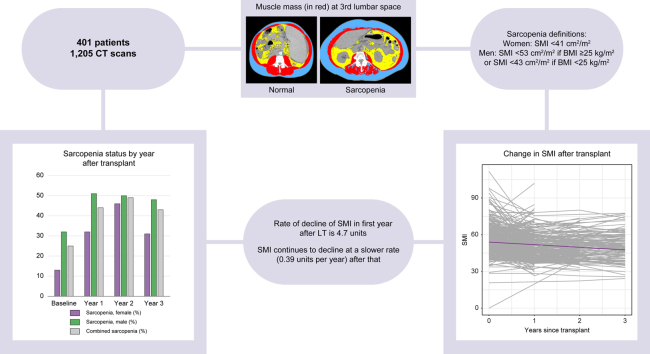
Abstract
Background & Aims
Sarcopenia has significant burden in cirrhosis and has been shown to worsen short-term post-liver transplantation (LT). This study aims to evaluate the long-term change in sarcopenia post-LT along with its associations and predictors.
Methods
A retrospective study of adult patients who underwent LT at a tertiary centre between 1/1/2009 and 12/31/2018. Relevant demographic and clinical data were collected. Skeletal muscle index (SMI) was calculated using standard of care computerised tomography (CT) scans pre- and post-LT. Sarcopenia was defined using previously established cut-points. The primary outcome was SMI change post-LT and secondary outcome was post-LT mortality.
Results
Out of 1165 patients, 401 met inclusion criteria (1,205 CT scans reviewed). The average age at transplant was 57 years; 63% were male. The average BMI was 28 kg/m2. Thirteen percent of females and 32% of males had sarcopenia pre-LT. Post-LT SMI declined by 4.7 cm2/m2 in the first year then by 0.39 cm2/m2 per year thereafter. Females had greater rate of decline in SMI after the first year compared with males (0.87 cm2/m2 per year vs. 0.17 cm2/m2 per year, respectively, p = 0.02). Post-LT physical rehabilitation, infection, and readmissions were not associated with SMI trajectory. At 3 years post-LT, 31% of females and 48% of males had sarcopenia. Baseline sarcopenia was the only predictor of long-term post-LT sarcopenia on multivariable analysis, but it was not associated with mortality.
Conclusions
Sarcopenia does not appear to resolve post-LT and likely worsens leading to nearly doubling its prevalence in those with long-term follow-up. Immediate post-LT physical rehabilitation was not associated with SMI trajectory in our cohort.
Impact and implications
The prevalence of sarcopenia is high among patients with cirrhosis; however, data are mixed on the impact of sarcopenia on post-liver transplant (LT) course and there have been no studies evaluating the long-term evolution of sarcopenia post-LT beyond 1 year. In this study, we analysed changes in muscle mass up to 3 years after transplant in 401 patients and found that sarcopenia did not resolve in most liver transplant recipients and skeletal muscle mass tended to worsen after transplant with the greatest decline in muscle mass in the first year post-LT. Interestingly, sarcopenia did not influence post-transplant outcomes. Future prospective studies are needed to further understand the natural course of sarcopenia post-LT to guide interventions aiming at reversing post-LT sarcopenia.
Keywords: Liver transplantation, Skeletal muscle index, Malnutrition, Hepatic decompensation
Graphical abstract
Highlights
-
•
The prevalence of sarcopenia is high among patients with cirrhosis.
-
•
Despite liver transplantation, sarcopenia in most patients never resolves afterwards.
-
•
Skeletal muscle mass worsens in the years following liver transplant with the worsening being most pronounced in the first year.
-
•
The degree of exposure to calcineurin inhibitors was not associated with evolution of sarcopenia post-LT.
-
•
There was no evidence that sarcopenia was associated with post-transplant mortality in this study.
Introduction
Sarcopenia is defined as progressive and generalised loss of skeletal muscle mass and function that occurs due to an imbalance between protein synthesis and breakdown. Although it is traditionally considered in the aging population, studies have shown that sarcopenia is highly prevalent in patients with cirrhosis with rates of up to 70%.1 The pathophysiology of sarcopenia in cirrhosis is multifactorial with contribution from portal hypertension complications, pro-inflammatory cytokines, hyperammonaemia, hypotestosteronaemia, and increased hepatic gluconeogenesis among others.2
Although the consensus definition of sarcopenia includes both muscle mass and function, most studies tend to focus on objective measures of muscle mass, using imaging techniques, in defining sarcopenia rather than assessing function, using performance-based measurements, which can be more subjective.3 Additionally, many use the terms frailty and sarcopenia interchangeably despite having differences in definition with frailty representing the syndrome of decreased physiologic reserve and increased vulnerability to health.3 Because of differences in measurement and definitions of sarcopenia, there has been variability in the impact on post-transplant short-term outcomes demonstrated in hepatology literature.4 Some studies demonstrated that pre-transplant sarcopenia is associated with increased intensive care unit (ICU) stay and hospital length of stay (LOS) as well as ventilator needs and infection risk after liver transplant (LT).5 Conversely, other studies showed no impact of sarcopenia on hospital LOS or hospitalisations in the first year post-LT.6 Despite the variability among studies, most have shown that pre-LT sarcopenia is associated with worse outcomes both pre- and post-LT.4,7,8
Although data appear to show that pre-LT sarcopenia is associated with impaired short- and long-term survival in patients with cirrhosis, there have been no studies evaluating the long-term evolution of sarcopenia post-LT beyond 1 year. Interestingly, animal studies showed that calcineurin inhibitors, the most commonly used long-term immunosuppression post-LT, can be associated with suppression of certain skeletal muscle proteins, which raises concern about persistence of sarcopenia post-LT.9 The aims of this study are to (i) evaluate the long-term changes in skeletal muscle mass after LT to understand whether it improves, persists, or even worsens, (ii) investigate the predictors, if any, of post-LT long-term changes in muscle mass, (iii) determine if there is an association between these changes and post-LT complications as well as mortality.
