Great hope for the future: CAR T-cell and bispecific antibody therapies in multiple myeloma.
Abstract
Historically, the outcomes for individuals with triple-class refractory and penta-drug refractory multiple myeloma (MM) have been poor because of a dearth of effective treatment options. However, the advent of chimeric antigen receptor (CAR) T-cell and T-cell redirecting bispecific antibody (BsAb) therapies has led to unprecedented response rates and durations of response in heavily relapsed/refractory (R/R) populations. Currently, two B-cell maturation antigen (BCMA)–directed CAR T-cell therapies (idecabtagene vicleucel and ciltacabtagene autoleucel) as well as one BCMA/CD3 BsAb (teclistamab) have been approved for late-line (greater than four previous lines) R/R MM in the United States. The purpose of this review is to analyze the recent data for these approved therapies as well as provide an overview of other related CAR T-cell and BsAb therapies under development, including non–BCMA-targeting agents. We review efficacy and safety considerations, with particular focus on cytokine release syndrome, neurotoxicity, and infection risk. The relative merits and limitations of each class of therapy are discussed, as well as the areas of unmet need with respect to optimal sequencing and supportive care measures. We examine the factors that challenge equitable access to these novel therapies across minoritized racial, ethnic, and socioeconomic populations. Although it is evident that CAR T-cell and BsAb therapies will transform treatment paradigms in MM for years to come, significant work remains to identify the optimal utilization of these novel therapies and ensure equitable access.
INTRODUCTION
The management of multiple myeloma (MM) is becoming increasingly complex as the number of therapeutic options increase. The immunomodulatory drugs (IMiDs), proteasome inhibitors (PIs), and anti-CD38 monoclonal antibodies (mAbs) have become mainstays of therapy for newly diagnosed disease and early lines of relapsed/refractory (R/R) disease. Although these three classes of drugs have markedly improved long-term outcomes, management of triple-class refractory disease (IMiDs, PIs, and anti-CD38 mAb) and penta-drug refractory disease (lenalidomide, pomalidomide, bortezomib, carfilzomib, and anti-CD38 mAb) has proven difficult with limited therapeutic options and short survival duration.1-4 In this context, the US Food and Drug Administration (FDA) approved several novel drugs, including selinexor (an XPO1 inhibitor), belantamab mafodotin (a B-cell maturation antigen [BCMA] antibody drug conjugate [ADC]), and melphalan flufenamide, all with overall response rates (ORRs) of approximately 25%-30% and median progression-free survival (PFS) durations of 3-4 months.5-7 Subsequently, both belantamab mafodotin and melphalan flufenamide were removed from the market.
Emerging into this therapeutic landscape are chimeric antigen receptor (CAR) T cells and T-cell–redirecting bispecific antibodies (BsAbs), therapies that directly harness the activity of T cells and show substantial efficacy in heavily pretreated patients. The BCMA-directed CAR T-cell product idecabtagene vicleucel (ide-cel) was approved by the FDA on March 27, 2021, with an indication for patients with R/R MM after four or more previous lines of therapy, including an IMiD, PI, and anti-CD38 mAb. On February 28, 2022, the FDA approved another BCMA-directed CAR T-cell product, ciltacabtagene autoleucel (cilta-cel), with the same indication. The anti-BCMA/CD3 BsAb, teclistamab, received approval by the FDA on October 25, 2022, again with the same indication. Of note, the phase II studies that led to the approval of these agents all enrolled patients with three or more previous lines of therapy.8-10 In this review, we provide an overview of the approved CAR T-cell/BsAb therapies as well as many of the therapies undergoing clinical trial investigation. We examine the relative merits and challenges associated with each class of therapy and examine the factors that challenge equitable access to these novel therapies and MM-related care in general.
CONTEXT
Key Objective
To provide an overview of the mechanisms of action, efficacy, toxicity, and issues surrounding equitable access to novel chimeric antigen receptor (CAR) T-cell and bispecific antibody (BsAb) therapies in multiple myeloma (MM).
Knowledge Generated
CAR T-cell and BsAb therapies targeting B-cell maturation antigen and other novel targets are yielding unprecedented responses in patients with heavily pretreated MM. Optimal sequencing of these agents, as well as strategies to limit toxicities such as cytokine release syndrome, neurotoxicity, and infections, remains to be determined.
Relevance (S. Lentzsch)
-
The review explores the pros and cons of the currently approved CAR T and bispecific products in MM for the multidisciplinary oncology community at large, including medical, radiation, and surgical oncologists.*
*Relevance section written by JCO Associate Editor Suzanne Lentzsch, MD, PhD.
MECHANISM OF ACTION
Both CAR T cells and T-cell–redirecting BsAbs harness the cytotoxic activity of T cells. Although it has long been recognized that T cells possess anti-MM activity,11,12 there are many mechanisms underlying T-cell dysfunction in MM.13 CAR T cells are designed to overcome some of these barriers by genetically modifying T cells such that the T cells are directed toward a specific tumor antigen (eg, BCMA) via an antigen-recognizing receptor coupled to signaling domains that lead to T-cell activation (Fig 1A). The commercially available products are derived from autologous T cells, although allogeneic CAR T cells are also under development. In brief, patients undergo leukapheresis of peripheral blood to obtain autologous T cells. Subsequently, these T cells are shipped to a central manufacturing laboratory (or, in some cases, for investigational products, are manufactured in-house) where they undergo engineering and expansion. Depending on the product and the manufacturing process, this might take anywhere from a few days to up to 8 weeks. Before receiving the finished engineered product, patients receive lymphodepleting chemotherapy (most commonly fludarabine and cyclophosphamide). Bridging chemotherapy is often administered during the manufacturing time period in an attempt to maintain disease control. CAR T-cell therapy has been referred to as a living drug as cells can expand in vivo, with variable durations of persistence.
FIG 1.
