Abstract
PURPOSE
The molecular classification of endometrial cancer (EC) has proven to have prognostic value and is predictive of response to adjuvant chemotherapy. Here, we investigate its predictive value for response to external beam radiotherapy (EBRT) and vaginal brachytherapy (VBT) in early-stage endometrioid EC (EEC).
METHODS
Data of the randomized PORTEC-1 trial (n = 714) comparing pelvic EBRT with no adjuvant therapy in early-stage intermediate-risk EC and the PORTEC-2 trial (n = 427) comparing VBT with EBRT in early-stage high-intermediate-risk EC were used. Locoregional (including vaginal and pelvic) recurrence-free survival was compared between treatment groups across the four molecular classes using Kaplan-Meier's methodology and log-rank tests.
RESULTS
A total of 880 molecularly classified ECs, 484 from PORTEC-1 and 396 from PORTEC-2, were included. The majority were FIGO-2009 stage I EEC (97.2%). The median follow-up was 11.3 years. No locoregional recurrences were observed in EC with a pathogenic mutation of DNA polymerase-ε (POLEmut EC). In mismatch repair–deficient (MMRd) EC, locoregional recurrence-free survival was similar after EBRT (94.2%), VBT (94.2%), and no adjuvant therapy (90.3%; P = .74). In EC with a p53 abnormality (p53abn EC), EBRT (96.9%) had a substantial benefit over VBT (64.3%) and no adjuvant therapy (72.2%; P = .048). In EC with no specific molecular profile (NSMP EC), both EBRT (98.3%) and VBT (96.2%) yielded better locoregional control than no adjuvant therapy (87.7%; P < .0001).
CONCLUSION
The molecular classification of EC predicts response to radiotherapy in stage I EEC and may guide adjuvant treatment decisions. Omitting radiotherapy seems to be safe in POLEmut EC. The benefit of radiotherapy seems to be limited in MMRd EC. EBRT yields a significantly better locoregional recurrence-free survival than VBT or no adjuvant therapy in p53abn EC. VBT is the treatment of choice for NSMP EC as it is as effective as EBRT and significantly better than no adjuvant therapy for locoregional tumor control.
INTRODUCTION
Endometrial cancer (EC) is currently classified into four molecular groups with a distinct tumor biology and prognosis. EC with a pathogenic mutation of DNA polymerase-ε (POLEmut EC) has an excellent prognosis, and EC with mismatch repair–deficient (MMRd EC) has an intermediate prognosis. EC with a p53 abnormality (p53abn EC) has a poor prognosis, whereas EC with no specific molecular profile (NSMP EC) has a stage-dependent prognosis.1,2
CONTEXT
Key Objective
To determine the predictive value of the molecular class of endometrial cancer (EC) for the efficacy of vaginal brachytherapy (VBT) and pelvic external beam radiotherapy (EBRT) in women with stage I endometrioid EC.
Knowledge Generated
Analyses of data from 880 women included in the randomized PORTEC-1 and PORTEC-2 radiotherapy trials showed at 5 years the following: (1) no locoregional recurrences in POLE-mutant EC regardless of adjuvant radiotherapy; (2) similar locoregional recurrence-free survival in mismatch repair–deficient EC after EBRT (94.2%), VBT (94.2%), and no adjuvant therapy (90.3%; P = .74); (3) significantly better locoregional recurrence-free survival in p53-abnormal EC with EBRT (96.9%), compared with VBT (64.3%) and no adjuvant therapy (72.2%; P = .048); and (4) significantly better locoregional recurrence-free survival in EC with no specific molecular profile, with both EBRT (98.3%) and VBT (96.2%) compared with no adjuvant therapy (87.7%; P < .0001).
Relevance (G.F. Fleming)
-
Systemic therapy for EC is being adapted for different TCGA groups, and this report adds to our knowledge about also adapting radiotherapy for biologic subsets.*
*Relevance section written by JCO Associate Editor Gini F. Fleming, MD.
Several studies have confirmed the independent prognostic impact of the molecular class.3-5 The molecular class also has predictive value for benefit of chemotherapy in women with high-risk EC.6 A number of international guidelines recently incorporated the molecular classification in risk assessment and adjuvant treatment recommendations, although mainly for high-risk EC.7-9 The molecular class might also have clinically relevant implications in (high-)intermediate-risk EC, for which adjuvant radiotherapy is often recommended.7-10
Whether the molecular classification has predictive value for benefit of radiotherapy has not yet been investigated. There are several studies that show differences between the molecular classes in the ways cancer cells respond to damage induced by radiation.11,12 For example, homologous recombination (HR)–deficient p53abn EC13,14 is unable to repair radiation-induced double-strand breaks, which might translate into radiosensitivity.15 MMRd EC has an impaired base mismatch repair apparatus, which is normally activated by radiotherapy-induced single-strand breaks.11 However, as these cancers are typically HR-proficient,16 it is uncertain whether this translates into a clinical benefit. The majority of NSMP ECs have an endometrioid histotype,3,6 of which 80% have aberrant activation of the PI3K/PTEN/AKT/mTOR-signaling pathway.11 Loss of PTEN is common17 and associated with increased radiosensitivity.18 Finally, it is likely that patients with POLEmut EC do not benefit from radiotherapy as recurrences are rare, even in the absence of adjuvant therapy.19
Here, we investigate the efficacy of radiotherapy across the four molecular classes of EC using data of two large, randomized radiotherapy trials in women with early-stage endometrioid EC.
METHODS
Study Population
Data of all women included in the intention-to-treat populations of the randomized PORTEC-1 (n = 714) and PORTEC-2 trials (n = 427) were used for this study. The design and outcomes of both trials have been published.20,21 In PORTEC-1, pelvic external beam radiotherapy (EBRT) was compared with no adjuvant treatment, and in PORTEC-2, vaginal brachytherapy (VBT) was compared with EBRT. From 1990 to 1997, PORTEC-1 trial recruited women with FIGO-1988 stage I endometrioid EC (EEC), grade 1-2 with deep myometrial invasion or grade 2-3 with superficial invasion. From 2000 to 2006, PORTEC-2 trial recruited women with EEC with high-intermediate-risk features, defined as (1) FIGO-1988 stage IB with age >60 years and grade 3, (2) stage IC with age >60 years and grade 1-2, or (3) stage IIA (except grade 3 with deep invasion).
Study Protocols (online only) of the PORTEC-1 and PORTEC-2 trials were approved by the medical ethics committees of all participating centers, and all patients provided informed consent.
Central Pathology Review and Molecular Analysis
Tumor characteristics and treatment outcomes were obtained from the trial databases. Tumor characteristics were based on the central pathology review that was performed after random assignment.3,22,23 This process identified a few women who were in hindsight not eligible. For this study, tumor stage was reclassified according to FIGO-2009.
For all EC, Sanger sequencing of exons 9 and 13 was performed for detection of pathogenic DNA-polymerase-ε exonuclease domain variants.24 Mismatch-repair status was determined using the Promega MSI analysis system (version 1.2) or immunohistochemistry (IHC) for the four mismatch-repair proteins.2 The p53 status was defined as abnormal if p53-IHC in >10% of tumor cells showed a null pattern, nuclear overexpression, or cytoplasmic expression.25 Details on primers and antibodies have been reported.3 The molecular class of EC was assigned according to WHO-2020 classification.1
Statistical Analysis
Statistical analyses were optimized for causal inference of any difference in treatment effects across the molecular classes. Exchangeability of treatment groups was preserved as much as possible by working with the intention-to-treat populations, excluding as few patients as possible and using the randomly allocated treatment.