Patients and methods
Study population
A single-centre, retrospective study was conducted including first liver-only transplants performed at Vanderbilt University Medical Center (VUMC) between January 1, 2009, and December 31, 2018. Patients ≥18 years old who underwent LT for a cirrhosis-related indication were included in the analysis. Patients without cirrhosis, with multi-organ transplant, and those without standard of care computerised tomography (CT) scans within 6 months pre-transplant or without any scans post-transplant were excluded. Patients who had their longitudinal post-LT care outside VUMC (i.e. at the Veterans Affairs Tennessee Valley Healthcare System) were excluded.
Study procedures and data collection
Demographic and clinical data were collected for all patients from the Vanderbilt Liver Transplant database and by manual chart review. The diagnosis of comorbidities such as hypertension (HTN), diabetes mellitus (DM), chronic kidney disease (CKD) was based on International Classification of Diseases, Ninth Revision and/or Tenth Revision (ICD-9/ICD-10) obtained from the research data warehouse. Trough levels of calcineurin inhibitors were obtained from the research data warehouse, as well. It is important to note that the Vanderbilt Transplant Center’s standard immunosuppression protocol after LT includes intravenous methylprednisolone followed by tacrolimus, mycophenolate, and prednisone. The initial trough goal for tacrolimus is generally 8–10 ng/ml in the first 3 months then 6–8 ng/ml for 3–12 months and then 4–7 ng/ml after 1 year. Most patients are off steroids by 90 days. The mycophenolate is often stopped after 90 days unless patients have rejection or renal insufficiency in which case it can be continued long-term for renal sparing. A small proportion of patients are treated with mechanistic target of rapamycin (mTOR) inhibitors at our centre.
CT scans within 6 months before LT and yearly after LT for up to 5 years were used in the analysis. If there were multiple CT scans in a certain year, the scan latest in that specific post-LT year was used for evaluation. Changes in CT-based skeletal muscle mass area (cm2) were calculated using automatic segmentation Slice-O-Matic software (Version 4.3, TomoVision, Montreal, Canada) and then edited manually by two analysts (EM, MG) who were blinded to the clinical data to assure complete and reliable quantification of morphometric measurements. At the Vanderbilt Diet, Body Composition, and Human Metabolism Core, the established performance for inter-observer precision error with CT skeletal muscle measurements is low at ≤1.8 cm2 or ≤1.5% (unpublished data). Total abdominal muscles including psoas, transversus abdominis, obliques, and rectus abdominis at the third lumbar vertebra (L3) were used to calculate skeletal muscle mass, as it has been the most investigated and validated measure of muscle mass in the general population and in patients with liver disease. The skeletal muscle index (SMI) was obtained by normalising the skeletal muscle mass for height. Sarcopenia was defined using sex and BMI cut-offs previously described.10 Sarcopenia was considered when SMI was <41 cm2/m2 for females and SMI <53 cm2/m2 if the BMI was 25 kg/m2 or greater or SMI <43 if the BMI was <25 kg/m2 for males. Data exist about potential SMI cut-offs that are specific for patients with cirrhosis, different than the cut-offs used in this study, which are widely accepted and published cut-points in the general population. As most images analysed this study were obtained post-transplant (i.e. in the absence of the state of cirrhosis), the study team decided to use the cut-offs previously established in general population as opposed to those proposed by prior studies to be specific for patients with cirrhosis.
The primary outcome was the change in muscle mass post-LT. Moreover, we investigated predictors of the primary outcome and its association with short-term (90 days) complications post-LT which included hospital LOS, surgery duration, days on ventilator after transplant surgery, readmission, T-cell mediated rejection (TCMR), undergoing physical rehabilitation, biliary complications, and infection episodes. The secondary outcome was mortality.