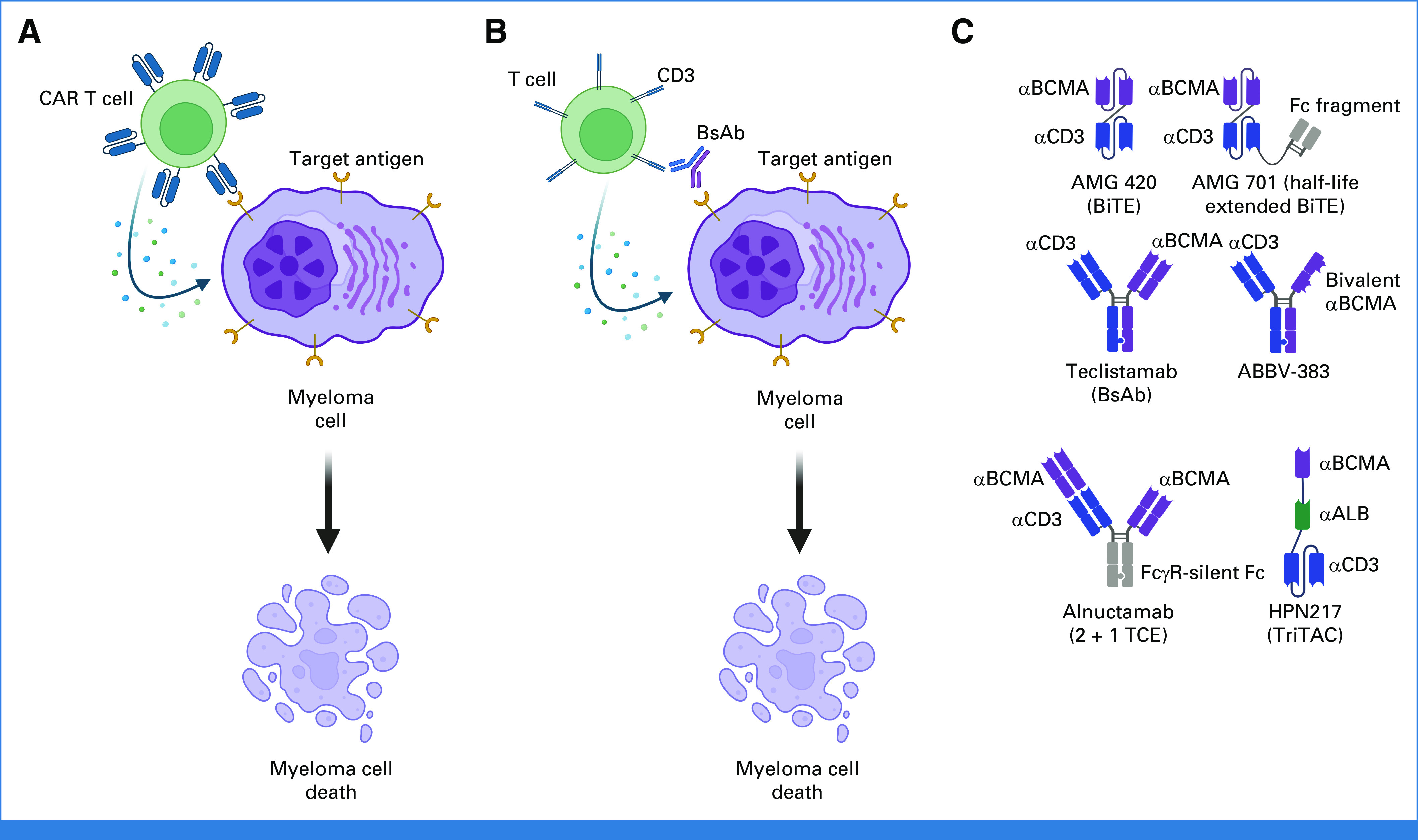
Mechanism of action of CAR T-cell and BsAb therapies in MM. (A) The engineered CAR T cells recognize and engage with the target antigen (eg, BCMA) on the surface of the myeloma cell, leading to activation of the CAR T cell with subsequent release of perforin, granzymes, and cytokines, leading to cytolysis of the tumor cell. (B) A BsAb simultaneously engages the target antigen (eg, BCMA) on the myeloma cell as well as CD3 on the T cell, leading to an immunologic synapse and cytolysis of the tumor cell via release of perforin, granzymes, and cytokines. (C) Examples of the designs of several types of T-cell–redirecting agents developed as anti-BCMA therapies in MM. Currently only teclistamab, a BsAb, has achieved FDA approval for the treatment of relapsed/refractory myeloma. BCMA, B-cell maturation antigen; BiTE, bispecific T-cell engager; BsAb, bispecific antibody; CAR, chimeric antigen receptor; FDA, US Food and Drug Administration; MM, multiple myeloma; TCE, T-cell engager; TriTAC, trispecific T-cell–activating construct.
Often referred to as off-the-shelf alternatives to CAR T-cell therapy, BsAbs and related T-cell redirecting agents take the approach of directing T cells to MM cells by simultaneously engaging both T cells and MM cells, creating an immunologic synapse (Fig 1B). Initial efforts in the field involved the bispecific T-cell engager AMG-420, composed of two linked single-chain variable fragments targeting BCMA and CD3 (Fig 1C). Although AMG-420 did show activity in a phase I first-in-human study in R/R MM (ORR of 31%; 70% at the maximum tolerated dose), this therapy was hindered by the need for a continuous IV infusion.14 Subsequent efforts focused on extended half-life derivatives such as pavurutamab (AMG 701; Fig 1C).15 Ultimately, the field turned to BsAbs (eg, teclistamab), which include an Fc region that provides stability and prolongs the agent's half-life permitting less frequent dosing (Fig 1C). Other variations include agents with two BCMA binding domains (eg, ABBV-383 and alnuctamab), trispecific T-cell–activating constructs that include an antialbumin domain for half-life extension (eg, HPN217) and modification of the Fc portion to minimize binding to FcγR and C1q (eg, alnuctamab; Fig 1C).
EFFICACY
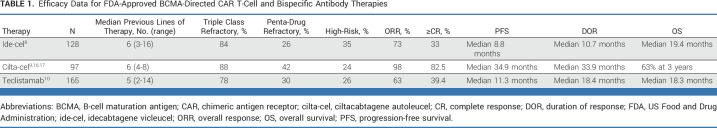
The accelerated approvals of ide-cel, cilta-cel, and teclistamab were based on phase I/II studies conducted in individuals with R/R MM who had not previously received BCMA-directed therapy. As shown in Table 1, most people with MM enrolled in these studies were heavily pretreated (median of five to six previous lines) with triple-class refractory disease, while a minority had penta-drug refractory disease. In this context, the ORRs of 73% (ide-cel), 98% (cilta-cel), and 63% (teclistamab) represented new benchmarks in this population with difficult-to-treat disease.
TABLE 1.
Efficacy Data for FDA-Approved BCMA-Directed CAR T-Cell and Bispecific Antibody Therapies
Several other ide-cel or cilta-cel studies have reported outcomes thus far. The CARTITUDE-2 cohort A evaluated patients (n = 20) with one to three previous lines of therapy (median of two previous lines, all triple class exposed, 40% triple-class refractory).18 The ORR was 95% with 85% achieving a complete response (CR) or better. The 6-month PFS rate was 90%. The KarMMa-2 cohort 2a enrolled participants experiencing disease progression within 18 months of frontline therapy (induction, autologous stem-cell transplant [ASCT], and lenalidomide-containing maintenance; n = 37). Forty-six percent of participants achieved a CR or better (the primary end point), and the median PFS was 11.4 months.19 The CARTITUDE-2 cohort B study also evaluated a functionally high-risk population—those who relapsed within 12 months of upfront ASCT or within 12 months from the start of induction (n = 19). The ORR was 100%, with 90% achieving CR or better and a 12-month PFS rate of 90%.20
Thus far, the full results of two confirmatory phase III studies have been reported. The KarMMa-3 study enrolled participants with two to four previous lines of therapy, randomizing in a 2:1 manner to ide-cel or one of five standard-of-care chemotherapy regimens. This study met its primary end point of PFS, with a median PFS of 13.3 months in the ide-cel group versus 4.4 months in the standard regimen arm (hazard ratio [HR], 0.49 [95% CI, 0.38 to 0.65]; P < .001).21 The CARTITUDE-4 trial enrolled patients with lenalidomide-refractory MM and one to three previous lines of therapy and randomized patients to either cilta-cel or standard of care (consisting of either daratumumab/pomalidomide/dexamethasone or pomalidomide/bortezomib/dexamethasone). This study also met its primary end point of PFS, with a median PFS not yet reached for the cilta-cel group versus 11.8 months in the standard-of-care group (HR, 0.26 [95% CI, 0.18 to 0.38]; P < .001).22
The results from real-world experiences with commercial ide-cel and cilta-cel are beginning to be reported. Hansen et al23 reported on the experience of 11 US institutions with ide-cel. The ORR was 84% with a median PFS of 8.5 months.23 Similarly, a report from a French registry (n = 49 patients infused with ide-cel) noted an ORR of 76% at 3 months along with a 3-month PFS rate of 82%.24 Hansen et al23 also reported on the experience of 12 US institutions with commercial cilta-cel. With brief follow-up (median of 5.8 months), the observed best ORR was 89% and the 6-month PFS rate was 79%.25
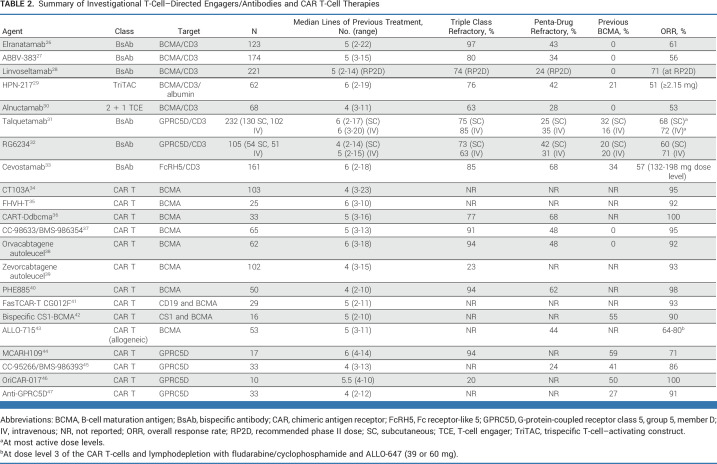
Although teclistamab represents the first BCMA-directed BsAb to obtain regulatory approval, there are multiple other BCMA-directed agents under development (Fig 1C; Table 2). Interestingly, response rates for all of these BCMA-/T-cell–directed agents have been strikingly consistent across studies thus far (range, 50%-60%). Whether all these agents will ultimately obtain regulatory approval and whether disease refractoriness to one agent equates to refractoriness to all agents in this group remains to be determined. Multiple other BCMA-directed CAR T-cell products are under investigation (Table 2), with some using innovative manufacturing procedures (eg, CC-98633/BMS-98635437), heavy chain-only BCMA binding domains (eg, FHVH-T35), dual antigen targeting (eg, bispecific CS1-BCMA42) or allogeneic T-cells (eg, ALLO-71543). All products have been reported to have high response rates (>60%), but follow-up for many of the studies is insufficient to gauge the durability of response.