The primary analysis of this study consists of two parts: (1) locoregional (vaginal and/or pelvic) recurrence-free survival by treatment across the molecular classes in PORTEC-1 and (2) pelvic recurrence-free survival by treatment across molecular classes in PORTEC-2. These end points were chosen to evaluate recurrences in locations that were exposed to radiotherapy in one treatment group and not exposed to radiotherapy in the other treatment group. Hence, in PORTEC-1, this comprises all recurrences in the pelvis, including in the lymph node regions and the vaginal vault, as no adjuvant therapy was compared with pelvic EBRT. In PORTEC-2, in which VBT was compared with EBRT, this only comprises pelvic recurrences as the vaginal vault region was irradiated in both treatment groups. For the secondary analysis, data of PORTEC-1 and PORTEC-2 were pooled to investigate locoregional recurrence-free survival by treatment across the molecular classes.
Time to recurrence was defined as the time from random assignment to recurrence, with censoring at last follow-up or death in the case of no recurrence. Actuarial survival times were estimated according to Kaplan-Meier's methodology and compared between groups using log-rank tests. Median follow-up was calculated by the reverse Kaplan-Meier method.26 Multivariable regression analysis was performed using a Cox, proportional hazards model stratified by trial with prespecified covariates on the basis of previous work,27 with the number of covariables adapted to minimize risk of overfitting. The model used all informative cases and excluded those with missing covariable data (only for lymphovascular space invasion [LVSI] 6.4% missingness). Model validation was performed by analysis of discrimination and indices of optimism determined by means of model fitting to 1,000 bootstrap resamples.28 Proportionality of hazards was evaluated by inspection of scaled Schoenfeld residuals.
Continuous variables were analyzed by either parametric or nonparametric methods depending on their distribution. Categorical variables were analyzed by nonparametric methods.
As a sensitivity analysis, the primary and secondary analyses were repeated using the competing risk method.29 Distant recurrences and deaths were considered competing events for both pelvic and locoregional recurrences. Cumulative incidences were estimated using a Fine and Gray model and compared between treatments across molecular classes using Gray's test.30
All P values were two-sided, and statistical significance was accepted at P < .05. Statistical analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). R packages used in this study included rms, survival, ggPlot2, survminer, and cmprsk.
RESULTS
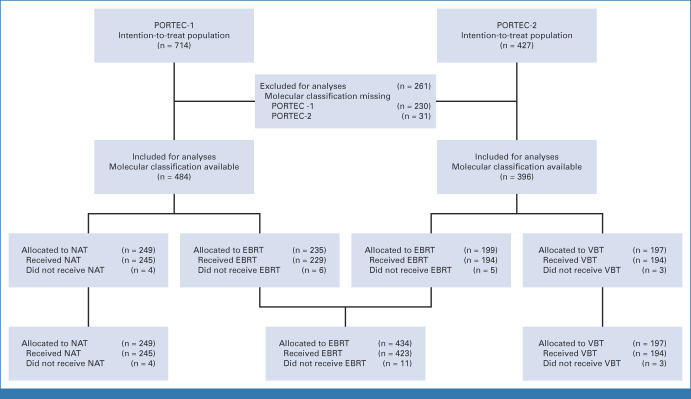
Of all women (n = 1141) included in the intention-to-treat populations of PORTEC-1 (n = 714) and PORTEC-2 (n = 427), 880 (77.1%) were molecularly classified and included in the current study (Fig 1). The included and excluded women had similar tumor characteristics and treatment outcomes (Appendix Tables A1 and A2, online only).
FIG 1.
CONSORT diagram. EBRT, external beam radiotherapy; NAT, no adjuvant therapy; VBT, vaginal brachytherapy.
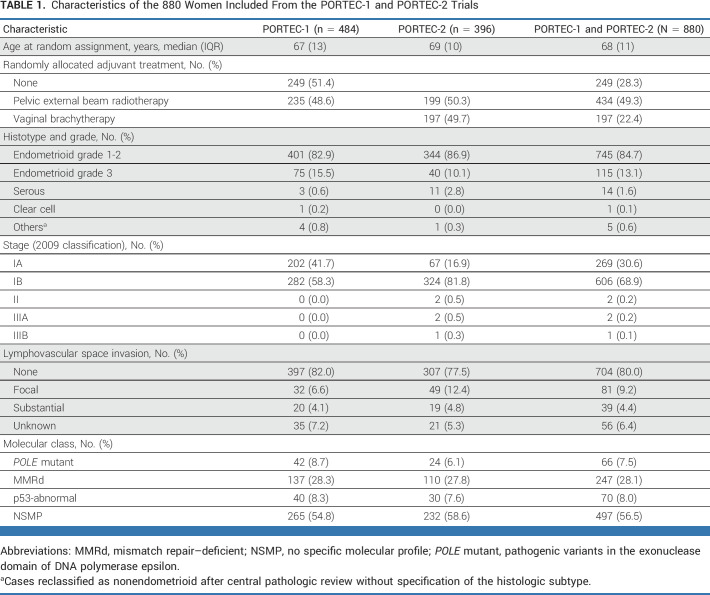
Characteristics of the 880 included women are presented in Table 1. Almost all had stage I endometrioid EC (97.2%). Few women with higher-stage disease (0.5%) and nonendometrioid histotypes (2.3%) were classified as such at central pathology review, which was performed after random assignment. Substantial LVSI was found in 39 women (4.4%). The most common molecular class was NSMP (56.5%), followed by MMRd (28.1%). Both POLEmut EC (7.5%) and p53abn EC (8.0%) were relatively rare. Substantial LVSI was present in one (1.5%) POLEmut EC, in 20 (8.1%) MMRd ECs, two (2.8%) p53abn ECs, and 19 (3.8%) NSMP ECs.
TABLE 1.
Characteristics of the 880 Women Included From the PORTEC-1 and PORTEC-2 Trials
The median follow-up was 12.4 years (95% CI, 12.0 to 12.8) in PORTEC-1, 10.5 years (95% CI, 10.2 to 10.7) in PORTEC-2, and 11.3 years (95% CI, 11.1 to 11.6) in the combined PORTEC-1 and PORTEC-2 cohort.
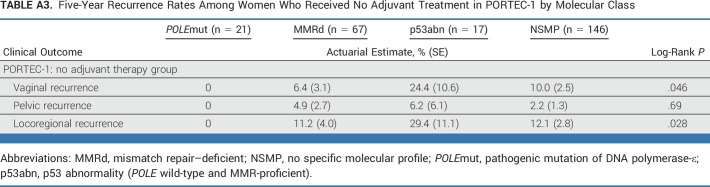
The 5-year risk of vaginal, pelvic, and locoregional recurrences without adjuvant therapy for each molecular class is presented in Appendix Table A3 (online only) on the basis of data of the PORTEC-1 trial. No recurrences were detected among women with POLEmut EC. In MMRd EC, vaginal recurrences (6.4%) were slightly more common than pelvic recurrences (4.9%). In p53abn EC, risk of vaginal recurrence was high (24.4%) and pelvic recurrences (6.2%) were also more common than in the other three molecular classes. In NSMP EC, vaginal recurrences were more common (10.0%) than pelvic recurrences (2.2%).
