Abstract
Background
Venovenous extracorporeal membrane oxygenation (VV ECMO) is frequently associated with deep sedation and neuromuscular blockades, that may lead to diaphragm dysfunction. However, the prevalence, risk factors, and evolution of diaphragm dysfunction in patients with VV ECMO are unknown. We hypothesized that the prevalence of diaphragm dysfunction is high and that diaphragm activity influences diaphragm function changes.
Methods
Patients with acute respiratory distress syndrome (ARDS) requiring VV ECMO were included in two centers. Diaphragm function was serially assessed by measuring the tracheal pressure in response to phrenic nerve stimulation (Ptr,stim) from ECMO initiation (Day 1) until ECMO weaning. Diaphragm activity was estimated from the percentage of spontaneous breathing ventilation and by measuring the diaphragm thickening fraction (TFdi) with ultrasound.
Results
Sixty-three patients were included after a median of 4 days (3–6) of invasive mechanical ventilation. Diaphragm dysfunction, defined by Ptr, stim ≤ 11 cmH2O, was present in 39 patients (62%) on Day 1 of ECMO. Diaphragm function did not change over the study period and was not influenced by the percentage of spontaneous breathing ventilation or the TFdi during the 1 week. Among the 63 patients enrolled in the study, 24 (38%) were still alive at the end of the study period (60 days).
Conclusions
Sixty-two percent of patients undergoing ECMO for ARDS related to SARS CoV-2 infection had a diaphragm dysfunction on Day 1 of ECMO initiation. Diaphragm function remains stable over time and was not associated with the percentage of time with spontaneous breathing.
ClinicalTrials.gov Identifier NCT04613752 (date of registration February 15, 2021).
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-023-01179-w.
Keywords: Diaphragm dysfunction, Weaning, Ventilator-induced diaphragm dysfunction, ECMO, ARDS
Background
Invasive mechanical ventilation aims to maintain adequate gas exchanges in acute respiratory distress syndrome (ARDS) while resting the respiratory muscles. Its current management is based on lung protective ventilation which combines tidal volume and plateau pressure reduction to limit the harmful effects of positive pressure ventilation on the alveoli, a phenomenon referred to as ventilator-induced lung injury (VILI) [1–3]. VILI is recognized to be the constellation of pulmonary consequences of mechanical ventilation that could potentially lead to an increase in the systemic inflammatory response and contribute to multiorgan failure [4]. A similar concern has also emerged about the potential adverse effects of invasive mechanical ventilation on the respiratory muscles. This entity was originally termed ventilator-induced diaphragmatic dysfunction which has been associated with prolonged duration of mechanical ventilation, difficult and prolonged weaning, and poor prognosis [5–7]. In severe ARDS refractory to conventional management, venovenous extracorporeal membrane oxygenation (VV ECMO) provides full extracorporeal blood oxygenation and carbon dioxide removal, to replace pulmonary function. To further limit the energy transmitted to the lungs by the mechanical ventilator, “ultra-lung-protective” ventilation reducing tidal ventilation, respiratory rate, and plateau and driving pressures is commonly used in combination with VV-ECMO [8]. Such a strategy requires heavy sedation which leads to a forced rest of the respiratory muscles, primarily the diaphragm, with a time-dependent dysfunction [9, 10], and atrophy [11, 12]. Additionally, prolonged time with non-invasive oxygenation strategies before ECMO may also expose these patients to excessive respiratory efforts [13] and subsequent diaphragm injury [14]. Although the influence of diaphragm dysfunction on the outcomes of invasively mechanically ventilated patients is well described [12], the diaphragmatic function of these patients with severe ARDS on ECMO has never been studied. Similarly, the impact of ultra-protective lung ventilation on the diaphragm function has never been studied. We aimed to report the prevalence, time course, and factors associated with diaphragm dysfunction in a population of severe ARDS on VV-ECMO with a particular focus on the influence of diaphragm activity resulting from spontaneous breathing on the diaphragm function changes. We hypothesized that the prevalence of diaphragm dysfunction is high and that diaphragm activity influences diaphragm function changes.
Methods
We conducted a prospective observational study in two intensive care units of the Pitié-Salpêtrière Hospital (Assistance Publique–Hôpitaux de Paris) over 8 months between March and October 2021. This study was approved by the ethical committee (Comité de Protection des Personnes du Sud-Est 5, RCB ID: 2019-A02637-50). Written informed consent was obtained from all patients’ relatives before inclusion. The study was registered on ClinicalTrials.gov (NCT04613752) prior inclusion of the first patient and followed the STROBE reporting guidelines for observational studies.
Patients
Inclusion criteria were: (1) patient with severe ARDS on VV ECMO for less than 24 h, (2) on pressure-controlled mechanical ventilation mode, and (3) sedated with a Richmond assessment scale (RASS) ≤ –2. Exclusion criteria were (1) age < 18 years, (2) known pregnancy, (3) contraindications to magnetic stimulation of the phrenic nerves (e.g., cardiac pacemaker or implanted defibrillator, cervical implants), and (4) expected death within 24 h.
Protocol
The participating ICUs are regional referral ECMO centers where patients are usually retrieved from non-ECMO centers after ECMO implantation by the mobile ECMO team [15]. As soon as ECMO started, all patients were ventilated using a V500 ventilator (Dräger®, Lübeck, Germany) in BIPAP/APRV mode with a constant driving pressure (plateau pressure minus PEEP) of 12–14 cmH2O (14, 15), a PEEP > 10 cmH2O, respiratory rate of 10–20 breaths/min and FiO2 to maintain SaO2 > 92% [3]. On ECMO, the level of sedation was monitored four times daily by the RASS. Spontaneous breathing on BIPAP/APRV was allowed. Of note, spontaneous breathing on BIPAP/APRV is not synchronized with the pressure cycle and could be expressed by the ratio of spontaneous minute ventilation to total minute ventilation. ECMO weaning criteria followed those applied during the EOLIA trial [3].
