Graphical Abstract
Keywords: Atrial Fibrillation, Polygenic Risk Score, High Density Mapping, Catheter Ablation, Left Atrial Substrate
Introduction
Genetic factors have an important role in AF with a familial predisposition or white ethnicity increasing the risk for incident AF. Rare genetic variants that are sufficient to cause AF have been associated with familial forms of AF. However, this accounts for a relatively small proportion of all AF cases(1). More frequently, AF occurs as a complex trait, with contributions from multiple factors including age, sex, background genetic variation and acquired clinical risk factors. Genome wide association studies (GWAS) have been used in recent years to identify common variants that influence disease susceptibility (2, 3). In order to refine AF risk prediction, suites of AF-associated single nucleotide variants (SNV) have been combined into polygenic risk scores for AF (AF-PRS). These scores have been strongly associated with prevalent and incident AF (4–7), and identify a substantially larger fraction of the population than is found by rare monogenic mutations(4, 8–10). However, knowledge gaps still exist about the relationship between AF phenotypes, clinical outcomes and the AF-PRS.
In this study, we sought to characterize the clinical, structural and electrical phenotype associated with an AF-PRS and the impact of AF-PRS on recurrence of AF following catheter ablation. We hypothesized that atrial remodeling due to high AF-PRS provides an ongoing substrate for arrhythmogenesis after initially successful ablation procedures.
Methods
Study design
The study protocol was approved by the Melbourne Health Research Ethics Committee and in accordance with the Helsinki Declaration. Two cohorts were recruited for this study. First, an AF cohort was recruited prospectively from a single tertiary center in Australia to study the impact of AF-PRS on the LA electrical substrate and clinical recurrence rates following catheter ablation. Second, a control cohort of individuals without history of AF were used from the ASPirin in Reducing Events in the Elderly (ASPREE) trial to compare the AF-PRS of a non-AF cohort to the AF cohort(11). Research associated with the genetic analysis of ASPREE study participants was approved by the Alfred Hospital Research Ethics Committee (Project 390/15, 27 August 2015). The ASPREE trial is registered (NCT01038583) and all institutions involved in human participant research received local institutional review board approvals.
Study Subjects
Eligible patients were required to have paroxysmal or persistent AF and referred for first-time AF ablation. Exclusion criteria were: structural heart disease (left ventricular ejection fraction <50% or valvular heart disease), inability to restore sinus rhythm, previous AF ablation, or amiodarone use. All patients provided written informed consent prior to their ablation procedure. The AF-PRS control group comprised healthy older individuals aged ≥ 70 years without a history of AF (n=12815; negative AF history confirmed by the treating physician assessment at enrolment) in the ASPREE trial.
Genotyping and Derivation of AF Polygenic Risk Scores
Peripheral blood samples were collected from AF cases and deoxyribonucleic acid (DNA) was extracted using the Illustra Nucleon BACC3 Genomic DNA Extraction Kit (GE Healthcare, Chicago, Illinois). DNA was extracted from ASPREE control participants using standard protocols as described previously(12). Patient DNA samples were then genotyped using the Axiom Precision Medicine Diversity Array (PMDA: v2.0; ThermoFisher Scientific, CA, USA). Control sample genotyping was undertaken using the same array but in a different laboratory (6). Quality metrics and variant calling were performed using in-house pipelines aligned to the human reference genome hg38. Sample ethnicity for both cohorts was determined using the 1000G whole-genome sequencing (WGS) cohort superpopulations as a reference. A set of intersecting variants between the PMDA data and 1000G WGS data was prepared using bcftools. Ancestry and Kinship Toolkit (AKT) was then used to compute principal components for the 1000G samples, with superpopulations forming distinct clusters in the first three principal components. Samples were then projected onto the 1000G plots using AKT and 1000G variant weights. PRS scoring was performed on imputed genotypes using plink (version 1.9), the sum option was passed to plink to compute the sum of all valid allele weight scores. A validated polygenic risk score for AF (AF-PRS; PGS000016) was calculated from a set of >6 million common sequence variants imputed from the PMDA genotype data (9). Rare variant analysis was not performed as this was not the focus of this current study.