Primary Analysis
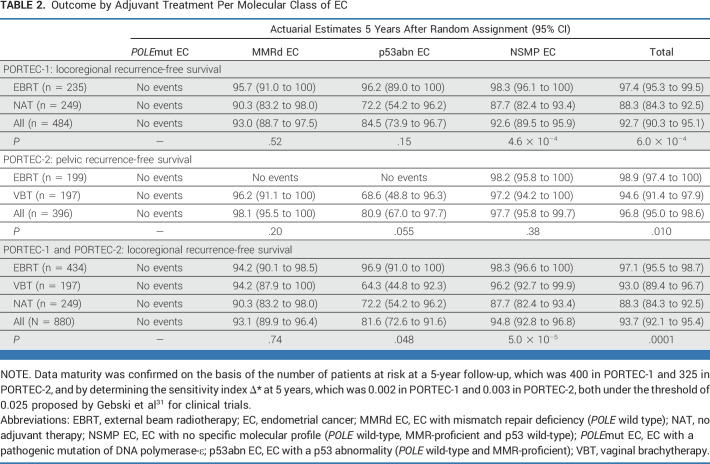
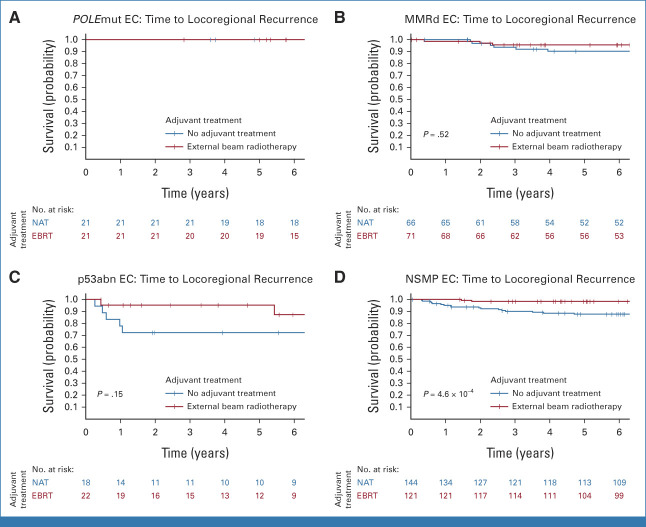
In the PORTEC-1 trial, 5-year locoregional recurrence-free survival was significantly better in women treated with EBRT (97.4%) than those with no adjuvant therapy (88.3%; P = 6.0 × 10−4; Fig 2A). Locoregional recurrence-free survival by adjuvant therapy for each molecular class is shown in Table 2 and Figure 3. No locoregional recurrences were observed among women with POLEmut EC. A small, but nonsignificant, benefit of EBRT (5-year locoregional recurrence-free survival 95.7%) over no adjuvant therapy (90.3%; P = .52) was found in MMRd EC. A substantial, but nonsignificant, benefit of EBRT (96.2%) compared with no adjuvant therapy (72.2%; P = .15) was observed in the 40 women with p53abn EC. A significant benefit of EBRT (98.3%) over no adjuvant therapy (87.7%; P = 4.6 × 10−4) was detected in NSMP EC.
FIG 2.
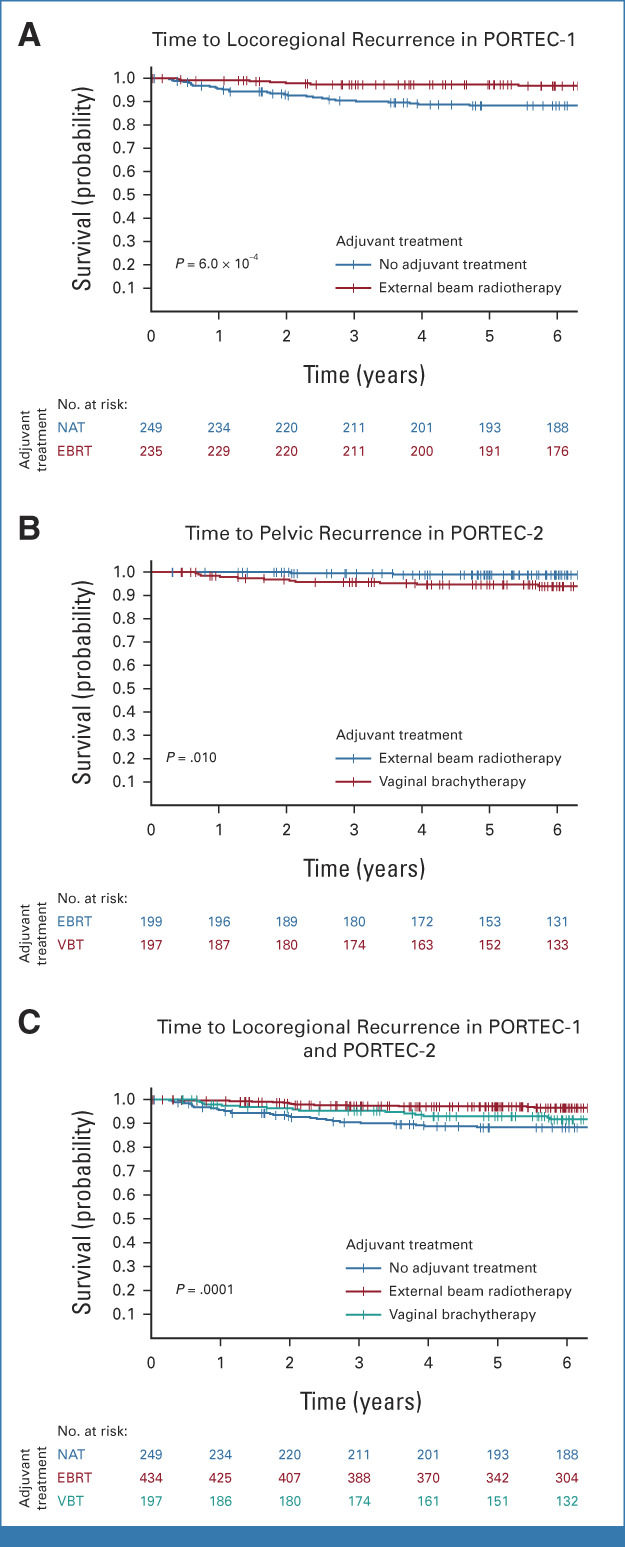
Time to recurrence by adjuvant treatment in the PORTEC-1 and PORTEC-2 trials. (A) Time to locoregional recurrence in PORTEC-1 by adjuvant treatment. (B) Time to pelvic recurrence in PORTEC-2 by adjuvant treatment. (C) Time to locoregional recurrence in the combined PORTEC-1 and -2 cohort by adjuvant treatment. EBRT, external beam radiotherapy; NAT, no adjuvant therapy; VBT, vaginal brachytherapy.
TABLE 2.
Outcome by Adjuvant Treatment Per Molecular Class of EC
FIG 3.
Time to locoregional recurrence per molecular class in PORTEC-1. (A) Time to locoregional recurrence in POLE-mutated endometrial cancer. (B) Time to locoregional recurrence in mismatch-repair deficient endometrial cancer. (C) Time to locoregional recurrence in p53 abnormal endometrial cancer. (D) Time to locoregional recurrence in no specific molecular profile endometrial cancer. EBRT, external beam radiotherapy; EC, endometrial cancer; MMRd EC, EC with mismatch repair deficiency (POLE wild-type); NAT, no adjuvant therapy; NSMP EC, EC with no specific molecular profile (POLE wild-type, MMR-proficient, and p53 wild-type); POLEmut EC, EC with a pathogenic mutation of DNA polymerase-ε; p53abn EC, EC with a p53 abnormality (POLE wild-type and MMR-proficient).
