Abstract
Background
The COVID-19 pandemic has affected the entire global healthcare system, including oncological care. This study investigated the effects of the COVID-19 pandemic on the diagnosis, stage, and treatment of esophagogastric cancer in the Netherlands.
Methods
Patients diagnosed in 2020 were divided into 5 periods, based on the severity of the COVID-19 pandemic in the Netherlands, and compared to patients diagnosed in the same period in the years 2017–2019. Patient characteristics and treatments were evaluated for esophageal cancer (EC) and gastric cancer (GC) separately.
Results
The number of esophagogastric cancer diagnoses decreased prominently during the first 2 months of the COVID-19 pandemic. During this period, a significantly higher percentage of GC patients was diagnosed with incurable disease (52.5% in 2017–2019 and 67.7% in 2020, p = 0.011). We observed a significant reduction in the percentage of patients with potentially curable EC treated with resection and neoadjuvant chemoradiotherapy (from 35.0% in 2017–2019 to 27.3% in 2020, p < 0.001). Also, patients diagnosed with incurable GC were treated less frequently with a resection (from 4.6% in 2017–2019 to 1.5% in 2020, p = 0.009) in the second half of 2020.
Conclusions
Compared to previous years, the number of esophagogastric cancer diagnoses decreased in the first 2 months of the COVID-19 pandemic, while an increased percentage of patients was diagnosed with incurable disease. Both in the curative and palliative setting, patients were less likely to be treated with a surgical resection.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00535-023-02009-3.
Keywords: COVID-19, Esophageal cancer, Gastric cancer, Incidence, Treatment
Introduction
Since December 2019, the Coronavirus Disease 2019 (COVID-19) pandemic caused by the SARS-CoV-2 virus has greatly impacted health care systems around the world. Many resources were preferentially dedicated to COVID-19 patients, forcing clinicians to make difficult triage decisions. In the Netherlands, the first COVID-19 patient was diagnosed on February 27, 2020 [1]. Subsequently, the Dutch government introduced measures to contain the virus spread and to protect its most vulnerable inhabitants [1]. From the second week of March 2020, these measures included social distancing, working from home, and closing schools and restaurants [2]. As in the rest of the world, the surge in COVID-19 patients increased pressure on standard care in the Netherlands. This resulted in major downscaling of standard care, including oncological care, especially during the first outbreak [3]. In April 2020, a significant decrease in the number of cancer diagnoses was observed [4–9]. This decrease was most likely caused by a combination of patient delay and doctor delay [4]. Patient delay occurs when an individual with symptoms postpones seeking medical attention, while doctor delay occurs when symptoms are not diagnosed timely. The latter can be due to postponed physical examinations as consultations were replaced by telehealth consultations, reduced diagnostic evaluation capacities, or halted diagnostic endoscopy programs, all of which occurred during the COVID-19 pandemic.
A diagnostic delay is of particular concern in cancers of the esophagus, esophagogastric junction, and stomach, together also known also as esophagogastric cancer. Esophagogastric cancer has a poor prognosis and accounted for 13.2% of all cancer deaths worldwide in 2020 [10]. Presenting symptoms of this rapidly progressive cancer type include fatigue, dysphagia, and weight loss [11]. Population screening esophagogastroduodenoscopy is not performed in the Netherlands, but screening is sporadically considered in men with chronic gastroesophageal reflux and two more risk factors, such as smoking or central obesity. In addition, screening is recommended every 3–5 years in patients with Barrett’s esophagus. Otherwise, patients are generally referred to a gastroenterologist for an endoscopy based on symptoms, such as anemia, weight loss, or dysphagia [12]. Diagnostic delay in esophagogastric cancer could result in more advanced disease at the time of diagnosis and the associated weight loss may also lead to poor tolerance of treatment with higher chances of complications. For instance, recent research has shown that underweight patients are more prone to develop postoperative complications after esophagectomy [13]. Downscaling during the COVID-19 pandemic may have resulted in postponed or different, potentially suboptimal, treatment options for patients with esophagogastric cancer. Altogether, the COVID-19 pandemic may have had a major impact on the care of patients with esophagogastric cancer. This study aimed to investigate the effects of the COVID-19 pandemic in 2020 on the diagnosis, stage, and type of treatment of esophagogastric cancer in the Netherlands.
Methods
Study population
For inclusion, patients diagnosed with esophageal cancer (EC) or gastric cancer (GC) between January 1, 2017 and December 31, 2020 were selected from the Netherlands Cancer Registry (NCR). The NCR is a nationwide population-based cancer registry that covers the entire Dutch population of more than 17 million people. The NCR is directly linked to the national pathological archive (PALGA) wherein all histologically confirmed cancer diagnoses are registered [14]. Every pathology laboratory in the Netherlands is part of PALGA, and all pathological reports are automatically transferred to the central PALGA database. Trained data managers routinely extract information on diagnosis, patient, tumor, and treatment characteristics from electronic medical records in the hospitals. Patients treated in foreign hospitals were not included for this study as the NCR only registers information from Dutch hospitals. This study included patients with all types of adenocarcinomas of the stomach and the esophagus, and all types of squamous cell carcinomas of the esophagus. Carcinomas not otherwise specified were also included, but patients with a histological diagnosis of a neuro-endocrine tumor or neuro-endocrine carcinoma, lymphoma, GIST, melanoma, or sarcoma were excluded.
Definitions
The year 2020 was divided into 5 periods based on the severity of the COVID-19 pandemic in the Netherlands. Period 1 spanned weeks 1–8 (i.e., before the COVID-19 pandemic); period 2 included weeks 9–12 (after the first COVID-19 case and before the lockdown); period 3 week 13–17 (during lockdown); period 4 weeks 18–26 (after lockdown and during scaling back up hospital care); and period 5 encompassed weeks 27–52 (stabilizing hospital care). Patients were categorized into one of the five time periods based on the date of diagnosis for all analyses.