AF Cohort Electroanatomic Mapping
All procedures were performed under general anaesthesia with periprocedural transoesophageal echocardiography to exclude LA thrombus. Following transseptal access, a 20 pole Lasso catheter (Biosense Webster, California, USA) was used to construct LA geometry and merge it with a pre procedural cardiac computed tomography using the CARTO3 electroanatomical mapping system (Biosense Webster). Voltage and activation maps were constructed during constant pacing from the distal coronary sinus at a fixed cycle length of 600 ms. Complete coverage of the entire LA geometry was performed and correlated to the computed tomography with a minimum of 1000 points collected using the Confidense algorithm (Biosense Webster). Strict criteria were used to account for the lack of tissue contact data on the multipolar catheter with point collection performed only by experienced operators after careful assessment of geometry and fluoroscopic motion. The operators performing left atrial substrate mapping were blinded to the AF PRS group allocations for each patient.
AF Cohort Ablation Procedure
Following mapping, wide antral circumferential pulmonary vein isolation (PVI) was performed. The primary procedural endpoint was entrance and exit block of all PVs following adenosine and 30-min waiting period. Posterior LA isolation was performed in persistent AF patients at the physician’s discretion. Cavo-tricuspid isthmus ablation was performed if the patient had clinically documented atrial flutter.
Electrogram Analysis
Electrogram analysis for each map was performed manually offline as previously described in detail(13, 14). Clinicians performing this offline signal analysis were blinded to the AF-PRS group allocations.
Global and Regional Atrial Voltage Analysis:
Segmental voltage analysis of the LA was performed by dividing the chamber into 6 segments: anterior, posterior, septal, lateral, roof, and inferior. The mean voltage of each region was calculated.
Conduction Velocity Analysis:
The mean LA and regional Conduction velocity (CV) was analysed in MATLAB as previously described using the polynomial algorithm(13). Atrial conduction slowing (defined as local CV of 10 to 20 cm/s) and conduction block (defined as <10 cm/s) were expressed as a proportion of total points in each region. Conduction heterogeneity was determined by calculating the coefficient of variation of CV.
Analysis of Complex Signals:
Complex signals were defined during pacing in sinus rhythm as electrograms with >3 deflections and >50 ms duration (fractionated potentials) or 2 separate deflections separate by an isoelectric interval (double potentials). The percentage of complex signals was expressed as a proportion of the number of complex signals divided by the total number of points.
Follow-up and Rhythm Monitoring
All patients were followed up for 24 months. AF rhythm monitoring was performed either via continuous rhythm monitoring using an implantable loop recorder (Reveal LINQ™, Medtronic) or a pre-existing dual chamber device; or via holter monitoring at 3-, 6-, and 12-months post ablation followed by 6 months intervals thereafter until study completion. Recurrence was defined as documented AF or atrial tachycardia >30 s following a 3-month blanking period.
Statistical Analysis
Data analysis was performed using SPSS software (version 26, IBM, New York). Normality of all quantitative variables was assessed using the Shapiro-Wilk test. Data are expressed as mean ± standard deviation (SD) unless otherwise stated. The AF-PRS in the control cohort were ranked in descending order of magnitude and divided into “bins” based on the top centile, decile, quintile and quartile of scores in order to determine the proportion of AF cases with scores above each of these different threshold levels. Given the normal distribution of the AF-PRS data, comparisons were made between subgroups of patient above and below the mean PRS score of the AF cohort (30.27) using Student’s t test. To compare the electroanatomic substrate differences between High vs Low AF-PRS groups, we first compared the global electroanatomic parameters and adjusted the P value by controlling for false discovery rate using the Benjamini-Hochberg procedure. If there were significant differences in the global electroanatomic parameters between the 2 groups, further comparisons were made of the regional electroanatomic parameters. Linear regression analysis was performed to determine univariate and multivariate predictors of CV heterogeneity and complex signals. Following univariable analysis, all predictors with P value <0.1 in addition to age and gender were included in a multivariate model. Survival curves for freedom from arrhythmia were determined using the Kaplan-Meier curve and compared using the log-rank test.
Results
Baseline Clinical and Genetic Characteristics
Ninety-five patients with AF were recruited (35 % females, mean age 60.1 ± 8.5 years). The demographics and clinical parameters for the AF cohort are summarized in Table 1. Ninety-three (98%) patients were confirmed to have European (n=90) or admixed European (n=3) ancestry, with 2 patients having East Asian or admixed South Asian ancestry. Overall results were similar when the latter 2 patients were excluded. We divided the AF cohort for comparison into 2 groups with AF-PRS values above and below the mean value, respectively. There were no differences between the high and low groups with regards to baseline characteristics (Table 1).
