Abstract
During the period from September 1996 through November 1996, 10 Dutch dairy farms were visited to collect fecal samples from all cattle present. The samples were examined for the presence of verocytotoxin (VT)-producing Escherichia coli (VTEC) of serogroup O157 (O157 VTEC) by immunomagnetic separation following selective enrichment. Cattle on 7 of the 10 dairy farms tested positive for O157 VTEC, with the proportion of cattle infected varying from 0.8 to 22.4%. On the seven farms positive for O157 VTEC, the excretion rate was highest in calves ages 4 to 12 months (21.2%). In a follow-up study, two O157 VTEC-positive farms and two O157 VTEC-negative farms identified in the prevalence study were revisited five times at intervals of approximately 3 months. Cattle on each farm tested positive at least once. The proportion of cattle infected varied from 0 to 61.0%. Excretion rates peaked in summer and were lowest in winter. Again, the highest prevalence was observed in calves ages 4 to 12 months (11.8%). O157 VTEC strains were also isolated from fecal samples from horses, ponies, and sheep and from milk filters and stable flies. O157 VTEC isolates were characterized by VT production and type, the presence of the E. coli attaching-and-effacing gene, phage type, and pulsed-field gel electrophoretic genotype. No overlapping strain types were identified among isolates from different farms except one. The predominance of a single type at each sampling suggests that horizontal transmission is an important factor in dissemination of O157 VTEC within a farm. The presence of more than one strain type, both simultaneously and over time, suggests that there was more than one source of O157 VTEC on the farms. Furthermore, this study demonstrated that the O157 VTEC status of a farm cannot be ascertained from a single visit testing a small number of cattle.
In recent years, verocytotoxin (VT)-producing Escherichia coli (VTEC) strains of serogroup O157 (O157 VTEC) have emerged as important human pathogens (5). They have been associated with a variety of human diseases, including mild diarrhea, hemorrhagic colitis, and the diarrhea-associated form of the hemolytic-uremic syndrome. The mechanisms by which O157 VTEC strains cause disease are not completely understood. Virulence factors contributing to the pathogenesis include the production of either or both of two phage-encoded toxins (VT1 and VT2) which are thought to cause the vascular endothelial damage observed in patients with hemorrhagic colitis and the hemolytic-uremic syndrome (34) and probably the formation of attaching-and-effacing lesions in the intestine of the host, as observed in experimentally infected animals (23). Since healthy domestic animals, in particular, ruminants like cattle, sheep, and goats, can harbor O157 VTEC and other VTEC in their feces, they are regarded as natural reservoirs of these organisms (3). O157 VTEC can be transmitted to humans through direct or indirect contamination of foods by fecal material. Undercooked ground beef and raw milk have most often been implicated in food-borne infections (1). In addition to consumption of contaminated foods, humans can become infected by direct transmission of O157 VTEC from infected animals or by secondary spread from person to person (1, 5).
Reported estimates of the prevalence of O157 VTEC in North American and European cattle range from 0 to almost 10% (1). The isolation rate is greatly influenced by factors such as the target population, the sampling strategy, and the screening method used. Furthermore, geographic and seasonal variations in prevalence may occur. Although knowledge about O157 VTEC has expanded, the interaction between these agents and the farm environment remains poorly understood. At present, data on the occurrence of O157 VTEC in cattle in The Netherlands are limited. In a recent survey, we isolated fecal O157 VTEC from approximately 10% of Dutch adult cattle (mainly dairy cattle) and from 0.5% of Dutch veal calves sampled at the major slaughterhouses of the country (15). An epidemiological survey on the occurrence of O157 VTEC in Dutch dairy herds has never been undertaken.
The aim of the present study was to investigate the occurrence of O157 VTEC on Dutch dairy farms. Following a point prevalence study on 10 farms, we conducted a longitudinal study on the maintenance and dissemination of O157 VTEC on 4 of these 10 farms. It was determined if fecal excretion of the pathogens by cattle was affected by age and season. Also, the distribution of different O157 VTEC strain types within a farm and within an animal and the length of shedding were studied.
MATERIALS AND METHODS
Study design.
Ten dairy farms were selected for the present study: five farms (farms I to V) were randomly chosen from a list of farms which in August 1996 had delivered for slaughter cattle that tested positive for fecal O157 VTEC, and five farms were randomly chosen from a list of farms which in August 1996 had delivered for slaughter cattle that tested negative for fecal O157 VTEC (farms VI to X) (15). The farms were visited during the period from September 1996 through November 1996. In a follow-up study, two farms that were O157 VTEC positive and two farms that were O157 VTEC negative on the initial sampling were revisited five times at intervals of approximately 3 months.
Collection of samples.
At each visit, attempts were made to sample all cattle present on the farm individually by digital rectal retrieval (ca. 50 g of feces). However, during the summer months no samples could be obtained by rectal palpation from cattle that were continuously at pasture. To identify individual cattle, ear tag numbers were recorded at the time that the feces were taken. Other animals, milk filters, silage, and water were randomly sampled at each visit. All samples collected were transferred to sterile containers and were immediately transported to the laboratory, where the microbiological examination was started within 20 h.
Isolation of O157 VTEC.
