Abstract
Sera from 210 patients with Lyme borreliosis (LB) were studied by an enzyme-linked immunosorbent assay (ELISA) based on a synthetic peptide (pepC10) comprising the C-terminal 10-amino-acid residues of OspC of Borrelia burgdorferi. We found that 36.3 and 45.0% of the serum samples from patients with erythema migrans (EM) and neuroborreliosis (NB), respectively, displayed immunoglobulin M (IgM) anti-pepC10 reactivities, while these samples rarely (≤8%) displayed IgG antibody reactivities. Sera from patients with acrodermatitis chronica atrophicans did not contain anti-pepC10 antibodies. The diagnostic performance of this newly developed peptide ELISA was compared with those of an ELISA based on the full-length recombinant OspC protein (rOspC) and a commercially available ELISA based on the B. burgdorferi flagellum (Fla). The sensitivity of the IgM pepC10 ELISA was slightly lower (P < 0.04) than that of the rOspC ELISA for EM patients (36.3 versus 43.8%), while there was no difference for NB patients (45.0 versus 48.0%). However, the optical density values obtained by the pepC10 ELISA were generally higher than those obtained by the rOspC ELISA, leading to a significantly better quantitative discrimination between seropositive patients with NB and controls (P < 0.008). The specificity of the pepC10 ELISA was similar to those of the rOspC ELISA and the Fla ELISA for relevant controls including patients with syphilis and mononucleosis. Although the overall diagnostic sensitivity of the Fla ELISA was superior, 8.8 and 12.0% of the EM and NB patients, respectively, were antibody positive only by the pepC10 ELISA. Thus, use of a diagnostic test for LB based on the detection of IgM antibodies to pepC10 and Fla has increased sensitivity for the diagnosis of early LB.
Lyme borreliosis (LB) is a multisystemic infection caused by the Borrelia burgdorferi sensu lato organisms comprising B. burgdorferi, Borrelia garinii, and Borrelia afzelii. The laboratory diagnosis of LB depends on the detection and quantitation of B. burgdorferi-specific antibodies in serum and CSF (14, 15, 17) because the direct demonstration of the organisms in clinical specimens by cultivation or PCR has a low sensitivity (3, 20, 22). Serological assays with whole B. burgdorferi cells as the antigen have a sensitivity that is acceptable for clinical use, but the specificity is low due to cross-reactivity to common antigens from other bacterial species (6, 24). This cross-reactivity may be eliminated by the use of purified single Borrelia antigens in the form of either native or recombinant proteins. For example, purified B. burgdorferi flagellum (Fla) is a highly sensitive and specific diagnostic antigen (13, 14, 19). Because the outer surface protein OspC elicits an early immune response in the human host, several attempts have been made to develop diagnostic assays based on recombinant OspC or on synthetic peptides derived from OspC (9, 11, 25, 28, 37, 42).
The relevance of OspC for the serodiagnosis of LB was first studied by Wilske et al. (41). However, in contrast to the B. burgdorferi Fla, which is conserved (18, 23, 31), nucleotide sequencing of ospC has disclosed that the deduced gene product is highly variable (18, 35, 40), a fact which could complicate its use as a test antigen. The degree of genetic and antigenic diversity between different OspC variants is high, even among strains belonging to the same genospecies. This is especially true for B. garinii isolates (34, 40), and on the basis of the results obtained with a panel of monoclonal antibodies, at least 13 different OspC serotypes could be identified among B. garinii strains (39). Patient sera with a strain-restricted anti-OspC antibody response have been identified (35, 40). However, such strain-restricted antibody responses seem rare and are not important from a diagnostic point of view (26, 38).
Motivated by our recent discovery that the conserved C terminus of OspC is widely recognized by immunoglobulin M (IgM) antibodies in sera from patients with neuroborreliosis (NB) (27), we have evaluated the diagnostic potential of an enzyme-linked immunosorbent assay (ELISA) based on a synthetic peptide corresponding to the C-terminal 10-amino-acid residues of OspC. This peptide-based ELISA performed at least as well as an ELISA based on rOspC and was well suited as a supplement to the Fla-based assay.
MATERIALS AND METHODS
Antigens.
Full-length recombinant OspC (rOspC) from B. garinii DK6 and the synthetic peptides PVVAESPKKP-CO2H (pepC10), corresponding to the C-terminal 10-amino-acid residues of OspC, and PVVAESPKNP-CO2H (modified pepC10) were produced as described previously (27).
Sera.
Sera from 210 patients with definite, active, and untreated LB were used in this study. They were divided into three groups according to clinical criteria.
(i) Sera from 60 consecutive Swedish patients and 20 consecutive Danish patients with EM.
The diagnosis of erythema migrans (EM) was based on clinical evidence according to the criteria of the Centers for Disease Control and Prevention and was always made by a dermatologist. The 60 Swedish patients were all seen and then illnesses were diagnosed by one of the authors (E.Å.). The diagnosis for the 20 Danish patients was further confirmed by culture of a skin biopsy specimen. The sera were collected from 1984 to 1992 from patients between 6 and 83 years of age (median age, 53 years). The disease duration was from 4 days to 26 weeks (median duration, 4 weeks).
