Abstract
Background:
Hematopoietic stem cell transplantation (HCT) is a curative treatment option for patients with hematologic conditions but presents many complications that must be managed as a complex, chronic condition. Mobile health applications (mHealth app) may permit tracking of symptoms in HCT. In seeking strategies to manage the complexities of HCT, our team collaborated with Sicklesoft Inc. to develop an mHealth app specifically for HCT patients to allow for daily evaluation of patient health, Technology Recordings to better Understand Bone Marrow Transplantation (TRU-BMT). The primary value of this application is that of potentially enhancing the monitoring of symptoms and general health of patients undergoing HCT, with the ultimate goal of allowing earlier detection of adverse events, earlier intervention, and improving outcomes.
Methods:
To first evaluate patient interest in mHealth apps, we designed and administered an interest survey to patients at the 2017 BMT-InfoNet reunion. As a follow-up to the positive feedback received, we began testing the TRU-BMT app in a Phase 1 pilot study. Thirty patients were enrolled in this single arm study and were given the TRU-BMT mHealth app on a smartphone device in addition to a wearable activity tracker. Patients were followed for up to 180 days, all the while receiving daily app monitoring.
Results:
Adherence to TRU-BMT was approximately 30% daily and 44% weekly, and greater adherence was associated with increased meal completion, decreased heart rate, and shorter hospital stay. TRU-BMT assessments of symptom severity were significantly associated with duration of hospital stay and development of chronic GVHD.
Conclusion:
Our findings suggest that using TRU-BMT throughout HCT transplant is feasible for patients and established a proof-of-concept for a future randomized control trial of the TRU-BMT application in HCT.
Keywords: Stem cell transplantation, activity tracking, mobile health application, mHealth, symptom monitoring, self-management
Introduction:
Hematopoietic stem cell transplant (HCT) is an intensive treatment with the potential to cure hematologic malignancies and other highly morbid diseases, but it is also associated with significant risks of morbidity and mortality1. Complications of HCT include disease relapse, graft-versus-host disease (GVHD), and infection leading to hospital and intensive care admissions1-3. Chemotherapy and immunosuppressive medications contribute to the challenges of anorexia, loss of muscle mass, and fatigue4. Furthermore, symptoms such as pain, fatigue, and depression are frequent and further decrease a patient’s quality of life and impact long-term HCT outcomes5, 6.
Effective monitoring and early intervention on patients’ self-reported symptoms have been effective at improving outcomes in malignancies outside of HCT, especially in the setting of complex care7-9. One study published by Basch et al. in 2017 demonstrated improved survival in metastatic cancer patients randomized to electronic symptom reporting versus the standard of care, attributing part of this difference to symptoms being detected sooner. This allowed for early intervention of both supportive and oncologic care, with the potential of providing better palliative relief and symptom control7. While the importance of symptom severity in HCT has previously been noted, whether detailed monitoring and tracking of symptoms translates into improved clinical outcomes is not yet known10.
Mobile Health Technology (mHealth) is defined as medical and public health practices supported by mobile devices11. mHealth apps specifically have the potential to enhance patient-provider communication and assessment through active and passive evaluation and tracking12. Smartphone mobile applications are able to record self-reported patient outcomes, while wearable devices such as the Apple Watch and Fitbit are able to collect real-time physiological data such as heart rate and step counts13, 14. While mHealth studies in HCT have been limited, a systematic review has shown that mHealth utilization is associated with improvement in management and symptom control of chronic diseases such as diabetes15. Furthermore, a systematic review of mHealth in patients with asthma has shown improved symptom control following utilization of technology16. Other studies, focused on the design and usability of these interventions for cancer patients, have found that mHealth interventions are associated with utility, convenience, improved access to personalized information, greater awareness of health, and an ability to interconnect with other users and health professionals17-21. Furthermore, telemedicine and mHealth are becoming key approaches to care for fragile patients (e.g. older adults and cancer patients) in order to minimize the risk associated with prolonged exposure to the hospital environment, a major consideration in the context of the COVID-19 pandemic22.
While there are numerous studies of mHealth interventions in the medical literature, none address mHealth use in adult patients undergoing HCT. There is limited research on the feasibility and validity of standardized electronic patient-reported outcomes for HCT patients6, 23, 24. As a population at risk of multiple treatment-related complications HCT patients may benefit from mHealth intervention and increased patient monitoring1, 2. Our approach of integrating mHealth into the care of HCT patients is rooted in the idea that, if successful, its implementation would lead to enhanced symptom and health monitoring, allowing earlier detection of adverse events, earlier intervention, and ultimately improving outcomes for HCT patients. Here we report results of an interest survey in HCT patients, which was used to inform a Phase 1 pilot of the Technology Recordings to better Understand Bone Marrow Transplantation (TRU-BMT) app.
Methods:
Pre-trial Survey
To further refine the design of TRU-BMT before evaluating the app and a wearable device in a Phase 1 clinical research study, we designed an interest survey with the Duke Behavioral Health and Survey Research Core. This survey prompted HCT survivors and caregivers to interact with the app and answer questions regarding the ease of use, how often (days per week) they would potentially use the app and an accompanying wearable device, and how helpful they perceived the app would be while undergoing transplant. The survey and study were approved by the Duke Health Institutional Review Board. No informed consent was required for this initial step, as this was conducted with entirely deidentified data.
Phase 1 Pilot
This study was aimed at assessing utilization and factors contributing to utilization and feasibility by enrolled patients in an iterative Phase 1 trial. Part I of the study spanned 2017-2018 and enrolled 20 subjects; based on feedback, the app was re-designed, and Part 2 began in 2018 and enrolled an additional 10 subjects. Patients were enrolled at or after HCT and followed through Day 180. Patients were provided with the wearable device and iPads pre-installed with the TRU-BMT app and instructed to use them daily over the study period. At the conclusion of the study, patients were asked to complete a feasibility and acceptability self-report survey. Feedback from patients and study team members was used to iteratively improve the TRU-BMT app between Part 1 and Part 2 studies. This phase 1 study was approved by the Duke Health Institutional Review Board and patients provided informed consent prior to their participation.