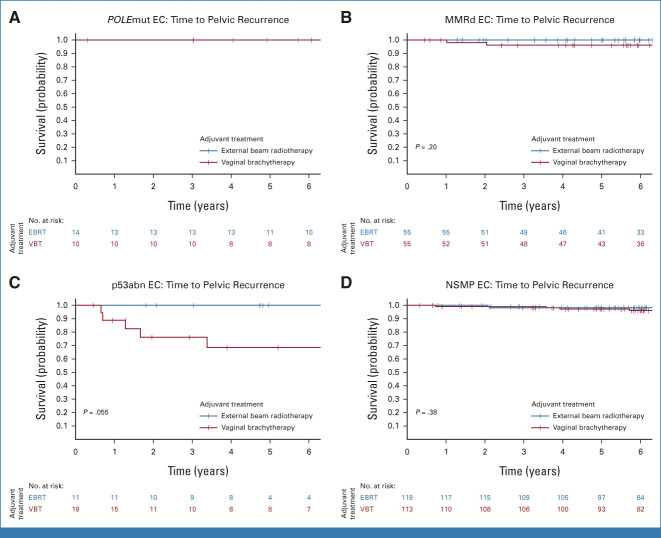
In the PORTEC-2 trial, no difference in 5-year vaginal recurrence-free survival was observed between the VBT arm (97.8%) and the EBRT arm (97.9%; P = .98). Pelvic recurrence-free survival was significantly better in the EBRT group (98.9%) than in the VBT group (94.6%; P = .010; Fig 2B). Five-year pelvic recurrence-free survival by adjuvant therapy across the four molecular classes is shown in Table 2 and Figure 4. None of the women with POLEmut EC had a pelvic recurrence. Women with MMRd EC who underwent VBT and those who received EBRT had a low risk of pelvic recurrence (96.2% v 100%; P = .20). None of the 11 women with p53abn EC in the EBRT group had a locoregional recurrence (100%) in contrast to five of 19 women in the VBT group (68.6%; P = .055). No differences in pelvic recurrence-free survival were observed between women with NSMP EC who underwent EBRT (98.2%) or VBT (97.2%; P = .38).
FIG 4.
Time to pelvic recurrence per molecular class in PORTEC-2. (A) Time to pelvic recurrence in POLE-mutated endometrial cancer. (B) Time to pelvic recurrence in mismatch-repair deficient endometrial cancer. (C) Time to pelvic recurrence in p53 abnormal endometrial cancer. (D) Time to pelvic recurrence in no specific molecular profile endometrial cancer. EBRT, external beam radiotherapy; EC, endometrial cancer; MMRd EC, EC with mismatch repair deficiency (POLE wild-type); NSMP EC, EC with no specific molecular profile (POLE wild-type, MMR-proficient, and p53 wild-type); POLEmut EC, EC with a pathogenic mutation of DNA polymerase-ε; p53abn EC, EC with a p53 abnormality (POLE wild-type and MMR-proficient); VBT, vaginal brachytherapy.
Secondary Analysis
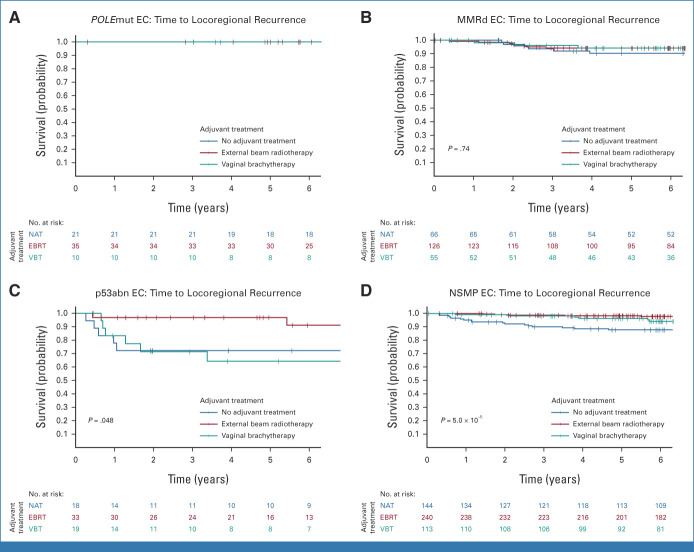
The 880 women included in the combined cohort of the PORTEC-1 and PORTEC-2 trials had been randomly assigned to EBRT (49.3%), VBT (22.4%), or no adjuvant treatment (28.3%), which resulted in significantly different 5-year locoregional recurrence-free survival rates of 97.1% (95% CI, 95.5 to 98.7), 93.0% (95% CI, 89.4 to 96.7), and 88.3% (95% CI, 84.3 to 92.5; P = .0001; Fig 2C), respectively. Table 2 and Figure 5 show the benefit of radiotherapy for each molecular class. Women with POLEmut EC had no recurrences and thus no benefit of radiotherapy. Women with MMRd EC who underwent EBRT (94.2%) or VBT (94.2%) had a small and nonsignificant benefit compared with those with no adjuvant therapy (90.3%; P = .74). In p53abn EC, locoregional recurrence-free survival with no adjuvant therapy (72.2%) and VBT (64.3%) was worse than that with EBRT (96.9%; P = .048). In NSMP EC, EBRT (98.3%) and VBT (96.2%) were associated with a significantly better locoregional recurrence-free survival than no adjuvant therapy (87.7%; P = 5.0 × 10−5).
FIG 5.
Time to locoregional recurrence per molecular class in PORTEC-1 and PORTEC-2. (A) Time to locoregional recurrence in POLE-mutated endometrial cancer. (B) Time to locoregional recurrence in mismatch-repair deficient endometrial cancer. (C) Time to locoregional recurrence in p53 abnormal endometrial cancer. (D) Time to locoregional recurrence in no specific molecular profile endometrial cancer. EBRT, external beam radiotherapy; EC, endometrial cancer; MMRd EC, EC with mismatch repair deficiency (POLE wild-type); NAT, no adjuvant therapy; NSMP EC, EC with no specific molecular profile (POLE wild-type, MMR-proficient, and p53 wild-type); POLEmut EC, EC with a pathogenic mutation of DNA polymerase-ε; p53abn EC, EC with a p53 abnormality (POLE wild-type and MMR-proficient); VBT, vaginal brachytherapy.
Multivariable analysis of locoregional recurrence-free survival of the combined PORTEC-1 and PORTEC-2 cohort showed that the interaction between the molecular class and the type of adjuvant therapy was not significant (P = .13). However, both molecular class and adjuvant therapy had independent prognostic value after correction for age, tumor grade, and the presence of LVSI (Appendix Table A4, online only).
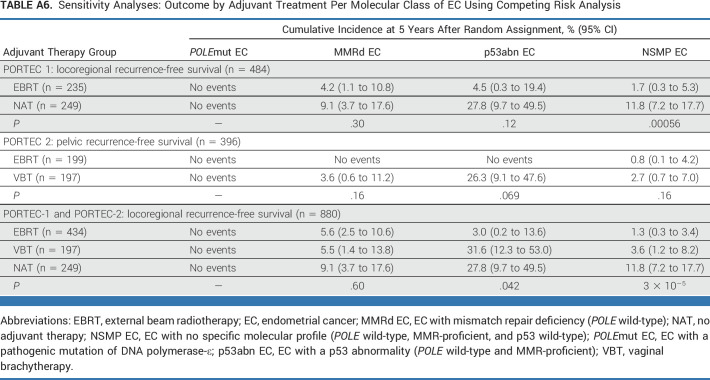
Sensitivity Analysis
An overview of the recurrence rates of first events in provided in Appendix Table A5 (online only). Results of the competing risk analyses are provided in Appendix Table A6 (online only). The cumulative incidences of pelvic and locoregional recurrences were similar to the estimates of locoregional and pelvic recurrence-free survival according to the Kaplan-Meier method. The comparisons of the treatment groups across molecular classes using Gray's test yielded the same findings as the comparisons by the log-rank test.