Diaphragm function assessment, diaphragm ultrasound, and lung ultrasound were all performed together within 24 h after VV-ECMO onset (Day 1) and thereafter repeated on ECMO Day 2, 3, 7, 10, 14, 21, and 28 until ECMO weaning or death, whatever occurred first. For those patients on continuous neuromuscular blockade (atracurium), the intravenous infusion was interrupted for at least 2 h (i.e., five half-lives) before diaphragm function assessment [16].
Diaphragm function assessment
Diaphragm function was defined as the capacity of the diaphragm to generate a negative intrathoracic pressure [17]. It was assessed by the changes in endotracheal tube pressure induced by bilateral phrenic nerve stimulation during airway occlusion (Ptr, stim). Phrenic nerve stimulation was performed by bilateral anterior magnetic stimulation, as described elsewhere [17–19]. Briefly, two figure-of-eight coils connected to a pair of Magstim® 200 stimulators (The Magstim Company, Whitland, UK) were positioned immediately posterior to the sternocleidomastoid muscles at the level of the cricoid cartilage. Bilateral phrenic nerve stimulation was performed while the endotracheal tube was manually occluded, and stimulations were delivered at the maximum intensity allowed by the stimulator (100%) known to result in supramaximal diaphragm contraction. The patients were studied in a standardized semi-recumbent position during a brief disconnection of the endotracheal tube from the ventilator. While the endotracheal tube was manually occluded, bilateral anterolateral magnetic stimulation was performed. The absence of active respiratory efforts was verified by checking the absence of a drop in airway pressure signal on the laptop screen. Two operators (MG, VJ) were required to achieve both stimulation and measurements. After positioning the coils, at least three stimulations were performed. Stimulations were separated by at least 60-s to avoid superposition. Patients were not reconnected to the ventilator between stimulations. Ptr,stim was defined as the amplitude of the negative pressure wave following stimulation, taken from baseline to peak. It was measured at the proximal external end of the endotracheal tube, using a linear differential pressure transducer (MP45 ± 100 cmH2O, Validyne, Northridge, Calif., USA). The pressure signal was sampled and digitized at 100 Hz (MP30, Biopac Systems, Santa Barbara, Calif., USA, or Powerlab, AD Instruments, Bella Vista, Australia) for offline data analysis. The average of three measures was considered during offline and blinded analysis. A Ptr,stim ≤ 11 cmH2O defined diaphragm dysfunction [13, 20].
Diaphragm ultrasound
Ultrasound was performed using two different machines in each ICU (Sparq ultrasound system, Phillips, Philips Healthcare, MA, USA, and CX50 Philips, Philips Healthcare, MA, USA) by two trained investigators (MG, VJ). Both operators had extensive experience in diaphragm ultrasound imaging and followed the same methodology to ensure the reliability of ultrasound recordings across participants. The methods to evaluate diaphragm thickness and thickening have been extensively detailed and validated elsewhere [20, 21]. Diaphragm ultrasound was conducted using a 10–15 MHz linear array transducer. Diaphragm thickness (including pleural and peritoneal membranes) was imaged on the right zone of apposition with the probe placed on the 8th to 10th intercostal space near the midaxillary line. The diaphragm was located as a muscular layer in-between two hyperechoic lines (i.e., the pleura and peritoneum), superficial to the liver. The thickening fraction (TFdi) was calculated offline as peak inspiration thickness minus end-expiratory thickness divided by end-expiratory thickness. All ultrasound measurements were repeated on at least three separate breaths and their averages were reported. The reproducibility of diaphragm ultrasound has been reported elsewhere and was not investigated in the present study [21, 22].
Lung ultrasound
Lung ultrasound was performed by two trained investigators (MG, VJ). A 2–4 MHz probe was used to scan the whole lung on both sides. The number of B-lines was counted on a rib short-axis scan between two ribs at each intercostal space of the upper and lower parts of the anterior, lateral, and posterior regions of the left and right chest wall (a total of 12 areas). For a given region of interest, points were allocated according to the observed ultrasound pattern: the presence of lung sliding with A lines or fewer than two isolated B lines = 0, multiple, well-defined B lines = 1, multiple coalescent B lines = 2, lung consolidation = 3 [23].
Data collection
Demographic data, severity scores, organ dysfunction–related variables, blood gas, ventilator settings, vasopressors, and inotropes doses were prospectively collected. Moreover, the proportion of spontaneous minute ventilation over the last 24 h was averaged at each diaphragm function assessment. Lastly, tracheostomy, ventilator-associated pneumonia, invasive mechanical ventilation duration, and ICU and hospital lengths of stay were also reported.
Statistical analysis
We followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) recommendations for reporting cohort studies [24]. Continuous variables were summarized with their median and inter-quartile range (IQR) and categorical variables using numbers and percentages (%). Wilcoxon rank sum test and Student test were used to compare patients’ characteristics according to the presence or absence of diaphragm dysfunction at ECMO day 1 (i.e., Ptr, stim ≤ 11 cmH2O) for variables on a continuous scale. The Pearson test and Fisher’s exact tests were used for comparisons of categorical values. Nominal p-values are reported and p-values < 0.05 were considered statistically significant. Linear regression was used to investigate the relationships between continuous variables. The averaged percentage of spontaneous breathing ventilation on APRV and the averaged TFdi were used as surrogates of the diaphragm activity over the first 7 days. Correlation between the averaged percentage of daily spontaneous breathing ventilation within the first 7 days and Ptr,stim on Day 7 was assessed. The same analysis was done for average TFdi during the first 7 days and Ptr,stim on Day 7.