Table 1:
Baseline demographic of AF patients.
| AF cohort (n=95) | High AF-PRS (n=42) | Low AF-PRS (n=53) | P value | |
|---|---|---|---|---|
| Age, years (SD) | 60.6 (8.5) | 60 (9) | 61 (8) | 0.6 |
| Female (%) | 34(35) | 13 (27) | 21 (43) | 0.1 |
| Persistent AF (%) | 49 (52) | 28 (59) | 21 (41) | 0.2 |
| Hypertension (%) | 33(35) | 14 (33) | 19 (36) | 0.9 |
| Diabetes Mellitus (%) | 7 (7) | 2 (5) | 5 (9) | 0.4 |
| Ischemic heart disease (%) | 10(10) | 5 (12) | 5 (9) | 0.3 |
| TIA/Stroke (%) | 5 (5) | 1 (2) | 4 (8) | 0.9 |
| CHA2-DS2-VASc score (SD) | 1.3 (1.1) | 1.2 (1.1) | 1.5 (1.04) | 0.2 |
| BMI, kg/m2 (SD) | 29.2 (4.6) | 29.8 (5) | 28.7 (4.1) | 0.3 |
| Obstructive sleep apnoea | 34 (36) | 15 (36) | 19 (36) | 0.9 |
| Family history of AF (%) | 31 (33) | 14 (33) | 17 (32) | 0.9 |
| LVEF, (SD) | 57 (5) | 57 (5) | 57 (6) | 0.7 |
| LA volume index, ml/m2 (SD) | 35.4 (10.9) | 33.9 (9) | 39.6 (12.5) | 0.1 |
| RA area, cm2 (SD) | 19.9 (5.2) | 19 (3.8) | 21.4 (6.4) | 0.1 |
The strong association between AF and AF-PRS reported previously was also seen in our cohort with mean AF-PRS in AF patients being significantly higher than that observed in healthy elderly individuals with no history of AF (p<0.0001) (Supplemental Figure 1), (9).
High Density Electroanatomical Mapping of the LA
Seventy-five of 95 (79%) patients who underwent PVI procedures also had high density electroanatomical mapping. (Tables 2, 3, and supplementary table 1).
Table 2:
Global Electrical Substrate Differences Between High and Low AF-PRS Groups.
| Electroanatomic Parameter | High AF-PRS | Low AF-PRS | P value | |
|---|---|---|---|---|
| Global Parameters | LA bipolar voltage | 1.9 (0.6) | 1.7 (0.6) | 0.4 |
| LA bipolar voltage heterogeneity | 0.85 (0.11) | 0.8 (0.1) | 0.02 | |
| Conduction velocity | 40 (16) | 44 (16) | 0.3 | |
| Conduction velocity heterogeneity | 0.44 (0.13) | 0.35 (0.08) | 0.0002 | |
| Slow & block points% | 31 (24) | 17 (23) | 0.02 | |
| Complex points% | 8.6 (7.2) | 6.2 (4.2) | 0.06 |
Table 3:
Regional Electroanatomic Substrate Differences Between High and Low AF-PRS.