Statistical analysis
Continuous variables were summarised using the median and interquartile range, and categorical variables were summarised using percentages. Baseline characteristics of the patients excluded due to lack of imaging pre- or post-transplant were compared to those included in the study using a standardised mean difference. To estimate the association of skeletal muscle mass with potential predictors, we used multivariable linear regression for SMI and multivariable logistic regression for presence of sarcopenia. Separate multivariable logistic regression models were fit for sarcopenia measured at baseline, year 1, year 2, year 3, year 4, and year 5. Each logistic regression model required 10–15 events per predictor. Because of subject attrition over time, later years included fewer potential predictors in multivariable models and figures included were restricted to 3 years post-LT. Results presented are conditional on having an available CT scan where outcomes were measured. Given changes in muscle mass in the first few months post-LT can be potentially confounded by frequent hospitalisations and high-dose immunosuppression in the early post-LT course, we performed sensitivity analyses where scans in the first 6 months were not included in the analysis for year 1 after LT. Mean changes in skeletal muscle mass over time were estimated using repeated measures linear regression and account for correlation arising from taking repeated measurements on the same subject over time using random effects. Multiplicative interaction terms of age, height, sex, and BMI with time since transplant were included in models to determine if the rate of change was modified by these covariates. Secondary analyses involved estimating the association between skeletal muscle mass and short-term complications, while controlling for potential confounders, including age, sex, BMI, aetiology of liver disease and comorbidities. We tested if short-term complications such as LT admission LOS, readmission within 90 days, rejection and infectious complications within 90 days were associated with future SMI trajectory using repeated measures linear regression. Cox regression and Kaplan–Meier curves were used to estimate the association of baseline sarcopenia with time to death. Subjects were censored at the date of their last known follow-up. Lastly, the association of median tacrolimus levels within the first 6 months after transplant with subsequent SMI trajectory (outcome) was estimated using multivariable, repeated measures linear regression.
Results
Baseline characteristics
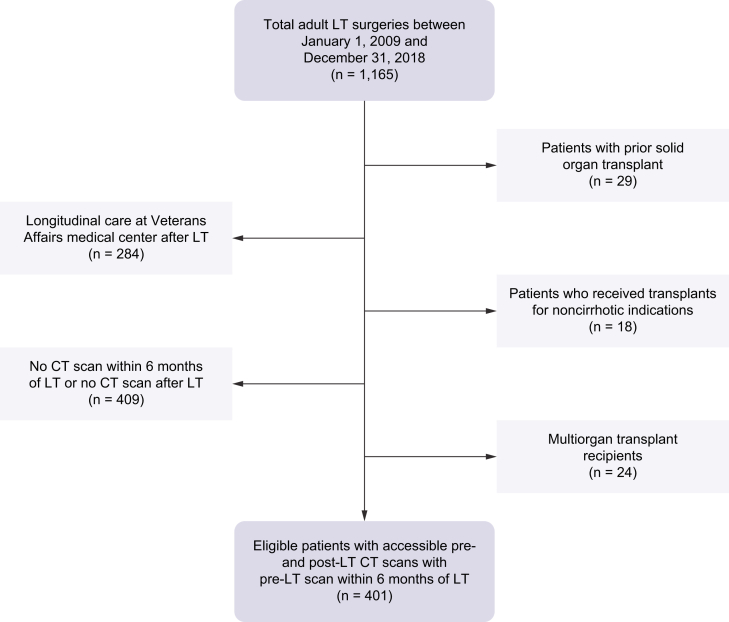
Out of 1,165 LTs during the study period, 401 patients met the inclusion criteria (Fig. 1). A total of 1,205 CT images were analysed. The average age was 57 years old, 63% were males, and 92% were white. Table 1 gives the baseline characteristics of study population. We compared the 409 patients that were excluded as a result of lack of pre-LT or post-LT imaging to the 401 included subjects with respect to age, liver disease aetiology, model for end-stage liver disease-sodium (MELD-Na), and sex. Excluded subjects had somewhat higher yet non-significant MELD-Na scores (25 vs. 22), and no other important differences were found (Table S1).
Fig. 1.
Flowchart of patient exclusions for the study cohort.
CT, computerised tomography, LT, liver transplant.
Table 1.
Baseline characteristics of cohort (N = 401), median (IQR) or N (%).
| Baseline characteristics | |
|---|---|
| Median age, years | 57 [51, 63] |
| Race | |
| Asian | 8 (2%) |
| Black | 21 (5%) |
| Caucasian | 368 (92%) |
| Hispanic | 3 (1%) |
| Male sex | 252 (63%) |
| Smoker | 43 (11%) |
| Chronic kidney disease | 27 (7%) |
| Diabetes mellitus | 36 (9%) |
| Median BMI at transplant | 28 [25, 33] |
| Aetiology of liver disease | |
| Non-alcoholic steatohepatitis | 108 (27%) |
| Alcohol-associated liver disease | 101 (25%) |
| Viral | 121 (30%) |
| Autoimmune | 37 (9%) |
| Other | 34 (8%) |
| Median MELD-Na at transplant | 22 [15, 29] |
| Ascites | 329 (82%) |
| Hepatic encephalopathy | 310 (77%) |
MELD-Na, model for end-stage liver disease-sodium.
The median follow-up for the study cohort was 6.5 years. The median time between pre-LT CT and transplantation was 50.5 days (IQR 21, 96). Sarcopenia affected 25% of patients pre-LT (13% females, 32% males). The median SMI pre-LT was 51 cm2/m2 for females and 55 cm2/m2 for males (Table 2). The variables that were significantly associated with baseline sarcopenia on multivariate analysis were a 10-year increase in age, male sex, and biologic MELD-Na at transplant with an odds ratio of 1.43 (1.09–1.93, p = 0.014), 2.94 (1.71–5.23, p <0.001) and 0.96 (0.93–0.98, p = 0.002, Table 3), respectively. There was no evidence that baseline sarcopenia was associated with comorbidities including DM, coronary artery disease (CAD), CKD, or HTN.