A 20-g portion of each sample was homogenized in a stomacher for 1 min with 180 ml of modified E. coli broth containing novobiocin (20 mg/liter; Sigma Chemical Co., St. Louis, Mo.) (mEC+n) (26). Milk filters and stable flies (one composite sample of about 30 flies per farm) were added to flasks containing 180 ml of mEC+n without being weighed and homogenized. After 6 to 8 h of incubation at 37°C on a rotary shaker (100 rpm), about 5 ml of each mEC+n culture was filtered through a piece of paper towel to remove particulate matter. Then, 1 ml of filtrate was added to 20 μl of magnetic beads coated with antibody to O157 (Dynal, Oslo, Norway), and immunomagnetic separation was performed according to the manufacturer’s instructions. Cultures containing samples of water were not filtered prior to immunomagnetic separation. The concentrates finally obtained were inoculated onto sorbitol-MacConkey agar (SMAC; Oxoid Ltd., Basingstoke, England) supplemented with cefixime (0.05 mg/liter) and potassium tellurite (2.5 mg/liter) (Dynal) (38). The plates were incubated at 37°C for 18 to 20 h. Sorbitol-nonfermenting colonies (up to 12 per sample) were selected for confirmation. The isolates were inoculated onto Levine’s eosin methylene blue agar (L-EMB; Oxoid) and onto SMAC supplemented with 4-methylumbelliferyl-β-d-glucuronide (MUG; 0.1 g/liter; Sigma) (25). Presumptive O157 VTEC isolates (typical E. coli metallic sheen on L-EMB and both sorbitol nonfermenting and β-glucuronidase negative on SMAC-MUG) were tested for agglutination with an E. coli O157 latex test kit (Oxoid). Isolates that gave a positive latex test result were confirmed to be E. coli by using an API 20E biochemical test strip (bioMérieux, Lyon, France) and were confirmed to be of serotype O157:H7 or serotype O157:H− (nonmotile) by serotyping at the National Institute of Public Health and the Environment, Bilthoven, The Netherlands (W. J. van Leeuwen). A maximum of three confirmed isolates from each positive sample were stored in glycerol-containing (10%) medium at −70°C. Recovery experiments demonstrated that O157 VTEC could be detected at inoculum levels of about 1 organism g−1 of feces (15).
Characterization of O157 VTEC isolates.
A single isolate from each of the positive samples was further characterized. Toxin production was determined by Vero cell culture assay. Colony sweeps of the isolates were grown overnight at 37°C (100 rpm) in Penassay broth (antibiotic medium 3; Difco Laboratories, Detroit, Mich.) containing mitomycin (0.2 mg/liter). Supernatants obtained by centrifuging the cultures at 10,000 × g for 10 min were filtered through 0.2-μm-pore-size membrane filters (Schleicher & Schuell, Dassel, Germany). Volumes (50 μl) of serial twofold dilutions of the filtrates were applied to confluent Vero cell monolayers and were evaluated for toxic activity as described by Karmali et al. (18). Toxin type and the presence of the E. coli attaching-and-effacing (eae) gene were determined by a multiplex PCR assay as described previously (16). Isolates were phage typed at the Laboratory for Enteric Pathogens, Central Public Health Laboratory, London, United Kingdom (B. Rowe). Genomic typing of the O157 VTEC isolates was performed by pulsed-field gel electrophoresis (PFGE) as described previously (15). Genomic DNAs were digested in agarose plugs with XbaI (10 U; Boehringer Mannheim, Mannheim, Germany). The resulting fragments were resolved by contour-clamped homogeneous electric field (CHEF) PFGE with a CHEF DR-II apparatus (Bio-Rad Laboratories, Richmond, Calif.) at a constant voltage of 200 V for 24 h at 13°C and a linearly ramped pulse time of 3 to 50 s. Interpretation of the PFGE patterns was performed by visual inspection. Patterns that differed by one or more fragment differences were considered to be different and were coded with different letters.
Statistical analysis.
In the prevalence study, we explored the dependence of the risk of being infected with O157 VTEC on the age of the cattle. Therefore, the animals were classified into the following groups: calves younger than 4 months, calves ages 4 to 12 months, heifers ages 1 to 2 years, cows ages 2 to 3 years, and adult cows older than 3 years. Because some farms may be more heavily infected than others, the risk of being infected may cluster within farms (the “units of observation”). To take this clustering into account, we carried out a logistic regression for dependent observations using the method of generalized estimating equations (GEE) (10). The GEE analysis was performed by the PROC GENMOD procedure in SAS release 6.12 (SAS Institute, Cary, N.C.). In the follow-up study, the units of observation were the individual cattle. Similarly, logistic regression by GEE was performed. The risk factors for O157 VTEC infection considered were season (analyzed per quarter), age (classified as described above), and farm. To confirm our analyses, we also tried to address the questions stated above using the conditional logistic regression (CLR) approach (33), with infection as the outcome variable. The CLR analysis was performed by the PROC PHREG procedure in SAS release 6.12. In the prevalence study, we used farm as a stratifying variable and age (classified as described above) as a covariable. In the follow-up study, we stratified by both individual animal and age to analyze the effect of season. Thus, cattle which were observed only once during the same age period were excluded from analysis. Similarly, cattle which were always either positive or negative during the same age period were also excluded from analysis. Analysis of the effect of age by CLR did not yield interpretable results because of the low number of usable observations after stratifying by both individual animal and season.
RESULTS
Prevalence study.
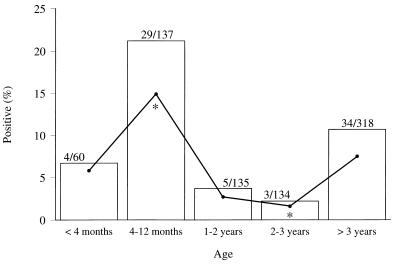
Cattle on 7 of the 10 dairy farms selected tested positive for O157 VTEC (Table 1). The proportion of cattle infected on the farms that yielded positive samples varied from 0.8 to 22.4%. None of the O157 VTEC-positive animals had diarrhea. Within the seven positive farms, the excretion rate was highest in calves ages 4 to 12 months (21.7%), with the prevalence in calves younger than 4 months of age being 6.7%, that in heifers ages 1 to 2 years being 3.7%, that in cows ages 2 to 3 years being 2.2%, and that in cattle older than 3 years being 10.7% (Fig. 1). By performing logistic regression by using GEE, only the prevalence of O157 VTEC excretion for calves ages 4 to 12 months was found to be statistically significantly higher (P < 0.05) than the prevalence of O157 VTEC excretion for cows ages 2 to 3 years (Fig. 1). By CLR, the following significant differences (P < 0.05) in excretion rates were found: the excretion rate of O157 VTEC for calves ages 4 to 12 months appeared to be statistically significantly higher than that for animals in all other age groups, and cattle older than 3 years were statistically significantly more often infected than heifers ages 1 to 2 years and cows ages 2 to 3 years (data not shown).
