ABSTRACT
Objectives:
This observational study analyzed telomerase reverse transcriptase (pTERT) mutations in 45 fine-needle aspiration (FNA) specimens obtained from thyroid nodules followed by postoperatively confirmation of papillary thyroid cancer (PTC) diagnosis, examining their relationship with clinicopathologic aspects and the BRAFV600E mutation.
Subjects and methods:
Clinical information was collected from patients who presented to Ribeirao Preto University Hospital for surgical consultation regarding a thyroid nodule and who underwent molecular testing between January 2010 to October 2012. Tests included a DNA-based somatic detection of BRAFV600E and pTERT mutations.
Results:
We found coexistence of pTERTC228T and BRAFV600E mutations in 8.9% (4/45) of thyroid nodules. All nodules positive for pTERT mutations were BRAFV600E positives. There was a significant association between pTERTC228T/BRAFV600E with older age and advanced stage compared with the group negative for either mutation.
Conclusions:
This series provides evidence that FNA is a reliable method for preoperative diagnosis of high-risk thyroid nodules. pTERTC228T/BRAFV600E mutations could be a marker of poor prognosis. Its use as a personalized molecular medicine tool to individualize treatment decisions and follow-up design needs to be further studied.
Keywords: TERT, BRAF V600E , papillary thyroid carcinoma
INTRODUCTION
Papillary thyroid cancer (PTC) risk stratification and prognostication has been normally placed on clinicopathologic aspects, which are usually unreliable and presurgically nonexistent (1). In recent years, molecularly established prognostication for PTC has been broadly advised (2–4). The role of BRAFV600E-mutation test in bettering the preoperative premonition of thyroid nodules US guided fine-needle aspiration (FNA) is dubious in terms of the prognostic accuracy of BRAFV600E mutations in PTC (5,6).
Telomerase reverse transcriptase (TERT) has been known to play a decisive role in cellular immortality by preserving the telomere length at the end of chromosomes and in encouraging other cellular functions such as proliferation and cell cycles (7). TERT gene promoter mutations (pTERT) increment the transcriptional activities of the TERT and have been connected to malignant tumors with superlative recurrence and lower survival in PTC (7,8). Only three studies preoperatively investigated pTERT mutations in PTC patients and proposed that the awareness of the mutation status might guide the amplitude of initial surgery (9–11). Coexistence of BRAFV600E and pTERT mutations leads to a more aggressive subgroup of PTC, whereas the two mutations alone have relatively less impact on the aggressiveness of PTC (12). This study preoperatively scrutinized high-risk thyroid nodules confirmed as PTC tumors for pTERT mutations and inspected their relationship with clinicopathologic features at the moment of the diagnosis and co-occurrence with the BRAFV600E-mutation.
SUBJECTS AND METHODS
FNA specimens
We have studied 59 consecutive patients with high-risk thyroid nodules after US evaluation, followed up at the Thyroid Outpatient Clinic of the Division of Endocrinology of the Ribeirao Preto Medical School of University of São Paulo, Brazil, who needed another FNA examination and were chosen in our hospital from January 2010 to October 2012. Inclusion criteria were: (1) TIRADS 4-6 at US, or (2) TIRADS 3 that meet at least one of the following criteria: the nodule grows during follow-up (more than a 50% change in volume or a 20% increase in at least two nodule dimensions with a minimal increase of 2 mm in solid nodules or in the solid portion of mixed cystic-solid nodules), patients with higher risk of malignancy like those exposed to previous radiation to the neck or family history of DTC, and (3) histologic confirmation of PTC after thyroidectomy and elective lymph node dissection. TNM classification was built according to the American Joint Committee on Cancer (AJCC) 8th edition (13,14). Genomic DNA from FNA specimens preoperatively obtained was isolated, and nested PCR was performed for direct genomic DNA sequencing to identify both the C228T and C250T pTERT mutations as previously described (9–11).