Participants
Survey participants were recruited at the 2017 BMT InfoNet Reunion and consisted of adult HCT survivors or primary caregivers of HCT patients. Participants spent approximately five minutes interacting with the TRU-BMT mHealth app on iPad or iPhone devices and were then prompted to complete a survey estimating the usefulness and feasibility of an app for patients undergoing HCT. For the Phase I study, adult patients (age 18-80 years) undergoing allogeneic HCT for malignant conditions were enrolled. Exclusion criteria included admission to the intensive care unit (ICU) at the time of enrollment and inability to fluently operate the mobile device. Of note, patients who undergo transplant with the Duke Adult Bone Marrow Transplant (ABMT) Center have the option of receiving care as an inpatient (typically when receiving myeloablative conditioning) or an outpatient (typically when receiving nonmyeloablative or reduced intensity conditioning) with daily visits to the ABMT day hospital. Patients who receive their care in the inpatient setting may stay in the hospital until engraftment, at which point they are discharged to receive outpatient care in the day hospital unless medical complications require that they stay in the hospital. Patients who receive their care in the outpatient setting live with an approved caregiver within close proximity to the hospital and undergo daily or several times a week visits for peri-transplant care, though they may still be admitted to our inpatient HCT unit in the setting of infection, GVHD, or other medical complications.
Mobile application, Mobile devices, and activity tracker
In collaboration with Sicklesoft Inc., members of the Duke University Adult Blood and Marrow Transplant Program (including nurses, advanced practice providers, and physicians) developed the mHealth app, Technology Recordings to better Understand Bone Marrow Transplantation (TRU-BMT) specifically for adult Duke University Medical Center HCT patients. TRU-BMT was designed to allow patients to enter their general health, food intake, stool count, exercise, sleep habits, stress, and symptoms and to receive an automatic report with information detailing trends over time. This app was programmed for the iOS operating system for Apple iPhone and iPad devices (Figure 1A); work is ongoing for an Android version. The study team provided an Apple iPad Mini ® (iPad) to report symptoms and health status via the study mobile app and an activity tracker (Microsoft Band or Apple Watch) to track patient movement and heart rate for each participant for the 180-day study period.
Figure 1.
Screen shots of TRU-BMT demonstrating the general health feature, weekly score card, and stool count tool.
Outcomes
The primary outcome of the Phase 1 study was adherence as measured by the percentage of days on study in which the patient utilized the app. App utilization was defined as the percentage of days on study in which the most commonly used app metric logged a response. Secondary outcomes were general health (GH), symptom severity (SS), meal completion, heart rate, steps, hospital stay, overall survival (OS), and relapse-free survival (RFS).
As this was the first study of mobile health (mHealth) in adult hematopoietic stem cell transplant patients, we did not design the study with a pre-specified threshold of adherence but instead assigned a status of adherent vs non-adherent by computing the mean of all individual adherences across Part 1 of the study. Subsequently, all patients were classified into two adherence groups: 1) adherent, the patient group that utilized the app more than the cohort mean and 2) non-adherent, the patient group that utilized the app less than the cohort mean. Primary and secondary outcomes were compared between adherent and non-adherent groups.
General Health (Figure 1B) was assessed on TRU-BMT using an electronic visual analog scale (VAS). GH assesses self-reported health status prompted by the question “In general, would you say your health today is?” TRU-BMT provides the user with a horizontal scale whose left and right endpoints are designated as “0” as the minimum value, and “10” as the maximum value. The user can move the slider to the position along the that best identifies his or her current status. As in the physical paper version of VAS, the TRU-BMT GH scale has no unit markings except for “0” and “10”. Subsequent to filling out all of the information described, a unified GH score out of 10 is automatically recorded and visible to the user.
Sleep quality and Stress are assessed on TRU-BMT using an electronic VAS. Sleep quality is prompted by the question “How would you rate the quality of sleep that you got last night?” while Stress is prompted by “How would you rate your stress today?”
Symptom Severity (Figure 1C) was assessed on TRU-BMT using an electronic VAS. SS assesses the self-reported symptom type (bleeding, blood in stool, blood in urine, blood in vomit, cold symptoms, constipation, diarrhea, fatigue, fever, headache, nausea, neuropathy, pain, shortness of breath, trouble urinating, vomiting), the intensity of the symptom (0-10), symptom improvement (new, resolved, better, same, worse), and whether the patient performed an intervention to address the symptom (none, deep breathing/used distraction/used relaxation, took medication). TRU-BMT provides the user with a horizontal scale similar to that described above. Meal completion (Figure 1D) was assessed on TRU-BMT as a percentage. The app provides self-reporting of percentage eaten of breakfast, lunch, and dinner. A patient eating all of his or her meal would have a score of 100% for that meal; on the contrary, no intake for that meal would equate to 0%.
Heart rate and Steps were assessed using the wearable device provided. The daily averages were calculated based on time stamps.
A feasibility and acceptability self-reported survey (Supplemental Figure) was conducted at the end of the study. The exit interview form included three types of assessments: technical feasibility, ease of use, and satisfaction/acceptability. Scores ranged from 0 to 4.
Statistical Analysis
Comparisons between adherent and non-adherent groups were done using Wilcoxon Rank Sum test for continuous variables, and Fisher’s exact test for categorical variables. The significance level was set at 0.05. Kaplan-Meier curves and Log-rank test were used for survival analysis. Logistic regression, Poisson regression, and Cox proportional hazard model were used to assess the transplant outcomes. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC)
Results
Interest survey:
A total of 32 surveys were collected at the Bone Marrow Transplant InfoNet Symposium from HCT survivors and caregivers. The median age was 62 (IQR 26-76) and 55% of participants were male. Of those surveyed, 73 percent had graduated from college or received some postgraduate education, and 27% had a high school education or some college coursework in addition to a high school diploma. Smartphone usage was prominent among this group, with 79% of participants reporting that they used their smartphone multiple times per day.