TABLE 1.
Isolation and characterization of fecal O157 VTEC strains from cattle from the 10 Dutch dairy farms investigated in the prevalence study
| Farm | Date of sampling | No. of cattle O157 VTEC positive/total no. of cattle (%) | Phage type | Result of Vero cell assay for VT | PCR results for the following:
|
PFGE pattern | No. of isolates | ||
|---|---|---|---|---|---|---|---|---|---|
| VT1 | VT2 | eae | |||||||
| I | Sept 1996 | 27/140 (19.3) | 34 | + | + | + | + | A | 23 |
| 34 | + | − | + | + | A | 2 | |||
| 31 | + | − | + | + | B | 1 | |||
| Rdnca | + | + | + | + | C | 1 | |||
| II | Sept 1996 | 15/67 (22.4) | 8 | + | + | + | + | D | 15 |
| III | Sept 1996 | 7/112 (6.3) | 31 | + | − | + | + | E | 7 |
| IV | Oct 1996 | 3/162 (1.9) | Rdnc | + | − | + | + | F | 3 |
| V | Oct 1996 | 17/83 (20.5) | 14 | + | − | + | + | G | 17 |
| VI | Oct 1996 | 1/120 (0.8) | 14 | + | − | + | + | H | 1 |
| VII | Nov 1996 | 5/100 (5.0) | 8 | + | + | + | + | I | 5 |
| VIII | Nov 1996 | 0/100 (0.0) | |||||||
| IX | Nov 1996 | 0/195 (0.0) | |||||||
| X | Nov 1996 | 0/73 (0.0) | |||||||
Rdnc, reacted with the phage set but did not correspond to a recognized phage type.
FIG. 1.
Fecal excretion of O157 VTEC by cattle from the seven farms that tested positive in the prevalence study relative to age of cattle. □, percentage of cattle found to be O157 VTEC positive in the age group; •, estimated percentage of O157 VTEC-positive cattle in the age group by GEE analysis; *, statistically significantly different (P < 0.05). Values above the bars are number of cattle found to be O157 VTEC positive/total number of cattle examined in the age group.
A single isolate from each of the 75 positive cattle was further characterized (Table 1). It appeared that isolates obtained from different farms were of distinct O157 VTEC strain types and isolates obtained from different cattle on the same farm were generally of the same strain type. However, among the isolates from farm I four distinct strain types were identified. To determine if different O157 VTEC strain types were simultaneously present in the two animals from which the strains characterized by PFGE patterns B and C were isolated, we also performed PCR and PFGE with the other two O157 VTEC isolates from each of these two animals. For both animals it appeared that all three isolates were of identical strain types (data not shown). Additionally, we performed a PCR with the other two O157 VTEC isolates from each of the two animals from which the VT2-producing isolates characterized by PFGE pattern A were isolated. It appeared that these isolates also contained VT2 genes only, with the exception that one isolate was positive for both the VT1 and VT2 genes (data not shown).
In addition to fecal samples from cattle, a total of 63 other samples were examined for the presence of O157 VTEC: droppings from pigs (n = 32), horses (n = 4), one pony, one sheep, one dog, and one rabbit; one sample of pig’s dung; milk filters (n = 7); samples of silage (n = 8); and samples of animal drinking water (n = 7). O157 VTEC strains were isolated from only one of the horses, the one sheep, and one of the milk filters (data not shown). The positive horse sample originated from farm II, and the positive sheep sample and milk filter originated from farm V. The isolates were of the same strain types as the cattle isolates obtained from the respective farms, as determined by phage typing, the Vero cell assay, the PCR assay, and PFGE.
Follow-up study.
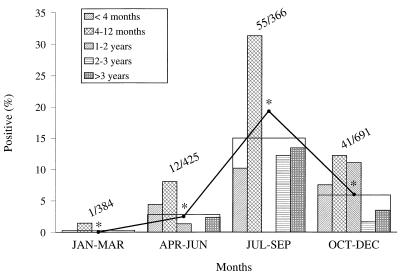
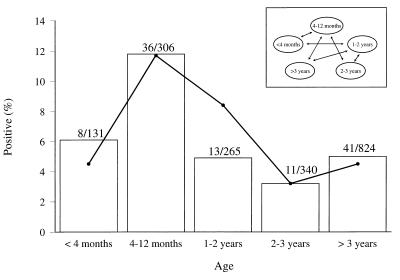
Two farms with cattle positive on the initial sampling (farm II and farm V) and two farms with cattle negative on the initial sampling (farm VIII and farm X) were revisited five times. However, farm X could not be visited in the early summer of 1997 since at the time classical swine fever was prevalent in the part of the country where farm X was located. The results of the isolation of O157 VTEC strains from cattle are summarized in Table 2. Table 3 presents the results for additional samples collected at the four farms. During the study period, the proportion of cattle infected on the farms varied from 0 to 61.0% (Table 2). None of the O157 VTEC-positive animals had diarrhea. Overall, 86 cattle were sampled once, 58 cattle were sampled twice, 82 cattle were sampled three times, 109 cattle were sampled four times, 104 cattle were sampled five times, and 77 cattle were sampled six times (data not shown). Ninety-three cattle tested positive for O157 VTEC: 78 had a single positive sample, 14 had two positive samples, and 1 had three positive samples. Six of the 14 cattle with two positive samples excreted the pathogens on two consecutive samplings. The remaining cattle with two positive samples tested negative at two or more samplings carried out in the period between the positive ones. The one animal with three positive samples had alternately positive and negative samples. Excretion rates peaked in summer and were lowest in winter (Fig. 2). Both by the GEE method (Fig. 2) and by the CLR method (data not shown), the quarterly differences in excretion rates were found to be statistically significant (P < 0.05). However, because of the low number of usable observations, the period from January to March did not yield interpretable results when the CLR method was used. Within each age group, shedding varied with a similar seasonal pattern (Fig. 2). However, heifers ages 1 to 2 years could not be sampled individually in the period from July to September because they were continuously at pasture. In agreement with the results of the prevalence study, the highest rate of excretion was observed in calves ages 4 to 12 months (11.8%) (Fig. 3). The highest prevalence in this group of animals was consistent in all seasons (Fig. 2). The results of the isolation of O157 VTEC from fecal samples obtained from cattle that could not be identified individually are included in Table 3. These samples were taken from cattle wearing no ear tag and from fresh cowpats in farm buildings (adult steers) and in pastures (heifers). Fourteen of the 16 positive samples were obtained from farm X, which previously always tested negative. The 18 samples from farm X were taken from the inner part of fresh cowpats in adjacent pastures in which heifers ages 1 to 2 years were grazing. In addition to feces from cattle, feces from horses, ponies, and sheep also occasionally tested positive for O157 VTEC (Table 3). Furthermore, the pathogens were isolated from milk filters and stable flies. O157 VTEC strains were not isolated from any of the fecal samples from pigs or from any of the samples of silage and water.