DISCUSSION
In this study, we determined the predictive value of the molecular classification of EC for response to radiotherapy using data of two large, randomized radiotherapy trials. A total of 880 women with stage I EEC who had been allocated to EBRT, VBT, or no adjuvant therapy were included for analyses. At 5 years, no locoregional recurrences were observed in women with POLEmut EC. In women with MMRd EC, EBRT and VBT yielded a small, nonsignificant benefit compared with no adjuvant therapy. For women with p53abn EC, locoregional recurrence-free survival was excellent after EBRT, but poor after VBT or no adjuvant therapy. In women with NSMP EC, VBT was as effective as EBRT and both yielded a significantly better locoregional control than no adjuvant therapy.
Although POLEmut is the rarest molecular class of EC, the evidence for excellent outcomes regardless of adjuvant therapy is accumulating.6,32,33 Even in the absence of adjuvant treatment, the risk of recurrence seems to be very low, as shown here in 21 women participating in the PORTEC-1 trial and previously in 26 women with high-grade POLEmut EC included in a Danish study.19 This supports prospective investigation of treatment de-escalation, which is currently ongoing in the PORTEC-4a trial (stage I-II EEC with high- to intermediate-risk features)34 and the POLEmut-BLUE trial (stage I-III EEC and non-EEC).35
This study found a small, but nonsignificant, benefit of adjuvant radiotherapy in women with stage I MMRd EEC, which is in line with a large, retrospective single-center study conducted in Finland.36 As these women have a relatively low risk of locoregional recurrence,3 the absolute benefit of EBRT might not outweigh the risk of toxicity.37,38 However, in early-stage MMRd EEC, substantial LVSI is associated with a significantly higher risk of recurrence and death.3 The PORTEC-4a trial currently investigates whether MMRd and NSMP EC with substantial LVSI benefit more from EBRT than standard VBT.34 A recent study among women with high-risk MMRd EC showed that grade 3 is not associated with an increased risk of recurrence,39 possibly because high-grade morphological features in MMRd EC result from its hypermutated genotype. Nonetheless, a retrospective study among 57 women with stage IB-II grade 3 MMRd EEC found a significantly better progression-free survival and overall survival with radiotherapy.40 Therefore, this study supports the current guidelines that recommend adjuvant VBT to reduce risk of vaginal recurrence.7,9 Further research to identify subgroups within stage I endometrioid MMRd EC that would benefit from EBRT is of interest.
Locoregional recurrence-free survival in early-stage p53abn EEC was poor (70.6% at 5 years) without adjuvant therapy. Although the numbers were limited, this study showed a large significant benefit of adjuvant EBRT over VBT and no adjuvant therapy. The size of the treatment effect seems to indicate that p53abn ECs are particularly radiosensitive. This is probably because p53abn ECs have high genomic instability16 and are often HR-deficient.13,14 Since radiotherapy and platinum-based chemotherapy synergistically act upon this, the combination of chemotherapy and radiotherapy should be considered for invasive stage I endometrioid p53abn EC.6
This study demonstrates that women with stage I endometrioid NSMP EC have significant benefit from adjuvant radiotherapy for locoregional disease control. This effect is probably the main driver of radiotherapy effects that have been observed in clinical trials20,41-43 as NSMP is the most common molecular class in early-stage EEC.3 Since vaginal recurrences are more common (approximately 10%) than pelvic recurrences (approximately 2%) and the toxicity profile of VBT is much milder than that of EBRT,37 VBT seems to be the preferred radiotherapeutic modality in these women. However, a recent study showed that estrogen receptor (ER)–negative-NSMP EC may represent a biologically distinct subgroup with a higher risk of recurrence.39 No subgroup analyses by ER status were performed in this study as only 14 of the 497 included NSMP ECs were ER-negative.
The assessment of the molecular classification is currently recommended for all women with high-risk EC.7-9 Several guidelines encourage molecular profiling also in women with (high-)intermediate-risk EC,7-9 and this and other studies show that it has clinically relevant implications. In 5%-10%, molecular testing will show POLEmut EC, which is associated with an excellent prognosis and should lead to consideration of de-escalation of adjuvant therapy.3,6-9,19,32 Around 30% will have MMRd EC, which requires in 70% further testing (for MLH1 promotor hypermethylation) and/or genetic counseling to detect Lynch syndrome (approximately 3% of all EC).44,45 In another 5%-10%, the molecular classification will reveal p53abn EC, which reclassifies these women to the high-risk group.7 For them, VBT alone is not sufficient for locoregional and distant disease control and chemotherapy and EBRT should be considered.6-9 In the remaining approximately 55%, molecular testing will show NSMP for which this study showed a clinically relevant difference in risk of locoregional recurrence between adjuvant radiotherapy and observation.
The body of evidence on the relevance of molecular testing in EC is growing, but clinical implementation remains challenging. Assessment of mismatch repair deficiency has become the standard of care in many countries because of the clinical importance of detecting Lynch syndrome, which is more effective by MMR IHC than age-based triage.44,46-48 IHC for p53 is increasingly performed as well, although often limited to selected patients, for example, with nonendometrioid histotypes. The main bottleneck, however, is DNA sequencing of the POLE gene, which is mainly performed by next-generation sequencing or Sanger sequencing, techniques that are expensive and/or time-consuming. The increasing number of places where POLE testing is offered and reimbursed indicates that many women in high-income countries will probably have access to molecular testing in this decade. Cheap alternatives for POLE sequencing have already been developed; examples are a quantitative polymerase chain reaction assay called QPOLE,49 a multiplex SNaPshot assay,50 and a droplet digital polymerase chain reaction assay.51 These tests may find their way to the clinic more rapidly and could make molecular testing and tailored adjuvant therapy available to women around the world.
To our knowledge, this is the first study to investigate the predictive value of the molecular classification for response to radiotherapy in early-stage EC using two randomized trials with high-quality long-term outcome data. Although an unprecedented number of 880 molecularly characterized ECs were included, some subgroup analyses were limited by insufficient power. For this reason, no further molecular class–specific analyses stratified by risk factors such as LVSI were performed. This study's design was optimized for the detection of a causal impact of the molecular class on treatment effects. Patients with protocol violations (such as nonendometrioid histotypes or treatment crossovers) were not removed from the analyses, which could have introduced some noise and may dilute differences between groups. The fact that the patterns of treatment effects across the four molecular classes were similar in the independent analyses of PORTEC-1 and PORTEC-2, and sensitivity analysis showing robustness against competing events, makes a strong case for transferability of this result. Nonetheless, external validation on another RCT comparing radiotherapy with no adjuvant therapy has not been performed. This could best be done for EC of the GOG-99,41 ASTEC/EN.5,41 and Swedish43 trials, which have not been molecularly characterized to our knowledge.