| Region | Electroanatomic Parameter | High AF-PRS | Low AF-PRS | P value |
|---|---|---|---|---|
| Posterior | LA bipolar voltage heterogeneity | 0.88 (0.18) | 0.84 (0.17) | 0.4 |
| Conduction velocity heterogeneity | 0.44 (0.19) | 0.35 (0.11) | 0.01 | |
| Slow & block points% | 36 (30) | 21 (28) | 0.04 | |
| Complex points% | 10.1 (11) | 6.1 (4.6) | 0.03 | |
|
| ||||
| Anterior | LA bipolar voltage heterogeneity | 0.77 (0.16) | 0.73 (0.15) | 0.4 |
| Conduction velocity heterogeneity | 0.38 (0.2) | 0.35 (0.15) | 0.4 | |
| Slow & block points% | 24 (32) | 16 (28) | 0.3 | |
| Complex points% | 4.8 (7.3) | 4.3 (4.5) | 0.7 | |
|
| ||||
| Lateral | LA bipolar voltage heterogeneity | 1.01 (0.38) | 0.86 (0.22) | 0.01 |
| Conduction velocity heterogeneity | 0.51 (0.25) | 0.35 (0.16) | 0.03 | |
| Slow & block points% | 43 (34) | 21 (29) | 0.004 | |
| Complex points% | 12 (12) | 8 (9) | 0.08 | |
|
| ||||
| Septal | LA bipolar voltage heterogeneity | 0.83 (0.2) | 0.82 (0.2) | 0.7 |
| Conduction velocity heterogeneity | 0.40 (0.2) | 0.35 (0.15) | 0.2 | |
| Slow & block points% | 28 (28) | 18 (27) | 0.1 | |
| Complex points% | 9.3 (7.8) | 6.6 (11) | 0.3 | |
|
| ||||
| Inferior | LA bipolar voltage heterogeneity | 0.85 (0.19) | 0.8 (0.17) | 0.2 |
| Conduction velocity heterogeneity | 0.55 (0.24) | 0.35 (0.16) | <0.001 | |
| Slow & block points% | 26.6 (26) | 14.4 (25.8) | 0.03 | |
| Complex points% | 9.6 (10.8) | 6.7 (7.5) | 0.2 | |
|
| ||||
| Roof | LA bipolar voltage heterogeneity | 0.8 (0.16) | 0.76 (0.18) | 0.4 |
| Conduction velocity heterogeneity | 0.42 (0.18) | 0.36 (0.14) | 0.1 | |
| Slow & block points% | 27 (32) | 13 (27) | 0.03 | |
| Complex points% | 5.2 (6.9) | 5.5 (4.7) | 0.9 | |
AF-PRS and LA bipolar voltage:
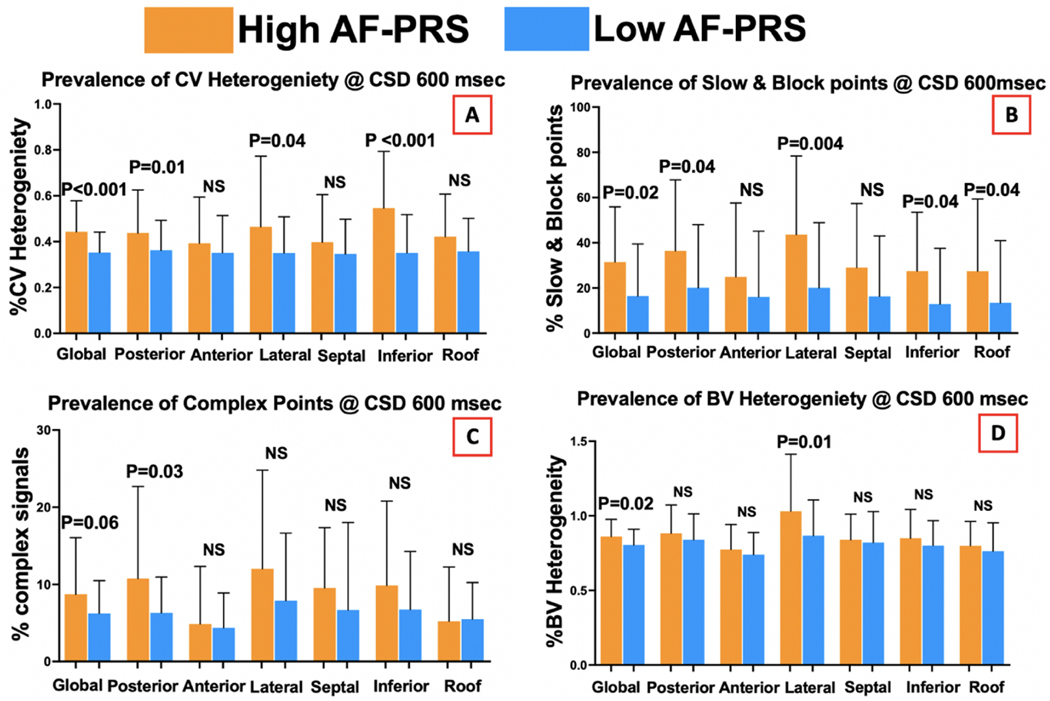
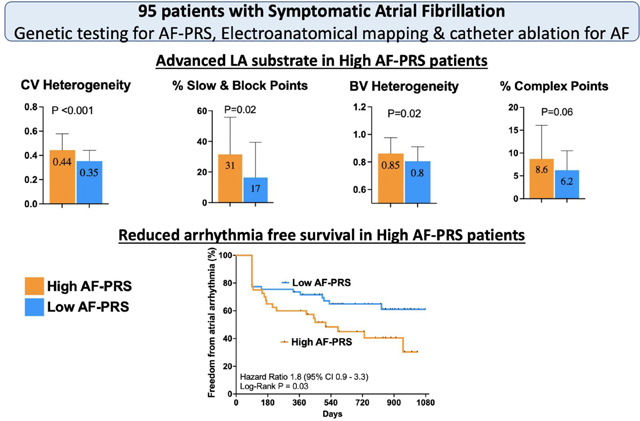
The mean global LA bipolar voltage was not different between the high and low AF-PRS groups (1.9 vs 1.7mV, p=0.4). However, bipolar voltage heterogeneity was higher in the high AF-PRS group (0.85 vs 0.80, p=0.02), which was most marked in the lateral segment (1.01 vs 0.86, p =0.01) (Figure 1).