(ii) Sera from 100 consecutive Danish patients with NB.
All patients with NB had been hospitalized in 1994 (58 males and 42 females between 4 and 80 years of age; median age, 49 years). NB was defined according to previously published criteria (16, 21). All the patients had documented cerebrospinal fluid pleocytosis and B. burgdorferi-specific intrathecal antibody synthesis. The combined finding of active cerebrospinal fluid inflammation and B. burgdorferi-specific intrathecal antibody synthesis yields a predictive value of >95% for active NB (12). Of the 100 patients, 48 recalled a previous EM-like skin lesion. The disease duration, defined as the time from the onset of neurological symptoms until the time that diagnostic blood and CSF samples were taken, ranged from 1 to 26 weeks (median duration, 3 weeks).
(iii) Sera from 30 Swedish patients with ACA.
Sera were obtained from 30 Swedish patients who had acrodermatitis chronica atrophicans (ACA) and who were between 36 and 89 years of age (median age, 61 years). The diagnosis of ACA was based on clinical appearance, histopathologic picture, and elevated serum IgG titers to Borrelia (4) and in every case was made by one of the authors (E.Å.). The disease duration ranged from 1 to 5 years (median duration, 2 years).
(iv) Control sera.
Control sera were from (i) 100 healthy Danish blood donors; (ii) 30 patients with early syphilis and very high IgM and/or IgG antibody levels (optical density [OD], >1.5) by the Reiter treponeme Fla ELISA (29, 30), a positive Wassermann reaction, a positive rapid plasma reagin test result, and a reactivity of ≥3+ in the fluorescent treponemal antibody absorption test; (iii) 20 patients with mononucleosis; (iv) 20 patients with clinical indications of salmonellosis; (v) 20 patients with clinical indications of leptospirosis; (vi) 23 patients with a positive test for the presence of rheumatoid factor; and (vii) 25 patients with anti-double-stranded DNA antibodies. All control sera were obtained from routine laboratories at the Statens Serum Institut.
ELISA with B. burgdorferi Fla.
Specific anti-Fla IgG and IgM antibody levels were measured by an indirect ELISA (code K416; Dako, Glostrup, Denmark) and a μ-capture ELISA (code K006; Dako) with purified B. burgdorferi Fla. Both ELISAs are commercially available and were used according to the manufacturer’s instructions. The results were expressed as OD values. In both assays the diagnostic cutoff levels were adjusted to a specificity of 98% based on the examination of blood from 100 healthy donors.
Indirect IgM and IgG ELISAs with recombinant OspC, pepC10, and modified pepC10.
Microtiter plates (Maxisorb; Nunc, Roskilde, Denmark) for IgM detection and polystyrene microtiter plates (Immunoplates code 2-69620; Nunc) for IgG detection were coated overnight at 4°C with rOspC diluted in 0.05 M bicarbonate (pH 9.6), blocked with 3% (wt/vol) milk powder in phosphate-buffered saline (PBS) for 1 h, and reacted with sera diluted 1/200 in 1% (wt/vol) milk powder in PBS–0.1% Tween 20 (PBST) for 2 h. The secondary antibody was a peroxidase-conjugated rabbit anti-human IgM or IgG (codes P-215 and P-214, respectively; Dako) diluted 1/1,000 and 1/8,000 in 1% (wt/vol) milk powder in PBST, respectively. After 1 h of incubation, bound secondary antibody was quantitated by coloring with o-phenylenediamine and H2O2 in citrate buffer (Sigma, St. Louis, Mo.) for 30 min. The OD at 492 nm was determined in a plate reader (EAR 400 AC-SLT; Labinstruments, Salzburg, Austria). The plates were washed extensively with PBST–0.5 M NaCl between each incubation step. The 98% specific diagnostic cutoff levels in these assays were ODs of 0.230 for IgM detection and 0.480 for IgG detection.
ELISAs with the synthetic peptide pepC10 were performed as follows. Microtiter plates were coated with streptavidin (2.5 μg/ml; S. Avidinii; Zymed, San Francisco, Calif.) in citrate buffer (pH 5) overnight at 4°C and were then incubated with biotinylated pepC10 (0.1 μg/ml) in 1% (wt/vol) milk powder in PBS containing 0.37 M NaCl and 0.5% (vol/vol) Tween 20 overnight at 4°C. After washing with PBST–0.5 M NaCl, serum samples diluted 1/200 in 1% (wt/vol) milk powder in PBST–0.7 M NaCl were added and the mixture was incubated for 2 h at room temperature, followed by incubation with peroxidase-conjugated rabbit anti-human IgM or IgG diluted 1/1,000 and 1/8,000 in 1% (wt/vol) milk powder in PBST. Color reactions were performed as described above for the rOspC ELISA. The 98% specific diagnostic cutoff levels in these assays were ODs of 0.450 for IgM detection and 0.240 for IgG detection.