In conclusion, the molecular classification is predictive of benefit from radiotherapy in women with stage-I EEC. Results of this study support decisions on adjuvant therapy. Omitting radiotherapy is safe in women with POLEmut EC and will reduce toxicity, improve quality of life, and reduce health care utilization and costs. The impact of both EBRT and VBT seems to be limited in stage I MMRd EC and was not significantly different from no adjuvant therapy in this study. However, this result could be due to insufficient power and should be prospectively validated. Women with stage I p53abn EC have a high risk of recurrence, but these cancers seem to be particularly radiosensitive. EBRT is recommended as it yields an excellent locoregional control, in contrast to VBT or no adjuvant therapy. Women with stage I NSMP EC have significant benefit from adjuvant radiotherapy. Here, VBT is the treatment of choice as it is as effective as EBRT but has a much milder toxicity profile. This implies that assessment of the molecular classification is needed to provide women with stage I EEC with the most suitable adjuvant treatment strategy.
ACKNOWLEDGMENT
The authors are grateful to the participants in the PORTEC-1 and PORTEC-2 trial, to the investigators and pathologists who recruited patients and collected samples, to the central data managers at the Comprehensive Cancer Center, the Netherlands-West, and to the data managers of the participating centers. The list of PORTEC Study Group participants is available in Appendix 1 (online only).
APPENDIX 1. THE PORTEC STUDY GROUP
PORTEC-1
University Hospital Rotterdam/Daniel den Hoed Cancer Center (C.L. Creutzberg, P.C.M. Koper, W.L.J. van Putten, R. Dercksen, M. van Lent, H. Beerman)
Catharina Hospital Eindhoven (M.L.M. Lybeert)
Medisch Spectrum Twente Enschede (J.J. Jobsen, J.H. Meerwaldt)
University Medical Center Utrecht (C.C. Wárlám-Rodenhuis)
Dr B Verbeeten, Institute Tilburg (K.A.J. De Winter)
Radiotherapy Institute Limburg (L.C.H.W. Lutgens)
University Hospital Groningen (A.C.M. van den Bergh)
Radiotherapy Institute Arnhem (E.M. van de Steen-Banasik)
Radiotherapy Institute Deventer (M.C. Stenfert Kroese)
University Hospital Nijmegen (L.A.M. Pop)
University Medical Center Amsterdam (L. Uitterhoeve)
Leiden University Medical Center (A.A. Snijders-Keilholz)
Netherlands Cancer Institute/A van Leeuwenhoek Huis Amsterdam (B.N.F.M. van Bunningen)
Westeinde Hospital The Hague (J.H. Biesta)
Leyenburg Hospital The Hague (F.M. Gescher)
R de Graaf Hospital Delft (J. Pomp)
University Hospital VU Amsterdam (O.W.M. Meijer)
Radiotherapy Institute Vlissingen (J.H. Tabak)
Radiotherapy Institute Leeuwarden (A. Slot)
PORTEC-2
University Medical Center Utrecht (I.M. Jürgenliemk-Schulz)
Medisch Spectrum Twente, Enschede (J.J. Jobsen)
MAASTricht Radiation Oncology Clinic, Maastricht (L.C.H.W. Lutgens)
Arnhem Radiotherapy Institute, Arnhem (E.M. van der Steen-Banasik)
Erasmus MC Rotterdam/Daniel den Hoed Cancer Center, Rotterdam (J.W.M. Mens)
Leiden University Medical Center, Leiden (C.L. Creutzberg, R.A. Nout, V.T.H.B.M. Smit)
Radiotherapy Institute Friesland, Leeuwarden (A. Slot)
Radiotherapy Institute Stedendriehoek en Omstreken, Deventer (M.C. Stenfert Kroese)
Netherlands Cancer Institute, Amsterdam (B.N.F.M. van Bunningen)
Catharina Hospital, Eindhoven (M.L.M. Lybeert)
University Medical Center Radboud, Nijmegen (J.W. Leer)
Sophia Hospital, Zwolle (P.R. Timmer)
VU Medical Center, Amsterdam (O.W.M. Meijer, B. van Triest)
University Medical Center Groningen (B. Pras)
Medical Centre Haaglanden, The Hague (R. Wiggenraad)
Academic Medical Center, Amsterdam (L. Uitterhoeve)
Haga Hospital, The Hague (F. Gescher, P.C.M. Koper)
R de Graaf Hospital, Delft (J. Pomp)
Zeeuwsch Radiotherapy Institute, Vlissingen (V.L.M. Coen)
TABLE A1.
Comparison of Included and Excluded PORTEC-1 Participants
TABLE A2.
Comparison of Included and Excluded PORTEC-2 Participants
TABLE A3.
Five-Year Recurrence Rates Among Women Who Received No Adjuvant Treatment in PORTEC-1 by Molecular Class
TABLE A4.
Predictors of Locoregional Recurrence-Free Survival in the Combined PORTEC-1 and PORTEC-2 Cohort
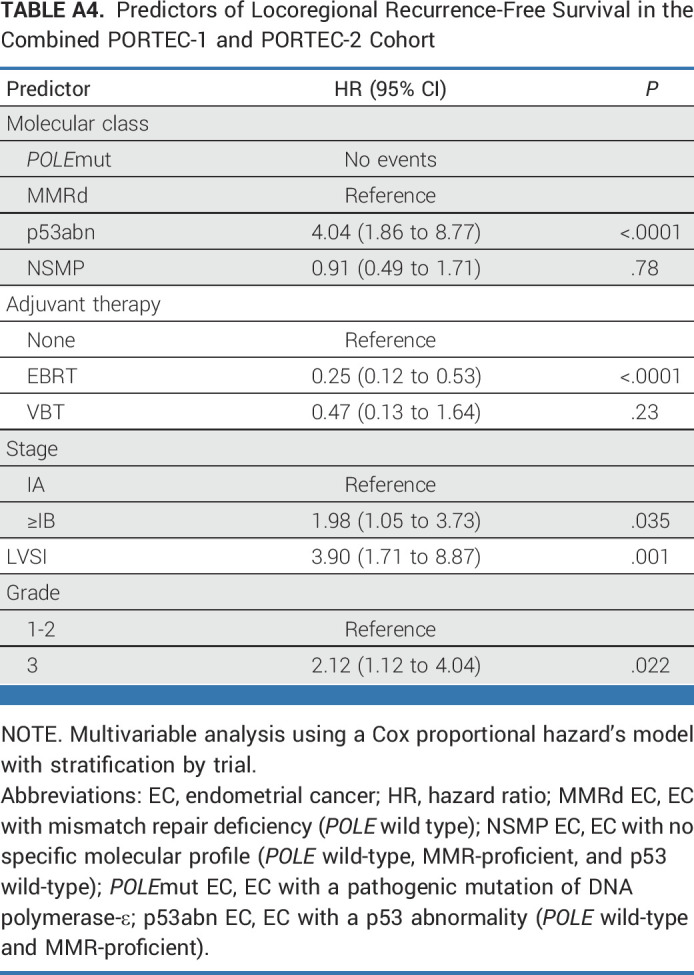
TABLE A5.
Overview of Failure Rates of First Events in the Combined PORTEC-1 and PORTEC-2 Cohort
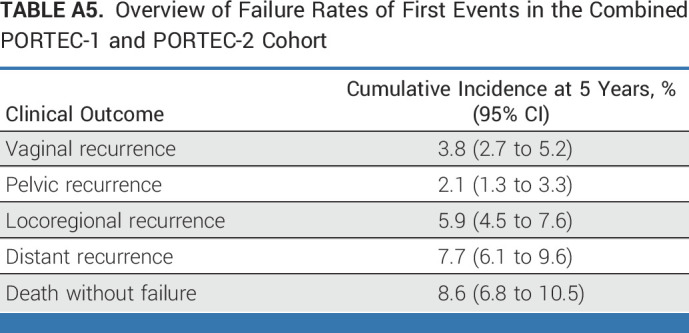
TABLE A6.