Figure 1:
Regional variation in the left atrial substrate between high and low AF-PRS groups.
AF-PRS and conduction velocity/conduction heterogeneity:
The mean LA CV was not different between the 2 groups (40 vs 44 cm/s, p=0.3). However, mean CV heterogeneity was higher in the high AF-PRS group (0.44 vs 0.35, p<0.001), particularly in the posterior (0.44 vs 0.35, p=0.01), inferior (0.55 vs 0.35, p<0.001), and lateral (0.51 vs 0.35, p=0.03) segments (Figure 1). Similarly, the high AF-PRS group had greater extent of conduction slowing or conduction block compared with the low AF-PRS group (31% vs 17%, p=0.02) with differences mostly present in the posterior (36% vs 21%, p=0.04), lateral (43% vs 21%, p=0.004), inferior (27% vs 14%, p=0.04), and roof (27% vs 13%, p=0.03) segments.
AF-PRS and electrogram fractionation:
There was a trend towards higher prevalence of complex fractionated electrograms and double potentials in the high 50% AF-PRS group compared with the low 50% AF-PRS group (8.6% vs 6.2%, p=0.06) (Figure 1). This was most marked in the posterior (10% vs 6%, p=0.03) segment of the LA.
AF-PRS and AF Phenotype:
When the degree of left atrial electrical substate was stratified according the AF-PRS group allocation and AF phenotype, more advanced markers of electrical remodelling were present amongst patients in the high AF PRS group with persistent AF (supplemental figure 2).
Predictors of Left Atrial Remodeling
Tables 4 show the predictors of advanced electrical substrate on univariate and multivariate analysis. AF-PRS emerged as an independent predictor for both CV heterogeneity and complex points.
Table 4:
Univariate and multivariate analysis of Conduction Heterogeneity and Complex Points.
| Conduction Heterogeneity | Complex points | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
|
| ||||||||
| Coefficient (95% CI) | P | Coefficient (95% CI) | P | Coefficient (95% CI) | P | Coefficient (95% CI) | P | |
| Age | −0.11 (−0.005–002) | 0.3 | −0.1 (−0.005−0.002) | 0.3 | −0.005 (−0.15−0.15) | 0.96 | 0.06 (−0.14−0.15) | 0.9 |
| Female | −0.31 (−0.06−0.04) | 0.7 | 0.076 (−0.03−0.07) | 0.5 | 0.23 (0.17–5.24) | 0.03 | 0.32 (1.06–6.43) | 0.007 |
| PRS | 0.31 (0.1−0.63) | 0.007 | 0.26 (0.02 – 0.55) | 0.03 | 0.24 (0.82–27.34) | 0.03 | 0.24 (0.83–25.66) | 0.03 |
| Persistent AF | 0.32 (0.02−0.12) | 0.003 | 0.21 (−0.009−0.11) | 0.09 | 0.29 (0.88–5.65) | 0.008 | 0.25 (0.09–5.49) | 0.04 |
| AF duration | 0.28 (0.001−0.001) | 0.02 | 0.21 (0−0.001) | 0.09 | 0.26 (0.003−0.051) | 0.02 | 0.15 (−0.009−0.45) | 0.18 |
| BMI | −0.02 (−0.01−0.006) | 0.8 | 0.13 (−0.11−0.48) | 0.2 | ||||