To reduce interassay variation, serial dilutions of two serum pools each containing eight serum samples with either high IgM or high IgG antibody titers against rOspC and pepC10 were included on every plate for construction of a standard reference curve. The OD values for all test samples were adjusted by use of the linear portion of this standard reference curve. The interassay variations of the pepC10 and rOspC ELISAs were determined by testing a positive control serum on 20 independent days. For the pepC10 ELISA the positive serum sample had a mean IgM OD value of 2.052 (standard deviation [SD], 0.386; coefficient of variation [CV], 18.8%) and a mean IgG OD value of 1.222 (SD, 0.192; CV, 15.7%). For the rOspC ELISA the positive serum sample had a mean IgM OD value of 1.829 (SD, 0.187; CV, 10.2%) and a mean IgG OD value of 0.993 (SD, 0.141; CV, 14.1%).
Statistics.
For comparison of the diagnostic sensitivities determined for the rOspC ELISA, the pepC10 ELISA, and the Fla ELISA and for comparison of the antibody levels obtained by the pepC10 ELISA and the rOspC ELISA, as well as those obtained by the pepC10 ELISA and the Fla ELISA, nonparametric methods were used, because these data were not normally distributed. McNemar’s test was used for the analysis of the diagnostic sensitivities obtained for the different assays. The abilities of the pepC10 ELISA and the rOspC ELISA to discriminate quantitatively between controls and seropositive patients were estimated. For each individual serum sample that was positive by both ELISAs, the distance from the achieved OD value to the cutoff level was calculated for both assays. These differences were compared by using Wilcoxon’s rank sum test for paired data. The diagnostic accuracies of the pepC10 ELISA and the rOspC ELISA were, furthermore, analyzed by empirical receiver operator characteristic (ROC) curves with MedCalc software (version 4.16a; Medcalc Software, San Francisco, Calif., Belgium).
RESULTS
Anti-pepC10 reactivities of sera from patients with LB.
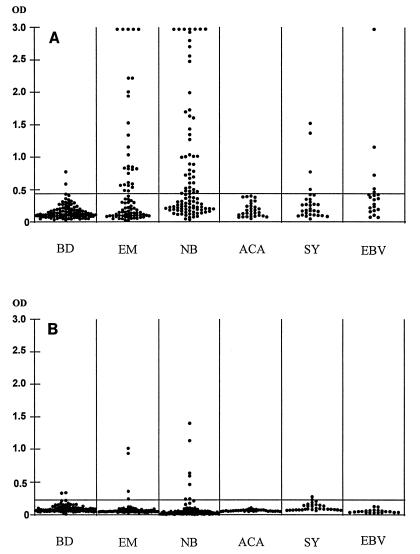
The sensitivity of the pepC10 ELISA for a specificity of 98% was examined with 210 serum samples from patients with LB. A positive IgM anti-pepC10 response was obtained for 36.3% (29 of 80), 45% (45 of 100), and 0% (0 of 30) of the patients with EM, NB, and ACA, respectively (Fig. 1A and Table 1). The IgG seropositivity rate was low (≤8%) for patients at all stages of LB (Fig. 1B and Table 1).
FIG. 1.
Anti-pepC10 IgM (A) and IgG (B) in sera from 100 blood donors (BD), 80 patients with EM, 100 patients with NB, 30 patients with ACA, 30 patients with syphilis (SY), and 20 patients with mononucleosis (Epstein-Barr virus [EBV] infection). The horizontal lines mark the diagnostic cutoff levels for 98% specificity.
TABLE 1.
IgM and IgG reactivities of sera from patients with LB to pepC10, rOspC, and Fla
| Patient group | No. (%) of serum samplesa reactive to the following:
|
|||||
|---|---|---|---|---|---|---|
| pepC10
|
rOspC
|
Fla
|
||||
| IgM | IgG | IgM | IgG | IgM | IgG | |
| EM (n = 80) | 29 (36.3) | 4 (5.0) | 35 (43.8) | 4 (5.0) | 30 (37.5) | 19 (23.8) |
| NB (n = 100) | 45 (45.0) | 8 (8.0) | 48 (48.0) | 6 (6.0) | 63 (63.0) | 52 (52.0) |
| ACA (n = 30) | 0 | 0 | 2 (6.7) | 1 (3.3) | 5 (16.7) | 30 (100) |
Sera with a response greater than the 98% percentile for 100 Danish blood donors.
Alanine replacement scanning of pepC10 has revealed a critical role for the PKKP sequence and its terminal carboxyl group for the binding of IgM antibodies from patients with LB (27). This motif is highly conserved because we have found only one B. garinii OspC variant with a Lys-to-Asn (K-to-N) substitution at position 206 (27). A peptide ELISA based on a peptide sequence with this substitution (see Materials and Methods) gave a positive response for only 35.5% (16 of 45) of the serum samples from patients with NB which tested positive by the pepC10 ELISA (data not shown). Only two of the serum samples which tested negative by the pepC10 ELISA displayed a positive reaction against the modified peptide, indicating that the inclusion of natural variants of the C-terminal epitope is not likely to significantly improve the diagnostic performance of the pepC10 ELISA.