TABLE 2.
Summary of Investigational T-Cell–Directed Engagers/Antibodies and CAR T-Cell Therapies
Notably, CAR T-cell and BsAb development has moved beyond targeting BCMA (Table 2). Of particular interest is the orphan G-protein-coupled receptor class C, group 5, member D (GPRC5D), which is highly expressed on plasma cells.48 Several phase I studies of GPRC5D-directed CAR T cells have shown high response rates (71%-100%).44-47 Likewise, the GPRC5D/CD3 BsAbs talquetamab and RG6234 have demonstrated ORRs in the 60%-70% range.31,32 Finally, Fc receptor-like 5 (FcRH5) has also proven to be a target of interest in MM.49 A phase I study of cevostamab, a BsAb directed against FcRH5/CD3, has shown an ORR of 57% at higher dose levels.33 The anti-GPRC5D and FcRH5 trials included some participants with previous BCMA-directed therapy, which is significant because this population is quickly becoming an area of unmet need.
TOXICITY
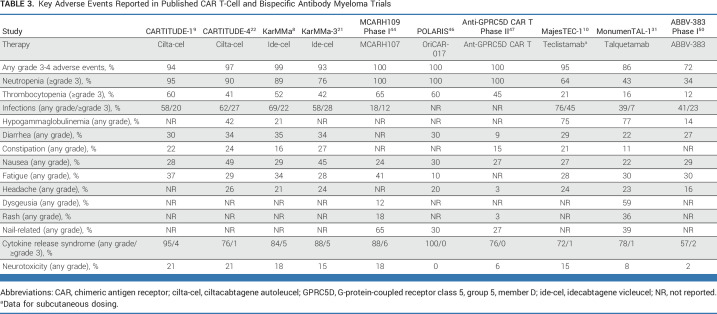
In addition to typical hematologic and nonhematologic toxicities of MM therapies, CAR T-cell and bispecific therapies have important classes of toxicity, including cytokine release syndrome (CRS) and immune-effector cell–associated neurotoxicity syndrome (ICANS; Table 3). CRS, characterized by fever, hypotension, hypoxia, and respiratory distress,51 occurs in about 90% of patients with MM receiving CAR T-cell therapy and around 70% of those receiving BsAbs (Table 3). CRS with the BsAbs appears to be generally lower in frequency and of lower grade than with CAR T-cell therapy, which may be in part due to the use of step-up dosing, wherein the BsAb is introduced at a very low dose and then escalated over several doses to reach the full planned dose. Clinical practice guidelines for managing CRS encourage early use of steroids and the interleukin-6 receptor antagonist tocilizumab.52 Prophylactic tocilizumab 2 hours before the first dose of cevostamab was explored, resulting in 36% of patients experiencing CRS, compared with 90% from a historical comparison cohort.53 Continued refinement of supportive care, including premedications, alternative dosing strategies, and prophylactic tocilizumab, may further reduce the incidence of this complication in patients receiving CAR T-cell and BsAb therapy.
TABLE 3.
Key Adverse Events Reported in Published CAR T-Cell and Bispecific Antibody Myeloma Trials
ICANS can include a range of symptoms and signs, including headache, confusion, somnolence, seizures, and coma. Strategies proposed for minimizing the development of ICANS include the use of bridging therapy to reduce tumor burden, intensive sustained monitoring, and early initiation of treatment for neurotoxicity.54 Our understanding of the impact of ICANS on patient function and quality of life (QoL) continues to evolve, particularly as delayed neurologic toxicities, such as Parkinsonism, movement disorders, cognitive impairment, cranial nerve palsies, and peripheral neuropathy, emerge as experience with CAR T-cell therapy grows.22,44,54,55
By nature, patients with advanced MM are severely immunocompromised and at risk for serious infections. High infection rates (including grade 3 or higher) were observed across many of the MM CAR T-cell and bispecific trials (Table 3), and grade 5 events have been reported as well.10,16,21,31,56 Rates of grade 3 or greater infections were approximately 20% with ide-cel or cilta-cel.8,9 The MajesTEC-1 trial reported a 45% grade 3 or higher rate of infections with teclistamab.10 In addition, there was a high incidence of COVID-19 infections, including 12 deaths due to COVID-19.10 Whether all BCMA-directed bispecific agents have similar risk of infection remains to be determined. Interestingly, in the talquetamab MonumenTAL-1 study, the rate of grade 3 or higher infection was only 7%,31 perhaps suggesting that the infection risk might be lower for GPRC5D-directed therapy than with BCMA-directed therapies.
In a real-world cohort of patients receiving ide-cel, over half of the patients experienced infections within the first 100 days.57 In this study, infections within the first 30 days tended to be bacterial and associated with cytopenias.57 Another group reported that within the first 3 months after BCMA CAR T-cell therapy there were primarily viral infections, with 95% developing hypogammaglobulinemia.58 Another retrospective analysis revealed high rates of infections in patients receiving BCMA BsAb therapy, with all responders experiencing severe hypogammaglobulinemia (IgG level <200 mg/dL).59 A recent pooled analysis of 1,185 participants in 11 trials of BsAbs demonstrated high rates of serious or even fatal infection: 24.5% grade 3/4 infection, including 10% grade 3/4 pneumonia.60 Of the reported deaths, 25% were due to infection. Opportunistic infections not typically observed in the MM population outside of the allogeneic transplant setting have been reported, including Pneumocystis jiroveci pneumonia infection and cytomegalovirus reactivation.21,31,56,61 There have also been reports of prolonged viral infections, including parvovirus and norovirus, in patients receiving BCMA-directed bispecific agents.62
Rates of grade 3/4 neutropenia appeared to be slightly higher in those treated with BCMA BsAbs versus non-BCMA BsAbs. Prolonged lymphopenia and hypogammaglobulinemia have been noted after BCMA CAR T-cell therapy.63 In the four studies reporting on hypogammaglobulinemia, the prevalence was 75%, with nearly half of the patients receiving intravenous immunoglobulin.60 As the majority of the clinical trials and commercial use of approved products have occurred during the ongoing COVID-19 pandemic, it is also not surprising that deaths from COVID-19 have been reported.10,31,56,64 The extent to which potential COVID-19–induced immune dysregulation, particularly T-cell dysfunction,65-67 affects susceptibility to other infections and MM responsiveness to CAR T-cell and BsAb therapies remains to be determined. Collaborations among MM clinicians, cellular/transplant specialists, and infectious disease specialists are needed to develop best practices for prophylaxis after CAR T and BsAb therapy.
It is worth noting that the development of new classes of agents targeting a novel antigen (GPRC5D) is accompanied by a new set of toxicities. GPRC5D is an orphan receptor whose physiologic function has not yet been clearly defined.48,68 Although GPRC5D is highly expressed on plasma cells, it is also expressed on cells within the hard keratinizing tissues and hair follicles of skin.48,68 Studies conducted to date using either GPRC5D-directed CAR T cells or BsAbs have reported skin and nail changes, rash, and oral-related adverse events, including dysgeusia, dry mouth, and dysphagia (Table 3), which are presumably related to GPRC5D expression in these tissues.31,32,44,45 Strategies to minimize these toxicities and improve long-term tolerability of these therapies are needed.