| Hypertension | −0.006 (−0.05−0.05) | 0.9 | −0.02 (−2.9–2.4) | 0.8 | ||||
| DM | 0.036 (−0.11−0.16) | 0.3 | 0.05 (−5.03–8.21) | 0.6 | ||||
| IHD | −0.31 (−0.09−0.07) | 0.7 | 0.01 (−3.72–4.25) | 0.8 | ||||
| CHADSVASC | −0.12 (−0.03−0.01) | 0.2 | −0.007 (−1.15–1.08) | 0.9 | ||||
| OSA | −0.003 (−0.05−0.05) | 0.9 | 0.23 (−2.37–3.02) | 0.8 | ||||
| Regular EtOH | −0.11 (−0.08−0.02) | 0.3 | −0.02 (−2.89–2.25) | 0.8 | ||||
| Familial AF | 0.05 (−0.04−0.07) | 0.6 | 0.19 (−0.43–5.02) | 0.09 | 0.14 (−0.92–4.4) | 0.2 | ||
| LVEF | 0.03 (−0.004−0.005) | 0.7 | 0.16 (−0.06–0.39) | 0.1 | ||||
| LA size | −0.01 (−0.004−0.004) | 0.8 | 0.004 (−0.16–0.16) | 0.9 | ||||
| LA Vol indexed | −0.03 (−0.005−0.004) | 0.8 | −0.01 (−0.2–0.18) | 0.9 | ||||
AF-PRS and Recurrence After AF Ablation
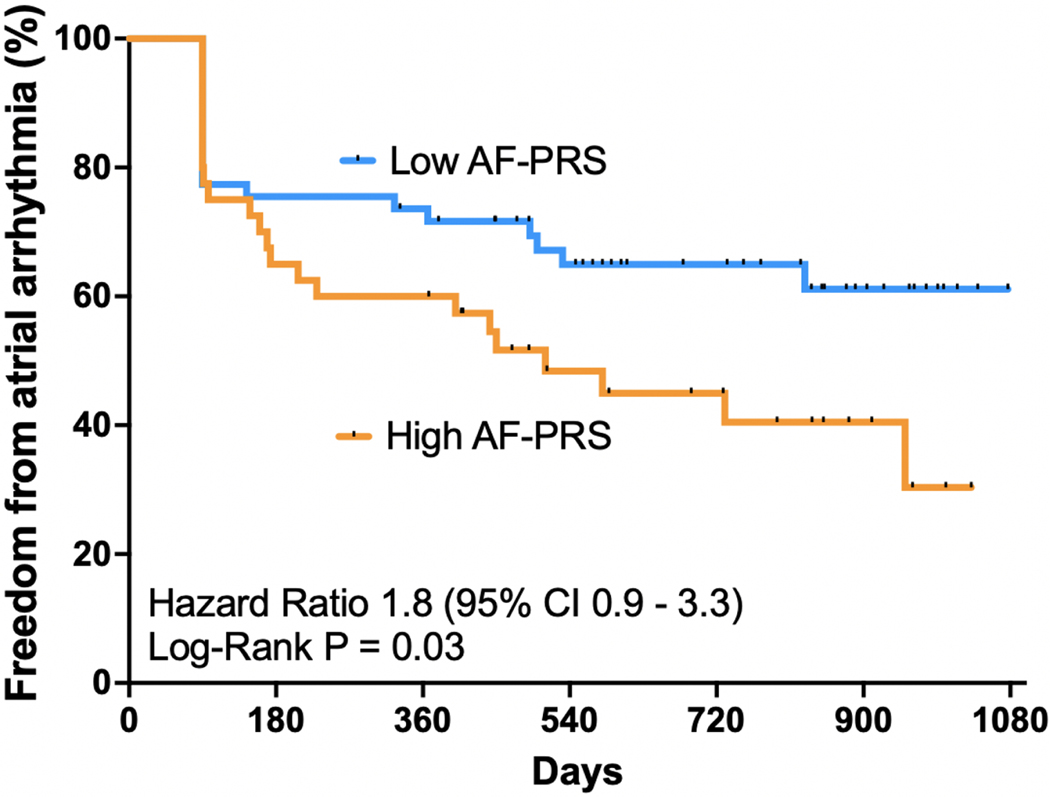
Successful PVI was achieved in 100% of patients (n=95). Total ablation time was similar between the groups (41 ± 11 vs 37 ± 10, p = 0.1). Similar rates of Cavotricuspid isthmus ablation were performed in both groups (33% vs 32%). Following the index AF ablation, 50/95 (53%) patients had continuous rhythm monitoring (92% via a loop recorder and 8% via a dual chamber pacemaker) whilst the remaining 46 patients (47%) had periodic 24-hour Holter monitoring. There was no significant difference in the mode of AF monitoring between the 2 groups. After a median follow up of 27 months (interquartile range: 19,31), 42 out of 95 (44%) patients had a clinical recurrence of AF with a mean time to recurrence of 514 ± 348 days (17 ± 11 months). Patients in the high AF-PRS value had lower atrial arrhythmia free survival compared to low AF-PRS patients (45% vs 64%; log-rank = 0. 03; Kaplan-Meier survival curve shown in Figure 2).
Figure 2:
Kaplan-Meier curve comparing single procedure freedom from atrial arrhythmia between high AF-PRS and low AF-PRS groups.
Discussion
Previous studies have shown robust statistical associations between AF-PRS and AF but the fundamental defects responsible for this have been elusive. Here we took advantage of a deeply phenotyped group of patients undergoing AF ablation procedures and showed that high AF-PRS values were associated with a distinctive pattern of electrical remodeling that predominantly involves the postero-lateral and inferior LA walls. Moreover, we found that patients with high AF-PRS had lower arrhythmia free survival post ablation. These results raise interesting questions about the factors that determine regional differences in atrial remodeling and their impact on post ablation outcomes.