FNA biopsy used 24-gauge needles fitted to a 10-mL syringe. Most of the material (about two thirds) from the needle was used for cytological examination, and the remaining amount was used for DNA isolation after needle washing with 1 mL of the phosphate buffer. Then the sample was stored and frozen for future DNA extraction, using 20-50 μL of DNA extraction buffer solution (50 mM Tris buffer, pH 8.3; 1 mM EDTA, pH 8.0; 5% Tween 20 and 100 μg/mL proteinase K) with 10% resin added to the samples and incubated at 56.8 °C for a minimum of 1 hour. After incubation, the tubes were heated to 100 °C for 10 minutes, followed by centrifugation to pellet the debris, and 5 μL of the supernatant was used in the PCR reaction.
BRAFV600E mutation analysis
PCR was performed to amplify the exon 15 of BRAF from the isolated DNA in 20 μL reaction volume containing 100 ng of genomic DNA, 7.5 pmol of each primer, 100 μm deoxynucleoside triphosphates (dNTPs), 5 μCi [α32P] dCTP, 1.5 mm MgCl2, Platinum TaqDNA polymerase high fidelity and buffer (Thermo Fisher Scientific, Waltham, MA, USA). The primer pair was designed flanking BRAF exon 15: 5‘ AAACTCTTCATAATGCTTGCTCTG3’ (sense) and 5‘GGCCAAAAATTTAATCAGTGGA 3’ (antisense). Quality confirmation of the PCR products was achieved by gel electrophoresis, and sequencing PCR was performed using the Veriti 96-Well Thermal Cycler (Thermo Fisher Scientific, Waltham, MA, USA).
pTERT mutation analysis
A fragment of the pTERT, which contained the sites for pTERTC250T and pTERTC228T mutations, was amplified by nested PCR on 50-100 ng of genomic DNA from FNA specimens. The first PCR used pair primers [5’ACGAACGTGGCCAGCGGCAG3’ (sense) and 5’CTGGCGTCCCTGCACCCTGG3’ (antisense)] in a 0,4 µM, 200 µM dNTPs, 1,5 mM MgCl2, PCRx Enhancer System 1X (Life Technologies, Carlsbad, CA), Taq DNA polimerase recombinant (Life Technologies, Carlsbad, CA), buffer and water (UltraPure™ DNase/RNase-Free Distilled Water, Life Technologies, Carlsbad, CA). It was performed with an initial denaturation at 94 °C for 5 minutes, followed by 40 cycles of 94 °C for 30 seconds, 62 °C annealing for 30 seconds, 72 °C elongation for 45 seconds and final completion with an elongation at 72 °C for 15 min. The second PCR used a dilution (1:50) of the first PCR product. The primers used were 5’ AGTGGATTCGCGGGCACAGA 3’ (sense) and 5’ CAGCGCTGCCTGAAACTC 3’ (antisense) in a 0,5 µM, 200 mM dNTPs, PCRx Enhancer System 1X (Life Technologies, Carlsbad, CA), Taq DNA polimerase Hot Start High Fidelity (Life Technologies, Carlsbad, CA), buffer and water to 50 µL of final volume. This PCR was performed with an initial denaturation at 98 °C for 3 minutes, followed by 35 cycles of 98 °C for 20 seconds, 66 °C annealing for 30 seconds, 72 °C elongation for 30 seconds and final completion with an elongation at 72 °C for 10 minutes.
Statistic analysis
For analysis of the relationship between tumor clinicopathologic features and presence of pTERT/BRAFV600E mutations, Pearson's chi-square test and Fisher's exact test were used. A linear-by-linear test was used to examine the association between T stage, N stage, AJCC stage, and mutations.