Esophagogastric cancer was classified into early carcinoma (cT1A or 1B, cN0 or cNX and cM0); potentially curable disease (cT1N + , cT2, cT3, cT4A, or cTX and cM0), palliative disease (cT4B or cM1), and unknown disease severity according to the TNM staging system for analysis of changes in disease stage [15]. Patients were classified in the EC or GC group based on the tumor location originally provided by PALGA. Cardia and junction tumors were classified as EC or GC based on treatment. Patients who underwent gastric resection were classified as GC, whereas patients who underwent esophageal resection and/or received chemoradiotherapy (CRT) and/or received any other treatments were grouped as EC.
In the Netherlands, treatment of esophagogastric cancer is selected based on the guidelines of the Dutch Federation of Medical Specialists [16, 17]. For patients with potentially curable EC, treatment selection is based on tumor stage for potential endoscopic resection, performance status, resectability of the tumor, operability of the patient, and patient preference [16]. Treatments were defined as follows; (1) neoadjuvant chemoradiotherapy with esophageal resection (nCRT-ER), in which patients were treated with chemotherapy and at least 37.8 Gy fractionated radiotherapy followed by resection, (2) definitive chemoradiotherapy (dCRT), in which patients were treated with chemotherapy and more than 41.4 Gy radiotherapy not followed by resection, (3) neoadjuvant chemoradiotherapy without resection (nCRT), in which patients were treated with chemotherapy and 37.8–41.4 Gy radiotherapy not followed by resection, (4) esophageal resection without neoadjuvant chemoradiotherapy (ER), and (5) other treatments.
For patients with potentially curable GC, treatment selection is based on tumor stage, performance status, operability of the patient, and patient preference [17]. Treatments were defined as follows; (1) chemotherapy with gastric resection (CT-GR), in which patients were treated with either neoadjuvant and/or adjuvant chemotherapy of any dosage combined with resection, (2) gastric resection without chemotherapy (GR), (3) chemotherapy without resection (CT), and (4) other treatments.
Survival in patients with incurable disease and a proper performance status can be improved with chemotherapy combined with targeted therapy. Surgery, short-course radiotherapy or placement of stents may be considered to palliate symptoms [16, 17]. Patients with incurable disease were classified into groups based on the most invasive treatment. Therefore, patients were classified either in the group undergoing resection with or without other treatment, the group undergoing chemotherapy (CT) with or without non-concomitant radiotherapy to alleviate symptoms, the group undergoing solely radiotherapy (RT) to alleviate symptoms, or the group undergoing other treatments such as best supportive care.
Time to start treatment was defined as the time from diagnosis until the start of any treatment indicated, including chemo (radio) therapy and resection. The time until direct resection was defined as the time from diagnosis until primary surgery in patients without any (neo) adjuvant treatment. The time until surgery was defined as the time between the end of neoadjuvant treatment and resection.
Statistical analysis
Comparisons were performed between the five periods in 2020 and the corresponding periods in 2017–2019. Tumor characteristics and treatments were displayed as counts and percentages and chi-squared tests were used to compare the percentages between groups. Treatments were compared separately for the potentially curable setting and the palliative setting for both EC and GC. The time between diagnosis, neoadjuvant treatment, and resection was represented as the mean with the standard deviation (SD). Times were assessed separately for the potentially curable and the palliative setting for both EC and GC. Two-sided unpaired Wilcoxon tests were used for comparison between 2017 and 2019 and 2020 for all periods. Two-year overall survival was analyzed using Cox regression per group (potentially curable EC, palliative EC, potentially curable GC, and palliative GC) and correcting for sex, age, comorbidities, and performance status. Survival data was censored after 731 days to ensure that the follow-up data of all patients were comparable. A p < 0.05 was considered statistically significant. All analyses were performed using R version 4.1.1 software [18].
