carcinomas. Eur J Cancer. 2018 ;92:40-7. [DOI] [PubMed]
- 37.Valderrabano P, McGettigan MJ, Lam CA, Khazai L, Thompson ZJ, Chung CH, et al. Thyroid Nodules with Indeterminate Cytology: Utility of the American Thyroid Association Sonographic Patterns for Cancer Risk Stratification. Thyroid. 2018 ;28(8):1004-12. [DOI] [PMC free article] [PubMed]
- 38.Szczepanek-Parulska E, Wolinski K, Stangierski A, Gurgul E, Ruchala M. Biochemical and ultrasonographic parameters influencing thyroid nodules elasticity. Endocrine. 2014 ;47(2): 519-27. [DOI] [PubMed]