It is estimated that more than 1.1 million Americans have died of COVID-19 disease as of March 2023.1 The pandemic has had significant additional impact to health by consuming health care resources and impeding care for other diseases. One study estimated that the decline in cancer screening, diagnosis, and treatment in just the first four months of the pandemic will lead to 10,000 additional deaths due to breast and colorectal cancer by 2030.2
THE TAKEAWAY
In the article that accompanies this editorial, Star et al3 document some of the significant impacts of the COVID-19 pandemic on health through impeding routine care for other diseases. The declines in the prevalence of cancer screening are startling and call for increased efforts to encourage health care providers and the general public to re-engage in using proven effective cancer control interventions.
In oncology, many studies have reported a prolonged decline in the prevalence of screening for cancers of the breast, cervix, colon, and prostate. The impact of these declines is yet to be determined.
To our knowledge, to date, the studies documenting the decline in screening prevalence are assessments of insurance claims or electronic medical records. These databases are nongeneralizable to the US population. They tend to be less representative of certain geographic areas, the poor, and certain racial and ethnic groups. In the article that accompanies this editorial, Star et al3 from the American Cancer Society use the National Health Interview Survey (NHIS), a nationally representative database, to show a downward trend in the prevalence of cancer screening for breast, cervical, and prostate cancer from the year before the epidemic (2019) to the second year of the epidemic (2021) in virtually all races and ethnicities and economic strata. Interestingly, the study finds a decline in use of colonoscopy screening for colorectal cancer with a rise in stool-based (especially stool DNA testing) colon cancer screening leading to the prevalence of colorectal screening remaining unchanged. Use of the stool-based test remained stable or increased especially among Americans with low education and income.
The National Health Interview Survey was established by the Centers for Disease Control and Prevention in 1957.4 Many consider it the gold standard in assessing American health trends. It is an annual, nationally representative, population-based survey. It has the advantage of generalizability not seen in other health surveys. Because it asks individuals about things in the past, there can be challenges with recall. In addition, the general population are also often unable to distinguish screening versus diagnostic tests.
The NHIS was done through in-person interviews before the pandemic. During the pandemic, a portion of the interviews were done by telephone. Response rates were lower in the 2021 survey, compared with 2019. It is not known how this affected results.4
Although the NHIS is done annually, the NHIS asked cancer screening questions every 5 years, before 2019. Fortunately, in 2019, a decision was made to ask about receipt of cancer screening, annually. The NHIS asks patients eligible for a specific cancer screening test on the basis of age and sex using commonly accepted recommendations.
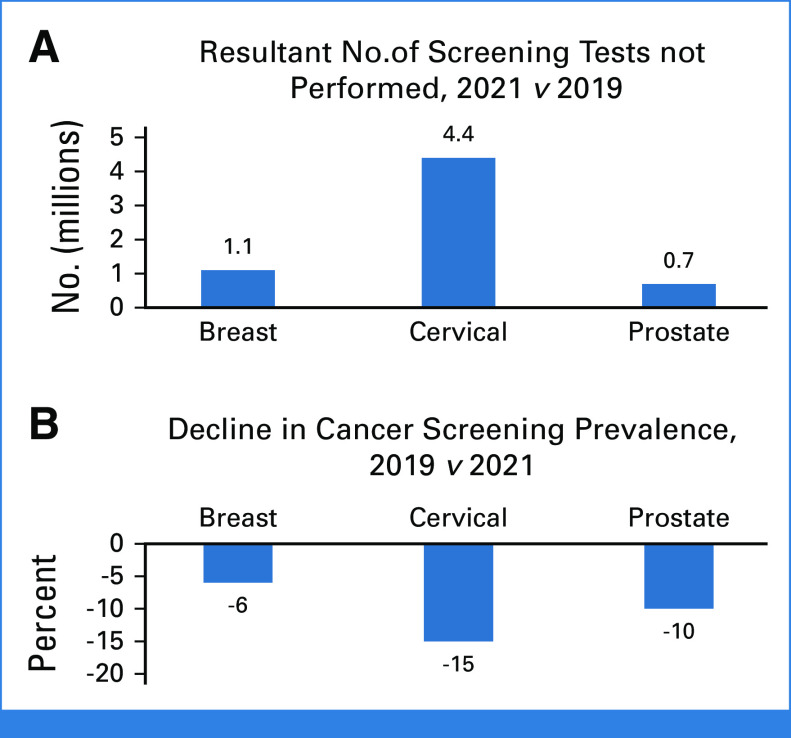
Assessing the prevalence of screening in 2019 and 2021, there were relative declines in the population from the prepandemic screening prevalence for breast cancer (6%), cervical cancer (15%), and prostate cancer (10%). This translated into 1.1 million, 4.4 million, and 0.7 million fewer Americans getting screened for breast, cervical, and prostate cancer, respectively (Fig 1).3 As noted above, the colorectal cancer screening prevalence remained stable. The prevalence of breast, cervical, colorectal, and prostate cancer screening in 2021 may represent a rebound from nadirs in 2020.
FIG 1.