Results
Baseline characteristics
In total, 15,715 patients were included in this study, of whom 9,959 EC patients and 5,756 GC patients (Table 1). Age, histology, stage, and comorbidities were evenly distributed between patients diagnosed in 2020 and patients diagnosed in 2017–2019. In 2020, there were significantly more female EC patients (p = 0.05) and significantly more GC patients had a worse performance status (p = 0.03). Additional patient characteristics for all periods separately are shown in Online Resource 1.
Table 1.
Baseline characteristics of 15.715 included patients with esophageal or gastric cancer
| Esophageal cancer | Gastric cancer | |||||
|---|---|---|---|---|---|---|
| 2017–2019 | 2020 | p-value | 2017–2019 | 2020 | p-value | |
| Patients | 7568 | 2391 | 4325 | 1431 | ||
| Sex | ||||||
| Female | 1945 (25.7) | 664 (27.8) | 0.048 | 1549 (35.8) | 515 (36.0) | 0.931 |
| Male | 5623 (74.3) | 1727 (72.2) | 2776 (64.2) | 916 (64.0) | ||
| Age | ||||||
| < 60 years | 1208 (16.0) | 367 (15.3) | 0.715 | 742 (17.2) | 272 (19.0) | 0.203 |
| 60–74 years | 3978 (52.6) | 1256 (52.5) | 1678 (38.8) | 527 (36.8) | ||
| > 74 years | 2382 (31.5) | 768 (32.1) | 1905 (44.0) | 632 (44.2) | ||
| Histology | ||||||
| Adenocarcinoma | 5542 (73.2) | 1738 (72.7) | 0.866 | 4281 (99.0) | 1410 (98.5) | 0.210 |
| Squamous cell carcinoma | 1966 (26.0) | 633 (26.5) | 0 (0.0) | 0 (0.0) | ||
| Other | 60 (0.8) | 20 (0.8) | 44 (1.0) | 21 (1.5) | ||
| Stage | ||||||
| Early carcinoma | 207 (2.7) | 67 (2.8) | 0.119 | 94 (2.2) | 32 (2.2) | 0.268 |
| Potentially curable | 4783 (63.2) | 1462 (61.1) | 1981 (45.8) | 621 (43.4) | ||
| Incurable | 2572 (34.0) | 857 (35.8) | 2246 (51.9) | 778 (54.4) | ||
| Unknown | 6 (0.1) | 5 (0.2) | 4 (0.1) | 0 (0.0) | ||
| Comorbidities | ||||||
| 0 | 3203 (44.6) | 1056 (45.0) | 0.818 | 1790 (44.1) | 625 (44.3) | 0.844 |
| 1 | 2360 (32.8) | 755 (32.1) | 1326 (32.7) | 468 (33.2) | ||
| 2 or more | 1623 (22.6) | 538 (22.9) | 945 (23.3) | 318 (22.5) | ||
| Performance status | ||||||
| ECOG 0 | 2222 (38.8) | 741 (38.2) | 0.057 | 958 (34.0) | 374 (35.8) | 0.031 |
| ECOG 1 | 2369 (41.4) | 764 (39.4) | 1129 (40.1) | 366 (35.1) | ||
| ECOG 2 | 745 (13.0) | 273 (14.1) | 428 (15.2) | 173 (16.6) | ||
| ECOG 3 or 4 | 385 (6.7) | 160 (8.3) | 302 (10.7) | 131 (12.5) | ||
All data are represented as n (%). Counts for 2017–2019 are shown as the sum of the 3 years. Percentages for 2017–2019 are shown as the average of the 3 years
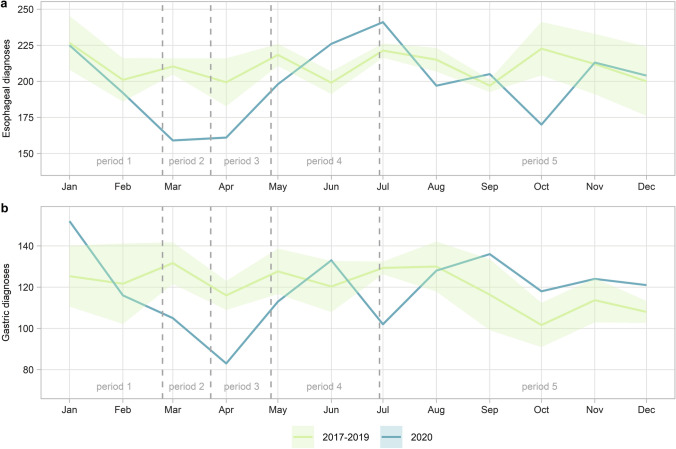
Incidence
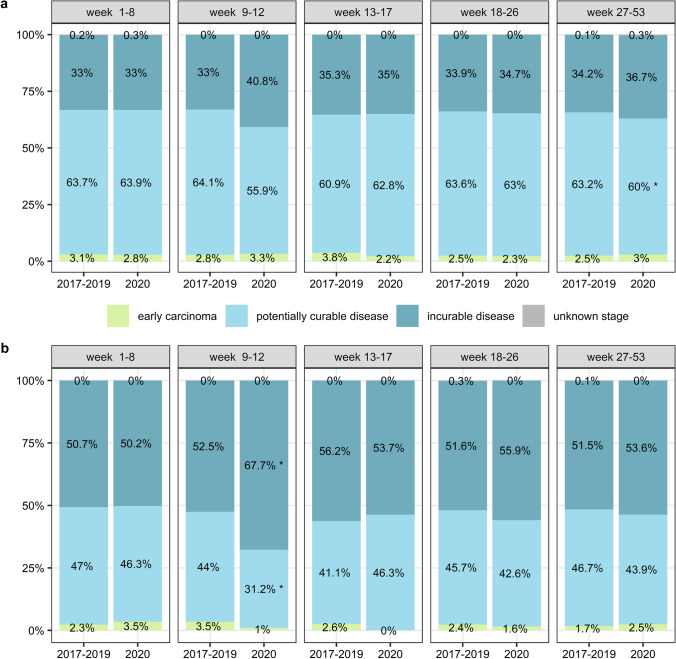
The absolute number of diagnoses decreased most prominently in the periods before and during the social lockdown (Fig. 1). The total incidence of EC in 2020 decreased with 5.2% compared to previous years (n = 2523 average in 2017–2019 and n = 2391 in 2020), whereas the incidence of GC diagnoses remained stable (n = 1442 average in 2017–2019 and n = 1431 in 2020). When analyzing the five separate periods, the percentage of GC patients diagnosed with incurable disease increased significantly (from 52.5% in 2017–2019 to 67.7% in 2020, p = 0.01) after the first COVID-19 patient was diagnosed in the Netherlands (Fig. 2). In the same period, we also observed a relative but not significant increase in EC patients diagnosed with incurable disease (from 33.0% in 2017–2019 to 40.8% in 2020, p = 0.09). No significant difference was found in the distribution of stages of disease at time of diagnosis over the entire year 2020 compared to 2017–2019.
Fig. 1.
Plot of the number of diagnoses in each month for the year 2020 compared to 2017–2019 where a shows the number of esophageal cancer diagnoses and b shows the number of gastric cancer diagnoses
Fig. 2.