Table 2.
Median skeletal muscle index and sarcopenia status yearly up to 3 years post-LT.
| N | Median SMI (female) | Median SMI (male) | Sarcopenia, female n (%) | Sarcopenia, male n (%) | Combined sarcopenia n (%) | |
|---|---|---|---|---|---|---|
| Baseline | 401 | 51 | 55 | 20/149 (13) | 80/252 (32) | 100/401 (25) |
| Year 1 | 334 | 45 | 50 | 40/124 (32) | 108/210 (51) | 148/334 (44) |
| Year 2 | 146 | 42 | 52 | 19/41 (46) | 53/105 (50) | 72/146 (49) |
| Year 3 | 112 | 46 | 52 | 11/35 (31) | 37/77 (48) | 48/112 (43) |
LT, liver transplant; SMI, skeletal muscle index.
Table 3.
Adjusted odds ratios (95% CI) summarising the multivariable association of covariates with presence of sarcopenia at four time points.
| Covariate | Baseline | Year 1∗ | Year 2 | Year 3 |
|---|---|---|---|---|
| Older age | 1.43 (1.09–1.93) | 1.32 (0.92–1.93) | 1.06 (0.67–1.68) | 1.62 (0.95–2.98) |
| MELD at LT | 0.96 (0.93–0.98) | |||
| DM pre-LT | 0.77 (0.31–1.75) | 0.73 (0.36–1.47) | 0.71 (0.32–1.56) | 0.31 (0.11–0.79) |
| CKD pre-LT | 0.24 (0.04–0.87) | 2.26 (0.61–9.66) | 1.37 (0.32–6.05) | |
| Male sex | 2.94 (1.71–5.23) | 2.11 (1.06–4.30) | 0.84 (0.36–2.00) | 0.98 (0.35–2.74) |
| Higher BMI | 1.00 (0.95–1.06) | 0.98 (0.91–1.05) | ||
| Any rehab within 90 days from LT | 2.02 (0.99–4.20) | 1.23 (0.55–2.76) | ||
| Tacrolimus Exposure | 0.93 (0.72–1.19) | 0.99 (0.74–1.33) | ||
| Baseline sarcopenia | 8.47 (3.80–20.70) | 5.78 (2.43–14.80) | 11.47 (4.01–37.91) |
The text in bold is statistically significant.
Year 1 scan done 180–-365 days after LT. CKD, chronic kidney disease; DM, diabetes mellitus; LT, liver transplant; MELD, model for end-stage liver disease.
On discharge after LT surgery, 50% went home, 21% went to an inpatient rehabilitation (IPR) facility, 27% went home with home physical therapy, and the remaining 2% (eight patients) went to a skilled nursing facility (two patients), long-term acute care centre (three patients) or died (three patients).
Primary outcome
Year 1
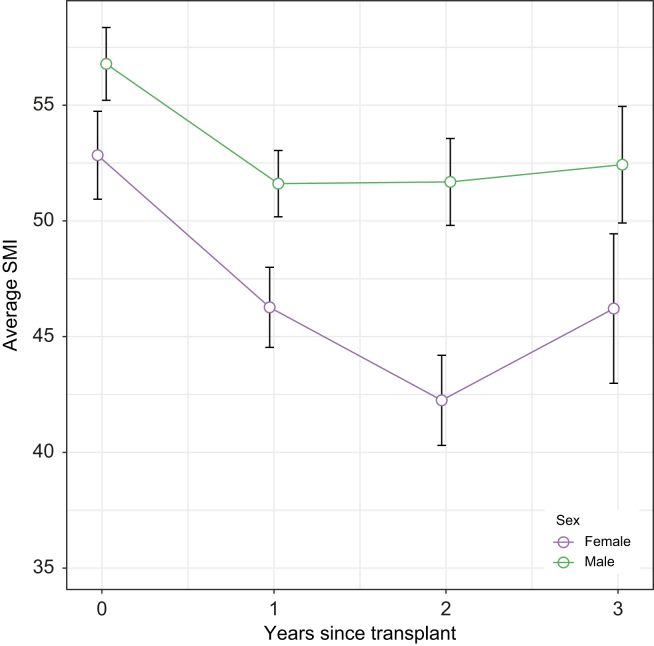
A total of 334 patients had CT within the first year post-LT of whom 218 patients had scans in the second 6 months post-LT (i.e. scans 180–365 days post-LT). Overall, the median SMI at 1 year after LT was 45 cm2/m2 for females and 50 cm2/m2 for males (Fig. 2), reflecting that 44% of patients were considered sarcopenic (32% females, 51% males, Table 2). On univariable analysis, male sex was associated with sarcopenia at 1-year post-LT with 73% of the sarcopenia group vs. 55% of the no sarcopenia group being males (p <0.001). Those with sarcopenia were older (58 vs. 56 years old, p = 0.003) and more likely to have had sarcopenia at baseline (46% vs. 11%, p <0.001). There was no impact for baseline DM, CAD, or HTN in the development of sarcopenia at 1 year. Similarly, there was no difference in sarcopenia at 1 year in those that developed DM in the first 6 months post-LT vs. those that did not (44% vs. 45%, p = 0.9). Prolonged stay on LT admission was not associated with subsequent increase in the risk for 1-year sarcopenia. Similar associations were observed when stratifying for those with CT scans 180–365 days post-LT. On multivariable analysis, baseline sarcopenia greatly increased the odds of sarcopenia at 1 year (odds ratio [OR] 8.47,95% CI 3.80–20.70, p <0.001, Table 3). In addition, controlling for baseline sarcopenia and other potential confounders, male sex was associated with increased odds of sarcopenia at 1 year (OR 2.11, CI 1.06–4.30, p = 0.037).