Due to the exploratory nature of our study and the lack of study references in that severe population, no formal sample size calculation was deemed necessary. We planned a convenient sample of at least 60 patients.
Results
Study population
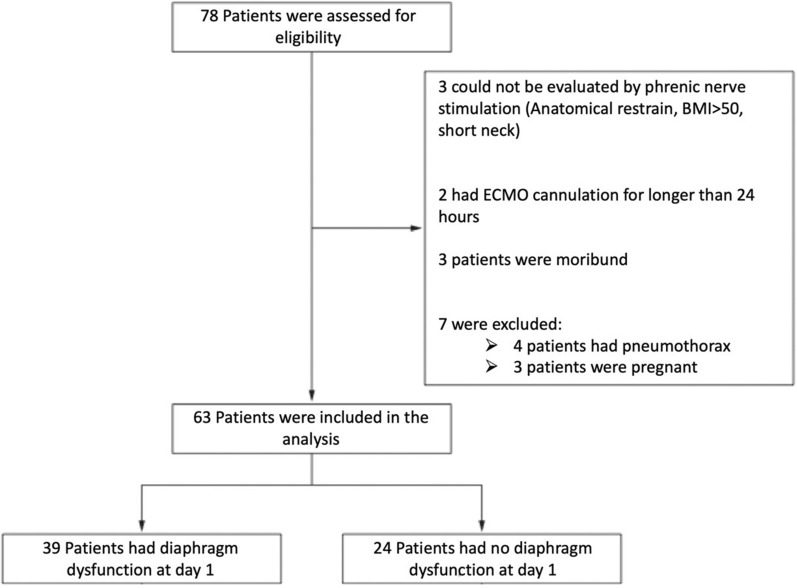
Between February 1st and July 31st, 2021, 78 patients with ARDS receiving VV ECMO were admitted to the two ICUs. All patients had COVID-19-related ARDS. 70 patients had all inclusion criteria. After excluding 7 patients, 63 patients were analyzed in the present study (Fig. 1). Their characteristics are reported in Table 1. Patients were predominantly male, with a median age of 53 years (42–59) and a median body mass index of 33 (29–37) kg/m2. Prior intubation, non-invasive ventilation, and high-flow nasal oxygen were used in 33 (54%) and 53 (85%) patients, respectively. Non-invasive respiratory support (either high-flow nasal oxygen or non-invasive ventilation) was provided for 4 (1–7) days before intubation and invasive mechanical ventilation was provided for 4 (3–6) days before ECMO. At ECMO onset, respiratory system compliance was 22 (16–25) mL/cmH2O, and driving pressure was 19 (16–21) cmH2O.
Fig. 1.
Flowchart
Table 1.
Pre-ECMO patients’ characteristics according to diaphragm dysfunction at ECMO day-1
| Variables | All patients (n = 63) | Diaphragm dysfunction (n = 39) | No diaphragm dysfunction (n = 24) | P value |
|---|---|---|---|---|
| Age, years, median (IQR) | 53 (42–59) | 55 (45–59) | 47 (40–54) | 0.049 |
| Female sex, n (%) | 16 (25) | 10 (26) | 6 (25) | 0.954 |
| Body mass index, kg/m2, median (IQR) | 33 (29–37) | 32 (29–37) | 34 (28–36) | 0.937 |
| Arterial hypertension, n (%) | 29 (46) | 22 (56) | 7 (30) | 0.035 |
| SAPS II, median (IQR) | 56 (45–66) | 58 (47–67) | 55 (39–61) | 0.191 |
| SOFA, median (IQR) | 12 (9–12) | 12 (9–13) | 12 (9–12) | 0.540 |
| Charlson ≥ 1, n (%) | 24 (38) | 15 (38) | 9 (38) | 0.800 |
| High flow nasal oxygen, n (%) | 53 (85) | 33 (87) | 20 (83) | 0.724 |
| Duration, days, median (IQR) | 5 (2–7) | 5 (1–7) | 5 (2–7) | 0.477 |
| Non-invasive ventilation, n (%) | 33 (54) | 21 (57) | 12 (50) | 0.539 |
| Duration, days, median (IQR) | 6 (2–7) | 6 (2–7) | 7 (4–9) | 0.438 |
| Duration of MV before ECMO, median (IQR) | 4 (3–6) | 4 (3–7) | 4 (2–5) | 0.593 |
| Length of stay in ICU before ECMO, days, median (IQR) | 10 (6–13) | 10 (6–11) | 9 (6–12) | 0.904 |
| Pre-ECMO ventilator settings | ||||
| Tidal volume, ml/kg PBW, median (IQR) | 6.0 (5.5–6.3) | 6.0 (5.7–6.4) | 5.9 (5.5–6.1) | 0.144 |
| Respiratory rate, min−1, median (IQR) | 30 (30–33) | 30 (30–32) | 32 (30–34) | 0.523 |
| Driving pressure, cmH2O, median (IQR) | 19 (16–21) | 18 (16–20) | 20 (20–25) | 0.006 |
| Positive end-expiratory pressure, cmH2O, median (IQR) | 12 (10–14) | 13 (12–15) | 12 (10–13) | 0.029 |
| Respiratory system compliance, mL/cmH2O, median (IQR) | 22 (16–25) | 23 (16–26) | 19 (12–22) | 0.005 |
| Corticosteroids, n (%) | 62 (98) | 38 (97) | 24 (100) | 0.735 |
| Neuromuscular blocking agents, n (%) | 62 (98) | 37 (97) | 24 (100) | 0.456 |
| Nitric oxide, n (%) | 22 (35) | 15 (39) | 7 (30) | 0.201 |
| Prone positioning, n (%) | 59 (95) | 34 (89) | 25 (100) | 0.398 |
| Criteria for VV-ECMO, n (%) | ||||
| PaO2/FiO2 < 50 for more than 3 h, n (%) | 12(19) | 9 (23) | 3 (13) | 0.707 |
| PaO2/FiO2 < 80 for more than 6 h, n (%) | 46 (73) | 26 (67) | 20 (83) | 0.233 |
| pH < 7.20 and PaCO2 > 70 mmHg for more than 6 h, n (%) | 13 (21) | 10 (26) | 3 (13) | 0.128 |
PBW Predicted Body Weight, MV Mechanical ventilation, VAP Ventilator-acquired pneumonia, ICU Intensive care unit, SAPS II Simplified Acute Physiology Score II, SOFA Sequential Organ Failure Assessment, IQR Inter-Quartile Range
Diaphragm function assessment on day 1
At ECMO day 1, Ptr,stim was 8.4 (5.1–12.5) cmH2O in the whole population. Thirty-nine (62%) patients had a diaphragm dysfunction on Day 1 (Ptr,stim 5.7 (3.3–8.1) cmH2O) whereas their counterparts had a Ptr,stim of 13.3 (12.0–15.6) cmH2O (Fig. 2). Patients with diaphragm dysfunction had more frequent hypertension and were older. Pre-ECMO non-invasive respiratory support and duration of invasive mechanical ventilation were similar between the two groups. Likewise, the dose of hypnotics, opioids, steroids, and norepinephrine was not different between groups (Table 2). By contrast, patients with diaphragm dysfunction had a significantly higher pre-ECMO PEEP level and a higher LUS score (Table 2).