AF-PRS and AF Substrate
Although the genetic association between AF-PRS and AF has been widely investigated, very little is known about the phenotypic features that are determined by AF-PRS. A study by Shoemaker et al. showed that patients with a high AF-PRS were younger and had fewer clinical risk factors for AF than those with low AF-PRS(15). These findings support a role for genetic risk in determining development of AF particularly in younger patients but do not explain why AF occurs. In our study, there was a trend towards more males and persistent AF in the high AF-PRS compared to low AF-PRS patients and there were on other significant differences in the traditional AF risk factors. The absence of significant differences between the 2 cohort could be due to the relatively smaller sample size compared to previous studies(15).
In the current study, patients with a higher AF-PRS had more advanced LA electrical remodeling parameters compared with patients with lower AF-PRS. This was manifest by regions of bipolar voltage heterogeneity, conduction slowing and heterogeneity, and complex fractionated electrograms. Such abnormal parameters have been associated with increased AF susceptibility in prior studies (16, 17). These changes were not uniformly distributed throughout the LA, and were mainly seen in the posterior, lateral, and inferior segments. Interestingly, this pattern of electrical remodeling with high AF-PRS is similar to that observed by Wong et al, in a study of the atrial phenotype in carriers of the SNV rs2200733, associated with the chromosome 4q25 AF GWAS locus(18). This SNV is located in a non-coding region of the genome, with the suspected target gene being the cardiac transcription factor, PITX2. This gene has an important role in left-right patterning during cardiac development and has been independently investigated as a candidate disease gene for AF. The predilection for posterolateral and inferior changes in conduction properties could reflect expression patterns of PITX2 or other factors that affect cell-cell coupling, resting membrane potential, and LA wall stress. In addition to AF-PRS, female sex and persistent AF were predictors of abnormal atrial electrical substrate, consistent with prior studies(14, 19).
AF-PRS and Clinical Outcomes After Catheter Ablation
Using a limited AF-PRS, prior studies have demonstrated an association with AF recurrence following cardioversion(20, 21). The association between AF-PRS and ablation outcomes remains poorly defined. Recently, AF-PRS demonstrated a significant association with AF recurrence after ablation in a Korean population(22). However, Shoemaker et al found no association between AF-PRS and post ablation outcomes at 12 months follow up(15). Our cohort had the advantage of more intensive rhythm monitoring (53% had continuous rhythm monitoring) and longer follow up (median 27 months). Patients with an AF-PRS above the mean PRS had lower arrhythmia free survival post ablation compared to patients with AF-PRS below the mean PRS for the cohort. This is most likely explained by proarrhythmogenic effects of the advanced LA electrical remodeling that is present in the higher AF-PRS group.
Study Limitations
As this is a small cohort, statistical power is limited. Therefore, subtle and clinically relevant differences in the clinical phenotype between the high and low AF-PRS group could have been present. larger studies are required to replicate our findings. Nevertheless, the data presented here are informative and hypothesis-generating. Our study cohort was restricted to patients with AF and hence we are unable to determine whether the atrial electrical changes observed might have been present prior to AF onset or represent remodeling induced by AF.
Conclusions
Amongst patients with AF, a higher AF-PRS is associated with more advanced LA electrical substrate with regions of increased CV heterogeneity, slowed conduction, complex fractionated signals and bipolar voltage heterogeneity. Moreover, patients with a higher AF-PRS had more AF recurrence following catheter ablation (Central Illustration).
Supplementary Material
Acknowledgement:
We would like to acknowledge the Victor Chang Cardiac Research Institute Innovation Centre, funded by the NSW Government for their collaboration to conduct this study.
Funding
The authors acknowledge funding support from the National Health and Medical Research Council (NHMRC) of Australia: Practitioner Fellowship (J.K), Research Scholarships (A.A., G.W., R.A., D.C., H.S.); Heart Foundation: Future Leader Fellowship (P.L.), Predictive Modelling Grant (D.F., J.M.K., P.M.K., R.J.); NSW Health: (D.F., R.J.). The ASPREE study was supported by a Flagship cluster grant (including the Commonwealth Scientific and Industrial Research Organisation, Monash University, Menzies Research Institute, Australian National University, University of Melbourne); and grants (U01AG029824 and U19AG062682) from the National Institute on Aging and the National Cancer Institute at the National Institutes of Health, by grants (334047 and 1127060) from the NHMRC, and by Monash University and the Victorian Cancer Agency.