Plot of the distribution of cancer stages stratified by the period of diagnosis where a shows the different stages of esophageal tumors and b shows the different stages of gastric tumors
Treatment
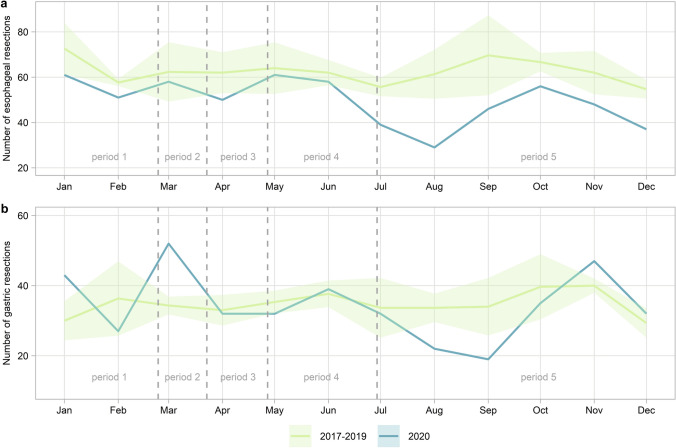
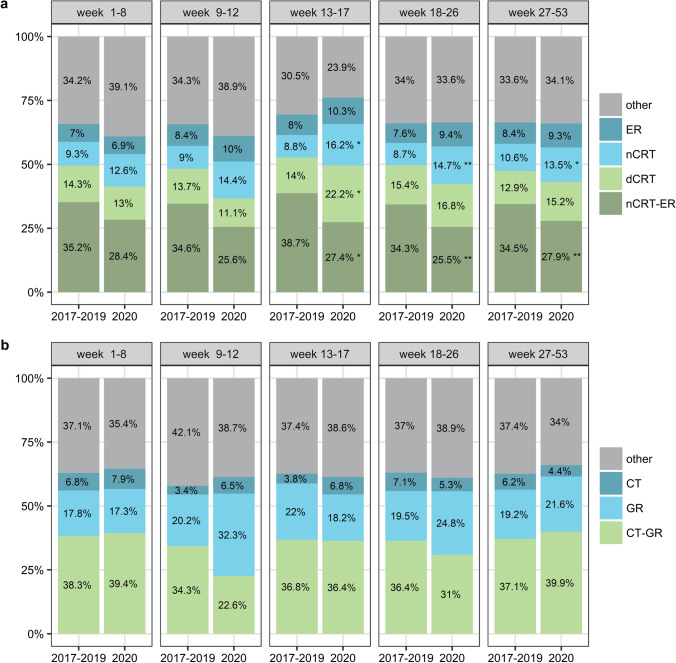
During the second half of 2020, a profound decrease in the number of resections was seen (Fig. 3). Over the entire year of 2020, we observed a significant decrease in the percentage of potentially curable EC patients treated with nCRT-ER (from 35.0% in 2017–2019 to 27.3% in 2020, p < 0.001) and a significant increase in the percentage of patients treated only nCRT (from 9.8% in 2017–2019 to 13.9% in 2020, p < 0.001). The difference in nCRT-ER and nCRT was most prominent in patients diagnosed during and after the lockdown (period 3, 4 and 5, Fig. 4a). At the same time, we found a significant increase in the use of dCRT during the lockdown (period 3, 14.0% in 2017–2019 compared to 22.2% in 2020, p = 0.04). This increase was seen both in patients with histologically diagnosed esophageal squamous cell carcinomas and adenocarcinomas (28.8% in 2017–2019 compared to 35.7% in 2020 and 8.6% in 2017–2019 and 14.7% in 2020 respectively). No difference in the percentage of patients treated with dCRT was observed over the whole year of 2020 compared to 2017–2019 (p = 0.10). For the patients with potentially curable GC, there was no significant difference in treatment over the entire year. However, a similar decreasing trend in CT-GR was observed after the first COVID-19 patient was diagnosed in the Netherlands (period 2, 34.3% in 2017–2019 compared to 22.6% in 2020, p = 0.209) with a concurrent increase in CT or GR alone (Fig. 4b). This concurrent increase is also evident by the peak of gastric resections in March (Fig. 3).
Fig. 3.
Plot of the number of resections in each month where a shows the number of esophageal resections and b shows the number of gastric resections
Fig. 4.
Plot of the overview of treatments given in the curable setting stratified by period of diagnosis where a shows the treatments of patients with potentially curable esophageal cancer and b shows the treatments in patients with potentially curable gastric cancer. nCRT-ER; neoadjuvant chemoradiotherapy with esophageal resection, dCRT; definitive chemoradiotherapy, nCRT; neoadjuvant chemoradiotherapy without resection, ER; esophageal resection without neoadjuvant chemoradiotherapy, CT-GR; gastric resection with (neo)adjuvant chemotherapy, GR; gastric resection without chemotherapy, CT; chemotherapy without resection
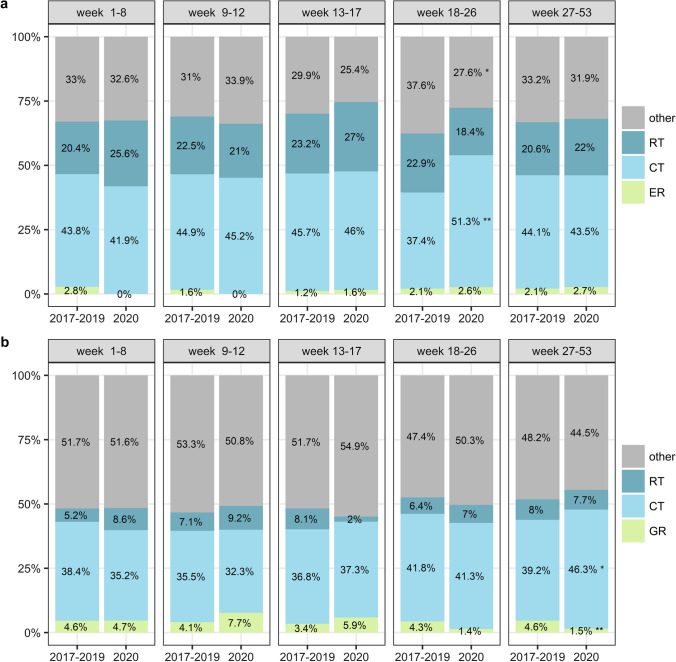
We observed a significant increase in the percentage of EC patients in the palliative setting treated with chemotherapy when healthcare was being upscaled in the Netherlands (period 4, from 37.4% in 2017–2019 to 51.3% in 2020, p = 0.004, Fig. 5a). At the same time, a decrease in the use of other treatments, such as best supportive care, was observed in this patient group (period 4, from 37.6% in 2017–2019 to 27.6% in 2020, p = 0.03, Fig. 5a). A similar pattern was seen in the group of GC patients in the palliative setting with relatively more patients being treated with chemotherapy in the second half of 2020 (period 5, from 39.2% in 2017–2019 to 46.3% in 2020, p = 0.02, Fig. 5b). Alternatively, in that same period, the percentage of patients with GC that underwent resection was significantly lower than in the years before (from 4.6% in 2017–2019 to 1.5% in 2020, p = 0.009, Fig. 5b). Over the whole year of 2020, no significant changes were found in the treatment of patients with incurable disease.
Fig. 5.