RESULTS
A total of 59 patients with confirmed PTC was enrolled, and 14 patients were excluded due PCR failure (n = 8) and 6 due to incomplete clinical data. Therefore, 45 thyroid nodules confirmed as PTC cases were included after histological re-review by two experienced pathologists. As reported in Table 1, 39 out of 45 patients (86.7%) were women, and the mean age for all the cases was 48.5 ± 14.33 years (range 16-78). Among those, 16 patients were over 55 years old (median) at the time of diagnosis. The median tumor size was 1.9 cm (range 0.6-6.8), mean 1.35 ± 1.48 cm, with 13 (29.5%) tumors smaller than 1 cm. More than half (75.6%) of PTC were unifocal; 4 cases had a focal extra thyroidal extension, and only 7 cases had lymph node metastases at diagnosis. Four distant metastases were observed. In all, 17 (38.6%) patients had locally advanced disease (AJCC stage III or IV), and the presence of capsular invasion was observed for 8 (17.8%) patients. In all, 66.7% (30/45) and 8.9% (4/45) of the cases were BRAFV600E-mutated and TERT-mutated, respectively.
Table 1. Clinical and pathological characteristics of 45 patients harboring thyroid nodules confirmed as papillary thyroid carcinoma and preoperatively submitted to molecular analysis of pTERT and BRAFV600E mutations.
| Variables | n | % | |
|---|---|---|---|
| Gender | |||
| Male | 6 | 13.3 | |
| Female | 39 | 86.7 | |
| Age, mean (sd) | 48.5 (14.33) | ||
| ≤ 55 | 23 | 51.1 | |
| > 55 | 22 | 48.9 | |
| Tumor size | |||
| ≤ 1 cm | 13 | 29.5 | |
| > 1 cm | 31 | 70.5 | |
| Multicentricity | |||
| Absent | 34 | 75.6 | |
| Present | 11 | 24.4 | |
| Extrathyroid extension | |||
| Absent | 41 | 91.1 | |
| Present | 4 | 8.9 | |
| Lymphnode metastases | |||
| Absent | 38 | 84.4 | |
| Present | 7 | 15.6 | |
| AJCC stage | |||
| I+II | 33 | 75.0 | |
| III+IV | 11 | 25.0 | |
| Capsular invasion | |||
| Absent | 37 | 82.2 | |
| Present | 8 | 17.8 | |
| BRAF status | |||
| Wild Type | 15 | 33.3 | |
| Mutated | 30 | 66.7 | |
| TERT status | |||
| Wild Type | 41 | 91.1 | |
| Mutated | 4 | 8.9 | |
Correlation between pTERT/BRAFV600E status and clinicopathological parameters
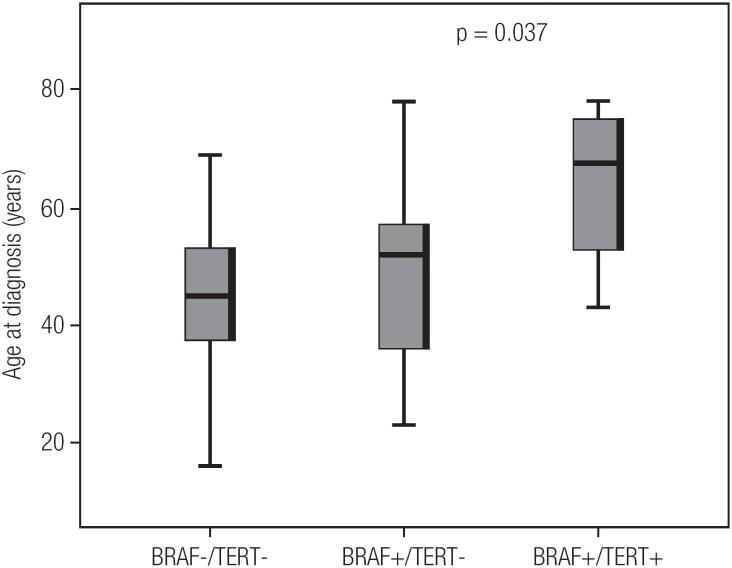
Four PTC tumors (two oncocytic, one classic and one trabecular) had pTERT mutations, and all additionally harbored the BRAFV600E mutation. There was no association between isolated BRAFV600E mutations and clinic-pathological parameters. However, the found pTERTC228T mutation was independently associated with advanced age (p = 0.02) and high AJCC stage (p = 0.03) (Table 2). Interestingly, three of four patients with concomitant BRAFV600E and pTERTC228T mutations were classified as stage III-IV. There was significant difference in age at diagnosis between wild type for both BRAFV600E and pTERTC228T mutations, only BRAFV600E positive and with concomitant BRAFV600E and pTERTC228T mutations patients. (p = 0.03) (Figure 1). However, in the follow-up evaluation, one patient had died, two present excellent response and another has indeterminated response to treatment at the study data snapshot.