Fig. 2.
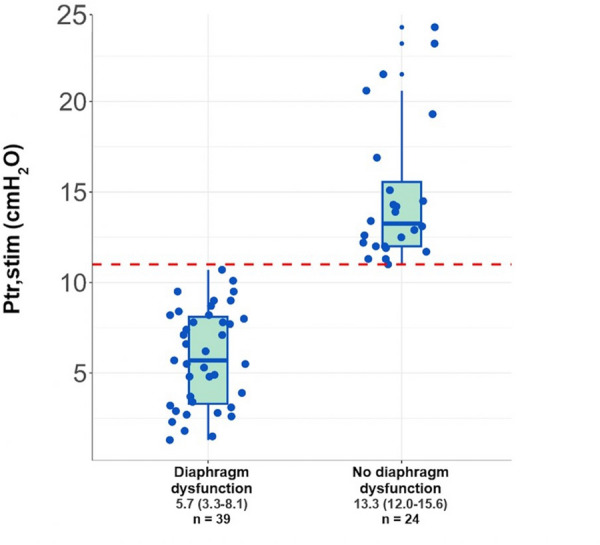
Individual Ptr,stim in patients with and without diaphragm dysfunction at ECMO Day 1. The horizontal red line represents the value of 11cmH2O defining the threshold value of diaphragm pressure generation in response to phrenic nerve stimulation
Table 2.
Patients’ characteristics at ECMO day 1 according to diaphragm dysfunction at ECMO day 1
| Variables | All patients (n = 63) | Diaphragm dysfunction (n = 39) | No diaphragm dysfunction (n = 24) | P value |
|---|---|---|---|---|
| Ventilator settings | ||||
| Tidal volume, ml/kg PBW | 2.8 (2.2–3.7) | 2.7 (2.2–3.6) | 3.0 (2.1–3.8) | 0.488 |
| Respiratory rate, /min | 20 (20–23) | 20 (20–22) | 21 (20–23) | 0.721 |
| Driving pressure, cmH2O | 14 (13–14) | 14 (12–14) | 14 (12–14) | 0.566 |
| Positive end-expiratory pressure, cmH2O | 12 (12–14) | 12 (12–14) | 12 (12–14) | 0.508 |
| Respiratory system compliance, mL/cmH2O | 14 (10–18) | 13 (10–18) | 16 (10–20) | 0.440 |
| Plateau pressure, cmH2O | 26 (24–28) | 26 (24–28) | 26 (25–28) | 0.833 |
| Hemodynamics | ||||
| Heart rate, min−1 | 81 (64–93) | 83 (65–94) | 74 (63–89) | 0.423 |
| Mean arterial pressure, mmHg | 73 (68–80) | 73 (68–78) | 74 (71–80) | 0.162 |
| Norepinephrine, µ/kg/min | 0.1 (0.0–0.34) | 0.1 (0.0–0.4) | 0.1 (0.0–0.3) | 0.658 |
| Arterial blood lactate, mmol/L | 1.8 (1.5–2.3) | 1.8 (1.5–2.4) | 1.8 (1.4–2.1) | 0.577 |
| Fluid balance of the last 24 h, ml | − 151 (− 709–1137) | 115 (− 753–1358) | − 252 (− 675–244) | 0.489 |
| Creatinine, µmol/l | 78 (62–127) | 94 (67–171) | 71 (49–99) | 0.028 |
| Lung ultrasound Score | 26 (23–27) | 26 (24–28) | 25 (22–26) | 0.008 |
| White cells count, G/l | 16 (12–20) | 16 (12–21) | 15 (10–20) | 0.577 |
| Procalcitonin, ng/l | 0.6 (0.3–3.7) | 1.1 (0.3–3.0) | 0.6 (0.2–4.1) | 0.326 |
| Cumulated dose of sedation | ||||
| Propofol, mg/day | 4800 (3600–5310) | 4800 (3600–4800) | 4800 (4500–6000) | 0.075 |
| Sufentanyl, µg/day | 480 (360–600) | 480 (360–600) | 480 (360–600) | 0.691 |
| Midazolam, mg/day | 480 (360–600) | 480 (360–540) | 480 (367–600) | 0.664 |
PBW predicted body weight
Time course of diaphragm function over the ICU stay
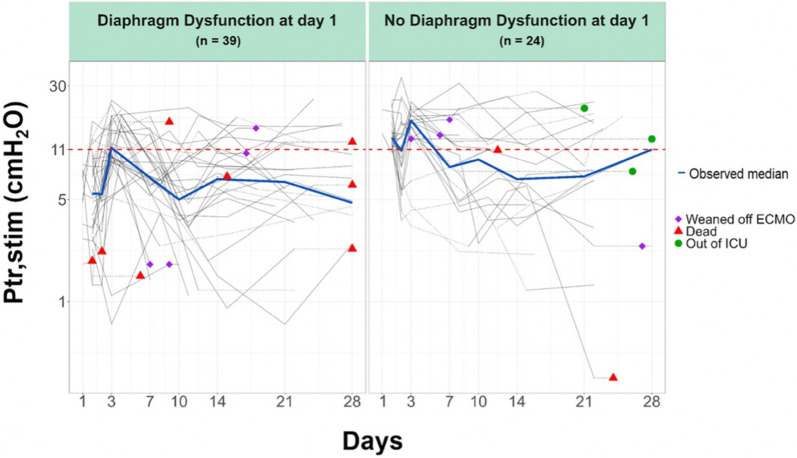
Ptr,stim was measured at ECMO days 1, 2, and 3 in 63, 51, and 47 patients, respectively. The numbers of patients at the time of the following assessments from D7 to D28 and at the time of ECMO weaning are presented in the online supplement (see Additional file 1: Table SDC-S1). The evolution of the diaphragm function over time was characterized by a large interindividual variability (Fig. 3). In the whole population, between the first (n = 63) and the last available (n = 12) diaphragm function assessment, Ptr,stim did not significantly change (from 8.4 (5.1–12.4) to 8.5 (5.1–13.2) cmH2O) (Fig. 3). Among the group of patients with diaphragm dysfunction at day 1, (n = 39), 21 had a Ptr, stim < 11cmH2O, 7 a Ptr, Stim ≥ 11cmH2O, 5 has not been evaluated, 4 were dead and 2 were weaned from ECMO on day 7. On the other hand, among patients without diaphragm dysfunction at day 1 (n = 24) 12 have Ptr,stim < 11cmH2O, 7 patients a Ptr, Stim ≥ 11cmH2O, 3 has not been evaluated, 1 was dead and 1 was weaned from ECMO on day 7.
Fig. 3.
Time course of diaphragm function (logarithm scale) over the ICU course according to the presence or absence of diaphragm dysfunction at ECMO day 1
Diaphragm activity evolution