The majority of respondents (76%) thought TRU-BMT was easy to use and 70% felt confident in its use after only a few minutes of testing. Daily symptom input was also perceived as a useful tool by 83% of survey respondents, and the majority of patients perceived that each feature would be very useful (Supplemental Table 2).
In examining how often TRU-BMT or a similar app would be used, 46% of participants stated they would use the app daily, and 89% would use the app at least three days per week. Similarly, 48% of participants reported they would use the wearable device to track step count and activity daily, with 89% of respondents reporting they would use the wearable device at least three times per week. Responses did not vary by level of education or age (Supplemental Table 1).
The research assistant who administered the survey collected qualitative feedback indicating the concern that daily use of an app such as TRU-BMT may depend on the medical status of the patient.
Phase 1 Demographics:
A total of thirty patients were included in the Phase 1 feasibility trial of TRU-BMT with demographics shown in Table 2. Median age for the cohort was 58 years (IQR 45-62) and 9 patients were female (30%). A level of education of four-year university or higher was attained by 15 patients (50%). The majority of patients received myeloablative conditioning (n=21, 70%) and most grafts came from peripheral blood (n=23, 74%). The majority of patients had KPS of 90-100 (n=22, 76%). Patients were enrolled in the study at a median of 0.5 days after transplant (Table 2).
Table 2.
Demographics for patients utilizing Technology Recordings to better Understand BMT (TRU-BMT)
| Empty Cell | Total (n = 30) |
Adherent (n = 11) |
Nonadherent(n = 19) | P value |
|---|---|---|---|---|
| Age, median (IQR) | 58 (45-62) | 61 (19-68) | 54 (19-66) | .33 |
| Gender | .69 | |||
| Female | 9 (30%) | 4 (36%) | 5 (26%) | |
| Male | 21 (70%) | 7 (64%) | 14 (74%) | |
| Race | .28 | |||
| Black | 3 (10%) | 0 (0%) | 3 (16%) | |
| White | 27 (90%) | 11 (100%) | 16 (84%) | |
| Hispanic | >.99 | |||
| No | 29 (97%) | 11 (100%) | 18 (95%) | |
| Unknown | 1 (3%) | 0 (0%) | 1 (5%) | |
| Education Level | .99 | |||
| 4-year college or higher | 15 (50%) | 6 (55%) | 9 (47%) | |
| Less than 4-year college | 15 (50%) | 5 (45%) | 10 (53%) | |
| Transplant Diagnosis | .20 | |||
| ALL/AML | 6 (20%) | 4 (36%) | 2 (11%) | |
| Lymphoma | 8 (27%) | 4 (36%) | 4 (21%) | |
| MDS/MPN | 11 (37%) | 2 (18%) | 9 (47%) | |
| Other | 5 (16%) | 1 (9%) | 4 (21%) | |
| Conditioning Regimen | .51 | |||
| Myeloablative | 21 (70%) | 9 (82%) | 12 (63%) | |
| Non-myeloablative | 6 (20%) | 2 (18%) | 4 (21%) | |
| Reduced Intensity | 3 (10%) | 0 (0%) | 3 (16%) | |
| Graft | .99 | |||
| BM | 4 (13%) | 1 (9%) | 3 (16%) | |
| Cord | 4 (13%) | 1 (9%) | 2 (11%) | |
| PBPC | 23 (74%) | 9 (82%) | 14 (74%) | |
| KPS | .14 | |||
| Unknown | 1 (3%) | 0 (0%) | 1 (5%) | |
| 80 | 7 (23%) | 2 (18%) | 5 (26%) | |
| 90 | 17 (57%) | 5 (46%) | 12 (63%) | |
| 100 | 5 (17%) | 4 (36%) | 1 (5%) | |
| HCT-CI (mean, range) | .42 | |||
| ≤ 3 | 21 (70%) | 9 (82%) | 12 (63%) | |
| >3 | 9 (30%) | 2 (18%) | 7 (37%) | |
| DRI | .89 | |||
| High | 2 (7%) | 1 (9%) | 1 (5%) | |
| Intermediate | 23 (77%) | 9 (82%) | 14 (74%) | |
| Low | 3 (10%) | 1 (9%) | 2 (11%) | |
| N/A | 2 (7%) | 0 (0%) | 2 (11%) | |
| Days from transplantation to enrollment, median (IQR) | 0.5 (0-5) | 0 (0-7) | 1 (0-4) | .40 |
ALL indicates acute lymphocytic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; MPN, myeloproliferative neoplasms; BM, bone marrow; PBPC, peripheral blood progenitor cell; KPS, Karnofsky performance score; DRI, Disease Risk Index.
Feasibility:
The threshold for daily adherence, as determined above, was set at 30%. As described in Table 2, eleven patients (37%) were categorized as adherent (daily GH response rate ≥ 30%), and 19 patients (63%) were categorized as non-adherent (daily GH response rate <30%). There were no statistically significant differences in age, gender, race, education level, transplant diagnosis, conditioning regimen, graft type, Karnofsky Performance Score (KPS), Hematopoietic Stem Cell Transplant Comorbidity Index (HCT-CI), Disease Risk Index (DRI), and days from transplant to enrollment between adherent and non-adherent groups (Table 2). While patients in the adherent group tended to be older, this difference was not statistically significant (median: 61 vs 54 years, p=0.33).