Comparison of IgM anti-pepC10 and anti-rOspC ELISAs.
In order to compare the pepC10 ELISA with an ELISA based on a full-length recombinant OspC protein, the 210 serum samples from patients with LB previously tested by the pepC10 ELISA were tested by ELISA for IgM and IgG antibodies to rOspC. For IgM anti-OspC, 43.8% (35 of 80), 48% (48 of 100), and 6.7% (2 of 30) of the serum samples from patients with EM, NB, and ACA, respectively, showed a positive reaction in the rOspC ELISA (Table 1). As for the peptide ELISA, IgG anti-OspC antibodies were rarely detected in sera from patients with LB by the rOspC ELISA (Table 1).
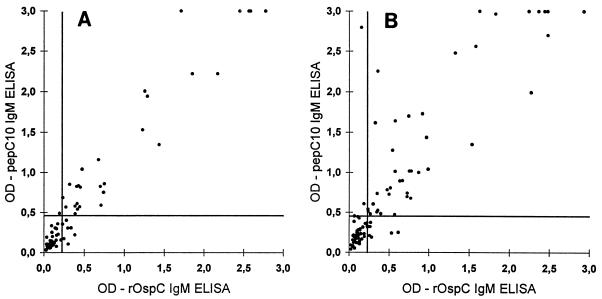
In Fig. 2A and B the antipeptide IgM response is plotted against the anti-rOspC response for individual serum samples from EM and NB patients. The pepC10 ELISA yielded higher OD signals than the rOspC ELISA (sum of the negative ranks is 1,296; P < 0.008; see legend to Fig. 2). The diagnostic sensitivity of the pepC10 ELISA, however, was slightly lower than the diagnostic sensitivity of the rOspC ELISA for EM patients (P < 0.04). This difference in diagnostic sensitivity is due to the fact that 7.5% (6 of 80) of the samples reacted only with rOspC. However, all of these sera gave rise to OD signals in the rOspC ELISA which were just above the cutoff level (Fig. 2A). For NB patients, the diagnostic sensitivities of the two assays were not different (P < 0.4).
FIG. 2.
Correlation of IgM anti-pepC10 and anti-rOspC measurement in sera from 80 patients with EM (A) and 100 patients with NB (B). The horizontal lines represent the cutoff of the IgM rOspC ELISA, and the vertical lines represents the cutoff of the IgM pepC10 ELISA. The pepC10 ELISA significantly improved the quantitative discrimination between control and seropositive samples, as estimated by comparing the distances of the achieved OD values from the cutoff level in each test by Wilcoxon’s rank sum test for paired data. In panel B, the sum of the negative ranks is 1,296 (P < 0.008).
The abilities of the pepC10 and the rOspC ELISAs to discriminate between LB patients and healthy blood donors was further analyzed by constructing empirical ROC curves. For LB patients with either EM or NB, the areas of the ROC curves are not different (Fig. 3A). However, for NB patients alone, the area of the ROC curve for the pepC10 ELISA was significantly larger than the area of the ROC curve for the rOspC ELISA (P = 0.037), indicating that the peptide ELISA gave the best discrimination (Fig. 3B). In the high-specificity part of the ROC curve, however, comparable sensitivities were found.
FIG. 3.
Empirical ROC curves of the performance of the anti-pepC10 and the IgM anti-rOspC ELISAs in discriminating between LB patients (patients with EM and NB) and controls (A) and in discriminating between patients with NB and controls (B). In panel A, there was no difference between the rOspC- and the pepC10-based immunoassays. In panel B, the pepC10 ELISA performed significantly better than the rOspC ELISA (P = 0.037).
Combined performance of pepC10 and Fla ELISAs.
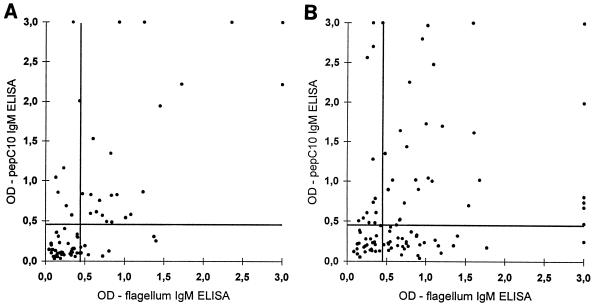
Because ELISAs based on Fla have been reported to have an improved diagnostic performance compared to the performance of assays based on sonic extracts (13, 14, 19), we decided to compare the performance of the pepC10 ELISA to that of the Fla ELISA. The IgM and IgG anti-Fla seropositivity rates for sera from patients with LB are presented in Table 1, and the IgM anti-pepC10 OD values are plotted against the IgM anti-Fla OD values for individual serum samples from patients with either EM or NB (Fig. 4A and B). If the peptide ELISA is used to supplement the Fla ELISA, the diagnostic sensitivity will increase from 37.5 to 46.3% for EM patients and from 63 to 75% for NB patients, with an expected increase in the false-positivity rate of from 2% to not more than 4%.