Beyond traditional adverse event reporting and grading, clinicians and researchers must also center their decision making about these agents from the perspectives of the lived experiences of patients who receive these therapies. This patient-centered focus would capture potential toxicities and other individual health outcomes, such as the impact of CAR T-cell therapies on patient-reported function and QoL. In addition, it could also unearth the potential barriers that limit equitable access to these therapies. Qualitative analyses of interview data collected from patients who received ide-cel or cilta-cel in clinical trials revealed overall positive patient-reported experiences, with the treatments meeting or exceeding their expectations and resulting in improvements in symptom burden and QoL.69,70 Complementary data using quantitative health-related QoL measures demonstrated longitudinal improvement in symptoms and QoL in individuals with MM receiving cilta-cel or ide-cel.71,72 Patient-reported outcome data were reported from a cohort in the MajesTEC-1 study and showed longitudinal improvements in global health status and reduction in pain with no change in fatigue or physical functioning.73 Similar data of patient experience and QoL with other BsAbs under development are awaited.
ACCESS TO CARE AND EQUITY IN REAL-WORLD SETTINGS
Despite the promising data supporting the efficacy of CAR T-cell and BsAb therapies among clinical trial participants and those fortunate enough to receive these therapies in the real world, the potential to see widening disparities in MM treatment access and key health outcomes, including survival, is of concern. Studies have shown disparities in MM treatment access on the basis of age, race/ethnicity, socioeconomic status, and residential neighborhood characteristics (eg, social vulnerability, which is considered to be the cumulative effects of poverty, housing, and transportation concerns, and large populations from minoritized racial/ethnic backgrounds).74-77 One database study of CAR T-cell therapy recipients with lymphoma, acute lymphoblastic leukemia, and MM identified disparities in access to this therapy among Black persons, those with government-funded health insurance (Medicare or Medicaid), and median household incomes <$40,000 in US dollars per year.78 Another study of 3,922 patients with diffuse large B-cell lymphoma also identified access barriers according to practice setting (academic v community-based).79 This early demonstration of disparities among CAR T therapy recipients provides an opportunity to identify equity-driven strategies and solutions that would improve access to clinical trials evaluating these products and help guide clinician decision making to ensure equitable access to these products with the anticipated expansion in availability.
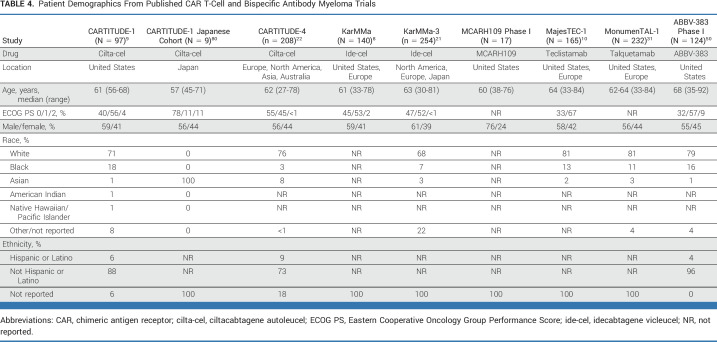
One early barrier clinicians would need to overcome would be the generalizability of early trial data, given that clinical trial populations are typically highly selected. Table 4 highlights what is known about the demographic characteristics of participants in published MM CAR T-cell and BsAb trials. Participants tended to be younger, with good performance status, and identified as non-Hispanic White. In addition, most studies excluded patients with various common comorbid conditions. Thus, clinicians are left with gaps in knowledge about the most effective treatments among those typically under-represented in clinical trials. Real-world data represent an important source of information in this setting. For example, Hansen et al23 recently reported on the outcomes of real-world patients who received commercial ide-cel in the United States. Notably, 75% of patients would have been ineligible for the KarMMa trial because of laboratory abnormalities, previous BCMA-directed therapy, other malignancies, or other conditions. Reassuringly, the reported ORR and PFS values were similar to those of the original trial.8,23
TABLE 4.
Patient Demographics From Published CAR T-Cell and Bispecific Antibody Myeloma Trials
Across most hematologic malignancies, older adults are underenrolled in clinical trials,81 a finding again seen among the published CAR T and BsAb trials highlighted here (Table 4). Exclusionary comorbidities typically include congestive heart failure, recent myocardial infarction, active infection, and second malignancies within the past 3-5 years. Nevertheless, even within these enrollment criteria, an older adult may have aging-associated vulnerabilities, raising a clinician's concern about their risk of toxicity. Although early data are encouraging that older patients selected for CAR T-cell therapy in real-world practice tolerate it similarly to younger patients,82 more widespread use of the therapy will reveal subgroups of people who may have poorer outcomes. For example, one recent study demonstrated that sarcopenia was associated with an increased risk of ICANS and longer length of hospital stay.83 Consistently gathering additional data (including geriatric assessment)84,85 about the baseline health of individuals enrolled in clinical trials of CAR T-cell and BsAbs will improve clinicians' insight into whether their patients are similar to those treated on trial and identify those who may be at increased risk for toxicity, allowing more informed shared decision making.
Barriers to racial equity in access to innovative therapies start during the clinical trial design phase and drug development process and persist after approval. A recent study demonstrated that only 36% of Black persons reside in US counties with available CAR T-cell trials.86 Although geographic location as a measure of availability is critical, Black persons with MM encounter several multilevel barriers such as clinician bias and stereotyping that influence the decision to offer trials to certain groups and perceived untrustworthiness and general distrust of the health care system and research enterprise, especially among the Black population.87-89 As mentioned above, a study of real-world patients, including those with MM, demonstrated that those who self-identified as Black race were less likely to receive CAR T-cell therapy.78 Gormley et al90 have suggested concrete steps to eliminating racial disparities in outcomes in MM. Among the first steps needed are multilevel interventions (eg, targeting patients, clinicians, the health care system, and community level) that address critical determinants of clinical trial enrollment for historically under-represented populations. Such interventions should target knowledge, attitudes, medical mistrust, and clinician-patient communication and include early and frequent collaboration with community representatives to ensure interventions are appropriately tailored and sustainable.
Another key step will require innovative approaches to facilitating access to CAR T cells and BsAbs after approval. Although CAR T-cell therapy is largely restricted to transplant centers at this point, BsAbs may be more easily implemented outside of tertiary care settings, although hospitalization is still required during initial treatment, as is access to tocilizumab. Areas for future exploration to improve access to therapy for rural populations could capitalize on technology such as wearable patient devices for early detection of CRS and ICANS.
SEQUENCING
The emergence of CAR T cells and BsAbs will give our patients additional therapeutic options, but how to best integrate these innovations into the therapeutic journey of the patient is not yet established. One published randomized trial has demonstrated the superiority of ide-cel over standard regimens in patients with two to four previous lines of therapy.21 Several other comparative and indirect treatment comparisons have similarly suggested the benefit of cilta-cel over standard options.91-93 Given that many of the emerging therapies target the same epitopes and trials excluded patients who had received therapy targeting those epitopes, the role of sequential therapies targeting the same molecule is not known.
The CARTITUDE-2 cohort C study enrolled individuals with previous triple class exposure as well as prior noncellular BCMA therapy (n = 20). The ORR was 60% with a median PFS of 9.1 months, both substantially lower than observed in the BCMA-naïve cohort of CARTITUDE-1.94 These data, in conjunction with real-world data of commercial ide-cel showing inferior outcomes in individuals previously treated with BCMA-directed therapies (median PFS of 3.2 [prior BCMA] v 9.0 [no prior BCMA] months; P = .0002),95 suggest that sequencing of BCMA-directed therapies matters. Intriguingly, data from the MajesTEC-1 cohort C study, which enrolled participants (n = 40) with previous BCMA therapy (either ADC or CAR T), showed an ORR of 53% for teclistamab.96 With a median follow-up period of 11.8 months, 71% of patients who had initially achieved a response were still maintaining their response.97 For the 15 patients with previous CAR T-cell exposure, the ORR was also 53%. A pooled analysis of patients enrolled in elranatamab clinical trials who had received previous BCMA-directed therapies showed an ORR of 46% (n = 87) and a median PFS of 5.5 months.98 For the subset of patients who had received previous BCMA CAR T-cell therapy, the ORR was 53% (n = 36) with a median PFS of 10.0 months.98 In aggregate, these data suggest that BCMA BsAb therapy may serve as effective salvage therapy in people whose disease progressed on BCMA CAR T-cell therapy.