TABLE 2.
Isolation and characterization of fecal O157 VTEC strains from cattle from the four Dutch dairy farms investigated in the follow-up study
| Farm | Date of sampling | No. of cattle O157 VTEC positive/total no. of cattle (%) | Phage type | Result of Vero cell assay for VT | PCR results for the following:
|
PFGE pattern | No. of isolates | ||
|---|---|---|---|---|---|---|---|---|---|
| VT1 | VT2 | eae | |||||||
| II | Sept 1996 | 15/67 (22.4) | 8 | + | + | + | + | D | 15 |
| Dec 1996 | 8/106 (7.5) | 8 | + | + | + | + | J | 7 | |
| 8 | + | + | + | + | D | 1a | |||
| Mar 1997 | 0/99 (0.0) | ||||||||
| Jun 1997 | 0/99 (0.0) | ||||||||
| Sept 1997 | 36/59 (61.0) | 21 | + | − | + | + | K | 35b | |
| 54 | + | − | + | + | L | 1 | |||
| Nov 1997 | 15/104 (14.4) | 21 | + | − | + | + | K | 15c | |
| V | Oct 1996 | 17/83 (20.5) | 14 | + | − | + | + | G | 17 |
| Jan 1997 | 1/83 (1.2) | 14 | + | − | + | + | G | 1d | |
| Apr 1997 | 2/79 (2.5) | 4 | + | − | + | + | M | 2e | |
| Jun 1997 | 9/52 (17.3) | 14 | + | − | + | + | N | 8f | |
| 48g | + | − | + | + | N | 1 | |||
| Sept 1997 | 2/38 (5.3) | 14 | + | − | + | + | O | 1h | |
| 54 | + | − | + | + | P | 1 | |||
| Nov 1997 | 0/41 (0.0) | ||||||||
| VIII | Nov 1996 | 0/100 (0.0) | |||||||
| Jan 1997 | 0/129 (0.0) | ||||||||
| Apr 1997 | 1/131 (0.8) | 4 | + | − | + | + | M | 1 | |
| Jul 1997 | 0/99 (0.0) | ||||||||
| Sept 1997 | 0/81 (0.0) | ||||||||
| Nov 1997 | 0/110 (0.0) | ||||||||
| X | Nov 1996 | 0/73 (0.0) | |||||||
| Feb 1997 | 0/73 (0.0) | ||||||||
| Apr 1997 | 0/64 (0.0) | ||||||||
| Sept 1997 | 2/22 (9.1) | 34 | + | − | + | + | Q | 2 | |
| Nov 1997 | 1/74 (1.4) | 34 | + | − | + | + | Q | 1 | |
Isolate from a calf 4 to 12 months of age which also tested positive in September 1996.
Including two isolates from adult cows >3 years of age which also tested positive in September 1996 and three isolates from cattle ages 2 to 3 years which also tested positive in December 1996.
Including one isolate from a calf younger than age 4 months and three isolates from calves 4 to 12 months of age which all also tested positive in September 1997, one isolate from a heifer 1 to 2 years of age which also tested positive in December 1996, and one isolate from a heifer 1 to 2 years of age which also tested positive in September 1996.
Isolate from a calf 4 to 12 months of age which also tested positive in October 1996.
Including an isolate from an adult cow which also tested positive in October 1996.
Including an isolate from a calf 4 to 12 months of age which also tested positive in October 1996.
Phage type 48 differs from phage type 14 in only one phage reaction.
Isolate from an adult cow which also tested positive in October 1996 and April 1997.
TABLE 3.
Isolation and characterization of O157 VTEC strains from additional samples collected at the four Dutch dairy farms investigated in the follow-up study
| Farm | Date of sampling | No. of samples O157 VTEC positive/no. of samples tested
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cattlea | Pigs | Horses | Ponies | Sheep | Stable flies | Milk filter | Silage | Animal drinking water | ||
| II | Sept 1996 | 1bc/2bc | 0/1 | |||||||
| Dec 1996 | 1d/1 | |||||||||
| Mar 1997 | 0/1 | 0/4 | 0/1 | 0/1 | 0/1 | |||||
| Jun 1997 | 0/1 | 0/2 | 0/1 | |||||||
| Sept 1997 | 0/1 | 3ec/3ec | 1e/1 | 1e/1 | 1e/1 | 0/1 | ||||
| Nov 1997 | 0/2 | 0/3 | 0/1 | 0/1 | 0/2 | 0/2 | ||||
| V | Oct 1996f | 0/10 | 1g/1 | 1g/1 | 0/2 | 0/1 | ||||
| Jan 1997h | 0/2i | 0/6 | 4g/5 | 0/1 | 0/3 | |||||
| Apr 1997 | 0/3j | 0/3 | 0/5 | 0/1 | ||||||
| Jun 1997 | 1k,l/5 | 0/5 | 1l/4 | 0/1 | ||||||
| Sept 1997 | 0/5m | 0/4 | 0/1 | 0/1 | ||||||
| Nov 1997 | 1n/7 | 0/5 | 0/2 | 0/9 | 0/1 | 0/2 | ||||
| VIII | Nov 1996 | 0/15 | 0/1 | 0/1 | 0/1 | |||||
| Jan 1997 | 0/1 | 0/1 | ||||||||
| Apr 1997 | 0/1 | 0/5 | 0/1 | 0/1 | ||||||
| Jul 1997 | 0/5 | 0/1 | 0/1 | |||||||
| Sept 1997 | 0/1 | 0/3 | 0/1 | 0/1 | 0/1 | |||||
| Nov 1997 | 0/5 | 0/1 | 0/1 | 0/1 | ||||||
| X | Nov 1996 | 0/7 | 0/1 | 0/1 | 0/1 | |||||
| Feb 1997 | 0/4 | 0/1 | 0/1 | 0/1 | ||||||
| Apr 1997 | 0/3 | 0/1 | 0/1 | |||||||
| Sept 1997 | 14o/18 | 0/4 | 0/1 | 0/2 | ||||||
| Nov 1997 | 0/1 | 0/1 | 0/1 | |||||||
Fecal samples from cattle which could not be identified individually since either the cattle wore no ear tag numbers or the samples were taken from fresh droppings in farm building (adult steers) or in pastures (heifers).