Footnotes
Disclosures: No conflict of interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Gladding PA, Legget M, Fatkin D, et al. Polygenic Risk Scores in Coronary Artery Disease and Atrial Fibrillation. Heart Lung Circ. 2020;29(4):634–40. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy MI, Abecasis GR, Cardon LR, et al. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet. 2008;9(5):356–69. [DOI] [PubMed] [Google Scholar]
- 3.Tam V, Patel N, Turcotte M, et al. Benefits and limitations of genome-wide association studies. Nat Rev Genet. 2019;20(8):467–84. [DOI] [PubMed] [Google Scholar]
- 4.Ellinor PT, Lunetta KL, Albert CM, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44(6):670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kertai MD, Mosley JD, He J, et al. Predictive Accuracy of a Polygenic Risk Score for Postoperative Atrial Fibrillation After Cardiac Surgery. Circ Genom Precis Med. 2021;14(2):e003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neumann JT, Riaz M, Bakshi A, et al. Prognostic Value of a Polygenic Risk Score for Coronary Heart Disease in Individuals Aged 70 Years and Older. Circ Genom Precis Med. 2021:Circgen121003429. [DOI] [PMC free article] [PubMed]
- 7.Weng LC, Preis SR, Hulme OL, et al. Genetic Predisposition, Clinical Risk Factor Burden, and Lifetime Risk of Atrial Fibrillation. Circulation. 2018;137(10):1027–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christophersen IE, Rienstra M, Roselli C, et al. Large-scale analyses of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat Genet. 2017;49(6):946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50(9):1219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubitz SA, Yin X, Lin HJ, et al. Genetic Risk Prediction of Atrial Fibrillation. Circulation. 2017;135(14):1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNeil JJ, Nelson MR, Woods RL, et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N Engl J Med. 2018;379(16):1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacaze P, Sebra R, Riaz M, et al. Medically actionable pathogenic variants in a population of 13,131 healthy elderly individuals. Genet Med. 2020;22(11):1883–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong GR, Nalliah CJ, Lee G, et al. Dynamic Atrial Substrate During High-Density Mapping of Paroxysmal and Persistent AF: Implications for Substrate Ablation. JACC Clin Electrophysiol. 2019;5(11):1265–77. [DOI] [PubMed] [Google Scholar]
- 14.Wong GR, Nalliah CJ, Lee G, et al. Sex-Related Differences in Atrial Remodeling in Patients With Atrial Fibrillation: Relationship to Ablation Outcomes. Circ Arrhythm Electrophysiol. 2022;15(1):e009925. [DOI] [PubMed] [Google Scholar]
- 15.Shoemaker MB, Husser D, Roselli C, et al. Genetic Susceptibility for Atrial Fibrillation in Patients Undergoing Atrial Fibrillation Ablation. Circ Arrhythm Electrophysiol. 2020;13(3):e007676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heida A, van der Does WFB, van Staveren LN, et al. Conduction Heterogeneity: Impact of Underlying Heart Disease and Atrial Fibrillation. JACC Clin Electrophysiol. 2020;6(14):1844–54. [DOI] [PubMed] [Google Scholar]
- 17.Polontchouk L, Haefliger JA, Ebelt B, et al. Effects of chronic atrial fibrillation on gap junction distribution in human and rat atria. J Am Coll Cardiol. 2001;38(3):883–91. [DOI] [PubMed] [Google Scholar]
- 18.Wong GR, Nalliah CJ, Lee G, et al. Genetic Susceptibility to Atrial Fibrillation Is Associated With Atrial Electrical Remodeling and Adverse Post-Ablation Outcome. JACC Clin Electrophysiol. 2020;6(12):1509–21. [DOI] [PubMed] [Google Scholar]
- 19.Giorgios T, Antonio F, Limite LR, et al. Bi-atrial characterization of the electrical substrate in patients with atrial fibrillation. Pacing Clin Electrophysiol. 2022. [DOI] [PMC free article] [PubMed]
- 20.Parvez B, Shoemaker MB, Muhammad R, et al. Common genetic polymorphism at 4q25 locus predicts atrial fibrillation recurrence after successful cardioversion. Heart Rhythm. 2013;10(6):849–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel S, Rudaka I, Rots D, et al. A Higher Polygenic Risk Score Is Associated with a Higher Recurrence Rate of Atrial Fibrillation in Direct Current Cardioversion-Treated Patients. Medicina (Kaunas). 2021;57(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choe WS, Kang JH, Choi EK, et al. A Genetic Risk Score for Atrial Fibrillation Predicts the Response to Catheter Ablation. Korean Circ J. 2019;49(4):338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.