Whether these lower response rates/response durations are a reflection of target-specific resistance mechanisms (eg, related to BCMA expression), T-cell fitness/exhaustion, or are simply a reflection of more heavily pretreated disease, remains to be determined. Until that time, there are substantial uncertainties regarding the optimal sequencing of these BCMA-directed therapies. As discussed earlier, GPRC5D- and FcRH5-directed CAR T-cell and/or BsAb therapies are showing efficacy in patients with previous BCMA-directed therapy,31-33,44,45 but again whether optimal sequencing should involve options within the same class (eg, BsAb to BsAb) or should involve switching classes (eg, CAR T-cell to BsAb) will need to be investigated.
PROS AND CONS
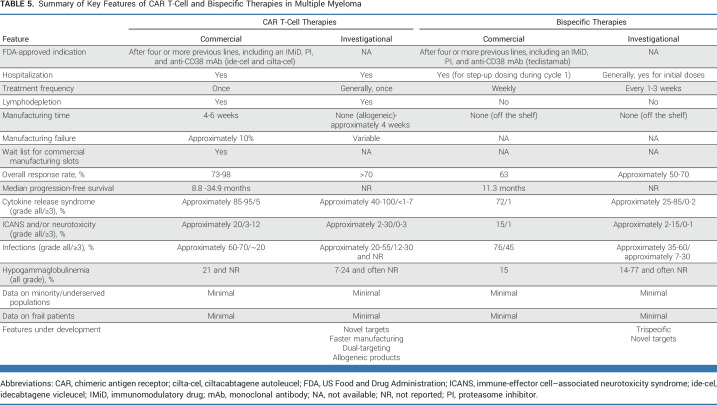
A commonly posed question to MM specialists is which patient would you recommend for BsAb therapy and which patient would you recommend for CAR T-cell therapy? Although there are a multitude of factors that should be considered, including patient preference, access to therapy, and disease-related features, for many patients, the reality is that they will likely receive both CAR T-cell and BsAb therapy at some point during their therapeutic journey. Table 5 provides a summary of the key features of both commercial and investigational products. A key advantage of CAR T-cell therapy is the current one and done approach, which permits responding patients to experience a true treatment-free interval. However, this practice might change in the future as several ongoing/planned studies are investigating consolidation/maintenance approaches post-CAR T. Likewise, although most bispecific therapies are currently being studied in a treat-until-progression manner, there is significant interest in determining whether fixed duration or response-directed duration approaches could limit treatment intensity. A common theme across recently reported CAR T-cell studies (both clinical trials and real-world studies) is the dropout of patients between time of apheresis and time of CAR T-cell administration, in part due to manufacturing failures and to disease progression/clinical decline. Thus, a key advantage of BsAb therapy over current CAR T-cell therapies is its off-the-shelf nature, permitting immediate initiation of treatment and of clear importance for patients with rapidly progressing disease. However, innovations in CAR T-cell manufacturing, such as reducing manufacturing to less than a week37 or using off-the-shelf allogeneic products,43 will be critical in permitting more timely administration of CAR T cells.
TABLE 5.
Summary of Key Features of CAR T-Cell and Bispecific Therapies in Multiple Myeloma
As discussed earlier, widespread utilization of both classes of therapies may be limited because of access and logistical issues. Currently, hospitalizations are required for both commercial CAR T-cell products as well as for the first three step-up doses of teclistamab. CAR T-cell therapy is primarily being administered in transplant centers; thus, biases or assumptions about CAR T-cell candidacy on the part of community oncologists might limit referral of potential candidates to the MM/CAR T specialists. Although outpatient administration of teclistamab could be more readily done in community infusion centers, the need for initial hospitalization, with access to personnel trained in recognizing/treating CRS/ICANS, may also prove a barrier. Prolonged cytopenias, hypogammaglobulinemia, and infection risk are attributes of both classes of therapies and will require intensive supportive care measures to ensure safety.
Finally, patient preferences will remain an important consideration in guiding patients through the newest therapies discussed here. A recent study found that most patients with R/R MM continue to prioritize response rates and overall survival.99 However, route of administration and toxicity profile remain important considerations to others and may inform recommendations for an individual. The current dearth of data regarding efficacy and safety of CAR T-cell and BsAb therapy in minority, underserved, older, or frail patients represents a significant limitation for the clinician who is counseling their patients about these therapies.
In conclusion, the field of MM is only at the beginning of its journey with CAR T-cell and BsAb (and other T-cell–redirecting agents) therapies, but clearly unprecedented response rates are being achieved in patient populations that previously had dismal outcomes. The rate at which new data are emerging from studies involving CAR T-cell and T-cell–redirecting therapies renders any review article outdated almost as soon as it is published. With current approval status of the commercial agents being for patients with four or more previous lines of therapy, and with most patients being penta-drug exposed/refractory by three lines (and many being triple-class refractory after one to two lines), there are significant therapeutic challenges in accessing the commercially available CAR T-cell and BsAb therapies. However, it is anticipated that approval indications may change in the future based off the results of KarMMa-3,21 CARTITUDE-4,22 and ongoing phase III studies, at which point the next challenge will become incorporating these agents into earlier lines of therapy. Likewise, with the expected eventual approval of non–BCMA-targeted agents, even more questions will arise as to the optimal sequencing of these therapies and resulting changes in treatment paradigms. Preliminary data showing remarkable efficacy of combining BsAbs (ie, teclistamab + talquetamab in the RedirecTT-1 trial100) raise even more questions about optimal use and sequencing of BsAbs. No matter what the future brings with respect to new therapeutic options, it is critical that the patient experience be documented and that proactive efforts are made toward ensuring equitable access to all of these innovative therapies.
Sarah A. Holstein
Consulting or Advisory Role: GlaxoSmithKline, Oncopeptides, Secura Bio, Takeda, Janssen Oncology, AbbVie
Research Funding: Oncopeptides (Inst), Bristol Myers Squibb/Celgene (Inst)
Tanya M. Wildes
Honoraria: Carevive Systems
Consulting or Advisory Role: Seagen, Carevive Systems, Sanofi
Research Funding: Janssen Oncology (Inst)
No other potential conflicts of interest were reported.