The isolate was characterized as being phage type 8, Vero cell assay positive, VT1 positive, VT2 positive, eae positive, and PFGE pattern D.
Including one fowl.
The isolate was characterized as being phage type 32, Vero cell assay positive, VT1 positive, VT2 positive, eae positive, and PFGE pattern S.
The isolates were characterized as being phage type 21, Vero cell assay positive, VT1 negative, VT2 positive, eae positive, and PFGE pattern K.
Additionally, fecal samples from a dog, a rabbit, and the pig’s dung were collected. All tested negative.
The isolates were characterized as being phage type 14, Vero cell assay positive, VT1 negative, VT2 positive, eae positive, and PFGE pattern G.
Additionally, fecal samples from a dog and the pig’s dung were collected. Both tested negative.
Including one adult steer.
All adult steers.
An adult steer.
The isolate was characterized as being phage type 14, Vero cell assay positive, VT1 negative, VT2 positive, eae positive, and PFGE pattern N.
Including three steers.
Sample taken from a fresh dropping in the pasture. The isolate was characterized as being phage type 14, Vero cell assay positive, VT1 negative, VT2 positive, eae positive, and PFGE pattern N.
Samples taken from fresh droppings in the pasture. Twelve of the isolates were characterized as being phage 34, Vero cell assay positive, VT1 negative, VT2 positive, eae positive, and PFGE pattern Q, and the remaining two were characterized as being phage type 2, Vero cell assay positive, VT1 negative, VT2 positive, eae positive, and PFGE pattern T.
FIG. 2.
Fecal excretion of O157 VTEC by cattle from the four farms examined in the follow-up study relative to season and age of cattle. Heifers ages 1 to 2 years could not be sampled in the period from July to September because they were continuously at pasture. Bars, total percentage of cattle found to be O157 VTEC positive in the quarter; •, estimated total percentage of O157 VTEC-positive cattle in the quarter by GEE analysis; *, statistically significantly different (P < 0.05). Values above the bars are number of cattle found to be O157 VTEC-positive/total number of cattle examined in the quarter.
FIG. 3.
Fecal excretion of O157 VTEC by cattle from the four farms examined in the follow-up study relative to age of cattle. □, percentage of cattle found to be O157 VTEC positive in the age group; •, estimated percentage of O157 VTEC-positive cattle in the age group by GEE analysis; ↔, statistically significant difference (P < 0.05) between estimated percentage of O157 VTEC-positive cattle in the age groups. Values above the bars are number of cattle found to be O157 VTEC positive/total number of cattle examined in the age group.
A single isolate from each of the positive samples was further characterized (Table 2 and Table 3). Again, it appeared that isolates obtained from different farms were of distinct O157 VTEC strain types. However, the two isolates from farm V and the one isolate from farm VIII isolated in April 1997 could not be distinguished (Table 2). Sometimes, more than one strain type was present simultaneously within a farm, but one type was predominant. The six cattle that tested positive on sequential samplings appeared to excrete isolates of a consistent strain type during the 3-month period (Table 2, footnotes a, c, and d). The remaining cattle with more than one positive sample excreted isolates of different strain types on different samplings (Table 2, footnotes b, c, e, f, and g). To determine if different O157 VTEC strain types were simultaneously present in the animals, we characterized the remaining two stored isolates for a selection of animals from farm II and farm V: the animal from which the strain characterized by PFGE pattern D was isolated in December 1996 (Table 2), the animal from which the strain characterized by PFGE pattern L was isolated in September 1997 (Table 2), the animal from which the strain characterized by PFGE pattern S was isolated in December 1996 (Table 3, footnote d), the two animals from which the two strains characterized by PFGE pattern M were isolated in April 1997 (Table 2), and the two animals from which the strains characterized by PFGE pattern O and PFGE pattern P were isolated in September 1997 (Table 2). For each of these selected animals, all three strains isolated from the same fecal sample were identified as being identical strain types (data not shown).
DISCUSSION
Our findings support those of previous farm studies indicating that the prevalence of O157 VTEC excretion is higher in immature cattle than in adult cattle (13, 14, 24, 36). The higher prevalence in younger animals is consistent with the higher numbers (numbers of CFU gram−1) and the longer duration of shedding observed in calves experimentally infected with O157 VTEC than in adult animals (9). The differences in excretion rates between age groups may be attributed to age-related differences in rumen function. Adult cattle possess a fully developed rumen, the largest of the four chambers of a ruminant’s stomach, where the combination of high concentrations of volatile fatty acids and a low pH inhibit the growth of O157 VTEC (30). Furthermore, the differences in excretion rates may reflect differences in diet, immune response, aspects of cattle management, or other unknown factors. While immature cattle are fed on a high-roughage diet, lactating cows are fed on a diet supplemented with concentrates high in nutrients. The diet of the youngest calves, those younger than 4 months, initially consists of milk or milk replacer, which over the course of time will gradually be replaced with roughage. The influence of diet on shedding of O157 VTEC by ruminants has been clearly demonstrated by experiments with experimentally inoculated sheep (20, 21). Kudva and colleagues (20, 21) hypothesized that diets high in nutrients and low in fiber induce a lower incidence of transmission and/or shedding of fewer O157 VTEC cells but do not induce clearance of the organisms from the intestine. Conversely, diets low in nutrients and high in fiber induce shedding of larger numbers of O157 VTEC and/or increased susceptibility to new intestinal colonization but also induced elimination of the organisms. High-nutrient and low-fiber feeds increase the concentrations of volatile fatty acids and decrease the pH in the ruminant gut, while low-nutrient and high-fiber feeds have the opposite effect. The relatively low rate of excretion observed in the youngest calves is consistent with the findings of Garber and colleagues (12). They reported that calves ages 8 weeks or older were three times more likely to shed O157 VTEC than calves less than 8 weeks old.