FIG. 4.
Correlation of IgM anti-pepC10 and anti-Fla measurements for 80 serum samples from patients with EM (A) and 100 patients with NB (B). The horizontal lines represents the cutoff of the IgM rOspC ELISA, and the vertical lines represents the cutoff of the IgM pepC10 ELISA.
To evaluate the diagnostic specificity of the pepC10 ELISA, we have examined 138 serum samples from control patients, including patients with syphilis and mononucleosis. As seen from Table 2, the overall specificity was in the same range as those for rOspC and the Fla ELISAs.
TABLE 2.
IgM reactivities of sera from patients with other diseases to pepC10, rOspC, and Fla
| Patient group | No. (%) of serum samples with IgM reactivities to the following:
|
||
|---|---|---|---|
| pepC10 | rOspC | Fla | |
| Syphilis (n = 30) | 4 (13.3) | 3 (10.0) | 2 (6.7) |
| Mononucleosis (n = 20) | 5 (20.0) | 8 (40.0) | 12 (60.0) |
| Salmonellosis (n = 20) | 0 | 1 (5.0) | 1 (5.0) |
| Leptospirosis (n = 20) | 1 (5.0) | 2 (10.0) | 0 |
| Rheumatoid factor positive (n = 23) | 0 | 2 (8.7) | 0 |
| Anti-double-stranded DNA positive (n = 25) | 0 | 0 | 0 |
DISCUSSION
Epitope mapping with recombinant proteins and synthetic peptides have identified a single major epitope within the C terminus of OspC which is recognized by IgM antibodies (27). In the present work we have evaluated the diagnostic performance of a peptide (pepC10) ELISA based on the C-terminal epitope of OspC and compared the diagnostic performance of this ELISA with the diagnostic performances of two established B. burgdorferi ELISAs based on either full-length rOspC or purified native B. burgdorferi Fla. The peptide-based ELISA performed at least as well as the ELISA based on rOspC and was well suited as a supplement to the Fla-based assay.
Synthetic peptides have been used as antigenic probes in diagnostic immunoassays, but almost only for the detection of antibodies to viruses, e.g., hepatitis C and E viruses and parvovirus type 19 (5, 8, 32). For the diagnosis of LB three attempts to use synthetic peptides in ELISAs have been reported (10, 33, 42). Gassmann et al. (10) and Schneider et al. (33) applied a variety of decapeptides covering the entire flagellin without identifying one or more peptides yielding a sufficient diagnostic performance. The success of the peptide ELISA approach depends mainly on the extent to which the synthetic peptides are able to mimic immunodominant epitopes within the native antigens. When used in solid-phase assays, either the peptides can be adsorbed directly onto the plastic of microtiter plates or they can be used when they are coupled to a carrier protein (36). In the peptide ELISA described here pepC10 was biotinylated at the N-terminal end and was coupled to streptavidin, although it may also be adsorbed directly onto the plastic surface.
The diagnostic sensitivity of the IgM pepC10 ELISA was slightly lower than that of the rOspC ELISA for EM patients (36.3 versus 43.8%), while there was no significant difference for NB patients (45 versus 48%). However, the OD values obtained by the pepC10 ELISA were generally higher than those obtained by the rOspC ELISA, thus leading to a significantly better quantitative discrimination between seropositive patients and controls (sum of the negative ranks, 1,296; P < 0.008). This is in accordance with the ROC curve analysis. The increase in OD signals obtained in the pepC10 ELISA compared to that obtained in the rOspC ELISA may be explained by a higher concentration of specific epitopes. Alternatively, the conformation of the peptide may be more favorable for antibody binding than the conformation of rOspC.
The diagnostic sensitivity of the pepC10 ELISA for IgM detection was comparable to that of the Fla ELISA for patients with EM (36.3 versus 37.5%) but lower for patients with NB (45 versus 63%). Since a significant number of serum samples were reactive in only one of the two assays, the addition of sera which tested positive only in the pepC10 ELISA to the Fla-positive sera increased the overall diagnostic sensitivity for IgM detection by 8.8 and 12% for patients with EM and NB, respectively. Thus, a diagnostic procedure that uses both the Fla ELISA and the pepC10 ELISA will have a better diagnostic performance than that of either ELISA used by itself.
In agreement with previous studies (1, 7, 37, 38, 41), we were unable to detect anti-OspC reactivity in sera from patients in the late stages of LB. In contrast, Fung et al. (9) and Magnarelli et al. (25) found a frequent IgG anti-OspC reactivity even in patients in the late stages of disease. The fact that pepC10 is widely recognized by IgM antibodies in sera from patients with EM or NB, as well as the lack of IgG recognition during all stages of LB, supports our recently proposed hypothesis that the immune response against this region of OspC is T-cell independent (27). The low frequency of IgG anti-OspC-positive sera is in accordance with four Western blotting studies performed with native whole-cell extracts (1, 7) or recombinant proteins (37, 38). More studies are needed to clarify the discrepant findings regarding the IgG anti-OspC response.