Fig. 2.
Average skeletal muscle index (SMI) with 95% CI for male and female patients at baseline and each year after LT up to 3 years post-LT.
For comparison across time or over sex, non-overlapping intervals are indicative of significant differences. LT, liver transplant.
On univariable analysis, the rates of sarcopenia at year 1 did not differ by rehabilitation type at discharge (p = 0.15); 40% for no rehabilitation, 51% for non-IPR rehabilitation and 42% for IPR. When SMI was analysed as a continuous outcome, there were no significant differences between the groups by type of rehab. In patients receiving IPR where LOS was known (i.e. an IPR associated with our medical centre), median LOS was not different in those with vs. without sarcopenia at 1 year (9 vs. 11 days, p = 0.08).
Given the impact of calcineurin inhibitors (CNIs) on muscle mass in the animal model,9 we analysed median tacrolimus trough level in day 1–180 after LT in relation to subsequent sarcopenia (180–365 days post-LT) and demonstrated no association overall (p = 0.32) or when further stratified by day 1–90 (p = 0.19) and day 91–180 (p = 0.091) of median tacrolimus trough levels. In 10 patients who were switched from tacrolimus to cyclosporine within the first 90 days, sarcopenia rates were 30% higher in those who switched vs. those who did not switch (80% vs. 50%, p = 0.06).
Year 2
A total of 146 patients had CT in year 2. The median SMI was 42 cm2/m2 for females and 52 cm2/m2 for males (Fig. 2). This corresponded to 49% of patients being considered sarcopenic (46% females 50% males, Table 2). On univariable analysis, older age (59 vs. 57 years old, p = 0.049) and baseline sarcopenia (44% vs. 15%, p <0.001) were associated with sarcopenia at year 2, whereas male sex and comorbidities were not. On multivariate regression, only baseline sarcopenia retained significance (OR 5.78 [2.43–14.80], p <0.001, Table 3). There was no difference in sarcopenia at year 2 in those that developed DM in the first 6 months post-LT vs. those that did not (48% vs. 51%, p = 0.7).
Year 3
A total of 112 patients had CT in year 3. The median SMI at year 3 post-LT was 46 cm2/m2 for females and 52 cm2/m2 for males (Fig. 2). This corresponded to 43% of patients being considered sarcopenic (31% females, 48% males, Table 2). On univariable analysis, older age (61 vs. 57 years old, p = 0.004), and baseline sarcopenia (58% vs. 9%, p <0.001) were associated with sarcopenia at year 3 while male sex and comorbidities were not. The presence of DM pre-LT was associated with less sarcopenia (52% vs. 27%, p = 0.009). On multivariate regression, only baseline sarcopenia retained significance (OR 11.47 [4.01–37.91], p <0.001, Table 3).
Years 4 and 5
Sarcopenia at years 4 and 5 was assessed but there were limited numbers of patients in those years (77 in year 4 and 38 in year 5) and, therefore, results were not included in the provided figures. The only associations with sarcopenia were older age (61 vs. 53 years old, p <0.001) and baseline sarcopenia (51% vs. 0%, p <0.001) at year 4 and older age (57 vs. 52 years old, p = 0.022) at year 5. The small sample size did not permit multivariable analysis for sarcopenia at years 4 or 5 post-transplant.
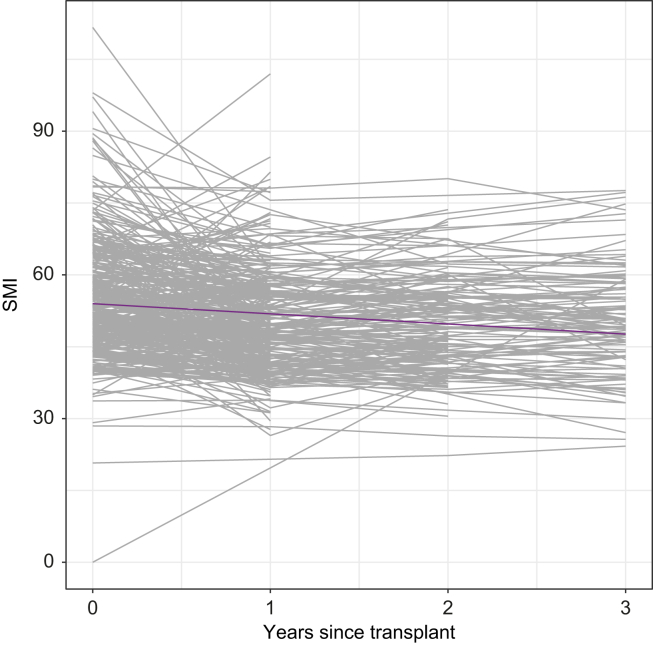
Post-transplant change in muscle mass
Among baseline characteristics, older age, male sex, and lower BMI were associated with lower baseline SMI. In our cohort, SMI declined in most patients throughout the observation period. The mean rate of decline was 4.7 cm2/m2 per year in the first year and then 0.39 cm2/m2 per year thereafter (Fig. 3). Although male and female patients had SMI decline by a similar amount in the first year (p = 0.63), the rate of decline in SMI after the first year was significantly greater in females compared with males (0.87 cm2/m2 per year in females vs. 0.17 cm2/m2 per year in males, p = 0.02; Fig. 2).