The evolution of diaphragm activity as estimated by the percentage of spontaneous breathing ventilation and Tfdi during the ECMO run is provided in the online supplement (see Additional file 1: Table SDC-S1b). From Day 1 to Day 7, the proportion of spontaneous breathing ventilation and Tfdi were low (see Additional file 1: Table SDC-S2). Linear regression did not identify a significant association between Ptr,stim on Day 7, and the mean percentage of spontaneous breathing ventilation between day 1 and day 6 (p = 0.680, see Additional file 1: Table SDC-2). Similarly, no correlation was found between Ptr,stim on Day 7, and the cumulative diaphragm thickening fraction since Day 1 (p = 0.698, see Additional file 1: Table SDC-S3). Lastly, % of spontaneous breathing did not differ between patients with Ptr,stim improvement at day 3 compared to those who did not (Additional file 1: Table SDC-S4).
Clinical outcomes
Among the 63 patients enrolled in the study, 24 (38%) were still alive on Day 60. One patient died after ECMO removal, and the 23 others were successfully separated from mechanical ventilation. Overall, the total duration of mechanical ventilation was 50 (37–71) days. While the differences were not significant, patients without diaphragm dysfunction on Day 1 had a shorter total duration of mechanical ventilation as compared to their counterparts (Table 3). Likewise, the duration of post-ECMO mechanical ventilation was shorter, but non-significantly so, in patients without diaphragm dysfunction on Day 1 as compared to their counterparts as well as the length of stay in the intensive care unit (Table 3). The number of ventilator-acquired pneumonia, length of stay in the ICU, and mortality were not different in patients with or without diaphragm dysfunction on Day 1. Similarly, no differences were reported according to the improvement or worsening of Ptr, stim on day 3 on ECMO (Additional file 1: Table SDC-S4).
Table 3.
Outcomes according to the presence of diaphragm dysfunction at ECMO Day 1
| All patients (n = 63) | Diaphragm dysfunction (n = 39) | No diaphragm dysfunction (n = 24) | P value | |
|---|---|---|---|---|
| Tracheostomy, n (%) | 25 (43) | 15 (41) | 10 (48) | 0.600 |
| Number of VAP episodes | 3 (2–4) | 3 (2–5) | 3 (2–4) | 0.397 |
| Total MV duration, days | 50 (37–71) | 50 (34–70) | 49 (38–73) | 0.845 |
| In survivors (n = 24), days | 60 (38–85) | 67 (47–82) | 42 (35–85) | 0.590 |
| MV post-ECMO, days | 20 (12–29) | 27 (14–31) | 14.5 (10.3–23) | 0.157 |
| In survivors (n = 24), days | 18 (12–29) | 27 (14–31) | 14 (9.5–21) | 0.124 |
| ECMO duration, days | 38 (18–56) | 36 (26–53) | 41 (18–57) | 0.705 |
| In survivors (n = 24), days | 32 (17–47) | 36 (18–45) | 27 (14–51) | 0.543 |
| ICU length of stay, days | 47 (1–83) | 61 (11–97) | 38 (1–54) | 0.298 |
| In survivors, days | 65 (46–101) | 86 (61–113) | 47 (40–89) | 0.134 |
| ICU Mortality, n (%) | 39 (62) | 26 (67) | 13 (54) | 0.321 |
MV Mechanical ventilation, VAP Ventilator acquired pneumonia, ICU Intensive care unit
Discussion
This study reporting on the diaphragm function, characteristics, and outcomes of 63 patients who received ECMO for severe ARDS shows that (1) 39 (62%) of this population had a diaphragm dysfunction on ECMO Day (1; 2) there was no association between diaphragm dysfunction on day 7 and the cumulative percentage of spontaneous breathing within the 1 week of ECMO and (3) diaphragm dysfunction was not associated with any clinical outcomes.