The seasonal variation in the prevalence of O157 VTEC-positive cattle observed in the follow-up survey has also been reported by others (7, 13, 14, 24). While younger cattle often were kept indoors year-round, cattle ages 1 year and older were at pasture from early summer through midautumn. Lactating cows were moved indoors only at the time of milking. The seasonal movements of cattle into farm buildings and out to pasture were accompanied by changes in diet. When cattle were kept indoors they were fed relatively more feeds high in nutrients, besides their regular diet based on silage, than when they were grazing in pastures. Referring to the findings of Kudva et al. (20, 21), these dietary changes may have contributed to the seasonal variation in O157 VTEC-positive cattle. In the course of the grazing season the risk of infection of animals that are at pasture will be increased because the pastures will increasingly be contaminated with feces from animals occupying the pastures. Furthermore, the warmer and more moist conditions of the summer months may favor the survival and growth of O157 VTEC in the environment.
No overlapping strain types were identified among isolates from different farms, with the exception of one: two isolates from farm V and the one isolate from farm VIII could not be distinguished. Although more than one distinct strain type was sometimes present simultaneously within a farm, one type always clearly predominated. Since VT production is encoded by phages, VT profiles can change over time (17). Therefore, all 25 isolates from farm I characterized by phage type 34 and PFGE pattern A can be considered as being of a single strain type (Table 1). The predominance of a single strain type supports the idea that horizontal transmission among animals is an important factor in the dissemination of O157 VTEC within a farm (11, 20, 21). Generally, calves ages 4 to 12 months were grouped together year-round in close contact indoors. Heifers were either together at pasture or grouped in close contact indoors. The same was true for lactating cows. However, 3 of the 10 farms (farm V, VI, and X) were organized more conventionally, with lactating cows having less close contact together indoors, being continuously individually tied. On all of the 10 farms, calves younger than 4 months of age were kept individually or in small groups of about three animals. Either all cattle shared the same farm building (farms I, VIII, and IX) or immature cattle and lactating cows were kept in separate buildings (farms II to VII and X). Cattle, horses, and sheep alternately occupied the same pastures. Furthermore, the horses on farm II were housed in the same building as cattle younger than 12 months of age. Management factors such as grouping of animals in close contact and communal housing may favor the horizontal transmission of O157 VTEC among animals within a farm (11, 12, 20, 21). Both Faith et al. (11) and Kudva et al. (21) isolated O157 VTEC strains that were identical to the strains isolated from animals from common water troughs. Therefore, they concluded that contaminated animal drinking water may be an important mode of dissemination of O157 VTEC among animals on farms. The isolation of identical O157 VTEC strains from stable flies and animals in the present study (farm II) suggests that flies may also be vehicles for transmission of the pathogens within a farm.
Although it cannot be ruled out, concurrent excretion of different O157 VTEC strains by individual animals was not likely to occur in the present study. The isolates further characterized were randomly selected from the three isolates stored from each sample. Since it appeared that the vast majority of isolates obtained from a single farm on the same date were of the same strain types, we decided to characterize only all three isolates stored from a single sample for a selection of animals (mainly those animals from which the initially selected isolate was not identical to the majority of isolates obtained from that farm on the same date) and found that all three isolates from a single sample were identical. However, Besser and colleagues (2) found multiple PFGE types in 2 of 12 bovine fecal samples from which multiple isolates were typed, with one sample containing two different types and the other sample containing three different types. Also, Faith and coworkers (11) reported that animals may harbor O157 VTEC strains that display different PFGE patterns: 7 of the 29 animals from which multiple isolates were typed appeared to harbor different O157 VTEC strains.
The longest period of excretion identified in the follow-up study was about 3 months. However, most O157 VTEC-positive cattle became culture negative within 3 months. Besser et al. (2) found that the duration of detected excretion of O157 VTEC by individual cattle was less than 1 month for 35 (63%) of 56 cattle. The length of excretion varied from 8 to at least 46 days in a study of Wells et al. (36). Experimental infection studies showed that fecal shedding of O157 VTEC varied widely among cattle of the same age group but persisted longer in calves than in adults. The feces of calves fed 1010 CFU of O157 VTEC were positive for 2 (8 of 8 calves), 7 (8 of 8), 14 (3 of 8), and 20 (2 of 8) weeks postinoculation, and the feces of adult steers were positive for 2 (9 of 9 adults), 7 (2 of 9), and 14 (1 of 9) weeks (9). In the present study, one to six O157 VTEC strain types were identified on each of the four farms over time. The transient nature of excretion by individual animals and the excretion of different strains at different times supports the idea of clearance of O157 VTEC followed by reinfection with different strains (2, 11, 29, 36, 39). However, persistent latent infection cannot be ruled out. The presence of more than one strain type on farms, both simultaneously and over time, suggests the presence of more than one source of O157 VTEC on the farms.