Table 2. Relationship of pTERT/BRAFV600E mutation and clinicopathologic aspects in 45 thyroid nodules specimens obtained by fine needle aspiration and postoperatively confirmed as papillary thyroid carcinoma.
| Variables | BRAF status, n (%) | P value | TERT status, n (%) | P value | |||
|---|---|---|---|---|---|---|---|
| Wild type | Mutated | Wild type | Mutated | ||||
| Gender | |||||||
| Male | 1 (6.7) | 5 (16.7) | 0.647 | 5 (12.2) | 1 (25.0) | 0.448 | |
| Female | 14 (93.3) | 25 (83.3) | 36 (87.8) | 3 (75.5) | |||
| Age, mean (sd) | 44.0 (14.35) | 50.7 (14.0) | 0.139 | 46.9 (13.5) | 64.0 (15.3) | 0.022 | |
| Tumor size | |||||||
| ≤ 1 cm | 3 (21.4) | 10 (33.3) | 0.498 | 13 (32.5) | 0 (0.0) | 0.302 | |
| > 1 cm | 11 (78.6) | 20 (66.7) | 27 (67.5) | 4 (100.0) | |||
| Multicentricity | |||||||
| Absent | 12 (80.0) | 22 (73.3) | 0.726 | 30 (73.2) | 4 (100.0) | 0.558 | |
| Present | 3 (20.0) | 8 (26.7) | 11 (26.8) | 0 (0.0) | |||
| Extrathyroid extension | |||||||
| Absent | 14 (93.3) | 27 (90.0) | 1.00 | 38 (92.7) | 3 (75.0) | 0.320 | |
| Present | 1 (6.7) | 3 (10.0) | 3 (7.3) | 1 (25.0) | |||
| Lymphnode metastases | |||||||
| Absent | 14 (93.3) | 24 (80.0) | 0.395 | 36 (87.8) | 2 (50.0) | 0.108 | |
| Present | 1 (6.7) | 6 (20.0) | 5 (12.2) | 2 (50.0) | |||
| AJCC stage | |||||||
| I+II | 12 (85.7) | 21 (70.0) | 0.456 | 33 (82.5) | 0 (0.0) | 0.002 | |
| III+IV | 2 (14.3) | 9 (30.0) | 7 (17.5) | 4 (100.0) | |||
| Capsular invasion | |||||||
| Absent | 13 (86.7) | 24 (80.0) | 0.699 | 34 (82.9) | 3 (75.0) | 0.557 | |
| Present | 2 (13.3) | 6 (20.0) | 7 (17.1) | 1 (25.0) | |||
Figure 1. Relationship between age at PTC diagnosis and mutation status.
CONCLUSION
We investigated the feasibility of combined BRAFV600E/pTERT mutations testing on routine FNA specimens and its prognostic value in US guide biopsied of high suspicious thyroid nodules. The BRAFV600E and pTERT mutations were found in a frequency of 66.7% and 8.9%, respectively. Indeed, we observed that BRAFV600E/pTERT mutation-positive thyroid nodules were detected only in cancers that behaved aggressively, representing 4/11 (36%) of advanced stage PTCs and harboring threatening clinic-pathological features such as lymph node metastases, extra-thyroidal invasion and distant metastases (Table 3).