Diaphragm dysfunction is a serious condition frequently encountered in critically ill patients exposed to invasive mechanical ventilation [12, 25]. Diaphragm dysfunction was present in 62% of our patients which is in line with previous studies [12, 25] using magnetic stimulation of the phrenic nerves [14]. However, the severity of diaphragm dysfunction in our patients (Ptr,stim 5.7 cmH2O) seemed worse as compared to the studies of Dres et al. (Ptr,stim 6.4 cmH2O) and Demoule et al. (Ptr,stim 6.3 cmH2O) [12, 14]. While multiorgan failure and sepsis have been previously reported as risk factors for diaphragm dysfunction [12, 26] this was not the case with our study. This could be explained by the homogeneity of our population with severe ARDS related to the same disease (i.e., SARS CoV-2 infection) with rare extrapulmonary organ dysfunction. Remarkably, the duration of invasive and non-invasive mechanical ventilation before Day 1 was not different between patients with and without diaphragm dysfunction on Day 1. These findings were unexpected since a previous study showed that both, low and excessive diaphragm activity induces diaphragm atrophy (but not necessarily diaphragm dysfunction) [17]. This suggests that neither respiratory muscles under assistance (before intubation under non-invasive oxygenation supports) nor over assistance (after intubation with controlled mechanical ventilation) could have influenced the diaphragm function in this ECMO population.
The level of PEEP before ECMO was significantly higher in patients with diaphragm dysfunction (13 cmH2O versus 11.5 cmH2O) which is in line with previous findings showing that PEEP causes changes in diaphragm geometry, especially muscle shortening, and decreases in vivo diaphragm contractile function [27, 28]. By displacing the diaphragm in the caudal direction and reducing the length of fibers, mechanical ventilation with high PEEP may induce longitudinal atrophy and subsequent diaphragm dysfunction [27]. If confirmed in further clinical studies, our findings could suggest that high PEEP level might be a risk factor for diaphragm dysfunction which may have important consequences when implementing diaphragm and lung protective ventilation strategies [14, 17]. We also found that the lung ultrasound score at D1 was higher in the group with diaphragm dysfunction (26 (24–28) vs. 24.5 (22–26) p = 0.008). These data suggest that the most severe parenchymal damage might be more likely associated with diaphragm dysfunction by exerting a mechanical constraint on the diaphragm geometry. This is a well-known mechanism already described in several physiological studies [28, 29]. In addition, the inflammatory component of the injured lung characterized by edema could be responsible for direct contiguous muscle damage. However, further investigations are still needed to confirm these hypotheses.
While diaphragm dysfunction has been associated with poor prognosis in several studies [12, 13], this association was not straightforward in our population. It could be explained by the very long duration of mechanical ventilation and ICU stay in this population in whom ICU survival is driven by many other potential contributors. Another possible explanation is the limited sample size of our ECMO population which precluded reaching statistical significance despite trends toward a worse prognosis for patients who had a diaphragm dysfunction with notably, a longer duration of mechanical ventilation, a longer duration of MV after ECMO weaning and ICU stay. While it does not imply causality, the association between diaphragm dysfunction and prolonged weaning seems consistent with previous data [13].
Our study was designed to serially measure the diaphragm function over time and to explore the role of diaphragm activity in its evolution. For this purpose, the proportion of spontaneous breathing ventilation and the diaphragm thickening fraction were used as surrogates of diaphragm activity. The evolution of the diaphragm function seems to remain stable over time for patients with and without diaphragm dysfunction on Day 1. It contrasts with previous studies reporting a time-dependent decline in diaphragm function with, however, limited sample sizes (< 10 patients) and a short follow-up. One can argue that ECMO might prevent the decline of diaphragm function over time and that ventilatory-induced diaphragm injury occurs before ECMO starts. The second important result is the lack of statistical correlation between the diaphragm activity and diaphragm function. A previous study reported that the inspiratory effort can modulate the diaphragm thickness [13], but the diaphragm function per se was not evaluated [13].
This study has several strengths. First, the population is remarkably homogeneous in terms of baseline characteristics, etiology of ARDS and ECMO, and ventilatory management which provides good external validity in a similar context. Second, we used the reference technique to measure the diaphragm function in mechanically ventilated patients. Third, we performed serial measurements of the diaphragm function with a standardized timing which allows a granular description of its evolution. Last, we performed the study in two centers inside a large academic hospital. This study has also some limitations. First, our study was conducted on COVID-19-related ARDS which may limit the generalizability of our results in non-COVID-19 ARDS on ECMO. Second, because of ICU beds constraint during the peak of the pandemic, the follow-up of the diaphragm function until extubation was difficult as patients were frequently re-transferred to their initial ICU after ECMO weaning. Third, the capacity of the diaphragm to generate pressure is influenced by the volume of the lungs and the lung volume likely increased as the patients recovered. Therefore, the conditions of measurement of the diaphragm function may have changed over the study which may have influenced Ptr,stim measurements. Fourth, we did not study diaphragmatic function in a control group (i.e., patients with COVID-19 on mechanical ventilation without ECMO). Fifth, our study has a limited sample size which challenges the generalization of our findings. Therefore, the impact on outcomes of the diaphragm function in patients on VV-ECMO still warrants further investigation. Because the role of COVID-19 itself with frequent isolated pulmonary dysfunction may explain our findings, further studies are needed in patients with non-COVID-related ARDS on ECMO.