(A) The number of breast, cervical, and prostate cancer screening tests performed in 2021 was less than that in 2019. (B) The proportional decline in screening for breast, cervical, and prostate cancer comparing 2019 screening rates with 2021 screening rates.
An increase in stool-based, especially stool DNA, screening offset the decline in colonoscopy screening. This may be due to the greater convenience of the stool test during a period of social distancing. Stool specimens are collected at home.
The increase may also be the result of a very successful advertising campaign. It is of note that there is only one stool DNA test in the US market. Its manufacturer has publicly stated in a trade publication and in its submissions to the US Securities and Exchange Commission that it viewed the COVID-19 pandemic as an opportunity to increase sales.5,6 As part of its business plan, it increased its direct-to-consumer advertising on television, digital, social, print, and other channels during the pandemic. To promote stool DNA testing, the company also provided support to advocacy organizations that promoted colorectal screening such as the American Cancer Society's National Colorectal Cancer Roundtable.
It is unfortunate that breast and cervical cancer screening and prostate cancer screening decision making and screening must be done in-person, and there were no concerted campaigns to encourage them.
The question about screening in the past year is appropriate when looking at changes from one year to another in the entire population. Accepted screening recommendations are breast cancer mammographic screening every 1 or 2 years, cervical cancer screening using the pap smear or HPV DNA every 3-5 years depending on the test used, and colorectal cancer screening can vary from annually to every 3 years or to every 10 years depending on the test. Because of questions about the efficacy of prostate cancer screening, most professional organizations do not outright recommend that all men get the test. They recommend shared or informed decision making after recognizing the potential benefits and known risks.
In addition to the question about getting a screening test in the past year, the NHIS has also asked about being up-to-date for screening for a specific cancer.7 In the 2015 NHIS survey, 53% of women, age older than 45 years, said they had had a mammogram in the past year (67.8% in the past 2 years); 81.4% of women, age 21-65 years, who have not had a hysterectomy were up-to-date for cervical screening; 62.6% of participants, age older than 50 years, were up-to-date for colorectal screening; and 34.4% of men, age older than 50 years, said they had been screened for prostate cancer in the past year. Only 17% of eligible men said they had undergone shared or informed decision making regarding prostate cancer screening.
These proportions indicate that there was a screening problem in the United States even before the COVID-19 pandemic. Taken with the study of Star et al,3 a significant number of American lives could be saved of breast, cervical, and colorectal cancer, if we could act together and increase the proportions screened and provide adequate diagnostics and treatment to all with a positive screening test. The findings support continued efforts to encourage interest in appropriate cancer screening. These efforts might take the form of public health initiatives (advertisements) aimed at the public and efforts to educate health care providers and staff. The data also support organized programs to encourage and remind patients to routinely undergo all the screening for which they are eligible.
Otis W. Brawley
Leadership: PDS Biotechnology, Incyte, Agilent, Lyell Immunopharma, Jackson Laboratory for Genomic Medicine
Honoraria: Genentech/Roche (less than $5,000 USD in a single calendar year), Grail ($5,000 USD or above in a single calendar year)
Consulting or Advisory Role: Genentech/Roche ($5,000 USD or above in a single calendar year), Grail ($5,000 USD or above in a single calendar year)
Travel, Accommodations, Expenses: Genentech/Roche
No other potential conflicts of interest were reported.
Footnotes
See accompanying Article, p. 4352
AUTHOR'S DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
On Cancer Screening During the COVID-19 Pandemic
The following represents disclosure information provided by the author of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Otis W. Brawley
Leadership: PDS Biotechnology, Incyte, Agilent, Lyell Immunopharma, Jackson Laboratory for Genomic Medicine
Honoraria: Genentech/Roche (less than $5,000 USD in a single calendar year), Grail ($5,000 USD or above in a single calendar year)
Consulting or Advisory Role: Genentech/Roche ($5,000 USD or above in a single calendar year), Grail ($5,000 USD or above in a single calendar year)
Travel, Accommodations, Expenses: Genentech/Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.CDC COVID Data Tracker, 2023. https://covid.cdc.gov/covid-data-tracker/#datatracker-home [Google Scholar]
- 2.Sharpless NE: COVID-19 and cancer. Science 368:1290, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Star J, Bandi P, Siegel RL, et al. : Cancer screening in the United States during the second year of the COVID-19 pandemic. J Clin Oncol 41:4352-4359, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Center for Health Statistics : National Health Interview Survey, 2019 and 2021. Hyattsville, MD, 2022 [Google Scholar]
- 5.Limburg PJ: As screening declines amid COVID-19, at-home stool DNA test for CRC gets high adherence in Medicare population. Washington, DC, The Cancer Letter. Paul Goldberg, 2020 [Google Scholar]
- 6.Exact Sciences Corporation Form 10K Annual Report to the US Securities and Exchange Commission. Washington DC, US Securities and Exchange Commission, 2022 [Google Scholar]
- 7.Goding Sauer A, Siegel RL, Jemal A, et al. : Current prevalence of major cancer risk factors and screening test use in the United States: Disparities by education and race/ethnicity. Cancer Epidemiol Biomarkers Prev 28:629-642, 2019 [DOI] [PubMed] [Google Scholar]