Plot of the overview of treatments given in the palliative setting stratified by period of diagnosis where a shows the treatments of patients with incurable esophageal cancer and b shows the treatments in patients with incurable gastric cancer. ER; esophageal resection with or without other treatments, GR; gastric resection with or without other treatments, CT; chemotherapy without resection and with or without other treatments, RT; radiotherapy without resection or chemotherapy
Time intervals
The time to start treatment was significantly shorter during and after the COVID-19 outbreak in the Netherlands both in the potentially curative setting and in the palliative setting for EC (Table 2) and GC patients (Table 3). This was most prominent for GC patients with potentially curable disease just before the lockdown (period 2, from 6.9 weeks in 2017–2019 to 5.0 weeks in 2020, p = 0.04, Table 3). In contrast, the time between nCRT and resection increased after the lockdown for EC patients with potentially curable disease (in period 4 from 11.1 weeks in 2017–2019 to 12.3 weeks in 2020, p = 0.03 and in period 5 from 11.0 weeks in 2017–2019 to 12.3 weeks in 2020, p < 0.001). There was no difference in the time until direct resection for patients suffering from GC or EC in any of the periods in 2017–2019 as compared to 2020.
Table 2.
Time between diagnosis and start treatment and time between neoadjuvant chemoradiotherapy and resection in esophageal cancer patients stratified by period
| Time from diagnosis to start treatment in patients with potentially curable disease | Time from diagnosis to start treatment in patients with incurable disease | Time from diagnosis to direct resection in patients with potentially curable disease | Time from end of nCRT to resection in patients with potentially curable disease | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017–2019 | 2020 | p | 2017–2019 | 2020 | p | 2017–2019 | 2020 | p | 2017–2019 | 2020 | p | |
| Week 1–8 | 6.18 (3.79) | 6.00 (3.46) | 0.539 | 5.00 (3.60) | 5.20 (2.64) | 0.635 | 9.68 (6.53) | 8.54 (6.02) | 0.766 | 11.17 (4.51) | 10.12 (3.31) | 0.058 |
| Week 9–12 | 6.23 (3.70) | 5.44 (2.60) | 0.069 | 4.88 (3.37) | 4.78 (4.10) | 0.881 | 26.75 (13.31) | 15.00 (21.21) | 0.435 | 11.55 (5.09) | 10.75 (4.47) | 0.464 |
| Week 13–17 | 6.74 (4.19) | 5.28 (2.09) | 0.001 | 4.87 (2.66) | 4.46 (2.37) | 0.339 | 11.08 (5.69) | 7.43 (2.65) | 0.320 | 11.82 (4.89) | 11.65 (4.44) | 0.848 |
| Week 18–26 | 6.47 (5.27) | 5.74 (2.81) | 0.034 | 5.23 (2.82) | 4.57 (2.29) | 0.027 | 8.96 (3.68) | 9.18 (6.12) | 0.934 | 11.13 (3.76) | 12.28 (5.16) | 0.027 |
| Week 27–53 | 6.32 (3.56) | 5.95 (3.13) | 0.017 | 4.91 (2.56) | 5.05 (2.83) | 0.418 | 12.05 (9.08) | 11.34 (6.18) | 0.809 | 10.95 (4.16) | 12.32 (4.53) | < 0.001 |
Data for 2017–2019 are shown as the average of the 3 years. All data are reported in number of weeks, represented by mean (SD)
nCRT; neoadjuvant chemoradiotherapy
Table 3.
Time between diagnosis and start treatment and time between neoadjuvant chemotherapy and resection in gastric cancer patients stratified by period
| Time from diagnosis to start treatment in patients with potentially curable disease | Time from diagnosis to start treatment in patients with incurable disease | Time from diagnosis to direct resection in patients with potentially curable disease | Time from end of neoadjuvant chemo to resection in patients with potentially curable disease | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017–2019 | 2020 | p | 2017–2019 | 2020 | p | 2017–2019 | 2020 | p | 2017–2019 | 2020 | p | |
| Week 1–8 | 6.60 (3.76) | 6.23 (3.40) | 0.397 | 5.14 (3.14) | 5.86 (5.04) | 0.186 | 7.85 (4.23) | 7.18 (2.95) | 0.500 | 7.31 (2.56) | 6.57 (3.50) | 0.128 |
| Week 9–12 | 6.93 (4.40) | 4.97 (3.92) | 0.044 | 5.27 (6.29) | 5.40 (3.23) | 0.906 | 6.92 (3.31) | 4.57 (4.75) | 0.080 | 7.54 (3.40) | 5.76 (1.48) | 0.176 |
| Week 13–17 | 7.48 (4.06) | 6.22 (4.17) | 0.116 | 5.63 (3.81) | 4.96 (2.24) | 0.416 | 9.33 (4.87) | 6.56 (6.97) | 0.170 | 7.50 (2.64) | 7.59 (3.61) | 0.914 |
| Week 18–26 | 7.30 (5.12) | 6.41 (2.98) | 0.127 | 6.01 (4.84) | 4.83 (3.52) | 0.048 | 9.04 (6.24) | 6.67 (3.55) | 0.063 | 7.24 (2.51) | 7.59 (2.76) | 0.469 |
| Week 27–53 | 7.22 (4.71) | 6.62 (4.01) | 0.060 | 5.50 (3.79) | 4.86 (2.87) | 0.020 | 7.96 (4.20) | 7.54 (5.53) | 0.505 | 7.24 (2.81) | 7.36 (3.09) | 0.671 |
Data for 2017–2019 are shown as the average of the 3 years. All data are reported in number of weeks, represented by mean (SD)
Overall survival
EC patients diagnosed with incurable disease in the period after the lockdown had a significantly better 2-year overall survival compared to patients diagnosed in the same period in previous years (period 4, HR = 0.78, 95% CI 0.62–0.98, p = 0.04). GC patients diagnosed with curable disease in the period just before the lockdown had a significantly worse 2-year overall survival compared to patients diagnosed in the same period in the previous years (period 2, HR = 1.87, 95% CI 1.03–3.41, p = 0.04). There was no difference in 2-year overall survival for EC patients diagnosed with potentially curable disease or GC patients diagnosed with incurable disease in any of the periods or when comparing the whole year 2020 to 2017–2019 (Online Resource 2).