Conclusion
Nearly two-thirds of patients undergoing ECMO for severe COVID-19-related ARDS had a severe diaphragm dysfunction on ECMO day 1. However, the diaphragmatic function remained stable over time and did not seem to be associated with any outcomes. Furthermore, there was no association between the presence of diaphragm dysfunction on Day 7 and the percentage of spontaneous breathing during the 1 week of ECMO.
Supplementary Information
Additional file1: Table SDC-S1. a Evolution of diaphragm function over time from D1 to weaning; b Evolution of diaphragm function and diaphragm activity as estimated by the percentage of spontaneous breathing ventilation and diaphragm thickening fraction. Table SDC-2. Impact of the cumulative percentage of spontaneous breathing ventilation on the diaphragm function on day 7. Table SDC-S3. Impact of the cumulative diaphragm thickening fraction on the diaphragm function at day 7. Table SDC-S4. Characteristics, pre ECMO management, spontaneous breathing and outcomes according to improvement or no improvement of Ptr, Stim between day 1 and day 3 on ECMO.
Acknowledgements
The authors thank all the nurses and caregivers for their active involvement in the management of patients during this exceptional epidemic situation and the staff of the Clinical Research Unit for their help in the conduct of the study.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- COVID-19
Coronavirus disease 2019
- ECMO
Extracorporeal membrane oxygenation
- FiO2
Fraction of inspired oxygen
- IBW
Ideal body weight
- ICU
Intensive care unit
- MV
Mechanical ventilation
- PaO2
Partial pressure of alveolar oxygen
- PBW
Predicted body weight
- PEEP
Positive end-expiratory pressure
- SAPS II
Simplified Acute Physiology Score II
- SARS-CoV-2
Severe acute respiratory distress syndrome coronavirus 2
- SOFA
Sequential Organ Failure Assessment
- SpO2
Oxygen saturation measured by pulse oximetry
Author contributions
MD and MS designed and coordinated the study. MD, MG, and VJ collected the patient’s data. MG, MS, and MD analyzed the patient’s data and wrote the manuscript. JR and LEH did the statistical analysis. All authors contributed to drafting the manuscript or critically revised it for important intellectual content and approved the final version of the manuscript.
Funding
Support was provided solely from institutional and/or departmental sources.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Comité de Protection des Personnes du Sud-Est 5 (RCB ID: 2019-A02637-50). Written informed consent was obtained from all patients’ relatives before inclusion. The study was registered on ClinicalTrials.gov (NCT04613752) prior inclusion of the first patient.
Consent for publication
Not applicable.
Competing interests
Martin Dres received personal fees from Lungpacer Medical Inc., Vancouver, Canada, support for attending meetings and/or travel from Lungpacer, outside the submitted work. Alexandre Demoule reports grants from the French Ministry of Health, Assistance publique–Hôpitaux de Paris, Lungpacer, Respinor, consulting fees from Respinor, Lungpace, Lowenstein, Tribunal administrative de Cergy, Liberate Medical, Payment or honoraria for lectures, presentations from Fisher & Paykel, Baxter, Getinge, Astra, Agence Européenne Informatique, Mindray, support for attending meetings and/or travel from Lungpacer, outside the submitted work. Thomas Similowski reports personal fees for consulting and teaching activities from ADEP Assistance, AstraZeneca France, Chiesi France, KPL consulting, Lungpacer Inc., OSO-AI, TEVA France, Vitalaire. He is a stock shareholder of startups Hephaï and Austral Dx. He is listed as an inventor on issued patents (WO2008006963A3, WO2012004534A1, WO2013164462A1) describing EEG responses to experimental and clinical dyspnea. Matthieu Schmidt reports lecture fees from Getinge, Drager, and Xenios outside the submitted work. Alain Combes reports grants from Getinge, and personal fees from Getinge, Baxter, and Xenios outside the submitted work. The other authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pham T, Rubenfeld GD. Fifty years of research in ards.the epidemiology of acute respiratory distress syndrome a 50th birthday review. Am J Respir Crit Care Med. 2017;195:860–870. doi: 10.1164/rccm.201609-1773CP. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt M, Franchineau G, Combes A. Recent advances in venovenous extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Curr Opin Crit Care. 2019;25:71–76. doi: 10.1097/MCC.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 3.Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 4.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 5.Jaber S, Jung B, Matecki S, Petrof BJ. Clinical review: ventilator-induced diaphragmatic dysfunction–human studies confirm animal model findings! Crit Care Lond Engl. 2011;15:206. doi: 10.1186/cc10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaber S, Petrof BJ, Jung B, Chanques G, Berthet J-P, Rabuel C, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183:364–371. doi: 10.1164/rccm.201004-0670OC. [DOI] [PubMed] [Google Scholar]
- 7.Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi Y-SA, et al. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med. 2003;167:120–127. doi: 10.1164/rccm.200210-1246OC. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M, Pham T, Arcadipane A, Agerstrand C, Ohshimo S, Pellegrino V, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome. an international multicenter prospective cohort. Am J Respir Crit Care Med. 2019;200:1002–1012. doi: 10.1164/rccm.201806-1094OC. [DOI] [PubMed] [Google Scholar]