Fig. 3.
Spaghetti plot of change in SMI over time since transplant. Each black line represents individual patients.
Purple line represents the average trend over time.
There were no associations between post-LT trajectory of SMI and liver disease aetiology, prior ascites, prior hepatic encephalopathy, biologic MELD, discharge to physical rehabilitation after LT admission, or number of post-LT admissions to a physical rehabilitation facility. Table 2, Table 4 demonstrate the large proportion of patients (up to 49%) with persistent or de novo sarcopenia on every post-LT year (through year 3) and the small proportion of patients (<20%) who had reversal of sarcopenia.
Table 4.
Evolution of sarcopenia in patients with scans at each time point.
| New sarcopenia | Persistent sarcopenia | Recovery from sarcopenia | Free of sarcopenia | |
|---|---|---|---|---|
| Year 1 | 29% (62/214) | 22% (47/214) | 4% (9/214) | 45% (96/214) |
| Year 2 | 7% (8/113) | 43% (49/113) | 12% (13/113) | 38% (43/113) |
| Year 3 | 9% (6/69) | 41% (28/69) | 7% (5/69) | 43% (30/69) |
Association of skeletal muscle mass and post-LT short-term complications
The rates of post-LT complications in the study groups are depicted in Table S2. It is notable that subjects with baseline sarcopenia had a statistically significant, clinically insignificant shorter index LOS compared with those without sarcopenia (7 days [IQR 6–11] vs. 8 days [IQR 6–16]; p = 0.038). Among the post-LT variables that reflect short-term complications post-LT, after controlling for age and sex, there was insufficient evidence for a meaningful association of sarcopenia with days on the ventilator. There was no association between SMI changes and LT surgery duration, post-LT LOS (in the ICU or in the hospital overall), readmissions, biliary or infectious complications, or TCMR.
Mortality
A total of 86 (21%) patients died during the observation period. There was no evidence that baseline sarcopenia was associated with mortality (HR 1.0, 95% CI 0.64–1.70). We had insufficient data on SMI or sarcopenia to model these variables as time varying covariates in the mortality analysis. There was significant association of pre-LT ascites (hazard ratio [HR] 3.0, 95% CI 1.3–6.8) and pre-LT HTN (HR 1.9, 95% CI 1.2–3.2) with post-LT mortality on univariable analysis. Median time to death was greater than 5 years as less than half of the subjects died during the observation period.
Discussion
This study provides new insights into the long-term evolution of skeletal muscle mass post-LT. Surprisingly, the SMI appears to continuously decline for at least 3 years after LT, but the decline is most pronounced in the first year post-LT. This decline has translated into increasing prevalence of sarcopenia long-term post-LT with strikingly low rates of recovery from sarcopenia. Baseline sarcopenia was consistently the most predictive factor for post-LT long-term sarcopenia. Interestingly, post-LT complications such as readmission or infectious complications did not impact change in SMI over time. Immediate physical rehabilitation post-LT index hospitalisation did not influence the subsequent changes in SMI either. Furthermore, baseline sarcopenia was not associated with post-LT mortality.