Raw cow’s milk has been associated several times with human O157 VTEC infection (4, 8, 19, 22, 35). The organisms have been isolated from samples of raw milk both from individual cattle (24, 37) and from bulk tanks (27). It has been suggested that the organisms are not being excreted in the milk but that contamination probably results from fecal contamination of milk as it is collected. The isolation of O157 VTEC from milk filters in the present study implies the presence of O157 VTEC in the respective bulk tanks. Based on the risk of the presence of O157 VTEC and other enteric pathogens in raw milk, people need to be strongly dissuaded from consuming raw milk. Finally, there is also a need for awareness that farmed or companion animals can be a direct vehicle of O157 VTEC infection in humans. Close contact with infected calves, horses, goats, and dogs has previously resulted in human infection (6, 28, 31, 32, 35).
By elucidating the epidemiology of O157 VTEC on farms, we hope to eventually identify strategies to reduce the risk of O157 VTEC-positive animals entering the food production system and, as a result, to reduce the risk of O157 VTEC infections in humans. The results of the present study indicate that the O157 VTEC status of a herd cannot be defined by testing only a limited number of cattle on a single visit. Further research is required to identify risk factors which promote fecal shedding of O157 VTEC. In addition, long-term reservoirs remain to be identified. Eventually, this all may lead to changes in farm management practices that may decrease the prevalence of O157 VTEC in farm animals and consequently the number of O157 VTEC-positive animals entering the food production chain.
ACKNOWLEDGMENTS
We thank all the farmers involved in this study for cooperation with this research.
The study was supported by the Prevention Fund (grant 28-2354).
REFERENCES
- 1.Armstrong G L, Hollingsworth J, Morris J G., Jr Emerging foodborne pathogens: Escherichia coli O157:H7 as a model of entry of a new pathogen into the food supply of the developed world. Epidemiol Rev. 1996;18:29–51. doi: 10.1093/oxfordjournals.epirev.a017914. [DOI] [PubMed] [Google Scholar]
- 2.Besser T E, Hancock D D, Pritchett L C, McRae E M, Rice D H, Tarr P I. Duration of detection of fecal excretion of Escherichia coli O157:H7 in cattle. J Infect Dis. 1997;175:726–729. doi: 10.1093/infdis/175.3.726. [DOI] [PubMed] [Google Scholar]
- 3.Beutin L, Geier D, Steinrück H, Zimmermann S, Scheutz F. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J Clin Microbiol. 1993;31:2483–2488. doi: 10.1128/jcm.31.9.2483-2488.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borczyk A A, Karmali M A, Lior H, Duncan L M C. Bovine reservoir for verotoxin-producing Escherichia coli O157:H7. Lancet. 1987;i:98. doi: 10.1016/s0140-6736(87)91928-3. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 5.Boyce T G, Swerdlow D L, Griffin P M. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med. 1995;333:364–368. doi: 10.1056/NEJM199508103330608. [DOI] [PubMed] [Google Scholar]
- 6.Chalmers R M, Salmon R L, Willshaw G A, Cheasty T, Looker N, Davies I, Wray C. Vero-cytotoxin-producing Escherichia coli O157 in a farmer handling horses. Lancet. 1997;349:1816. doi: 10.1016/s0140-6736(05)61697-2. [DOI] [PubMed] [Google Scholar]
- 7.Chapman P A, Siddons C A, Cerdan Malo A T, Harkin M A. A 1-year study of Escherichia coli O157 in cattle, sheep, pigs and poultry. Epidemiol Infect. 1997;119:245–250. doi: 10.1017/s0950268897007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman P A, Wright D J, Higgins R. Untreated milk as a source of verotoxigenic E. coli O157. Vet Rec. 1993;133:171–172. doi: 10.1136/vr.133.7.171. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 9.Cray W C, Jr, Moon H W. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl Environ Microbiol. 1995;61:1586–1590. doi: 10.1128/aem.61.4.1586-1590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diggle P J, Liang K Y, Zeger S L. Analysis of longitudinal data. New York, N.Y: Oxford University Press Inc; 1996. [Google Scholar]
- 11.Faith N G, Shere J A, Brosch R, Arnold K W, Ansay S E, Lee M S, Luchansky J B, Kaspar C W. Prevalence and clonal nature of Escherichia coli O157:H7 on dairy farms in Wisconsin. Appl Environ Microbiol. 1996;62:1519–1525. doi: 10.1128/aem.62.5.1519-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garber L P, Wells S J, Hancock D D, Doyle M P, Tuttle J, Shere J A, Zhao T. Risk factors for fecal shedding of Escherichia coli O157:H7 in dairy calves. J Am Vet Med Assoc. 1995;207:46–49. [PubMed] [Google Scholar]
- 13.Hancock D D, Besser T E, Kinsel M L, Tarr P I, Rice D H, Paros M G. The prevalence of Escherichia coli O157:H7 in dairy and beef cattle in Washington state. Epidemiol Infect. 1994;113:199–207. doi: 10.1017/s0950268800051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock D D, Besser T E, Rice D H, Herriott D E, Tarr P I. A longitudinal study of Escherichia coli O157 in fourteen cattle herds. Epidemiol Infect. 1997;118:193–195. doi: 10.1017/s0950268896007212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heuvelink A E, Van Den Biggelaar F L A M, De Boer E, Herbes R G, Melchers W J G, Huis In ’T Veld J H J, Monnens L A H. Isolation and characterization of verocytotoxin-producing Escherichia coli O157 strains from Dutch cattle and sheep. J Clin Microbiol. 1998;36:878–882. doi: 10.1128/jcm.36.4.878-882.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heuvelink A E, Van De Kar N C A J, Meis J F G M, Monnens L A H, Melchers W J G. Characterization of verocytotoxin-producing Escherichia coli O157 isolates from patients with the haemolytic uraemic syndrome in Western Europe. Epidemiol Infect. 1995;115:1–14. doi: 10.1017/s0950268800058064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karch H, Meyer T, Rüssmann H, Heesemann J. Frequent loss of Shiga-like toxin genes in clinical isolates of Escherichia coli upon subcultivation. Infect Immun. 1992;60:3464–3467. doi: 10.1128/iai.60.8.3464-3467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karmali M A, Petric M, Lim C, Fleming P C, Arbus G S, Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 19.Keene W E, Hedberg K, Herriott D E, Hancock D D, McKay R W, Barrett T J, Fleming D W. A prolonged outbreak of Escherichia coli O157:H7 infections caused by commercially distributed raw milk. J Infect Dis. 1997;176:815–818. doi: 10.1086/517310. [DOI] [PubMed] [Google Scholar]