Discussion
This analysis including data from all hospitals in the Netherlands describes the effect of the COVID-19 pandemic on the incidence, stage, and treatment of patients with esophagogastric cancer. We observed a profound decrease in the number of esophagogastric cancer diagnoses in the first 2 months of the COVID-19 pandemic. Comparable decreases in the incidence of esophagogastric cancer during the pandemic were observed in Japan, Italy, and France. [19–21] Similar to observations made in colorectal cancer, we observed that the decrease in number of GC diagnoses was fully compensated in the second half of 2020. [9] We suspect that reports of fewer cancer diagnoses in the Dutch media, and the call to patients to seek medical care may have boosted this catch-up effect. However, for unknown reasons, this catch-up did not fully compensate for the number of esophageal cancer diagnoses, resulting in a decreased incidence of 5.2% in 2020 compared to previous years. Potentially, the catch-up of EC diagnoses continued in 2021, but we performed a preliminary analysis that did not support this hypothesis.
Among the patients diagnosed during the first COVID-19 wave, a relatively high percentage was diagnosed with incurable gastric cancer, while a relatively low percentage was diagnosed with curable disease. A comparable decline in number of diagnoses and redistribution to higher disease stage was reported in the Netherlands for prostate cancer, breast cancer, and colorectal cancer [7–9]. Potentially, patients with relatively few and not alarming symptoms were less likely to visit a general practitioner during the start of the COVID-19 pandemic. This is supported by the findings of Lantinga et al. [22], who found a decrease in the total number of endoscopies and cancer diagnoses in that period but an increase in the percentage of patients undergoing an endoscopy in whom a malignancy was suspected. Although the percentage of patients diagnosed with curable disease was lower, detection of early carcinoma did not differ between 2017 and 2019 and 2020.
Similar to previous findings in colorectal cancer and across all cancer types, we found a significant impact of the COVID-19 pandemic on the type of treatment given both in the potentially curative setting and in the palliative setting for esophagogastric cancer patients [23, 24]. We detected a decrease in the number of gastric and esophageal resections 5months after the lockdown. This may very well have been a direct result of the decrease in the number of diagnoses during the lockdown, since the time between diagnosis and resection in these patients is often around 5 months. A possible explanation for the decrease in the number of resections could be the increase in the number of interval metastases in patients diagnosed during and after the social lockdown, resulting in an increase in neoadjuvant treatment without surgery.
Another possible explanation for the decrease of treatment with nCRT followed by resection compared to nCRT alone is an increase in wait-and-see approach. The wait-and-see approach was investigated in the Dutch Surgery As Needed for Oesophageal Cancer (SANO) trial, where EC patients with a clinical complete response after nCRT underwent active surveillance (and surgical resection only when residual tumor was found in the absence of distant metastases) [25]. The stepped-wedge design of this trial resulted in more patients being treated with nCRT alone in 2020 compared to the previous years. However, we would expect the effect to be significant in the entire year if the decrease in nCRT with resection would only be explained by the SANO trial. The fact that the decrease in resections was only significant for patients diagnosed during and after the lockdown suggests that the COVID-19 pandemic also impacted treatment. Possibly, there was a reluctance to perform surgical procedures during the pandemic, because these require resources that were redirected to care for COVID-19 patients. There are two separate observations that support this notion. First, we found a relative increase in patients treated with dCRT for patients diagnosed with potentially curable EC compared to nCRT with surgery. Second, we also observed a relative reduction in palliative gastric resections performed in the second half of 2020 for patients diagnosed with incurable GC. Of note, surgical care during the COVID-19 pandemic was not associated with an increase in postoperative complications, neither pulmonary nor other [26, 27].
The differences in treatment during the COVID-19 pandemic did partially influence survival. Patients diagnosed with incurable EC were treated more frequently with chemotherapy in the months after the social lockdown. A concurrent decrease in EC patients receiving other treatments such as best supportive care in the palliative setting was found. This shift in treatment toward chemotherapy resulted in a significantly better 2-year overall survival for EC patients in the palliative setting. As mentioned before, patients diagnosed with incurable GC in the second half of 2020 were less likely to undergo a palliative gastric resection. We observed a concurrent increase in the percentage of incurable GC patients treated with chemotherapy. This decrease in palliative resections did not impact overall survival, as a gastrectomy for advanced gastric cancer is known to have no added survival benefit [28]. For patients with potentially curable GC, there was a decreasing trend in CT-GR with a concurrent increase in GR alone after the first COVID-19 patient was diagnosed in the Netherlands. This increase in GR alone for potentially curable GC is also visible by the peak of GR in March. This shift toward GR alone instead of CT-GR is a possible explanation for the significantly poorer 2-year survival for patients with potentially curable GC diagnosed just before the lockdown. For potentially curable EC patients, there was a significant decrease in nCRT-ER in the second half of 2020 and a concurrent increase dCRT. In spite of this, there was no difference in 2-year overall survival. Treating esophageal cancer with nCRT followed by resection has a 2-year survival of 65% while treatment with dCRT has a 2-year overall survival of 55% [29, 30]. However, this difference increases over time, where nCRT with a resection has a 3-year survival of 58% while treatment with dCRT has a 3-year survival of 42%. Thus, with the current relatively short follow-up, long-term survival differences cannot be ruled out.
We also observed differences in the time to and between treatments. Interestingly, we found that the time between diagnosis and start of treatment was significantly shorter for most patients in 2020. Possibly the decrease in the number of patients diagnosed with esophagogastric cancer allowed for faster scheduling in the outpatient clinic. The clinical relevance of starting treatment 1 week sooner is debatable, as our results also show that overall survival was not different for almost all subpopulations.
While time between diagnosis and start of treatment was significantly shorter, time to surgery after neoadjuvant treatment was significantly longer in the second half of 2020. Since the effect of longer time to surgery was not present during the first half of 2020, it is again unlikely that the SANO trial is the only explanation for the increased time to surgery. Probably, scheduling difficulties occurred in the second half of 2020 due to upscaling of healthcare. In line with the existing literature, we found no impact on overall survival for a longer time to surgery after nCRT [31–33].
This study is the first to show the effect of the COVID-19 pandemic on the incidence, stage, and treatment of esophagogastric cancer. It allowed for a nationwide investigation of not only the incidence and stage of patients with esophagogastric cancer but also the types of treatment that were given. A limitation may be the relatively low number of reference years, which posed some statistical difficulties when comparing the absolute number of diagnoses and resections. We chose these reference years, since they were most comparable due to the changing epidemiology of esophagogastric cancer in the previous years [34]. This approach is also frequently used in other studies [7–9]. Another limitation is the relatively short follow-up data of 1–2 years. It will be interesting to analyze the association between the differences in treatment that are observed in this study and survival data with a longer follow-up.
