ABSTRACT
Atrial fibrillation (AF) is a rare complication of multisystem inflammatory syndrome in children (MIS-C) associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. A 10-year-old boy with a history of SARS-CoV-2 infection 10 weeks before presentation developed AF following the onset of MIS-C. The patient presented with high fever, conjunctival congestion, erythematous throat, and a diffuse erythematous macular rash involving the face and both legs, in addition to respiratory distress and shock requiring oxygen and vasopressor support. Echocardiography revealed poor left ventricular contractility and normal-appearing coronary vessels. The patient received intravenous immunoglobulin, pulse methylprednisolone, and aspirin. AF resolved with synchronized cardioversion and the patient’s clinical condition subsequently improved. This case reports a rare phenomenon of AF in a case of MIS-C. Further research is required to verify the association.
Keywords: Atrial fibrillation, multisystem inflammatory syndrome in children, severe acute respiratory syndrome coronavirus 2
INTRODUCTION
Multisystem Inflammatory Syndrome in Children (MIS-C) is a postinfectious syndrome linked to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Cardiac involvement is present in approximately 80% of cases of MIS-C and can include ventricular dysfunction, valvular regurgitation, coronary artery dilation, arrhythmia, and conduction abnormalities.[1] Electrocardiographic abnormalities are present in approximately half of the patients with MIS-C, with the most common manifestation being changes in the ST-segment and T-waves.[2] In this case report, we present a child with MIS-C and atrial fibrillation (AF).
CASE REPORT
A 10-year-old male of African ethnicity presented with a 5-day history of high-grade fever, conjunctival congestion, erythematous throat, and a diffuse erythematous macular rash involving the face and both legs [Figure 1a and b]. The patient had a previous SARS-CoV-2 infection 10 weeks before presentation. On admission, the patient’s vital signs were as follows: temperature of 39.6°C, heart rate of 150/min, respiratory rate of 28/min, blood pressure of 81/44 mmHg, and oxygen saturation of 99% in room air. No lymphadenopathy was present, and there were no signs of neurological involvement. The patient received a fluid bolus of normal saline and was initiated on vasopressor support with epinephrine and norepinephrine, as well as high-flow nasal oxygen for respiratory distress. Echocardiography revealed poor left ventricular contractility with an ejection fraction of 35% and normal-appearing coronary vessels. A diagnosis of MIS-C associated with SARS-CoV-2 infection was made, and the patient received intravenous immunoglobulin (2 g/kg), pulse methylprednisolone (20 mg/kg/day for 3 days), and aspirin (5 mg/kg/day). Ceftriaxone was also administered to cover the possibility of sepsis.
Figure 1.
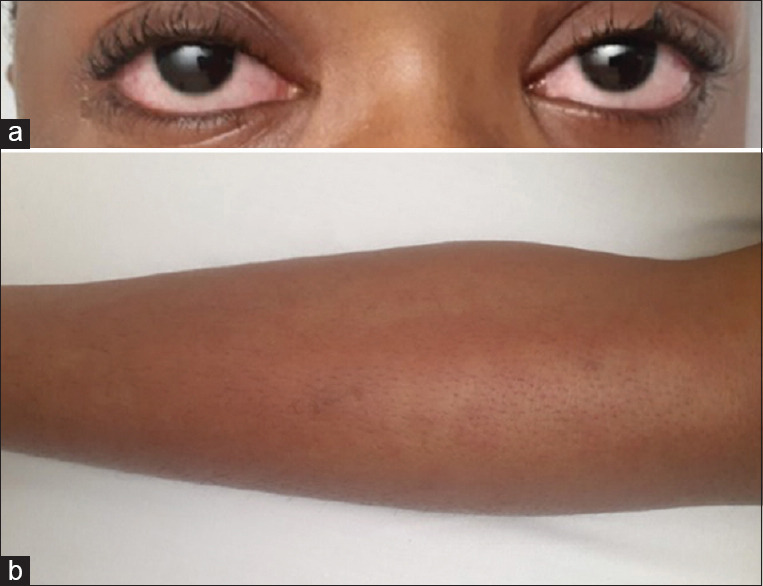
(a) Conjunctival congestion (Clinical photograph taken with permission), (b) Diffuse erythematous macular rash on the leg (Clinical photograph taken with permission)
Initial laboratory findings showed a hemoglobin level of 10.7 g/dL, white blood cell count of 4630/ml with a neutrophilic predominance of 87%, platelet count of 1.14 lakh/microliter, C-reactive protein (CRP) of 195 mg/L, procalcitonin of 8.68 ng/mL, D-dimer of 5.35 micrograms/mL, fibrinogen of 450 mg/dL, ferritin of 256.8 ng/mL, lactate dehydrogenase of 381 U/L, creatine kinase myocardial band of 0.80 ng/mL, and high-sensitivity troponin I of 0.22 ng/mL. Prothrombin time/international normalized ratio, partial thromboplastin time, and liver and renal function tests were normal. The respiratory swab was negative for SARS-CoV-2 by reverse transcription polymerase chain reaction, and anti-SARS-CoV-2 spike protein and trimeric S immunoglobulin G were positive.