In the report by Yu et al. (42), two B-cell epitopes were identified within the variable region of OspC and were tested for the suitability of their use in ELISAs. However, with a combined conjugate only few serum samples displayed IgM and IgG antibody responses against the two OspC peptides. The importance of the C-terminal epitope of OspC was not recognized in that study, possibly because the synthetic peptides used by Yu et al. (42) were coupled to bovine serum albumin through a cysteine residue in the carboxyl terminus. Since we have previously shown that the carboxy terminal group is critical for the binding of human IgM anti-OspC antibodies, the lack of a free carboxyl group may explain why Yu et al. (42) did not identify the C-terminal epitope.
One of the purposes of a peptide ELISA is to increase specificity by eliminating potentially cross-reactive epitopes present in the full-length protein and in remaining contaminants in the purified protein. Accordingly, we have examined the level of cross-reactivity in sera from patients with diseases which may give rise to a false-positive signal in serological assays for LB. The reactivities of sera from patients with syphilis were unexpected because a Blast search (2) did not reveal homology between ospC and nucleotide sequences in the Treponema pallidum genome (data not shown) and because previous studies have identified few serum samples from patients with syphilis which reacted with full-length OspC (9, 26, 28). This phenomenon remains unexplained; however, from a clinical point of view this cross-reactivity does not constitute a major problem because it is possible to differentiate between patients with syphilis and LB on the basis of differences in clinical symptoms and the results of the nontreponemal tests (Wassermann reaction, rapid plasma reagin test, and Venereal Disease Research Laboratory test) (29, 30). The high number of false-positive reactions for patients with acute mononucleosis was not surprising. The etiological agent, Epstein-Barr virus, causes polyclonal B-cell stimulation, which may comprise B cells producing antibodies against many antigens including proteins from B. burgdorferi. The reactivities of sera from patients with other bacterial infections and with autoimmune diseases were negligible.
The results presented here demonstrate that pepC10 is a potentially useful antigen for use in tests for the early detection of LB: (i) it is recognized in patients with EM and NB, (ii) the epitope exhibits minor and, from a serological viewpoint, unimportant strain variations, (iii) the diagnostic sensitivity and specificity of the pepC10 ELISA are high, (iv) the peptide is easy to produce, (v) it improves the possibilities for standardization of the detection of anti-OspC antibodies, and (vi) the use of the pepC10 ELISA in combination with the Fla ELISA constitutes an improved diagnostic assay for the detection of early LB.
ACKNOWLEDGMENTS
We thank H. Hasselager for technical assistance and K. Krogfeldt and A. Wiik for providing the control sera.
This work was supported by the Research Center for Medical Biotechnology under the Danish Biotechnology Research Program.
REFERENCES
- 1.Aguero-Rosenfeld M E, Nowakowski J, McKenna D F, Carbonaro C A, Wormser G P. Serodiagnosis in early Lyme disease. J Clin Microbiol. 1993;31:3090–3095. doi: 10.1128/jcm.31.12.3090-3095.1993. . (Erratum, 32:860, 1994.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Amouriaux P, Assous M, Margarita D, Baranton G, Saint-Girons I. Polymerase chain reaction with the 30-kb circular plasmid of Borrelia burgdorferi B31 as a target for detection of the Lyme borreliosis agents in cerebrospinal fluid. Res Microbiol. 1993;144:211–219. doi: 10.1016/0923-2508(93)90046-5. [DOI] [PubMed] [Google Scholar]
- 4.Asbrink E. Acrodermatitis chronica atrophicans. Clin Dermatol. 1993;11:369–375. doi: 10.1016/0738-081x(93)90092-q. [DOI] [PubMed] [Google Scholar]
- 5.Bresters D, Reesink H W, Cuypers H T, Jansen P L, Mauser-Bunschoten E P, van-der-Poel C L, Lelie P N. Sensitivity of an anti-HCV core peptide ELISA. J Med Virol. 1992;37:187–191. doi: 10.1002/jmv.1890370307. [DOI] [PubMed] [Google Scholar]
- 6.Bruckbauer H R, Preac-Mursic V, Fuchs R, Wilske B. Cross-reactive proteins of Borrelia burgdorferi. Eur J Clin Microbiol Infect Dis. 1992;11:224–232. doi: 10.1007/BF02098084. [DOI] [PubMed] [Google Scholar]
- 7.Dressler F, Ackermann R, Steere A C. Antibody responses to the three genomic groups of Borrelia burgdorferi in European Lyme borreliosis. J Infect Dis. 1994;169:313–318. doi: 10.1093/infdis/169.2.313. [DOI] [PubMed] [Google Scholar]