SUPPORT
S.J.G.: National Cancer Institute Grant No. 5-K12-CA120780-13; PI: William Kim and National Institute on Aging Grant No. 1 R03 AG074030-01; PI: S.J.G.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Chimeric Antigen Receptor T-Cell and Bispecific Antibody Therapy in Multiple Myeloma: Moving Into the Future
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sarah A. Holstein
Consulting or Advisory Role: GlaxoSmithKline, Oncopeptides, Secura Bio, Takeda, Janssen Oncology, AbbVie
Research Funding: Oncopeptides (Inst), Bristol Myers Squibb/Celgene (Inst)
Tanya M. Wildes
Honoraria: Carevive Systems
Consulting or Advisory Role: Seagen, Carevive Systems, Sanofi
Research Funding: Janssen Oncology (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bal S, Malek E, Kansagra A, et al. : Treatment outcomes of triple class refractory multiple myeloma: A benchmark for new therapies. Leukemia 36:877-880, 2022 [DOI] [PubMed] [Google Scholar]
- 2.Mateos MV, Weisel K, De Stefano V, et al. : LocoMMotion: A prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma. Leukemia 36:1371-1376, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill SK, Unawane R, Wang S, et al. : I-OPen: Inferior outcomes of penta-refractory compared to penta-exposed multiple myeloma patients. Blood Cancer J 12:138, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goel U, Charalampous C, Kapoor P, et al. : Defining drug/drug class refractoriness vs lines of therapy in relapsed/refractory multiple myeloma. Blood Cancer J 13:11, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chari A, Vogl DT, Gavriatopoulou M, et al. : Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med 381:727-738, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Lonial S, Lee HC, Badros A, et al. : Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol 21:207-221, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Richardson PG, Oriol A, Larocca A, et al. : Melflufen and dexamethasone in heavily pretreated relapsed and refractory multiple myeloma. J Clin Oncol 39:757-767, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munshi NC, Anderson LD Jr, Shah N, et al. : Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 384:705-716, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Berdeja JG, Madduri D, Usmani SZ, et al. : Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 398:314-324, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Moreau P, Garfall AL, van de Donk N, et al. : Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med 387:495-505, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhodapkar MV, Krasovsky J, Olson K: T cells from the tumor microenvironment of patients with progressive myeloma can generate strong, tumor-specific cytolytic responses to autologous, tumor-loaded dendritic cells. Proc Natl Acad Sci U S A 99:13009-13013, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raitakari M, Brown RD, Sze D, et al. : T-cell expansions in patients with multiple myeloma have a phenotype of cytotoxic T cells. Br J Haematol 110:203-209, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Dhodapkar MV: The immune system in multiple myeloma and precursor states: Lessons and implications for immunotherapy and interception. Am J Hematol 98:S4-S12, 2023. (suppl 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topp MS, Duell J, Zugmaier G, et al. : Anti-B-cell maturation antigen BiTE molecule AMG 420 induces responses in multiple myeloma. J Clin Oncol 38:775-783, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Harrison SJ, Minnema MC, Lee HC, et al. : A phase 1 first in human (FIH) study of AMG 701, an anti-B-cell maturation antigen (BCMA) half-life extended (HLE) BiTE® (bispecific T-cell engager) molecule, in relapsed/refractory (RR) multiple myeloma (MM). Blood 136:28-29, 2020 [Google Scholar]
- 16.Martin T, Usmani SZ, Berdeja JG, et al. : Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol 41:1265-1274, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Y, Martin TG, Usmani SZ, et al. : CARTITUDE-1 final results: Phase 1b/2 study of ciltacabtagene autoleucel in heavily pretreated patients with relapsed/refractory multiple myeloma. J Clin Oncol 41, 2023 (suppl; abstr 8009) [Google Scholar]
- 18.Einsele H, Cohen AD, Delforge M, et al. : P08: CARTITUDE-2 update: Ciltacabtagene autoleucel, a B-cell maturation antigen–directed chimeric antigen receptor T-cell therapy, in lenalidomide-refractory patients with progressive multiple myeloma after 1-3 prior lines of therapy. Hemasphere 6:15, 2022 [Google Scholar]
- 19.Usmani S, Patel K, Hari P, et al. : KarMMa-2 cohort 2a: Efficacy and safety of idecabtagene vicleucel in clinical high-risk multiple myeloma patients with early relapse after frontline autologous stem cell transplantation. Blood 140:875-877, 2022. 35709354 [Google Scholar]
- 20.Van De Donk NW, Agha M, Cohen AD, et al. : Ciltacabtagene autoleucel (cilta-cel), a BCMA-directed CAR-T cell therapy, in patients with multiple myeloma (MM) and early relapse after initial therapy: CARTITUDE-2 cohort B 18-month follow-up. Blood 140:7536-7537, 2022 [Google Scholar]
- 21.Rodriguez-Otero P, Ailawadhi S, Arnulf B, et al. : Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N Engl J Med 388:1002-1014, 2023 [DOI] [PubMed] [Google Scholar]
- 22.San-Miguel J, Dhakal B, Yong K, et al. : Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N Engl J Med 389:335-347, 2023 [DOI] [PubMed] [Google Scholar]
- 23.Hansen DK, Sidana S, Peres LC, et al. : Idecabtagene vicleucel for relapsed/refractory multiple myeloma: Real-world experience from the myeloma CAR T consortium. J Clin Oncol 41:2087-2097, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferment B, Lambert J, Caillot D, et al. : French early nationwide idecabtagene vicleucel (ide-cel) chimeric antigen receptor (CAR) T-cell therapy experience in patients with relapsed/refractory multiple myeloma (FENIX): An IFM study from the DESCAR-T registry. Blood 140:4668-4670, 2022 [DOI] [PubMed] [Google Scholar]
- 25.Hansen DK, Patel KK, Peres LC, et al. : Safety and efficacy of standard of care (SOC) ciltacabtagene autoleucel (cilta-cel) for relapsed/refractory multiple myeloma (RRMM). J Clin Oncol 41, 2023 (suppl; abstr 8012) [Google Scholar]
- 26.Bahlis NJ, Tomasson MH, Mohty M, et al. : Efficacy and safety of elranatamab in patients with relapsed/refractory multiple myeloma naïve to B-cell maturation antigen (BCMA)-Directed therapies: Results from cohort a of the magnetismm-3 study. Blood 140:391-393, 2022 [Google Scholar]
- 27.Voorhees PM, D'Souza A, Weisel K, et al. : A phase 1 first-in-human study of abbv-383, a BCMA × CD3 bispecific T-cell-redirecting antibody, as monotherapy in patients with relapsed/refractory multiple myeloma. Blood 140:4401-4404, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HC, Bumma N, Richter JR, et al. : LINKER-MM1 study: Linvoseltamab (REGN5458) in patients with relapsed/refractory multiple myeloma. J Clin Oncol 41, 2023 (suppl; abstr 8006) [Google Scholar]
- 29.Abdallah A-O, Cowan AJ, Leleu X, et al. : Updated interim results from a phase 1 study of HPN217, a half-life extended tri-specific T cell activating construct (TriTAC®) targeting B cell maturation antigen (BCMA) for relapsed/refractory multiple myeloma (RRMM). Blood 140:7284-7285, 2022 [Google Scholar]
- 30.Wong SW, Bar N, Paris L, et al. : Alnuctamab (ALNUC; BMS-986349; CC-93269), a B-cell maturation antigen (BCMA) × CD3 T-cell engager (TCE), in patients (pts) with relapsed/refractory multiple myeloma (RRMM): Results from a phase 1 first-in-human clinical study. Blood 140:400-402, 2022 [Google Scholar]
- 31.Chari A, Minnema MC, Berdeja JG, et al. : Talquetamab, a T-cell-redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med 387:2232-2244, 2022 [DOI] [PubMed] [Google Scholar]
- 32.Carlo-Stella C, Mazza R, Manier S, et al. : RG6234, a GPRC5DxCD3 T-cell engaging bispecific antibody, is highly active in patients (pts) with relapsed/refractory multiple myeloma (RRMM): Updated intravenous (IV) and first subcutaneous (SC) results from a phase I dose-escalation study. Blood 140:397-399, 2022 [Google Scholar]
- 33.Trudel S, Cohen AD, Krishnan AY, et al. : Cevostamab monotherapy continues to show clinically meaningful activity and manageable safety in patients with heavily pre-treated relapsed/refractory multiple myeloma (RRMM): Updated results from an ongoing phase I study. Blood 138:157, 2021 [Google Scholar]