On the 2nd day of hospitalization, the patient’s signs of inflammatory shock resolved and fever subsided, but he developed chest pain and an electrocardiogram (ECG) showed AF with right bundle branch block (RBBB) and ST-segment and T-wave changes in multiple chest leads [Figure 2]. The high-sensitivity troponin I had raised to 1.53 ng/mL, CRP to 254 mg/L, and B-type natriuretic peptide (BNP) 102.49 pg/mL. The electrocardiographic abnormalities were attributed to ongoing myocardial injury. Anti-inflammatory therapy was intensified with a single dose of infliximab and prophylactic doses of low-molecular weight heparin added to prevent atrial thrombosis. Cardioversion was delayed until inflammation had subsided. Despite improvement in the patient’s general condition and decline in inflammatory markers, electrocardiographic abnormalities persisted. After 2 days of onset of AF, synchronized cardioversion was performed under intravenous sedation with ketamine, resulting in a return to sinus rhythm after a single dose of 0.5 J/kg.
Figure 2.
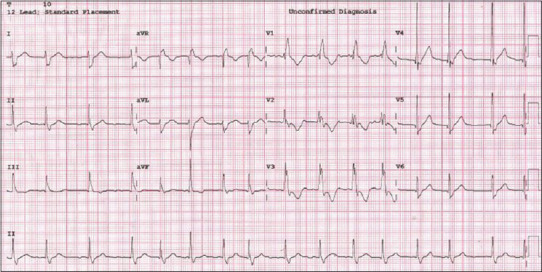
ECG showing the atrial fibrillation, along with RBBB and ST-segment and T-wave changes in multiple chest leads. ECG: Electrocardiogram, RBBB: Right bundle branch block
Following pulse therapy with methylprednisolone and a decline in inflammatory markers such as CRP, BNP, troponin I, and procalcitonin, the dose of methylprednisolone was reduced. Echocardiography at discharge showed normal cardiac function with a left ventricular ejection fraction of 65%, trivial mitral regurgitation, and no pericardial effusion. The ECG at this time showed sinus rhythm with frequent premature ventricular contractions and resolution of RBBB, ST-segment and T-wave changes [Figure 3]. The patient was discharged after 10 days of hospitalization and received tapering doses of steroids and aspirin for 4 weeks, as well as prophylactic doses of low-molecular-weight heparin for 2 weeks.
Figure 3.
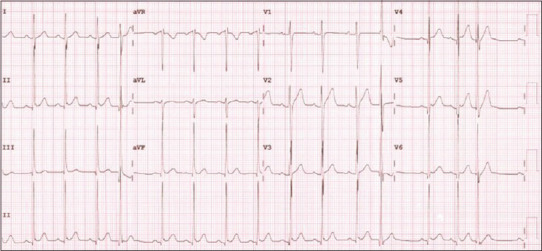
ECG showing sinus rhythm with frequent premature ventricular contractions. ECG: Electrocardiogram
Two weeks following discharge, the patient remained asymptomatic but continued to have frequent junctional beats on the ECG. Repeat echocardiography showed normal function with mild tricuspid regurgitation and mild pulmonary insufficiency.