Sensitivity Analyses: Outcome by Adjuvant Treatment Per Molecular Class of EC Using Competing Risk Analysis
Nanda Horeweg
Research Funding: Varian Medical Systems (Inst)
Remi A. Nout
Consulting or Advisory Role: Merck KGaA (Inst)
Research Funding: Varian Medical Systems (Inst), Elekta (Inst), Accuray (Inst)
Stephanie M. de Boer
Research Funding: Varian Medical Systems
Carien L. Creutzberg
Consulting or Advisory Role: Merck (Inst)
Research Funding: Elekta (Inst), Varian Medical Systems (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the European Society for Gynecological Oncology (ESGO) conference, Berlin, Germany, October 27-30, 2022 and the European Society for Radiotherapy and Oncology (ESTRO) conference, Vienna, Austria, May 12-16, 2023.
SUPPORT
Supported by grants from the Dutch Cancer Society (CKTO 90-01 and CKTO 2001-04, respectively). This study was supported by a translational research project grant from the Dutch Cancer Society (UL2012-5719). N.H. was supported by a Dutch Cancer Society Grant (KWF-13400). T.B. was supported by a Dutch Cancer Society Young Investigator Award.
CLINICAL TRIAL INFORMATION
NCT00376844 (PORTEC-2)
Contributor Information
Collaborators: C.L. Creutzberg, P.C.M. Koper, W.L.J. van Putten, R. Dercksen, M. van Lent, H. Beerman, M.L.M. Lybeert, J.J. Jobsen, J.H. Meerwaldt, C.C. Wárlám-Rodenhuis, K.A.J. De Winter, L.C.H.W. Lutgens, A.C.M. van den Bergh, E.M. van de Steen-Banasik, M.C. Stenfert Kroese, L.A.M. Pop, L. Uitterhoeve, A.A. Snijders-Keilholz, B.N.F.M. van Bunningen, J.H. Biesta, F.M. Gescher, J. Pomp, O.W.M. Meijer, J.H. Tabak, A. Slot, I.M. Jürgenliemk-Schulz, E.M. van der Steen-Banasik, J.W.M. Mens, R.A. Nout, V.T.H.B.M. Smit, J.W. Leer, P.R. Timmer, B. van Triest, B. Pras, R. Wiggenraad, F. Gescher, and V.L.M. Coen
DATA SHARING STATEMENT
The syntax used for data analysis are available upon request. Requests for data sharing can be addressed, with a scientific study proposal, to the corresponding author within 15 years from the date of publication. The PORTEC study group will evaluate the request. Depending on the specific research proposal, the PORTEC study group will determine when, for how long, for which specific purposes, and under which conditions the requested data can be made available, subject to ethical consent.
AUTHOR CONTRIBUTIONS
Conception and design: Nanda Horeweg, Remi Nout, Carien L. Creutzberg
Administrative support: Nanda Horeweg
Provision of study materials or patients: Ina M. Jürgenliemk-Schulz, Ludy C.H.W. Lutgens, Jan J. Jobsen, Marie A.D. Haverkort, Jan Willem M. Mens, Annerie Slot, Ellen Stelloo, Vincent T.H.B.M. Smit, Tjalling Bosse, Carien L. Creutzberg
Collection and assembly of data: Nanda Horeweg, Remi A. Nout, Ina Jürgenliemk-Schulz, Ludy C.H.W. Lutgens, Jan J. Jobsen, Marie A.D. Haverkort, Jan Willem M. Mens, Annerie Slot, Bastiaan G. Wortman, Stephanie M. de Boer, Ellen Stelloo, Karen W. Verhoeven-Adema, Hein Putter, Vincent T.H.B.M. Smit, Tjalling Bosse, Carien L. Creutzberg
Data analysis and interpretation: Nanda Horeweg, Remi A. Nout, Ina Jürgenliemk-Schulz, Ludy C.H.W. Lutgens, Jan J. Jobsen, Marie A.D. Haverkort, Jan-Willem Mens, Annerie Slot, Bastiaan G. Wortman, Stephanie M. de Boer, Hein Putter, Vincent T.H.B.M. Smit, Tjalling Bosse, Carien L. Creutzberg
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Molecular Classification Predicts Response to Radiotherapy in the Randomized PORTEC-1 and PORTEC-2 Trials for Early-Stage Endometrioid Endometrial Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Nanda Horeweg
Research Funding: Varian Medical Systems (Inst)
Remi A. Nout
Consulting or Advisory Role: Merck KGaA (Inst)
Research Funding: Varian Medical Systems (Inst), Elekta (Inst), Accuray (Inst)
Stephanie M. de Boer
Research Funding: Varian Medical Systems
Carien L. Creutzberg
Consulting or Advisory Role: Merck (Inst)
Research Funding: Elekta (Inst), Varian Medical Systems (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.WHO Classification of Tumours Editorial Board : WHO classification of tumours (ed 5), in Female Genital Tumours, Lyon, France, International Agency for Research on Cancer. Volume 4, 2020, pp 252-266 [Google Scholar]
- 2.Vermij L, Smit V, Nout R, et al. : Incorporation of molecular characteristics into endometrial cancer management. Histopathology 76:52-63, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stelloo E, Nout RA, Osse EM, et al. : Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res 22:4215-4224, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Kommoss S, McConechy MK, Kommoss F, et al. : Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol 29:1180-1188, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Talhouk A, McConechy MK, Leung S, et al. : Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 123:802-813, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Leon-Castillo A, de Boer SM, Powell ME, et al. : Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: Impact on prognosis and benefit from adjuvant therapy. J Clin Oncol 38:3388-3397, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Concin N, Matias-Guiu X, Vergote I, et al. : ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer 31:12-39, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Oaknin A, Bosse TJ, Creutzberg CL, et al. : Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 33:860-877, 2022 [DOI] [PubMed] [Google Scholar]
- 9.Harkenrider MM, Abu-Rustum N, Albuquerque K, et al. : Radiation therapy for endometrial cancer: An American Society for Radiation Oncology Clinical Practice Guideline. Pract Radiat Oncol 13:41-65, 2023 [DOI] [PubMed] [Google Scholar]
- 10.Koh WJ, Abu-Rustum NR, Bean S, et al. : Uterine neoplasms, version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 16:170-199, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Sorolla MA, Parisi E, Sorolla A: Determinants of sensitivity to radiotherapy in endometrial cancer. Cancers 12:1906, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biau J, Chautard E, Verrelle P, et al. : Altering DNA repair to improve radiation therapy: Specific and multiple pathway targeting. Front Oncol 9:1009, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jonge MM, Auguste A, van Wijk LM, et al. : Frequent homologous recombination deficiency in high-grade endometrial carcinomas. Clin Cancer Res 25:1087-1097, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Marquard AM, Eklund AC, Joshi T, et al. : Pan-cancer analysis of genomic scar signatures associated with homologous recombination deficiency suggests novel indications for existing cancer drugs. Biomark Res 3:9, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saffari B, Bernstein L, Hong DC, et al. : Association of p53 mutations and a codon 72 single nucleotide polymorphism with lower overall survival and responsiveness to adjuvant radiotherapy in endometrioid endometrial carcinomas. Int J Gynecol Cancer 15:952-963, 2005 [DOI] [PubMed] [Google Scholar]
- 16.The Cancer Genome Atlas Research Network : Integrated genomic characterisation of endometrial carcinoma. Nature 2:67-73, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Risinger JI, Hayes K, Maxwell GL, et al. : PTEN mutation in endometrial cancers is associated with favorable clinical and pathologic characteristics. Clin Cancer Res 4:3005-3010, 1998 [PubMed] [Google Scholar]
- 18.Yard BD, Adams DJ, Chie EK, et al. : A genetic basis for the variation in the vulnerability of cancer to DNA damage. Nat Commun 7:11428, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leon-Castillo A, Horeweg N, Peters EEM, et al. : Prognostic relevance of the molecular classification in high-grade endometrial cancer for patients staged by lymphadenectomy and without adjuvant treatment. Gynecol Oncol 164:577-586, 2022 [DOI] [PubMed] [Google Scholar]