This study had some findings in agreement with prior work and other findings that differed. Specifically, the finding of SMI worsening in the year after LT is consistent with earlier literature.11,12 Bhanji et al.11 evaluated 293 patients with pre-LT scans, of which 50% had sarcopenia. A total of 161 patients had post-LT scans and the authors noted 98 patients (61%) having sarcopenia of whom 25 (26%) had de novo sarcopenia. They did not find a difference in 1-year mortality in those with or without sarcopenia. Tsien et al.12 evaluated 53 patients and noted 33 patients (62%) with sarcopenia pre-LT and 46 (87%) with sarcopenia post-LT. Our study not only had similar findings of lack of sarcopenia recovery in 1 year, but also found persistent decline in SMI beyond 1-year post-LT. Consequently, the overall prevalence of sarcopenia in the years following LT nearly doubled and approached 50% reflecting high rates of persistent sarcopenia and de novo sarcopenia with minimal rates of recovery from sarcopenia. Importantly, there are some studies that note an initial decline in muscle mass but with at least relative improvement at 1 year which differs from our findings.13,14 They were both small studies (72 patients and 14 patients) and used different approaches to measure muscle mass (multifrequency body composition analyser and dual-energy x-ray absorptiometry). It is important to note that the wide range of sarcopenia prevalence amongst studies is likely attributable to the various methods used to measure muscle mass and the lack of a standardised definition for sarcopenia. Another limitation for the prior studies is the smaller sample size which may have led to inflated or underappreciated prevalence and associations of sarcopenia that may not be reproducible. This may account for why some studies find morbidity and mortality differences, whereas others do not.6,11
This study differs from some of the prior studies as we did not find associations of sarcopenia with most early post LT complications or all-cause mortality.4,5,7,8 Index LOS (7 days [IQR 6–11] in those with sarcopenia vs. 8 days [IQR 6–16] in those without sarcopenia; p = 0.038) demonstrated statistically significant, clinically insignificant difference in our study. In addition, there was no evidence of meaningful or reproducible association of SMI with days on ventilator. Conversely, DiMartini et al.7 concluded that skeletal muscle mass was a more significant predictor of ICU and total hospital LOS and intubation days in males rather than females and a predictor of post-LT survival in males but not in females. In a systemic review by Ooi et al.,5 pre-LT sarcopenia was associated with post-LT complications including infection risk, longer ventilatory dependency and ICU LOS. A systemic review of CT-assessed skeletal muscle mass on outcomes of LT candidates found that sarcopenia was consistently associated with worse post-LT mortality with an HR of 1.84 and there was less consistent evidence of it being associated with infectious complications.4 A study by Golse et al.8 used psoas muscle area to define sarcopenia and noted worse 1- and 5-year survival in those with baseline sarcopenia.8 Lastly, prior work has demonstrated improved exercise capacity and muscle strength with exercise-based interventions after LT, whereas our work did not note improvement in SMI with physical rehabilitation.15 This may be because of the fact that we were able to assess only immediate post-LT physical rehabilitation rather than physical rehabilitation after full recovery from LT (e.g. after 6 months post-LT).
Ultimately, we acknowledge that our results relatively differ from other studies but concluding definite effects of sarcopenia on morbidity and mortality is challenging given variable definitions and modes of measurements across studies. However, given our large sample size which enabled us to control for many potential confounders that can affect sarcopenia and its potential sequelae, our study provides informative results that pave the path for prospective investigation in this sphere, with standardised definitions and measurements of sarcopenia.
Our study demonstrates a provocative observation and raises a question of why skeletal muscle mass does not recover long-term after LT. Although advancing age can be an explanation, at least in some study patients, for the rates of worsening sarcopenia, the rate noted in the SMI analysis (4.7 cm2/m2 decline in the first year post-LT) is more than what has been observed in terms of annual decline in skeletal muscle mass in the elderly general population,16,17 which suggests additional contributors in this patient population. In daily clinical practice, patients feel better after LT with increased energy, appetite, and mobility. Therefore, persistent, or worsening sarcopenia is unexpected and intriguing at the same time. It is noteworthy that CNIs are the most prescribed immunosuppressive agents after LT. Interestingly, CNIs appear to upregulate myostatin which is a well-known inhibitor of muscle-related protein synthesis.12 In fact, a CNI-related phenomenon may be the only conceivable explanation for the lack of recovery of cirrhosis-related sarcopenia post-LT. Although CNI impact on muscles has been demonstrated in an animal model, it is unknown if this effect is dependent or independent of extent of exposure to these medications.9 Our data did not demonstrate an association between the magnitude of exposure to tacrolimus, measured through trough levels, and sarcopenia after LT. This may suggest that the impact of CNI on muscles may be dose independent. However, more research is needed to fully investigate the role of immunosuppressive agents on muscle mass. Although there are data regarding corticosteroids and mTOR inhibitors effect on muscle mass, these were not investigated in the current study. Nearly all patients were off steroids within the first 90 days after transplant, per our institutional protocol, and there were only 26 patients prescribed mTOR inhibitors during the study period. Therefore, the utility of analysing impact of corticosteroids on the long-term SMI assessment would be limited given the universal protocol for expedited taper and short-term use of this medication in our centre. In addition, we could not have a sufficient sample size for mTOR inhibitor-related analysis given its infrequent use in our patient cohort and given the variable times of change from CNI to mTOR inhibitor, per clinical indication.
The strengths of our study include, but are not limited to, large sample size as it is the largest study to date assessing post-LT long-term evolution of sarcopenia and its impact on long-term mortality. The granularity of the analysed data is another strength of our study. This granularity offered a unique opportunity to analyse the impact of the extent of immunosuppression exposure on the course of sarcopenia post LT. In view of the lack of universal cut-offs for muscle mass to define sarcopenia in patients with cirrhosis, we have pursued a robust analysis approach that has taken two pathways that complement each other; analysing sarcopenia based on the SMI cut-offs (i.e. categorical analysis) derived from populations without cirrhosis and analysing the changes in the values of SMI post-LT as a continuum, while accounting for known clinically important covariates in this population. Both approaches have demonstrated the same striking finding that most patients with sarcopenia fail to have muscle recovery long-term after transplant.