- 8.Fridell E, Cohen B J, Wahren B. Evaluation of a synthetic-peptide enzyme-linked immunosorbent assay for immunoglobulin M to human parvovirus B19. J Clin Microbiol. 1991;29:1376–1381. doi: 10.1128/jcm.29.7.1376-1381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung B P, McHugh G L, Leong J M, Steere A C. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun. 1994;62:3213–3221. doi: 10.1128/iai.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gassmann G S, Jacobs E, Deutzmann R, Gobel U B. Analysis of the Borrelia burgdorferi GeHo fla gene and antigenic characterization of its gene product. J Bacteriol. 1991;173:1452–1459. doi: 10.1128/jb.173.4.1452-1459.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber M A, Shapiro E D, Bell G L, Sampieri A, Padula S J. Recombinant outer surface protein C ELISA for the diagnosis of early Lyme disease. J Infect Dis. 1995;171:724–727. doi: 10.1093/infdis/171.3.724. [DOI] [PubMed] [Google Scholar]
- 12.Hansen K. Lyme neuroborreliosis: improvements of the laboratory diagnosis and a survey of epidemiological and clinical features in Denmark 1985–1990. Acta Neurol Scand Suppl. 1994;151:1–44. [PubMed] [Google Scholar]
- 13.Hansen K, Asbrink E. Serodiagnosis of erythema migrans and acrodermatitis chronica atrophicans by the Borrelia burgdorferi flagellum enzyme-linked immunosorbent assay. J Clin Microbiol. 1989;27:545–551. doi: 10.1128/jcm.27.3.545-551.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen K, Hindersson P, Pedersen N S. Measurement of antibodies to the Borrelia burgdorferi flagellum improves serodiagnosis in Lyme disease. J Clin Microbiol. 1988;26:338–346. doi: 10.1128/jcm.26.2.338-346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen K, Lebech A M. Lyme neuroborreliosis: a new sensitive diagnostic assay for intrathecal synthesis of Borrelia burgdorferi-specific immunoglobulin G, A, and M. Ann Neurol. 1991;30:197–205. doi: 10.1002/ana.410300212. [DOI] [PubMed] [Google Scholar]
- 16.Hansen K, Lebech A M. The clinical and epidemiological profile of Lyme neuroborreliosis in Denmark 1985–1990. A prospective study of 187 patients with Borrelia burgdorferi specific intrathecal antibody production. Brain. 1992;115:399–423. doi: 10.1093/brain/115.2.399. [DOI] [PubMed] [Google Scholar]
- 17.Hansen K, Pii K, Lebech A M. Improved immunoglobulin M serodiagnosis in Lyme borreliosis by using a mu-capture enzyme-linked immunosorbent assay with biotinylated Borrelia burgdorferi flagella. J Clin Microbiol. 1991;29:166–173. doi: 10.1128/jcm.29.1.166-173.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jauris-Heipke S, Fuchs R, Motz M, Preac-Mursic V, Schwab E, Soutschek E, Will G, Wilske B. Genetic heterogenity of the genes coding for the outer surface protein C (OspC) and the flagellin of Borrelia burgdorferi. Med Microbiol Immunol Berlin. 1993;182:37–50. doi: 10.1007/BF00195949. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson M. Western immunoblot and flagellum enzyme-linked immunosorbent assay for serodiagnosis of Lyme borreliosis. J Clin Microbiol. 1990;28:2148–2150. doi: 10.1128/jcm.28.9.2148-2150.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlsson M, Hovind-Hougen K, Svenungsson B, Stiernstedt G. Cultivation and characterization of spirochetes from cerebrospinal fluid of patients with Lyme borreliosis. J Clin Microbiol. 1990;28:473–479. doi: 10.1128/jcm.28.3.473-479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristoferitsch W. Neurological manifestations of Lyme borreliosis: clinical definition and differential diagnosis. Scand J Infect Dis Suppl. 1991;77:64–73. [PubMed] [Google Scholar]
- 22.Lebech A M, Hansen K. Detection of Borrelia burgdorferi DNA in urine samples and cerebrospinal fluid samples from patients with early and late Lyme neuroborreliosis by polymerase chain reaction. J Clin Microbiol. 1992;30:1646–1653. doi: 10.1128/jcm.30.7.1646-1653.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luft B J, Pawagi S, Jiang W, Fiseene S, Gorevic P D, Dunn J. Analysis and expression of the Borrelia burgdorferi P/Gau fla gene: identification of heterogeneity with the B31 strain. FEMS Microbiol Lett. 1992;72:63–67. doi: 10.1016/0378-1097(92)90490-f. [DOI] [PubMed] [Google Scholar]
- 24.Magnarelli L A, Anderson J F, Johnson R C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987;156:183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- 25.Magnarelli L A, Fikrig E, Padula S J, Anderson J F, Flavell R A. Use of recombinant antigens of Borrelia burgdorferi in serologic tests for diagnosis of lyme borreliosis. J Clin Microbiol. 1996;34:237–240. doi: 10.1128/jcm.34.2.237-240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathiesen M J, Hansen K, Axelsen N H, Halkier S L, Theisen M. Analysis of the human antibody response to OspC of Borrelia burgdorferi sensu stricto, Borrelia garinii, and Borrelia afzelii. Microbiol Immunol. 1996;185:121. doi: 10.1007/s004300050021. [DOI] [PubMed] [Google Scholar]