- 20.Creutzberg CL, van Putten WL, Koper PC, et al. : Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 355:1404-1411, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Nout RA, Smit VT, Putter H, et al. : Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet 375:816-823, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Scholten AN, van Putten WL, Beerman H, et al. : Postoperative radiotherapy for stage 1 endometrial carcinoma: Long-term outcome of the randomized PORTEC trial with central pathology review. Int J Radiat Oncol Biol Phys 63:834-838, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Bosse T, Peters EE, Creutzberg CL, et al. : Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer—A pooled analysis of PORTEC 1 and 2 trials. Eur J Cancer 51:1742-1750, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Leon-Castillo A, Britton H, McConechy MK, et al. : Interpretation of somatic POLE mutations in endometrial carcinoma. J Pathol 250:323-335, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermij L, Leon-Castillo A, Singh N, et al. : p53 immunohistochemistry in endometrial cancer: clinical and molecular correlates in the PORTEC-3 trial. Mod Pathol 35:1475-1483, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schemper M, Smith TL: A note on quantifying follow-up in studies of failure time. Control Clin Trials 17:343-346, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Horeweg N, de Bruyn M, Nout RA, et al. : Prognostic integrated image-based immune and molecular profiling in early-stage endometrial cancer. Cancer Immunol Res 8:1508-1519, 2020 [DOI] [PubMed] [Google Scholar]
- 28.Harrell FE: Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY, Springer, 2001 [Google Scholar]
- 29.Scrucca L, Santucci A, Aversa F: Competing risk analysis using R: An easy guide for clinicians. Bone Marrow Transpl 40:381-387, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988 [Google Scholar]
- 31.Gebski V, Garés V, Gibbs E, et al. : Data maturity and follow-up in time-to-event analyses. Int J Epidemiol 47:850-859, 2018 [DOI] [PubMed] [Google Scholar]
- 32.McAlpine JN, Chiu DS, Nout RA, et al. : Evaluation of treatment effects in patients with endometrial cancer and POLE mutations: An individual patient data meta-analysis. Cancer 127:2409-2422, 2021 [DOI] [PubMed] [Google Scholar]
- 33.McConechy MK, Talhouk A, Leung S, et al. : Endometrial carcinomas with POLE exonuclease domain mutations have a favorable prognosis. Clin Cancer Res 22:2865-2873, 2016 [DOI] [PubMed] [Google Scholar]
- 34.van den Heerik A, Horeweg N, Nout RA, et al. : PORTEC-4a: International randomized trial of molecular profile-based adjuvant treatment for women with high-intermediate risk endometrial cancer. Int J Gynecol Cancer 30:2002-2007, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.RAINBO Research Consortium: Refining adjuvant treatment in endometrial cancer based on molecular features: The RAINBO clinical trial program. Int J Gynecol Cancer 33:109-117, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loukovaara M, Pasanen A, Butzow R: Mismatch repair protein and MLH1 methylation status as predictors of response to adjuvant therapy in endometrial cancer. Cancer Med 10:1034-1042, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nout RA, Putter H, Jurgenliemk-Schulz IM, et al. : Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: First results of the randomized PORTEC-2 trial. J Clin Oncol 27:3547-3556, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Nout RA, van de Poll-Franse LV, Lybeert ML, et al. : Long-term outcome and quality of life of patients with endometrial carcinoma treated with or without pelvic radiotherapy in the post operative radiation therapy in endometrial carcinoma 1 (PORTEC-1) trial. J Clin Oncol 29:1692-1700, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Vermij L, Jobsen JJ, Léon-Castillo A, et al. : Prognostic refinement of NSMP high-risk endometrial cancers using oestrogen receptor immunohistochemistry. Br J Cancer 128:1360-1368, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reijnen C, Kusters-Vandevelde HVN, Prinsen CF, et al. : Mismatch repair deficiency as a predictive marker for response to adjuvant radiotherapy in endometrial cancer. Gynecol Oncol 154:124-130, 2019 [DOI] [PubMed] [Google Scholar]
- 41.Keys HM, Roberts JA, Brunetto VL, et al. : A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 92:744-751, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Group AES, Blake P, Swart AM, et al. : Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): Pooled trial results, systematic review, and meta-analysis. Lancet 373:137-146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorbe B, Horvath G, Andersson H, et al. : External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium-risk endometrial carcinoma—A prospective randomized study. Int J Radiat Oncol Biol Phys 82:1249-1255, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Post CCB, Stelloo E, Smit V, et al. : Prevalence and prognosis of Lynch syndrome and sporadic mismatch repair deficiency in endometrial cancer. J Natl Cancer Inst 113:1212-1220, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan NAJ, McMahon R, Tobi S, et al. : The proportion of endometrial tumours associated with Lynch syndrome (PETALS): A prospective cross-sectional study. PLoS Med 17:e1003263, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aiyer KTS, Doeleman T, Ryan NA, et al. : Validity of a two-antibody testing algorithm for mismatch repair deficiency testing in cancer; a systematic literature review and meta-analysis. Mod Pathol 35:1775-1783, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan N, Wall J, Crosbie EJ, et al. : Lynch syndrome screening in gynaecological cancers: Results of an international survey with recommendations for uniform reporting terminology for mismatch repair immunohistochemistry results. Histopathology 75:813-824, 2019 [DOI] [PubMed] [Google Scholar]
- 48.Snowsill TM, Ryan NAJ, Crosbie EJ: Cost-effectiveness of the Manchester approach to identifying Lynch syndrome in women with endometrial cancer. J Clin Med 9:1664, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van den Heerik ASVM, Ter Haar NT, Vermij L, et al. : QPOLE: A quick, simple, and cheap alternative for POLE sequencing in endometrial cancer by multiplex genotyping quantitative polymerase chain reaction. JCO Glob Oncol 10.1200/GO.22.00384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devereaux KA, Steiner DF, Ho C, et al. : A multiplex SNaPshot assay is a rapid and cost-effective method for detecting POLE exonuclease domain mutations in endometrial carcinoma. Int J Gynecol Pathol 41:541-551, 2022 [DOI] [PubMed] [Google Scholar]
- 51.Kim G, Lee SK, Suh DH, et al. : Clinical evaluation of a droplet digital PCR assay for detecting POLE mutations and molecular classification of endometrial cancer. J Gynecol Oncol 33:e15, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The syntax used for data analysis are available upon request. Requests for data sharing can be addressed, with a scientific study proposal, to the corresponding author within 15 years from the date of publication. The PORTEC study group will evaluate the request. Depending on the specific research proposal, the PORTEC study group will determine when, for how long, for which specific purposes, and under which conditions the requested data can be made available, subject to ethical consent.