Table 3. Correlation of pTERT/BRAFV600E mutations and clinicopathologic aspects in 45 thyroid nodules specimens obtained by fine needle aspiration and postoperatively confirmed as papillary thyroid carcinoma.
| Variables | BRAF/TERT status, n (%) | |||
|---|---|---|---|---|
| BRAF-/TERT- | BRAF+/TERT- | BRAF+/TERT+ | ||
| Gender | ||||
| Male | 14 (93.3) | 22 (84.6) | 3 (75.0) | |
| Female | 1 (6.7) | 4 (15.4) | 1 (25.0) | |
| P valuea = 0.407 | P valueb = 0.636 | P valuec = 0.386 | ||
| Age, mean (sd) | 44.0 (14.35) | 48.7 (12.9) | 64.0 (15.3) | |
| P valuea = 0.042 | P valueb = 0.881 | P valuec = 0.037 | ||
| Tumor size | ||||
| ≤ 1 cm | 3 (21.4) | 10 (38.5) | 0 (0.0) | |
| > 1 cm | 11 (78.6) | 16 (61.5) | 4 (100.0) | |
| P valuea = 0.284 | P valueb = 0.316 | P valuec = 1.00 | ||
| Multicentricity | ||||
| Absent | 12 (80.0) | 18 (69.2) | 4 (100.0) | |
| Present | 3 (20.0) | 8 (30.8) | 0 (0.0) | |
| P valuea = 0.592 | P valueb = 0.716 | P valuec = 1.00 | ||
| Extrathyroid extension | ||||
| Absent | 14 (93.3) | 24 (92.3) | 3 (75.0) | |
| Present | 1 (6.7) | 2 (7.7) | 1 (25.0) | |
| P valuea = 0.509 | P valueb = 1.00 | P valuec = 0.386 | ||
| Lymphnode metastases | ||||
| Absent | 14 (93.3) | 22 (84.6) | 2 (50.0) | |
| Present | 1 (6.7) | 4 (15.4) | 2 (50.0) | |
| P valuea = 0.139 | P valueb = 0.636 | P valuec = 0.097 | ||
| AJCC stage | ||||
| I+II | 12 (85.7) | 21 (80.8) | 0 (0.0) | |
| III+IV | 2 (14.3) | 5 (19.2) | 4 (100.0) | |
| P value a = 0.004 | P valueb = 1.00 | P valuec = 0.005 | ||
| Capsular invasion | ||||
| Absent | 13 (86.7) | 21 (80.8) | 3 (75.0) | |
| Present | 2 (13.3) | 5 (19.2) | 1 (25.0) | |
| P valuea = 0.861 | P valueb = 1.00 | P valuec = 0.530 | ||
Global test.
BRAF-/TERT- vs BRAF+/TERT- comparasion.
BRAF-/TERT- vs BRAF+/TERT+ comparasion.
We did not evaluate the degree of concordance between matched FNA and formalin-fixed, paraffin-embedded samples. The mutation analysis sensitivity can be compromised by using lavage fluid once the amount and composition of the cellular content is unknown, potentially leading to discordance between matched FNA and formalin-fixed, paraffin-embedded. As a result, we were not able to measure the mutation false-negative and false-positive rates on FNA preparations. Indeed, strategies such as real-time Light Cycler PCR and fluorescence melting curve analysis might be superior.
In conclusion, preoperative determination of BRAFV600E and pTERT mutations status can be easily performed on cytologic preparation using lavage fluids collected from needle rinsing. The presence of the BRAFV600E/pTERT mutations could be a marker of poor prognosis in elderly population, although the absence of the mutation may not yet be considered an index of good prognosis to individualize treatment decisions and follow-up protocol. Preoperative knowledge of the BRAFV600E/pTERT mutations status would help determine the extent of surgery for thyroid nodules. Disease free or overall survival is still unclear.