TRU-BMT usage
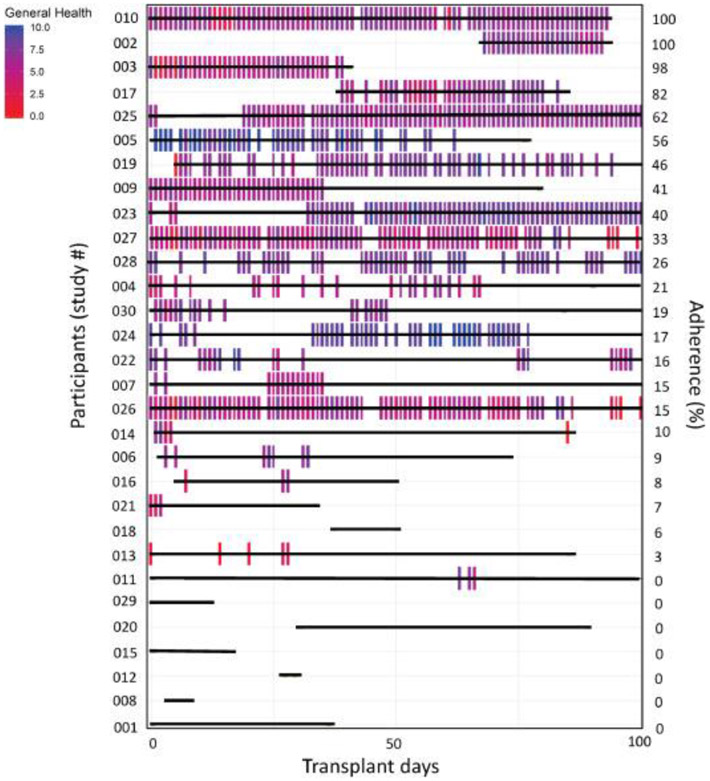
Patients were longitudinally tracked for TRU-BMT app utilization and daily GH scores (Figure 2). The level of utilization of each feature of TRU-BMT is shown in Table 3. The most utilized daily TRU-BMT app feature was GH at 30.4% of days, followed by appetite at 26.7%, stool consistency at 25.7%, and symptom severity at 23.1%. Daily activity tracker utilization was higher than app usage at 58%. The weekly utilization of TRU-BMT app features was GH at 43.6%, appetite at 40.2%, stool consistency at 37.1%, and symptom severity at 35.6%. Weekly activity tracker utilization was higher than app usage at 83%.
Figure 2.
Patient daily utilization (bars) and adherence to TRU-BMT up to Day 100 by participant.
Table 3.
Daily utilization of different app functions in TRU-BMT
| Empty Cell | Mean % Days Reporting (n = 30) |
Mean % Weeks Reporting |
|---|---|---|
| Activity tracker usage | 58.0 | 83.1 |
| General health (sleep quality, stress, sleep hours) | 30.4 | 43.6 |
| Meals | 26.7 | 40.2 |
| Stool consistency | 25.7 | 37.1 |
| Symptom severity | 23.1 | 35.6 |
Cohort transplant outcomes
Median OS for the combined cohort was 339 days (IQR 229-725 days), median RFS was 329 days (IQR 184-725 days), and median hospital stay was 12 days (IQR 2-23 days). 10 patients (33%) experienced acute GVHD of grade 2 or higher, while 16 patients (53%) experienced chronic GVHD of moderate grade or higher. The median TRU-BMT GH score was 6.4, median SS was 5.0, and median meal completion score was 88%. The median activity tracker-measured HR was 86 bpm, with an average of 2750 daily steps.
Associations between Adherence and Outcomes (Table 4)
Table 4.
Outcomes for Adherent Versus Nonadherent Patients in TRU-BMT
| Empty Cell | Total (n = 30) | Adherent (n = 11) |
Nonadherent (n = 19) |
P value |
|---|---|---|---|---|
| Overall survival (days), median (IQR) | 339 (229-725) | 327 (238-704) | 365 (185-736) | .22 |
| Relapse-free survival, median (IQR) | 329 (184-725) | 322 (202-704) | 343 (166-736) | .26 |
| Hospital days, median (IQR) | 11.5 (2-23) | 2 (0-18) | 16 (7-27) | .03 |
| aGVHD (no. of patients) | .70 | |||
| <2 | 20 (67%) | 8 (73%) | 12 (63%) | |
| ≥2 | 10 (33%) | 3 (27%) | 7 (37%) | |
| cGVHD (no. of patients) | .47 | |||
| < moderate | 14 (47%) | 4 (36%) | 10 (53%) | |
| ≥ moderate | 16 (53%) | 7 (64%) | 9 (47%) | |
| GH average score, median (IQR) | 6.4 (5.36-7.11) | 6.77 (6.03-7.98) | 5.52 (5.25-6.67) | .09 |
| Stress, median (IQR) | 4.16 (2.49-5.67) | 4.1 (2.23-6.05) | 4.18 (2.75-5.61) | .86 |
| Sleep quality, median (IQR) | 5.48 (5.12-6.66) | 5.46 (4.58-7.53) | 5.5 (5.3-5.93) | .78 |
| Sleep hours, median (IQR) | 7.47 (6.33-7.81) | 7.47 (6.52-7.8) | 7.47 (5.75-8) | .82 |
| SS avg. score, median (IQR) | 5 (4.4 - 5.73) | 5.23 (4.1 - 6.08) | 5 (4.43 - 5.5) | .69 |
| Meals (% completion), median (IQR) | 88 (80-96) | 95 (88-98) | 81 (64-88) | .004 |
| Heart rate, median (IQR) | 85.7 (77.8-89.1) | 79.65 (73.75-85.7) | 89.1 (82.1-100.3) | .004 |
| Steps (daily), median (IQR) | 2750 (1989-3759) | 2370 (1566-3119) | 3149.5 (2194-4264.5) | .22 |
aGVHD (Acute Graft-versus-Host Disease), cGVHD (Chronic Graft-versus-Host Disease), GH (General Health), SS (Symptom Severity)
Adherence was associated with lower heart rate (median: 80 vs 89 bpm, p=0.004) greater meal completion (95% vs 81%, p=0.004), and shorter hospital stay (median: 2 vs 16 days, p=0.03). There was also a trend toward higher GH (median: 6.8 vs 5.5 score, p=0.09), though not statistically significant. We did not detect a statistical difference for adherent and non-adherent patients in OS and RFS (median: 327 vs 365 and 322 vs 343, p=0.22 and p=0.26), acute GVHD (3 (27.3%) vs 7 (37%) patients, p=0.7020) or chronic GVHD (7(64%) vs 9 (47%) patients, p=0.47).