- 34.Li C, Wang D, Fang B, et al. : Updated results of FUMANBA-1: A phase 1b/2 study of a novel fully human B-cell maturation antigen-specific CAR T cells (CT103A) in patients with relapsed and/or refractory multiple myeloma. Blood 140:7435-7436, 2022 [Google Scholar]
- 35.Mikkilineni L, Manasanch EE, Natrakul D, et al. : T cells expressing a fully-human anti-BCMA chimeric antigen receptor with a heavy-chain-only antigen-recognition domain exhibit rapid and durable activity against multiple myeloma. Blood 140:7433-7434, 2022 [Google Scholar]
- 36.Frigault M, Rosenblatt J, Dhakal B, et al. : Phase 1 study of CART-ddBCMA for the treatment of subjects with relapsed and/or refractory multiple myeloma. Blood 140:7439-7440, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa LJ, Kumar SK, Atrash S, et al. : Results from the first phase 1 clinical study of the B-cell maturation antigen (BCMA) Nex T chimeric antigen receptor (CAR) T cell therapy CC-98633/BMS-986354 in patients (pts) with relapsed/refractory multiple myeloma (RRMM). Blood 140:1360-1362, 2022 [Google Scholar]
- 38.Mailankody S, Jakubowiak AJ, Htut M, et al. : Orvacabtagene autoleucel (orva-cel), a B-cell maturation antigen (BCMA)-directed CAR T cell therapy for patients (pts) with relapsed/refractory multiple myeloma (RRMM): Update of the phase 1/2 EVOLVE study (NCT03430011). J Clin Oncol 38, 2020 (suppl; abstr 8504) [Google Scholar]
- 39.Chen W, Fu C, Fang B, et al. : Phase Ⅱ study of fully human BCMA-targeting CAR-T cells (zevorcabtagene autoleucel) in patients with relapsed/refractory multiple myeloma. Blood 140:4564-4565, 2022 [Google Scholar]
- 40.Sperling AS, Derman BA, Nikiforow S, et al. : Updated phase I study results of PHE885, a T-charge manufactured BCMA-directed CAR-T cell therapy, for patients (pts) with r/r multiple myeloma (RRMM). J Clin Oncol 41, 2023 (suppl; abstr 8004) [Google Scholar]
- 41.Du J, Fu W-J, Jiang H, et al. : Updated results of a phase I, open-label study of BCMA/CD19 dual-targeting fast CAR-T GC012F for patients with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol 41, 2023 (suppl; abstr 8005) [Google Scholar]
- 42.Li C, Wang X, Wu Z, et al. : Bispecific CS1-BCMA CAR-T cells are clinically active in relapsed or refractory multiple myeloma: An updated clinical study. Blood 140:4573-4574, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mailankody S, Matous JV, Liedtke M, et al. : Universal updated phase 1 data highlights role of allogeneic anti-BCMA ALLO-715 therapy for relapsed/refractory multiple myeloma. Blood 140:4620-4622, 2022 [Google Scholar]
- 44.Mailankody S, Devlin SM, Landa J, et al. : GPRC5D-targeted CAR T cells for myeloma. N Engl J Med 387:1196-1206, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bal S, Kocoglu MH, Nadeem O, et al. : Clinical activity of BMS-986393 (CC-95266), a G protein-coupled receptor class C group 5 member D (GPRC5D)-targeted chimeric antigen receptor (CAR) T cell therapy, in patients with relapsed and/or refractory (R/R) multiple myeloma (MM): First results from a phase 1, multicenter, open-label study. Blood 140:883-885, 2022 [Google Scholar]
- 46.Zhang M, Wei G, Zhou L, et al. : GPRC5D CAR T cells (OriCAR-017) in patients with relapsed or refractory multiple myeloma (POLARIS): A first-in-human, single-centre, single-arm, phase 1 trial. Lancet Haematol 10:e107-e116, 2023 [DOI] [PubMed] [Google Scholar]
- 47.Xia J, Li H, Yan Z, et al. : Anti-G protein-coupled receptor, class C group 5 member D chimeric antigen receptor T cells in patients with relapsed or refractory multiple myeloma: A single-arm, phase Ⅱ trial. J Clin Oncol 41:2583-2593, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith EL, Harrington K, Staehr M, et al. : GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med 11:eaau7746, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elkins K, Zheng B, Go M, et al. : FcRL5 as a target of antibody-drug conjugates for the treatment of multiple myeloma. Mol Cancer Ther 11:2222-2232, 2012 [DOI] [PubMed] [Google Scholar]
- 50.D'Souza A, Shah N, Rodriguez C, et al. : A phase I first-in-human study of ABBV-383, a B-cell maturation antigen × CD3 bispecific T-cell redirecting antibody, in patients with relapsed/refractory multiple myeloma. J Clin Oncol 40:3576-3586, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris EC, Neelapu SS, Giavridis T, et al. : Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol 22:85-96, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santomasso BD, Nastoupil LJ, Adkins S, et al. : Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J Clin Oncol 39:3978-3992, 2021 [DOI] [PubMed] [Google Scholar]
- 53.Trudel S, Bahlis NJ, Spencer A, et al. : Pretreatment with tocilizumab prior to the CD3 bispecific cevostamab in patients with relapsed/refractory multiple myeloma (RRMM) showed a marked reduction in cytokine release syndrome incidence and severity. Blood 140:1363-1365, 2022 [Google Scholar]
- 54.Cohen AD, Parekh S, Santomasso BD, et al. : Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J 12:32, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Oekelen O, Aleman A, Upadhyaya B, et al. : Neurocognitive and hypokinetic movement disorder with features of Parkinsonism after BCMA-targeting CAR-T cell therapy. Nat Med 27:2099-2103, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bumma N, Richter J, Brayer J, et al. : Updated safety and efficacy of REGN5458, a BCMAxCD3 bispecific antibody, treatment for relapsed/refractory multiple myeloma: A phase 1/2 first-in-human study. Blood 140:10140-10141, 2022 [Google Scholar]
- 57.Logue JM, Peres LC, Hashmi H, et al. : Early cytopenias and infections after standard of care idecabtagene vicleucel in relapsed or refractory multiple myeloma. Blood Adv 6:6109-6119, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lancman G, Shyu M, Metzger M, et al. : Timing and nature of infections in multiple myeloma patients treated with anti-BCMA CAR-T cells. Blood 140:7198-7199, 2022 [Google Scholar]
- 59.Lancman G, Parsa K, Rodriguez C, et al. : Infections and severe hypogammaglobulinemia in multiple myeloma patients treated with anti-BCMA bispecific antibodies. Blood 140:10073-10074, 2022 [Google Scholar]
- 60.Mazahreh F, Mazahreh L, Schinke C, et al. : Risk of infections associated with the use of bispecific antibodies in multiple myeloma: A pooled analysis. Blood Adv 7:3069-3074, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lesokhin AM, Arnulf B, Niesvizky R, et al. : Initial safety results for MagnetisMM-3: A phase 2 trial of elranatamab, a B-cell maturation antigen (BCMA)-CD3 bispecific antibody, in patients (pts) with relapsed/refractory (R/R) multiple myeloma (MM). J Clin Oncol 40, 2022 (suppl; abstr 8006) [Google Scholar]
- 62.Palmen B, Hari P, D'Souza A, et al. : Protracted viral infections in patients with multiple myeloma receiving bispecific T-cell engager therapy targeting B-cell maturation antigen. Haematologica 10.3324/haematol.2023.283003 [epub ahead of print on April 13, 2023] [DOI] [PMC free article] [PubMed]
- 63.Richard S, Lancman G, Thibaud S, et al. : Immune recovery post BCMA CAR-T: Implications for infection prophylaxis and vaccinations. Blood 140:7444-7446, 2022 [Google Scholar]
- 64.Chari A, Touzeau C, Schinke C, et al. : Talquetamab, a G protein-coupled receptor family C group 5 member D × CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma (RRMM): Phase 1/2 results from MonumenTAL-1. Blood 140:384-387, 2022 [Google Scholar]
- 65.Phetsouphanh C, Darley DR, Wilson DB, et al. : Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol 23:210-216, 2022 [DOI] [PubMed] [Google Scholar]
- 66.Wiech M, Chroscicki P, Swatler J, et al. : Remodeling of T cell dynamics during long COVID is dependent on severity of SARS-CoV-2 infection. Front Immunol 13:886431, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Otto Walter L, Cardoso CC, Santos-Pirath ÍM, et al. : T cell maturation is significantly affected by SARS-CoV-2 infection. Immunology 169:358-368, 2023 [DOI] [PubMed] [Google Scholar]