DISCUSSION
AF is a rare complication of MIS-C associated with SARS-CoV-2 infection. Previous case reports have demonstrated the association between AF and MIS-C, as well as the potential for AF to recur in the presence of high inflammatory markers. Santi et al. reported AF in a 17-year-old boy who initially required cardioversion with 100 J and 150 J, and with the recurrence on the next day required cardioversion with 150 J and a bolus dose of amiodarone to revert to sinus rhythm.[3] In our case, the presence of high inflammatory markers was correlated with the occurrence of arrhythmia. To prevent the recurrence of arrhythmia and minimize the need for multiple cardioversions, we considered it necessary to control inflammation. As a consequence, we delayed cardioversion until inflammation had decreased. Man Singh et al. reported junctional tachycardia in a 12-year-old girl diagnosed with MIS-C, which resolved after treatment with intravenous immunoglobulin infusion.[4] This suggests that the atrioventricular node could also trigger an arrhythmia with inflammation in MIS-C.
The mechanism by which AF occurs in MIS-C is not well understood, but it may be related to cardiomyocyte inflammatory signaling.[5] Therefore, control of inflammation is important for the normalization of rhythm and prevention of recurrence. Initial immunomodulatory therapy for MIS-C typically includes glucocorticoids and intravenous immunoglobulin, with the option for intensification with higher-dose glucocorticoids, infliximab, or anakinra in nonresponsive cases.[6]
There is a less consensus on the use of anticoagulation, either prophylactic or therapeutic, in patients with MIS-C without large coronary artery aneurysms or moderate-to-severe left ventricular dysfunction, although marked elevations in D-dimer levels may suggest an increased risk for thrombosis.[6] Furthermore, AF per se poses the risk of intracardiac thrombosis. In this case, intensification with infliximab and prophylactic doses of low molecular weight heparin and aspirin were used. Studies involving the measurement of peak left atrial longitudinal strain by atrial deformation analysis have shown that this modality could assist in the diagnosis of MIS-C, especially when abnormalities could not be detected by conventional echocardiogram.[7]
CONCLUSION
This case reports a rare phenomenon of AF in a case of MIS-C. Further research is required to verify the association.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Acevedo L, Piñeres-Olave BE, Niño-Serna LF, Vega LM, Gomez IJ, Chacón S, et al. Mortality and clinical characteristics of multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 in critically ill patients: An observational multicenter study (MISCO study) BMC Pediatr. 2021;21:516. doi: 10.1186/s12887-021-02974-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mannarino S, Raso I, Garbin M, Ghidoni E, Corti C, Goletto S, et al. Cardiac dysfunction in multisystem inflammatory syndrome in children: An Italian single-Center study. Ital J Pediatr. 2022;48:25. doi: 10.1186/s13052-021-01189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santi AD, Aquino P, Dorfman M. Atrial fibrillation in a child with COVID-19 infection. Cardiol Young. 2021;31:318–21. doi: 10.1017/S1047951120003893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Man Singh J, Palting RL, Bratincsak A. Junctional tachycardia due to multisystem inflammatory syndrome in children with SARS-CoV-2 infection in a 12-year-old female. Cardiol Young. 2021;31:1021–3. doi: 10.1017/S1047951120005016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nattel S, Heijman J, Zhou L, Dobrev D. Molecular basis of atrial fibrillation pathophysiology and therapy: A translational perspective. Circ Res. 2020;127:51–72. doi: 10.1161/CIRCRESAHA.120.316363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hospitalized Pediatric Patients: Therapeutic Management of MIS-C. COVID-19 Treatment Guidelines. [[Last accessed on 2022 Aug 21]]. Available from: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-children/hospitalized-pediatric-patients—therapeutic-management-of-mis-c/
- 7.Krishna MR, Sennaiyan UN. Peak left atrial longitudinal strain: A potential diagnostic entity in children with multi-inflammatory syndrome in children. Ann Pediatr Cardiol. 2021;14:393–6. doi: 10.4103/apc.apc_18_21. [DOI] [PMC free article] [PubMed] [Google Scholar]