TRU-BMT measurements are associated with transplant outcomes
As shown in Table 5, adherence to TRU-BMT is associated with a shorter hospital stay (IRR; 2.1; 95% CI: 1.61-2.75; p=<0.001). Lower SS was significantly associated with decreased odds of moderate or higher cGVHD (OR: 0.24; 95% CI: 0.06-0.87; p=0.003). Lower GH trended toward decreased odds of grade 2-4 aGVHD (OR: 0.43; 95% CI: 0.13-1.43; p=0.10) and moderate or higher cGVHD (OR: 0.43; 95% CI: 0.14-1.39; p=0.10).
Table 5.
TRU-BMT Multivariable Assessments of Three Covariates (GH, SS, and Adherence) With Transplant Outcomes
| Empty Cell | Hospital Stay | Overall Survival | Relapse-free survival |
aGVHD | cGVHD |
|---|---|---|---|---|---|
| GH | 1.06 (0.96-1.17)* | 0.864 (0.353-2.114) | 0.902 (0.34-2.393) | 0.43 (0.13-1.43) | 0.43 (0.14-1.39) |
| SS | 0.9 (0.82-0.99)* | 0.946 (0.489-1.831) | 0.927 (0.496-1.733) | 0.98 (0.42-2.26) | 0.24 (0.06-0.87)* |
| Nonadherence | 2.1 (1.61-2.75)* | 0.316 (0.052-1.898) | 0.314 (0.053-1.871) | 0.54 (0.06-4.95) | 0.12 (0.01-2.07) |
Statistical analysis: Poisson regression model with incident rate ratio (95% CI) for hospital stay; Cox proportional hazard model with hazard ratio (95% CI) for overall survival and relapse-free survival; logistic regression model with odds ratio (95% CI) for aGVHD and cGVHD.
P value <.05.
Patient self-reported satisfaction/feasibility
Self-reported satisfaction/feasibility survey results are summarized in Table 5. A score of “0” indicates the lowest rating, and a score of “4” indicates the highest rating in the respective section. Mean technical feasibility, was rated at 3.0 (out of 4). Patients rated ease of use at an average of 3.1 (out of 4). Patients rated satisfaction/acceptability at an average of 2.2 (out of 4).
Discussion
Remote assessments using mHealth have proven effective in multiple settings; benefits include prevention of unscheduled medical services, encouraging patient behavioral changes and increased self-management, increased data exchange across providers, and improved patient-provider communication and patient trust25-27. Considering the growing importance of telemedicine and the use of technology to perform remote patient monitoring due to the COVID-19 pandemic, mHealth may facilitate data exchange and patient-provider communication and improve outcomes28, 29.
Herein, we present results from use of the TRU-BMT mHealth app and a wearable device at our center. To our knowledge, this is the first prospective study of an mHealth app and wearable device for adult patients undergoing HCT. Feedback from survey participants prior to our Phase 1 study suggested there is interest among the HCT patients and the caregiver population for an mHealth application designed to improve patient wellness. Our survey found that patients believe such an app will be useful and half report willingness to use it daily throughout their transplant (Supplemental Table 1). This led to our study in which the combination of the TRU-BMT app with the wearable device allowed for detailed, continuous, and quantitative assessment of both subjective and objective endpoints including stress, sleep, symptom severity, meal completion, and heart rate. As detailed below, we found that adherence to TRU-BMT was similar to other published reports of mHealth, and several clinical endpoints measured by TRU-BMT were associated with HCT outcomes that suggest prognostic relevance for TRU-BMT.
Adherence to mHealth applications is defined by variable time periods of measurement in the medical literature, including weekly, bimonthly, and monthly30. We found that adherence in our study was 30% for daily use of the TRU-BMT application, 43% for weekly use, and 58% for the wearable device. These results are similar to the 27-45% rate of adherence published in other studies of mHealth applications31, 32. Furthermore, we report similar feasibility and acceptability values to that of a smaller study in pediatric HCT patients despite a much older age group33. Additionally, adherence did not vary by age, suggesting TRU-BMT can be used in the increasing number of older patients undergoing HCT34.
TRU-BMT adherence may be impacted by health status, particularly in the HCT population given the significant hospitalization rate and the risk of multi-system complications such as GVHD. Patients who were adherent to TRU-BMT tended to have significantly shorter hospital stays, and adherent patients tended to have statistically significant improved meal completion and lower heart rate. These metrics are particularly important for patients undergoing HCT. Meal completion is a feasible surrogate marker for caloric intake, and malnutrition was notably one of the most common symptoms following transplant associated with gastrointestinal toxicity35-38. Data from the activity tracker was included in the study to inform clinical care by providing valuable physiologic data that will further inform providers regarding the patient’s overall health. The physiologic data is meant to be complementary to the self-reporting questionnaires within the TRU-BMT app. Heart rate data may be an early indicator of HCT complications. Elevated heart rate is related to subclinical loss of cardiac reserve39 and poor physical fitness27. In normotensive patients, elevated HR measured by continuous HR tracking is predictive of cardiovascular and noncardiovascular death26, and elevated heart rate has been identified as a predictor of in-hospital mortality risk for HCT patients40, 41. The relationship between adherence and the markers of patient health, such as hospitalization days (p=0.03), chronic graft-vs-host disease (p=0.09), coupled with the direction of effect for meal completion (p=0.004) and heart rate (p=0.004), indicate that TRU-BMT utilization may be associated with the health of participants.