In conclusion, the incidence of esophagogastric cancer diagnoses decreased significantly during the COVID-19 pandemic in the Netherlands. Relatively more incurable patients with worse performance scores were diagnosed. In addition, we found evidence that fewer surgical resections were performed.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Netherlands Cancer Registry (NCR) for providing the data and their data managers for collecting that data. The authors acknowledge the members of the COVID and Cancer-NL Consortium: Prof. Dr. S. Siesling, Department of Research and Development, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht and Technical Medical Centre, Department of Health Technology and Services Research, Twente University, Enschede; Dr. J.C. van Hoeve, Department of Research and Development, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht; prof. dr. M.A.W. Merkx, Department of Research and Development, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht; IQ Healthcare, Radboud University Nijmegen Medical Centre, Nijmegen; Prof. Dr. N.J. de Wit, Department of General Practice, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht (UMCU), Utrecht University, Utrecht; Dr. C.W. Helsper, Department of General Practice, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht (UMCU), Utrecht University, Utrecht; M.Sc. I. Dingemans, Dutch Federation of Cancer Patient Organisations (NFK), Utrecht; Prof. Dr. I.D. Nagtegaal, Department of Pathology, Radboud University Nijmegen Medical Centre, Nijmegen, on behalf of the Automated Pathology Archive (PALGA); Drs. M. van der Schaaf, Department of Insight and Innovation, Dutch Hospital Data (DHD), Utrecht; Prof. Dr. C.H. van Gils, Department of Clinical Epidemiology, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht (UMCU), Utrecht University, Utrecht; prof. dr. H.C.P.M. van Weert, Department of General Practice, Amsterdam Public Health, Amsterdam UMC location AMC, Amsterdam.
Funding
This study has been funded by ZonMw (Grant No. 10430022010014). The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Declarations
Conflict of interest
Peter Siersema receives unrestricted grants from Pentax (Japan), Norgine (UK), Motus GI (USA), MicroTech (China), and The eNose Company (The Netherlands), and is in the advisory board of Motus GI (USA) and Boston Scientific (USA). All fees paid to the institute and unrelated to the present study. Camiel Rosman has received research grants from Johnson & Johnson and Medtronic. All fees paid to the institute and unrelated to the present study. Nicole C.T. van Grieken reported receiving grants from the Dutch Cancer Society, Cancer Center Amsterdam, and The Netherlands Organisation for Health Research and Development, and serving on an advisory board for Bristol-Myers Squibb and Merck Sharp and Dohme. All fees paid to the institute and unrelated to the present study. Mark I. van Berge Henegouwen is consultant for Mylan, Johnson & Johnson, Alesi Surgical, BBraun, and Medtronic, and received unrestricted research grants from Stryker. All fees paid to the institute and unrelated to the present study. Dr. Maarten F. Bijlsma received research funding from Celgene and Lead Pharma and has acted as a consultant for Servier. All fees paid to the institute and unrelated to the present study. Rob HA Verhoeven has received research grants from Roche and Bristol-Myers Squibb. All fees paid to the institute and unrelated to the present study. M. Verheij reported receiving grants from the Dutch Cancer Society, the Dutch Colorectal Cancer Group, and Hoffmann La Roche. All fees paid to the institute and unrelated to the present study. Prof. Dr. Hanneke W. M. van Laarhoven has served as a consultant or in an advisory role for: Amphera, AstraZeneca, Beigene, BMS, Daiichy-Sankyo, Dragonfly, Eli Lilly, MSD, Nordic Pharma, Servier. Prof. dr. Hanneke W. M. van Laarhoven has received research funding and/or medication supply from: Bayer, BMS, Celgene, Janssen, Incyte, Eli Lilly, MSD, Nordic Pharma, Philips, Roche, Servier. Prof. Dr. Hanneke W. M. van Laarhoven served a speaker role for: Astellas, Benecke, Daiichy-Sankyo, JAAP, Medtalks, Novartis, Travel Congress Management B.V. Prof. Dr. Hanneke W. M. van Laarhoven is employed and performs a managing role at: Amsterdam UMC, The Netherlands (Head of the Department of Medical Oncology) Honorary: ESMO (Chair Upper GI Faculty). None of these parties were involved in the current study. All other authors have no conflict of interest to report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Benthe H. Doeve and Jeanne A. C. Bakx Share first authorship.
Rob H. A. Verhoeven and Hanneke W. M. van Laarhoven Share senior authorship.
References
- 1.RIVM. Patient with novel coronavirus COVID-19 in the Netherlands. 2020. https://www.rivm.nl/node/152811. Accessed 08 Mar 2022.
- 2.Government of the Netherlands. March 2020: Measures against spread of coronavirus, intelligent lockdown. 2020. https://www.government.nl/latest/news/2020/03/12/new-measures-to-stop-spread-of-coronavirus-in-the-netherlands. Accessed 08 Mar 2022.