- 68.Inoue S, Nambu T, Shimomura T: The RAIG family member, GPRC5D, is associated with hard-keratinized structures. J Invest Dermatol 122:565-573, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Shah N, Delforge M, San-Miguel J, et al. : Patient experience before and after treatment with idecabtagene vicleucel (ide-cel, bb2121): Qualitative analysis of patient interviews in the KarMMa trial. Leuk Res 120:106921, 2022 [DOI] [PubMed] [Google Scholar]
- 70.Cohen AD, Hari P, Htut M, et al. : Patient perceptions regarding ciltacabtagene autoleucel treatment: Qualitative evidence from interviews with patients with relapsed/refractory multiple myeloma in the CARTITUDE-1 study. Clin Lymphoma Myeloma Leuk 23:68-77, 2023 [DOI] [PubMed] [Google Scholar]
- 71.Martin T, Lin Y, Agha M, et al. : Health-related quality of life in patients given ciltacabtagene autoleucel for relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b-2, open-label study. Lancet Haematol 9:e897-e905, 2022 [DOI] [PubMed] [Google Scholar]
- 72.Delforge M, Shah N, Miguel JSF, et al. : Health-related quality of life with idecabtagene vicleucel in relapsed and refractory multiple myeloma. Blood Adv 6:1309-1318, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martin TG, Moreau P, Usmani SZ, et al. : Health-related quality of life in patients with relapsed/refractory multiple myeloma (RRMM) treated with teclistamab, a B-cell maturation antigen (BCMA) × CD3 bispecific antibody: Patient-reported outcomes in MajesTEC-1. J Clin Oncol 40, 2022 (suppl; abstr 8033) [Google Scholar]
- 74.Peres LC, Hansen DK, Maura F, et al. : The knowns and unknowns of disparities, biology, and clinical outcomes in Hispanic and Latinx multiple myeloma patients in the U.S. Semin Oncol 49:3-10, 2022 [DOI] [PubMed] [Google Scholar]
- 75.Ganguly S, Mailankody S, Ailawadhi S: Many shades of disparities in myeloma care. Am Soc Clin Oncol Educ Book 39:519-529, 2019 [DOI] [PubMed] [Google Scholar]
- 76.Marinac CR, Ghobrial IM, Birmann BM, et al. : Dissecting racial disparities in multiple myeloma. Blood Cancer J 10:19, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grant SJ, Jansen M, Kuo T-M, et al. : Cross-sectional analysis of clinical trial availability and North Carolina neighborhood social vulnerability. JCO Oncol Pract 19:e248-e262, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahmed N, Shahzad M, Shippey E, et al. : Socioeconomic and racial disparity in chimeric antigen receptor T cell therapy access. Transpl Cell Ther 28:358-364, 2022 [DOI] [PubMed] [Google Scholar]
- 79.Snyder S, Chung KC, Jun MP, et al. : Access to chimeric antigen receptor T cell therapy for diffuse large B cell lymphoma. Adv Ther 38:4659-4674, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ri M, Suzuki K, Ishida T, et al. : Ciltacabtagene autoleucel in patients with relapsed/refractory multiple myeloma: CARTITUDE-1 (phase 2) Japanese cohort. Cancer Sci 113:4267-4276, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanapuru B, Singh H, Kwitkowski V, et al. : Older adults in hematologic malignancy trials: Representation, barriers to participation and strategies for addressing underrepresentation. Blood Rev 43:100670, 2020 [DOI] [PubMed] [Google Scholar]
- 82.Reyes KR, Huang C-Y, Lo M, et al. : Safety and efficacy of BCMA-targeted CAR-T therapy in geriatric patients with multiple myeloma. Blood 140:5105-5107, 2022 [Google Scholar]
- 83.Parker N, Modi K, Villanueva R, et al. : Sarcopenia prevalence and influence on the development of toxicity and length of stay in patients with relapsed and refractory myeloma treated with commercial anti-BCMA CART cells. Blood 140:4688-4690, 2022 [Google Scholar]
- 84.Magnuson A, Van der Walde N, McKoy JM, et al. : Integrating geriatric assessment measures into national cancer Institute clinical trials. J Natl Cancer Inst Monogr 2022:142-150, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Le-Rademacher J, Mohile S, Unger J, et al. : Trial design considerations to increase older adult accrual to National Cancer Institute clinical trials. J Natl Cancer Inst Monogr 2022:135-141, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alqazaqi R, Schinke C, Thanendrarajan S, et al. : Geographic and racial disparities in access to chimeric antigen receptor-T cells and bispecific antibodies trials for multiple myeloma. JAMA Netw Open 5:e2228877, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grant SJ, Jean-Baptiste M, Moore M, et al. : “If you don't trust your doctor that much…you'd feel less confident doing a research study”: Factors influencing Black patient participation in hematology trials. Blood 140:924-925, 2022 [Google Scholar]
- 88.Al Hadidi S, Schinke C, Thanendrarajan S, et al. : Enrollment of Black participants in pivotal clinical trials supporting US Food and Drug Administration approval of chimeric antigen receptor-T cell therapy for hematological malignant neoplasms. JAMA Netw Open 5:e228161, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grant SJ, Jean-Baptiste M, Moore M, et al. : “You have your knowledge, but I have my knowledge of my body”: The hematologist-patient relationship and enrollment of Black participants in clinical trials. Blood 140:930-931, 2022 [Google Scholar]
- 90.Gormley N, Fashoyin-Aje L, Locke T, et al. : Recommendations on eliminating racial disparities in multiple myeloma therapies: A step toward achieving equity in healthcare. Blood Cancer Discov 2:119-124, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mateos MV, Weisel K, Martin T, et al. : Adjusted comparison of outcomes between patients from CARTITUDE-1 versus multiple myeloma patients with prior exposure to PI, IMiD and anti-CD38 antibody from the prospective, multinational LocoMMotion study of real-world clinical practice. Haematologica 108:2192-2204, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin T, Krishnan A, Yong K, et al. : Comparative effectiveness of ciltacabtagene autoleucel in CARTITUDE-1 versus physician's choice of therapy in the Flatiron Health multiple myeloma cohort registry for the treatment of patients with relapsed or refractory multiple myeloma. EJHaem 3:97-108, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Costa LJ, Hari P, Berdeja JG, et al. : Meta-analysis of ciltacabtagene autoleucel versus physician's choice therapy for the treatment of patients with relapsed or refractory multiple myeloma. Curr Med Res Opin 38:1759-1767, 2022 [DOI] [PubMed] [Google Scholar]
- 94.Cohen AD, Mateos M-V, Cohen YC, et al. : Efficacy and safety of cilta-cel in patients with progressive multiple myeloma after exposure to non-cellular anti-BCMA immunotherapy. Blood 140:4646-4648, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ferreri CJ, Hildebrandt MAT, Hashmi H, et al. : Idecabtagene vicleucel (ide-cel) chimeric antigen receptor (CAR) T-cell therapy in patients with relapsed/refractory multiple myeloma (RRMM) who have received a prior BCMA-targeted therapy: Real world, multi-institutional experience. Blood 140:1856-1858, 2022 [Google Scholar]
- 96.Touzeau C, Krishnan AY, Moreau P, et al. : Efficacy and safety of teclistamab (tec), a B-cell maturation antigen (BCMA) × CD3 bispecific antibody, in patients (pts) with relapsed/refractory multiple myeloma (RRMM) after exposure to other BCMA-targeted agents. J Clin Oncol 40, 2022 (suppl; abstr 8013) [Google Scholar]
- 97.Touzeau C, Krishnan A, Moreau P, et al. : S184: Evaluating teclistamab in patients with relapsed/refractory multiple myeloma following exposure to other b-cell maturation antigen (BCMA)-targeted agents. HemaSphere 6:85-86, 2022 [Google Scholar]
- 98.Nooka AK, Lesokhin AM, Mohty M, et al. : Efficacy and safety of elranatamab in patients with relapsed/refractory multiple myeloma (RRMM) and prior B-cell maturation antigen (BCMA)-directed therapies: A pooled analysis from MagnetisMM studies. J Clin Oncol 41, 2023 (suppl; abstr 8008) [Google Scholar]
- 99.Popat R, Ailawadhi S, Kleinman D, et al. : Treatment preferences of patients with relapsed or refractory multiple myeloma (RRMM) in the United States, United Kingdom, France, Spain, Italy, and Germany: Results from a discrete choice experiment. Blood 140:7211-7213, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cohen YC, Morillo D, Gatt ME, et al. : First results from the RedirecTT-1 study with teclistamab (tec) + talquetamab (tal) simultaneously targeting BCMA and GPRC5D in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol 41, 2023 (suppl; abstr 8002) [Google Scholar]