Intrapatient variability in TRU-BMT adherence further supports the potential correlation of patient health and adherence. As can be noticed from Figure 2, there is an extent of intrapatient variability in TRU-BMT adherence. Specifically, two patients in our study that classify as adherent exemplify the trend described: Pt. 23 and Pt. 25. Both patients were adherent early in the days following their HCT; however, both patients experienced early complications, and the period in which the patients were experiencing these complications paralleled the time in which they were non-adherent to TRU-BMT. The patients resumed entries in the app around the time of discharge and symptom resolution. Additionally, it is worth noting that there was a subset of participants (n=6) that utilized the application over 60% daily, and over 80% weekly. This group of “super users” had a notably shorter median hospital stay (2 vs 12 days), a higher overall survival (418 vs 339 days), and a lower frequency of grade 2 or higher acute GVHD (17% vs 33%) than the general cohort. This provides support for participant health as a factor impacting adherence to TRU-BMT. It is important to consider that the least ill patients may have been most adherent due to their health status and ability to interact with the TRU-BMT app. While some could argue the opposite, that high adherence may contribute to better health in this subset, there is insufficient evidence in this preliminary study to determine causation and further investigations are needed.
TRU-BMT measurements may also correlate with HCT outcomes, suggesting prognostic utility. While HCT clinicians currently use the HCT-CI to predict risk of TRM related to HCT42, studies on HCT-CI have reported the inability to predict non-relapse mortality (NRM) and OS43, 44; furthermore, HCT-CI is a pre-HCT tool and this assessment cannot be updated continuously over the course of HCT. While studies have suggested biomarkers such as ST245 and REG3A46 are associated with development of GVHD and decreased survival, these are not yet widely used in the clinical setting, with barriers including access and cost. In contrast, subjective measurements of symptoms can be performed continuously and in HCT patients and have been previously associated with HCT outcomes10. We found that TRU-BMT’s assessment of symptom severity as well as non-adherence to TRU-BMT were associated with duration of hospital stay. Similar to prior studies suggesting a link between quality of life and symptom distress to GVHD10, we found that higher GH was associated with numerically lower risk for acute and chronic GVHD, though with our small sample size this was not statistically significant (0.43 and 0.43, p=0.102 and p=0.104, respectively), and that a lower SS score was associated with lower risk for chronic GVHD (0.24, p=0.003). Notably, this single-arm feasibility study does not clearly suggest a causative relationship between TRU-BMT metrics and HCT outcomes, however these correlations indicate relationships that should be explored further in larger studies.
In addition to being clinically relevant, TRU-BMT was also acceptable to patients. Ease of use was rated as 3.1 out of 4 and 79% of patients reported no significant problems with the combination of TRU-BMT and the wearable device. While the average score for satisfaction/acceptability was 2.2, of the n=7 patients with low ratings for utilization of the devices or accessibility of the app, n=5 (71%) had complaints that were related to technical issues that can be fixed for later iterations of the application such as difficulty entering data for past dates (n=3) and technical difficulties with TRU-BMT (n=2).
Our study has limitations. While the median timing of enrollment was shortly after HCT, there were five patients who enrolled later (median day 41), potentially introducing bias as these patients may have been already past the most difficult part of HCT. Of note, patients also utilized two types of wearable devices in the study: Microsoft Band for Part 1 (n=20) and Apple Watch for Part 2 (n=10). Adherence, while similar to the general literature, was lower than expected from our pre-study survey. We found that adherence to the activity tracker was much higher, suggesting that to the degree possible incorporating automated assessments based on objective data may allow for more consistent measurements in the future, and that passive input, rather than active symptom entry, may be more feasible for patients. This finding is helpful in understanding that moving forward, as we implement mHealth for HCT patients, it will be valuable to incorporate automated assessments to complement self-reported applications.
The level of adherence, although comparable to similar studies, could be improved moving forward. Exit interviews indicated that a significant portion of participants (20%) noted that they used TRU-BMT rarely or less, indicating need to improve the number of reminders or creating a more effective type of reminder notification. As a team, we will strive to integrate further updates into program implementation that will be aimed at optimizing TRU-BMT adherence. Firstly, we will plan to modify the application by integrating additional push reminders to the mobile device prompting the user to utilize the application (one in the morning and one in the evening). This modification is meant to address the participant feedback addressing the need for “more numerous and effective reminders.” Secondly, we will plan to integrate in-person reminders to utilize the application for patients that are hospitalized. This is especially important to address the population whose adherence is most strongly affected: patients with longer hospitalizations. Lastly, we note that our single-arm study with small sample size of 30 was likely a limiting factor in identifying outcomes that correlate with TRU-BMT metrics as well as adherence; to this end we plan to enroll a Phase 2 trial of TRU-BMT that will evaluate HCT outcomes in patients randomized between standard of care and TRU-BMT.
In conclusion, we found that the use of TRU-BMT in adult patients undergoing HCT was feasible with reasonable adherence similar to reports in the literature. TRU-BMT assessments provided clinically useful data with potential prognostic significance, and TRU-BMT was acceptable to patients. Our findings suggest that using an mHealth app and activity tracker throughout the complex management of HCT is feasible, provides utility to the patient and healthcare team, and suggests proof-of-concept for a future randomized control trial of the TRU-BMT application in HCT. Our study has provided us with preliminary evidence that integrating TRU-BMT as an adult HCT mHealth intervention may help clinicians recognize transplant outcomes early on, such as chronic GVHD. Future studies comparing TRU-BMT to standard-of-care may be able to further elucidate this relationship. As we plan our phase 2 study, the constructive data gathered in this Phase 1 study will guide us towards optimizing the implementation of this program so that we may assess its clinical value in early intervention driven by symptom and health monitoring.