- 3.van Ballegooijen H, Goossens L, Bruin RH, et al. Concerns, quality of life, access to care and productivity of the general population during the first 8 weeks of the coronavirus lockdown in Belgium and the Netherlands. BMC Health Serv Res. 2021;21:227. doi: 10.1186/s12913-021-06240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinmohamed AG, Visser O, Verhoeven RH, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IKNL. Fewer cancer diagnoses in 2020 due to COVID-19 crisis in the Netherlands: first decrease in thirty years. 2021. https://iknl.nl/en/news/fewer-cancer-diagnoses-in-2020-due-to-covid-19-cri. Accessed 14 Jun 2022
- 6.Uyl-de Groot CA, Schuurman MS, Huijgens PC, et al. Fewer cancer diagnoses during the COVID-19 epidemic according to diagnosis, age and region. TSG Tijdschrift Voor Gezondheidswetenschappen. 2021;99:1–8. doi: 10.1007/s12508-020-00289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eijkelboom AH, de Munck L, Vrancken Peeters M-JTFD, et al. Impact of the COVID-19 pandemic on diagnosis, stage, and initial treatment of breast cancer in the Netherlands: a population-based study. J Hematol Oncol. 2021;14:64. doi: 10.1186/s13045-021-01073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deukeren DV, Heesterman BL, Roelofs L, et al. Impact of the COVID-19 outbreak on prostate cancer care in the Netherlands. Cancer Treat Res Commun. 2022;31:100553. doi: 10.1016/j.ctarc.2022.100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toes-Zoutendijk E, Vink G, Nagtegaal ID, et al. Impact of COVID-19 and suspension of colorectal cancer screening on incidence and stage distribution of colorectal cancers in the Netherlands. Eur J Cancer. 2022;161:38–43. doi: 10.1016/j.ejca.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 11.Arnal MJD, Arenas ÁF, Arbeloa ÁL. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol WJG. 2015;21:7933. doi: 10.3748/wjg.v21.i26.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaheen NJ, Falk GW, Iyer PG, et al. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Off J Am College Gastroenterol ACG. 2016;111:30–50. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gooszen JA, Eshuis WJ, Blom RL, et al. The effect of preoperative body mass index on short-term outcome after esophagectomy for cancer: a nationwide propensity score–matched analysis. Surgery. 2022 doi: 10.1016/j.surg.2022.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Casparie M, Tiebosch A, Burger G, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Anal Cell Pathol. 2007;29(1):19–24. doi: 10.1155/2007/971816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. Hoboken: John Wiley & Sons; 2017. [Google Scholar]
- 16.Dutch Federation of Medical Specialists. Oesofaguscarcinoom. 2023. https://richtlijnendatabase.nl/richtlijn/oesofaguscarcinoom/oesofaguscarcinoom_-_startpagina.html. Accessed 22 Mar 2023.
- 17.Dutch Federation of Medical Specialists. Maagcarcinoom. 2023. https://richtlijnendatabase.nl/richtlijn/maagcarcinoom/algemeen.html. Accessed 22 Mar 2023.
- 18.Team RC, R: A language and environment for statistical computing. 2013.
- 19.Kempf E, Lamé G, Layese R, et al. New cancer cases at the time of SARS-Cov2 pandemic and related public health policies: a persistent and concerning decrease long after the end of the national lockdown. Eur J Cancer. 2021;150:260–267. doi: 10.1016/j.ejca.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torzilli G, Viganò L, Galvanin J, et al. A snapshot of elective oncological surgery in Italy during COVID-19 emergency: pearls, pitfalls, and perspectives. Ann Surg. 2020;272:e112. doi: 10.1097/SLA.0000000000004081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horita N. Impact of the COVID-19 pandemic on cancer diagnosis and resection in a COVID-19 low-burden country: Nationwide registration study in Japan. Eur J Cancer. 2022;165:113–115. doi: 10.1016/j.ejca.2022.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lantinga MA, Theunissen F, Ter Borg PC, et al. Impact of the COVID-19 pandemic on gastrointestinal endoscopy in the Netherlands: analysis of a prospective endoscopy database. Endoscopy. 2021;53:166–170. doi: 10.1055/a-1272-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riera R, Bagattini ÂM, Pacheco RL, et al. Delays and disruptions in cancer health care due to COVID-19 pandemic: systematic review. JCO Glob Oncol. 2021;7:311–323. doi: 10.1200/GO.20.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kempf E, Priou S, Lamé G, et al. Impact of two waves of Sars-Cov2 outbreak on the number, clinical presentation, care trajectories and survival of patients newly referred for a colorectal cancer: a French multicentric cohort study from a large group of university hospitals. Int J Cancer. 2022;150:1609–1618. doi: 10.1002/ijc.33928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eyck BM, van der Wilk BJ, Noordman BJ, et al. Updated protocol of the SANO trial: a stepped-wedge cluster randomised trial comparing surgery with active surveillance after neoadjuvant chemoradiotherapy for oesophageal cancer. Trials. 2021;22:1–6. doi: 10.1186/s13063-021-05274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borgstein AB, Brunner S, Hayami M, et al. Safety of esophageal cancer surgery during the first wave of the COVID-19 pandemic in Europe: a multicenter study. Ann Surg Oncol. 2021;28:4805–4813. doi: 10.1245/s10434-021-09886-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Graaff MR, Hogenbirk RN, Janssen YF, et al. Impact of the COVID-19 pandemic on surgical care in the Netherlands. British J Surg. 2022 doi: 10.1093/bjs/znac301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujitani K, Yang H-K, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309–318. doi: 10.1016/S1470-2045(15)00553-7. [DOI] [PubMed] [Google Scholar]
- 29.Hulshof MC, Geijsen ED, Rozema T, et al. Randomized study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer (ARTDECO Study) J Clin Oncol. 2021;39:2816–2824. doi: 10.1200/JCO.20.03697. [DOI] [PubMed] [Google Scholar]
- 30.Eyck BM, van Lanschot JJB, Hulshof MC, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol Off J Am Soc Clin Oncol. 2021 doi: 10.1200/JCO.20.03614. [DOI] [PubMed] [Google Scholar]
- 31.Van der Werf L, Dikken J, van der Willik E, et al. Time interval between neoadjuvant chemoradiotherapy and surgery for oesophageal or junctional cancer: a nationwide study. Eur J Cancer. 2018;91:76–85. doi: 10.1016/j.ejca.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro J, van Hagen P, Lingsma HF, et al. Prolonged time to surgery after neoadjuvant chemoradiotherapy increases histopathological response without affecting survival in patients with esophageal or junctional cancer. Ann Surg. 2014;260:807–814. doi: 10.1097/SLA.0000000000000966. [DOI] [PubMed] [Google Scholar]
- 33.Nilsson K, Klevebro F, Rouvelas I, et al. Surgical morbidity and mortality from the multicenter randomized controlled NeoRes II trial: standard versus prolonged time to surgery after neoadjuvant chemoradiotherapy for esophageal cancer. Ann Surg. 2020;272:684–689. doi: 10.1097/SLA.0000000000004340. [DOI] [PubMed] [Google Scholar]
- 34.Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol. 2013 doi: 10.1016/j.semradonc.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.