- 27.Mathiesen M J, Holm A, Christiansen M, Hansen K, ¥stergaard S, Theisen M. The dominant epitope of Borrelia garinii outer surface protein C recognized by sera from patients with neuroborreliosis has a surface-exposed conserved structural motif. Infect Immun. 1998;66:4073–4079. doi: 10.1128/iai.66.9.4073-4079.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padula S J, Dias F, Sampieri A, Craven R B, Ryan R W. Use of recombinant OspC from Borrelia burgdorferi for serodiagnosis of early Lyme disease. J Clin Microbiol. 1994;32:1733–1738. doi: 10.1128/jcm.32.7.1733-1738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedersen N S, Petersen C S, Axelsen N H. Enzyme-linked immunosorbent assay for detection of immunoglobulin M antibody against the Reiter treponeme flagellum in syphilis. J Clin Microbiol. 1982;16:608–614. doi: 10.1128/jcm.16.4.608-614.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen N S, Petersen C S, Vejtorp M, Axelsen N H. Serodiagnosis of syphilis by an enzyme-linked immunosorbent assay for IgG antibodies against the Reiter treponeme flagellum. Scand J Immunol. 1982;15:341–348. doi: 10.1111/j.1365-3083.1982.tb00657.x. [DOI] [PubMed] [Google Scholar]
- 31.Picken R N. Polymerase chain reaction primers and probes derived from flagellin gene sequences for specific detection of the agents of Lyme disease and North American relapsing fever. J Clin Microbiol. 1992;30:99–114. doi: 10.1128/jcm.30.1.99-114.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qi Z, Cui D, Pan W, Yu C, Song Y, Cui H, Arima T. Synthesis and application of hepatitis E virus peptides to diagnosis. J Virol Methods. 1995;55:55–66. doi: 10.1016/0166-0934(95)00045-v. [DOI] [PubMed] [Google Scholar]
- 33.Schneider T, Lange R, Ronspeck W, Weigelt W, Kolmel H W. Prognostic B-cell epitopes on the flagellar protein of Borrelia burgdorferi. Infect Immun. 1992;60:316–319. doi: 10.1128/iai.60.1.316-319.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theisen M, Borre M, Mathiesen M J, Mikkelsen B, Lebech A M, Hansen K. Evolution of the Borrelia burgdorferi outer surface protein OspC. J Bacteriol. 1995;177:3036–3044. doi: 10.1128/jb.177.11.3036-3044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Theisen M, Frederiksen B, Lebech A M, Vuust J, Hansen K. Polymorphism in ospC gene of Borrelia burgdorferi and immunoreactivity of OspC protein: implications for taxonomy and for use of OspC protein as a diagnostic antigen. J Clin Microbiol. 1993;31:2570–2576. doi: 10.1128/jcm.31.10.2570-2576.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van-Regenmortel M H. Synthetic peptides versus natural antigens in immunoassays. Ann Biol Clin Paris. 1993;51:39–41. [PubMed] [Google Scholar]
- 37.Wilske B, Fingerle V, Herzer P, Hofmann A, Lehnert G, Peters H, Pfister H W, Preac-Mursic V, Soutschek E, Weber K. Recombinant immunoblot in the serodiagnosis of Lyme borreliosis. Comparison with indirect immunofluorescence and enzyme-linked immunosorbent assay. Med Microbiol Immunol Berlin. 1993;182:255–270. doi: 10.1007/BF00579624. [DOI] [PubMed] [Google Scholar]
- 38.Wilske B, Fingerle V, Preac-Mursic V, Jauris-Heipke S, Hofmann A, Loy H, Pfister H W, Rossler D, Soutschek E. Immunoblot using recombinant antigens derived from different genospecies of Borrelia burgdorferi sensu lato. Med Microbiol Immunol Berlin. 1994;183:43–59. doi: 10.1007/BF00193630. [DOI] [PubMed] [Google Scholar]
- 39.Wilske B, Jauris-Heipke S, Lobentanzer R, Pradel I, Preac-Mursic V, Rossler D, Soutschek E, Johnson R C. Phenotypic analysis of outer surface protein C (OspC) of Borrelia burgdorferi sensu lato by monoclonal antibodies: relationship to genospecies and OspA serotype. J Clin Microbiol. 1995;33:103–109. doi: 10.1128/jcm.33.1.103-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilske B, Preac-Mursic V, Schierz G, Busch K V. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentrbl Bakteriol Mikrobiol Hyg Reihe A. 1986;263:92–102. doi: 10.1016/s0176-6724(86)80108-0. [DOI] [PubMed] [Google Scholar]
- 42.Yu Z, Carter J M, Sigal L H, Stein S. Multi-well ELISA based on independent peptide antigens for antibody capture. Application to Lyme disease serodiagnosis. J Immunol Methods. 1996;198:25–33. doi: 10.1016/0022-1759(96)00140-8. [DOI] [PubMed] [Google Scholar]