Supplementary Material
Table 1.
HCT Patients and Caregivers Report mHealth App User Experience
| Survey prompt | Response | ||||
|---|---|---|---|---|---|
| Strongly Agree |
Somewhat Agree |
Neither Agree nor Disagree |
Somewhat Disagree |
Strongly Disagree |
|
| “TRU-BMT was easy to use” | 16 (49%) | 10 (30%) | 0 (0%) | 4 (12%) | 3 (9%) |
| “I think most people could learn to use TRU-BMT quickly” | 15 (44%) | 13 (38%) | 1 (3%) | 1 (3%) | 4 (12%) |
| “I felt confident using TRU-BMT” | 13 (40%) | 10 (30%) | 5 (15%) | 1 (3%) | 4 (12%) |
| “I think using the application and activity tracker daily would be helpful for tracking symptoms and pain” | 16 (46%) | 10 (29%) | 4 (11%) | 4 (11%) | 1 (3%) |
Highlights.
Mobile health (mHealth) is a novel approach to supplement current standards for management of HCT complications
This is the first study to address feasibility of mHealth utilization in adult HCT patients through the use of an HCT-specific app: TRU-BMT
The survey and Phase 1 study indicate both interest and feasibility for mHealth utilization in HCT management
Adherence to mHealth is variable and associated with patient health markers, an important consideration for Phase 2 and 3 studies
Tracking of symptom severity associates with transplant outcomes
Acknowledgments
We thank the medical staff, fellows, and nursing staff who provided the care, as well as the patients and families who allowed us to conduct this study. We would additionally like to thank Dr. Sandra Sue Stinnett, Associate Professor of Biostatistics and Bioinformatics for technical advising regarding statistical methodology.
Financial Disclosure:
This work was supported by funding from the National Institute of Aging: NIA P30-AG028716-13 Mini #6 (to A.D.S) and the American Society of Hematology: ASH Scholar Award to A.D.S.
Footnotes
Conflicts of Interest: Jude Jonassaint is an officer of Sicklesoft.
Uncategorized References
- 1.Arnaout K, Patel N, Jain M, El-Amm J, Amro F, Tabbara IA. Complications of allogeneic hematopoietic stem cell transplantation. Cancer Invest. 2014;32:349–362. [DOI] [PubMed] [Google Scholar]
- 2.Rimkus C Acute complications of stem cell transplant. Semin Oncol Nurs. 2009;25:129–138. [DOI] [PubMed] [Google Scholar]
- 3.Bayraktar UD, Shpall EJ, Liu P, et al. Hematopoietic cell transplantation-specific comorbidity index predicts inpatient mortality and survival in patients who received allogeneic transplantation admitted to the intensive care unit. J Clin Oncol. 2013;31:4207–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appelbaum FR, Forman SJ, Negrin RS, Antin JH. Thomas' hematopoietic cell transplantation : stem cell transplantation. Fifth edition. ed:1 online resource. [Google Scholar]
- 5.Anderson KO, Giralt SA, Mendoza TR, et al. Symptom burden in patients undergoing autologous stem-cell transplantation. Bone Marrow Transplant. 2007;39:759–766. [DOI] [PubMed] [Google Scholar]
- 6.Wood WA, Deal AM, Abernethy A, et al. Feasibility of frequent patient-reported outcome surveillance in patients undergoing hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basch E, Deal AM, Dueck AC, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA. 2017;318:197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basch E, Deal AM, Kris MG, et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J Clin Oncol. 2016;34:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nipp RD, El-Jawahri A, Ruddy M, et al. Pilot randomized trial of an electronic symptom monitoring intervention for hospitalized patients with cancer. Ann Oncol. 2019;30:274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bevans MF, Mitchell SA, Barrett JA, et al. Symptom distress predicts long-term health and well-being in allogeneic stem cell transplantation survivors. Biol Blood Marrow Transplant. 2014;20:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. mHealth: new horizons for health through mobile technologies: second global survey on eHealth. Geneva, Switzerland: 2011. [Google Scholar]
- 12.Cheong IY, An SY, Cha WC, et al. Efficacy of Mobile Health Care Application and Wearable Device in Improvement of Physical Performance in Colorectal Cancer Patients Undergoing Chemotherapy. Clin Colorectal Cancer. 2018;17:e353–e362. [DOI] [PubMed] [Google Scholar]
- 13.Heintzman ND. A Digital Ecosystem of Diabetes Data and Technology: Services, Systems, and Tools Enabled by Wearables, Sensors, and Apps. J Diabetes Sci Technol. 2015;10:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leahy AB, Feudtner C, Basch E. Symptom Monitoring in Pediatric Oncology Using Patient-Reported Outcomes: Why, How, and Where Next. Patient. 2018;11:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenwood DA, Gee PM, Fatkin KJ, Peeples M. A Systematic Review of Reviews Evaluating Technology-Enabled Diabetes Self-Management Education and Support. J Diabetes Sci Technol. 2017;11:1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui CY, Walton R, McKinstry B, Jackson T, Parker R, Pinnock H. The use of mobile applications to support self-management for people with asthma: a systematic review of controlled studies to identify features associated with clinical effectiveness and adherence. J Am Med Inform Assoc. 2017;24:619–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis SW, Oakley-Girvan I. Achieving value in mobile health applications for cancer survivors. J Cancer Surviv. 2017;11:498–504. [DOI] [PubMed] [Google Scholar]
- 18.Darlow S, Wen KY. Development testing of mobile health interventions for cancer patient self-management: A review. Health Informatics J. 2016;22:633–650. [DOI] [PubMed] [Google Scholar]