- 9.Petrof BJ, Jaber S, Matecki S. Ventilator-induced diaphragmatic dysfunction. Curr Opin Crit Care. 2010;16:19–25. doi: 10.1097/MCC.0b013e328334b166. [DOI] [PubMed] [Google Scholar]
- 10.Vassilakopoulos T, Petrof BJ. Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;169:336–341. doi: 10.1164/rccm.200304-489CP. [DOI] [PubMed] [Google Scholar]
- 11.Hussain SN, Simkus G, Roussos C. Respiratory muscle fatigue: a cause of ventilatory failure in septic shock. J Appl Physiol Bethesda Md. 1985;1985(58):2033–2040. doi: 10.1152/jappl.1985.58.6.2033. [DOI] [PubMed] [Google Scholar]
- 12.Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, et al. Diaphragm dysfunction on admission to the intensive care unit. prevalence, risk factors, and prognostic impact-a prospective study. Am J Respir Crit Care Med. 2013;188:213–219. doi: 10.1164/rccm.201209-1668OC. [DOI] [PubMed] [Google Scholar]
- 13.Dres M, Dubé B-P, Mayaux J, Delemazure J, Reuter D, Brochard L, et al. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med. 2017;195:57–66. doi: 10.1164/rccm.201602-0367OC. [DOI] [PubMed] [Google Scholar]
- 14.Dres M, Goligher EC, Dubé B-P, Morawiec E, Dangers L, Reuter D, et al. Diaphragm function and weaning from mechanical ventilation: an ultrasound and phrenic nerve stimulation clinical study. Ann Intensive Care. 2018;8:53. doi: 10.1186/s13613-018-0401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lebreton G, Schmidt M, Ponnaiah M, Folliguet T, Para M, Guihaire J, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med. 2021;9:851–862. doi: 10.1016/S2213-2600(21)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beemer GH, Bjorksten AR, Crankshaw DP. Pharmacodynamics of atracurium during propofol, thiopentone and opioid anaesthesia. Br J Anaesth. 1990;65:675–683. doi: 10.1093/bja/65.5.675. [DOI] [PubMed] [Google Scholar]
- 17.Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, et al. Evolution of diaphragm thickness during mechanical ventilation. impact of inspiratory effort. Am J Respir Crit Care Med. 2015;192:1080–1088. doi: 10.1164/rccm.201503-0620OC. [DOI] [PubMed] [Google Scholar]
- 18.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 19.Akoumianaki E, Vaporidi K, Georgopoulos D. The injurious effects of elevated or nonelevated respiratory rate during mechanical ventilation. Am J Respir Crit Care Med. 2019;199:149–157. doi: 10.1164/rccm.201804-0726CI. [DOI] [PubMed] [Google Scholar]
- 20.Goligher EC, Brochard LJ, Reid WD, Fan E, Saarela O, Slutsky AS, et al. Diaphragmatic myotrauma: a mediator of prolonged ventilation and poor patient outcomes in acute respiratory failure. Lancet Respir Med. 2019;7:90–98. doi: 10.1016/S2213-2600(18)30366-7. [DOI] [PubMed] [Google Scholar]
- 21.Laveneziana P, Albuquerque A, Aliverti A, Babb T, Barreiro E, Dres M, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J. 2019;53:1801214. doi: 10.1183/13993003.01214-2018. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society/European Respiratory Society ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 23.Tuinman PR, Jonkman AH, Dres M, Shi Z-H, Goligher EC, Goffi A, et al. Respiratory muscle ultrasonography: methodology, basic and advanced principles and clinical applications in ICU and ED patients-a narrative review. Intensive Care Med. 2020;46:594–605. doi: 10.1007/s00134-019-05892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Dres M, Goligher EC, Heunks LMA, Brochard LJ. Critical illness-associated diaphragm weakness. Intensive Care Med. 2017;43:1441–1452. doi: 10.1007/s00134-017-4928-4. [DOI] [PubMed] [Google Scholar]
- 26.Lecronier M, Jung B, Molinari N, Pinot J, Similowski T, Jaber S, et al. Severe but reversible impaired diaphragm function in septic mechanically ventilated patients. Ann Intensive Care. 2022;12:34. doi: 10.1186/s13613-022-01005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindqvist J, van den Berg M, van der Pijl R, Hooijman PE, Beishuizen A, Elshof J, et al. Positive end-expiratory pressure ventilation induces longitudinal atrophy in diaphragm fibers. Am J Respir Crit Care Med. 2018;198:472–485. doi: 10.1164/rccm.201709-1917OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansen D, Jonkman AH, de Vries HJ, Wennen M, Elshof J, Hoofs MA, et al. Positive end-expiratory pressure affects geometry and function of the human diaphragm. J Appl Physiol Bethesda Md. 1985;2021(131):1328–1339. doi: 10.1152/japplphysiol.00184.2021. [DOI] [PubMed] [Google Scholar]
- 29.Similowski T, Yan S, Gauthier AP, Macklem PT, Bellemare F. Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med. 1991;325:917–923. doi: 10.1056/NEJM199109263251304. [DOI] [PubMed] [Google Scholar]
- 30.Hermans G, Agten A, Testelmans D, Decramer M, Gayan-Ramirez G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit Care Lond Engl. 2010;14:R127. doi: 10.1186/cc9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file1: Table SDC-S1. a Evolution of diaphragm function over time from D1 to weaning; b Evolution of diaphragm function and diaphragm activity as estimated by the percentage of spontaneous breathing ventilation and diaphragm thickening fraction. Table SDC-2. Impact of the cumulative percentage of spontaneous breathing ventilation on the diaphragm function on day 7. Table SDC-S3. Impact of the cumulative diaphragm thickening fraction on the diaphragm function at day 7. Table SDC-S4. Characteristics, pre ECMO management, spontaneous breathing and outcomes according to improvement or no improvement of Ptr, Stim between day 1 and day 3 on ECMO.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.