- 20.Kudva I T, Hatfield P G, Hovde C J. Effect of diet on the shedding of Escherichia coli O157:H7 in a sheep model. Appl Environ Microbiol. 1995;61:1363–1370. doi: 10.1128/aem.61.4.1363-1370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kudva I T, Hunt C W, Williams C J, Nance U M, Hovde C J. Evaluation of dietary influences on Escherichia coli O157:H7 shedding by sheep. Appl Environ Microbiol. 1997;63:3878–3886. doi: 10.1128/aem.63.10.3878-3886.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin M L, Shipman L D, Potter M E, Wachsmuth I K, Wells J G, Hedberg K, Tauxe R V, Davis J P, Arnoldi J, Tilleli J. Isolation of Escherichia coli O157:H7 from dairy cattle associated with two cases of haemolytic uraemic syndrome. Lancet. 1986;ii:1043. doi: 10.1016/s0140-6736(86)92656-5. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 23.McKee M L, Melton-Celsa A R, Moxley R A, Francis D H, O’Brien A D. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect Immun. 1995;63:3739–3744. doi: 10.1128/iai.63.9.3739-3744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mechie S C, Chapman P A, Siddons C A. A fifteen month study of Escherichia coli O157:H7 in a dairy herd. Epidemiol Infect. 1997;118:17–25. doi: 10.1017/s0950268896007194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okrend A J G, Rose B E, Bennett B. A screening method for the isolation of Escherichia coli O157:H7 from ground beef. J Food Prot. 1990;53:249–252. doi: 10.4315/0362-028X-53.3.249. [DOI] [PubMed] [Google Scholar]
- 26.Okrend A J G, Rose B E, Matner R. An improved screening method for the detection and isolation of Escherichia coli O157:H7 from meat, incorporating the 3M Petrifilm™ test kit-HEC for hemorrhagic Escherichia coli O157:H7. J Food Prot. 1990;53:936–940. doi: 10.4315/0362-028X-53.11.936. [DOI] [PubMed] [Google Scholar]
- 27.Padhye N V, Doyle M P. Rapid procedure for detecting enterohemorrhagic Escherichia coli O157:H7 in food. Appl Environ Microbiol. 1991;57:2693–2698. doi: 10.1128/aem.57.9.2693-2698.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parry S M, Salmon R L, Willshaw G A, Cheasty T, Lund L J, Weardon P, Quoraishi A H, Fitzgerald T. Haemorrhagic colitis in child after visit to farm visitor centre. Lancet. 1995;346:572. doi: 10.1016/s0140-6736(95)91407-2. [DOI] [PubMed] [Google Scholar]
- 29.Rahn K, Renwick S A, Johnson R P, Wilson J B, Clarke R C, Alves D, McEwen S, Lior H, Spika J. Persistence of Escherichia coli O157:H7 in dairy cattle and the dairy farm environment. Epidemiol Infect. 1997;119:251–259. doi: 10.1017/s0950268897007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen M A, Cray W C, Jr, Casey T A, Whipp S C. Rumen contents as a reservoir of enterohemorrhagic Escherichia coli. FEMS Microbiol Lett. 1993;114:79–84. doi: 10.1016/0378-1097(93)90145-r. [DOI] [PubMed] [Google Scholar]
- 31.Renwick S A, Wilson J B, Clarke R C, Lior H, Borczyk A A, Spika J, Rahn K, McFadden K, Brouwer A, Copps A, Anderson N G, Alves D, Karmali M A. Evidence of direct transmission of Escherichia coli O157:H7 infection between calves and a human. J Infect Dis. 1993;168:792–793. doi: 10.1093/infdis/168.3.792. [DOI] [PubMed] [Google Scholar]
- 32.Shukla R, Slack R, George A, Cheasty T, Rowe B, Scutter J. Escherichia coli O157 infection associated with a farm visitor centre. Commun Dis Rep. 1995;5:R86–R90. [PubMed] [Google Scholar]
- 33.Stokes M E, Davis C S, Koch G G. Categorical data analysis using the SAS system. Cary, N.C: SAS Institute; 1995. [Google Scholar]
- 34.Tesh V L, O’Brien A D. The pathogenic mechanisms of Shiga toxin and Shiga-like toxins. Mol Microbiol. 1991;5:1817–1822. doi: 10.1111/j.1365-2958.1991.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 35.Trevena W B, Willshaw G A, Cheasty T, Wray C, Gallagher J. Verocytotoxin-producing E. coli O157 infection associated with farms. Lancet. 1996;347:60–61. doi: 10.1016/s0140-6736(96)91593-7. [DOI] [PubMed] [Google Scholar]
- 36.Wells J G, Shipman L D, Greene K D, Sowers E G, Green J H, Cameron D N, Downes F P, Martin M L, Griffin P M, Ostroff S M, Potter M E, Tauxe R V, Wachsmuth I K. Isolation of Escherichia coli serotype O157:H7 and other Shiga-like-toxin-producing E. coli from dairy cattle. J Clin Microbiol. 1991;29:985–989. doi: 10.1128/jcm.29.5.985-989.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright D J, Chapman P A, Siddons C A. Immunomagnetic separation as a sensitive method for isolating Escherichia coli O157 from food samples. Epidemiol Infect. 1994;113:31–39. doi: 10.1017/s0950268800051438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zadik P M, Chapman P A, Siddons C A. Use of tellurite for the selection of verocytotoxigenic Escherichia coli O157. J Med Microbiol. 1993;39:155–158. doi: 10.1099/00222615-39-2-155. [DOI] [PubMed] [Google Scholar]
- 39.Zhao T, Doyle M P, Shere J, Garber L. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl Environ Microbiol. 1995;61:1290–1293. doi: 10.1128/aem.61.4.1290-1293.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]