Acknowledgements:
this study was supported by Fapesb (Fundação de Amparo à Pesquisa no Estado da Bahia, Edital 011/2013; TOU RED010/2013). FAEPA (Fundação de Amparo ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto).
Funding Statement
Financial support: this work was supported in part by grants Fapesb (Fundação de Amparo à Pesquisa no Estado da Bahia, Edital 011/2013; TOU RED010/2013). FAEPA (Fundação de Amparo ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto).
Footnotes
Financial support: this work was supported in part by grants Fapesb (Fundação de Amparo à Pesquisa no Estado da Bahia, Edital 011/2013; TOU RED010/2013). FAEPA (Fundação de Amparo ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto).
REFERENCES
- 1.Nikiforov YE. Thyroid cancer in 2015: Molecular landscape of thyroid cancer continues to be deciphered. Nat Rev Endocrinol. 2016;12(2):67-8. [DOI] [PubMed]
- 2.Yip L, Ferris RL. Clinical application of molecular testing of fine-needle aspiration specimens in thyroid nodules. Otolaryngol Clin North Am. 2014;47(4):557-71. [DOI] [PMC free article] [PubMed]
- 3.Nikiforov YE, Yip L, Nikiforova MN. New strategies in diagnosing cancer in thyroid nodules: impact of molecular markers. Clin Cancer Res. 2013;19(9):2283-8. [DOI] [PubMed]
- 4.Xing M. BRAF V600E mutation and papillary thyroid cancer. JAMA. 2013;310(5):535. [DOI] [PMC free article] [PubMed]
- 5.Kim SW, Lee JI, Kim JW, Ki CS, Oh YL, Choi YL, et al. BRAFV600E mutation analysis in fine-needle aspiration cytology specimens for evaluation of thyroid nodule: a large series in a BRAFV600E-prevalent population. J Clin Endocrinol Metab. 2010;95(8):3693-700. [DOI] [PubMed]
- 6.Marotta V, Sciammarella C, Colao AA, Faggiano A. Application of molecular biology of differentiated thyroid cancer for clinical prognostication. Endocr Relat Cancer. 2016;23(11):R499-R515. [DOI] [PubMed]
- 7.Kim TH, Kim YE, Ahn S, Kim JY, Ki CS, Oh YL, et al. TERT promoter mutations and long-term survival in patients with thyroid cancer. Endocr Relat Cancer. 2016;23(10):813-23. [DOI] [PubMed]
- 8.Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer. 2016;23(3):R143-55. [DOI] [PMC free article] [PubMed]
- 9.Crescenzi A, Trimboli P, Modica DC, Taffon C, Guidobaldi L, Taccogna S, et al. Preoperative Assessment of TERT Promoter Mutation on Thyroid Core Needle Biopsies Supports Diagnosis of Malignancy and Addresses Surgical Strategy. Horm Metab Res. 2016;48(3):157-62. [DOI] [PubMed]
- 10.Liu R, Xing M. Diagnostic and prognostic TERT promoter mutations in thyroid fine-needle aspiration biopsy. Endocr Relat Cancer. 2014;21(5):825-30. [DOI] [PMC free article] [PubMed]
- 11.Lee SE, Hwang TS, Choi YL, Han HS, Kim WS, Jang MH, et al. Prognostic Significance of TERT Promoter Mutations in Papillary Thyroid Carcinomas in a BRAF(V600E) Mutation-Prevalent Population. Thyroid. 2016;26(7):901-10. [DOI] [PubMed]
- 12.Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32(25):2718-26. [DOI] [PMC free article] [PubMed]
- 13.Brierley JD, Panzarella T, Tsang RW, Gospodarowicz MK, O’Sullivan B. A comparison of different staging systems predictability of patient outcome. Thyroid carcinoma as an example. Cancer. 1997;79(12):2414-23. [PubMed]
- 14.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. [DOI] [PMC free article] [PubMed]