- 19.Bender JL, Yue RY, To MJ, Deacken L, Jadad AR. A lot of action, but not in the right direction: systematic review and content analysis of smartphone applications for the prevention, detection, and management of cancer. J Med Internet Res. 2013;15:e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson K, Burford O, Emmerton L. Mobile Health Apps to Facilitate Self-Care: A Qualitative Study of User Experiences. PLoS One. 2016;11:e0156164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birkhoff SD, Smeltzer SC. Perceptions of Smartphone User-Centered Mobile Health Tracking Apps Across Various Chronic Illness Populations: An Integrative Review. J Nurs Scholarsh. 2017;49:371–378. [DOI] [PubMed] [Google Scholar]
- 22.Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020:1357633X20916567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stover A, Irwin DE, Chen RC, et al. Integrating Patient-Reported Outcome Measures into Routine Cancer Care: Cancer Patients' and Clinicians' Perceptions of Acceptability and Value. EGEMS (Wash DC). 2015;3:1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green AK, Reeder-Hayes KE, Corty RW, et al. The project data sphere initiative: accelerating cancer research by sharing data. Oncologist. 2015;20:464–e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coye MJ, Haselkorn A, DeMello S. Remote patient management: technology-enabled innovation and evolving business models for chronic disease care. Health Aff (Millwood). 2009;28:126–135. [DOI] [PubMed] [Google Scholar]
- 26.Palatini P, Julius S. Heart rate and the cardiovascular risk. J Hypertens. 1997;15:3–17. [DOI] [PubMed] [Google Scholar]
- 27.Kannel WB, Wilson P, Blair SN. Epidemiological assessment of the role of physical activity and fitness in development of cardiovascular disease. Am Heart J. 1985;109:876–885. [DOI] [PubMed] [Google Scholar]
- 28.Davis MP, Dreicer R, Walsh D, Lagman R, LeGrand SB. Appetite and cancer-associated anorexia: a review. J Clin Oncol. 2004;22:1510–1517. [DOI] [PubMed] [Google Scholar]
- 29.Laviano A, Meguid MM, Rossi-Fanelli F. Cancer anorexia: clinical implications, pathogenesis, and therapeutic strategies. Lancet Oncol. 2003;4:686–694. [DOI] [PubMed] [Google Scholar]
- 30.Judson TJ, Bennett AV, Rogak LJ, et al. Feasibility of long-term patient self-reporting of toxicities from home via the Internet during routine chemotherapy. J Clin Oncol. 2013;31:2580–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker S, Kribben A, Meister S, Diamantidis CJ, Unger N, Mitchell A. User profiles of a smartphone application to support drug adherence--experiences from the iNephro project. PLoS One. 2013;8:e78547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Min YH, Lee JW, Shin YW, et al. Daily collection of self-reporting sleep disturbance data via a smartphone app in breast cancer patients receiving chemotherapy: a feasibility study. J Med Internet Res. 2014;16:e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaughn J, Gollarahalli S, Shaw RJ, et al. Mobile Health Technology for Pediatric Symptom Monitoring: A Feasibility Study. Nurs Res. 2020;69:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Souza A ZX. Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT). CIBMTR. Houston, Texas: Center for International Blood & Marrow Transplant Research; 2019. [Google Scholar]
- 35.Walrath M, Bacon C, Foley S, Fung HC. Gastrointestinal side effects and adequacy of enteral intake in hematopoietic stem cell transplant patients. Nutr Clin Pract. 2015;30:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuji S, Einsele H, Savani BN, Kapp M. Systematic Nutritional Support in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Biol Blood Marrow Transplant. 2015;21:1707–1713. [DOI] [PubMed] [Google Scholar]
- 37.Yavuzsen T, Davis MP, Walsh D, LeGrand S, Lagman R. Systematic review of the treatment of cancer-associated anorexia and weight loss. J Clin Oncol. 2005;23:8500–8511. [DOI] [PubMed] [Google Scholar]
- 38.Bevans MF, Mitchell SA, Marden S. The symptom experience in the first 100 days following allogeneic hematopoietic stem cell transplantation (HSCT). Support Care Cancer. 2008;16:1243–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kannel WB, Kannel C, Paffenbarger RS, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–1494. [DOI] [PubMed] [Google Scholar]
- 40.Price KJ, Thall PF, Kish SK, Shannon VR, Andersson BS. Prognostic indicators for blood and marrow transplant patients admitted to an intensive care unit. Am J Respir Crit Care Med. 1998;158:876–884. [DOI] [PubMed] [Google Scholar]
- 41.Yang TM, Wang PN, Kao KC, Huang CC, Tsai YH, Hsieh MJ. Outcome of hematopoietic stem cell recipients who were mechanically ventilated and admitted to intensive care units. J Formos Med Assoc. 2007;106:295–301. [DOI] [PubMed] [Google Scholar]
- 42.Berro M, Arbelbide JA, Rivas MM, et al. Hematopoietic Cell Transplantation-Specific Comorbidity Index Predicts Morbidity and Mortality in Autologous Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;23:1646–1650. [DOI] [PubMed] [Google Scholar]
- 43.Guilfoyle R, Demers A, Bredeson C, et al. Performance status, but not the hematopoietic cell transplantation comorbidity index (HCT-CI), predicts mortality at a Canadian transplant center. Bone Marrow Transplant. 2009;43:133–139. [DOI] [PubMed] [Google Scholar]
- 44.Nakaya A, Mori T, Tanaka M, et al. Does the hematopoietic cell transplantation specific comorbidity index (HCT-CI) predict transplantation outcomes? A prospective multicenter validation study of the Kanto Study Group for Cell Therapy. Biol Blood Marrow Transplant. 2014;20:1553–1559. [DOI] [PubMed] [Google Scholar]
- 45.Vander Lugt MT, Braun TM, Hanash S, et al. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N Engl J Med. 2013;369:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson RP, Khawaja MR, Perkins SM, et al. Prognostic biomarkers for acute graft-versus-host disease risk after cyclophosphamide-fludarabine nonmyeloablative allotransplantation. Biol Blood Marrow Transplant. 2014;20:1861–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.