Our study has some limitations. First, the retrospective nature for this study carries an inherent limitation. Second, the timing of the CT scans post-LT was not the same for all patients given that scans were done for standard of care indications at variable time points. In addition, conceptually, there could be bias as those patients that were sicker may have been getting more imaging. Furthermore, some patients may have had MRI instead of CT either pre-LT or post-LT and were not included. However, our analysis of baseline characteristics in the excluded group found comparable age, sex distribution, and liver disease aetiology. In fact, the comparison revealed that the excluded group had statistically comparable and numerically higher MELD-Na, which argues against the idea that the study population may be a sicker group at least initially. Given the large similarity between included and excluded groups in age and sex (the two variables significantly associated with baseline sarcopenia in this study) and in view of our finding that baseline sarcopenia is most predictive of long-term sarcopenia post-LT, our cohort was likely representative of this patient population. However, other unmeasured variables could have demonstrated differences between included and excluded cohorts and future prospective studies that also include patient lifestyle (e.g. activity level and nutrition) would be needed.
In conclusion, although sarcopenia is prevalent in patients with cirrhosis and expected to resolve after curing end-stage liver disease with LT, this study showed that sarcopenia rarely resolves in LT recipients on long-term follow up. Moreover, we found a progressive decline in muscle mass over time after LT with the greatest rate of decline within the first year followed by more subtle decline in the years that follow. Baseline sarcopenia did not have a negative impact on long-term mortality post-LT in this cohort. Immediate post-LT physical rehabilitation was not protective of long-term post-LT sarcopenia in this study. Prospective multicentre studies are needed to further evaluate the natural course of sarcopenia post-LT, its impact on outcomes, and the interventions needed for reversal of post-LT sarcopenia.
Authors’ contributions
Study concept, study design, data interpretation, and drafting the manuscript: SB, MI, HJS. Data collection: BR, EBo, ER, EM, MG, AS, JA, EBr, AD, SA. Analysis and interpretation of data: JCS. Critical revision of the manuscript and study supervision: MI, HJS. Drafting final manuscript: all authors.
Financial support
The authors received no financial support to produce this manuscript.
Conflicts of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank the staff of the Vanderbilt Diet, Body Composition, and Human Metabolism Core under the direction of Dr Silver for CT scan morphometric analysis.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100881.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Bhanji R.A., Montano-Loza A.J., Watt K.D. Sarcopenia in cirrhosis: looking beyond the skeletal muscle loss to see the systemic disease. Hepatology. 2019;70:2193–2203. doi: 10.1002/hep.30686. [DOI] [PubMed] [Google Scholar]
- 2.Ebadi M., Bhanji R.A., Mazurak V.C., Montano-Loza A.J. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54:845–859. doi: 10.1007/s00535-019-01605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai J.C., Tandon P., Bernal W., Tapper E.B., Ekong U., Dasarathy S., et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1611–1644. doi: 10.1002/hep.32049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Vugt J.L.A., Levolger S., de Bruin R.W.F., van Rosmalen J., Metselaar H.J., Ijzermans J.N.M. Systematic review and meta-analysis of the impact of computed tomography–assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transpl. 2016;16:2277–2292. doi: 10.1111/ajt.13732. [DOI] [PubMed] [Google Scholar]
- 5.Ooi P.H., Hager A., Mazurak V.C., Dajani K., Bhargava R., Gilmour S.M., et al. Sarcopenia in chronic liver disease: impact on outcomes. Liver Transpl. 2019;25:1422–1438. doi: 10.1002/lt.25591. [DOI] [PubMed] [Google Scholar]
- 6.Aby E.S., Lee E., Saggi S.S., Viramontes M.R., Grotts J.F., Agopian V.G., et al. Pretransplant sarcopenia in patients with NASH cirrhosis does not impact rehospitalization or mortality. J Clin Gastroenterol. 2019;53:680–685. doi: 10.1097/MCG.0000000000001109. [DOI] [PubMed] [Google Scholar]
- 7.DiMartini A., Cruz R.J., Jr., Dew M.A., Myaskovsky L., Goodpaster B., Fox K., et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl. 2013;19:1172–1180. doi: 10.1002/lt.23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golse N., Bucur P.O., Ciacio O., Pittau G., Sa Cunha A., Adam R., et al. A new definition of sarcopenia in patients with cirrhosis undergoing liver transplantation. Liver Transpl. 2017;23:143–154. doi: 10.1002/lt.24671. [DOI] [PubMed] [Google Scholar]
- 9.Dunn S.E., Burns J.L., Michel R.N. Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem. 1999;274:21908–21912. doi: 10.1074/jbc.274.31.21908. [DOI] [PubMed] [Google Scholar]
- 10.Martin L., Birdsell L., Macdonald N., Reiman T., Clandinin M.T., McCargar L.J., et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 11.Bhanji R.A., Takahashi N., Moynagh M.R., Narayanan P., Angirekula M., Mara K.C., et al. The evolution and impact of sarcopenia pre- and post-liver transplantation. Aliment Pharmacol Ther. 2019;49:807–813. doi: 10.1111/apt.15161. [DOI] [PubMed] [Google Scholar]
- 12.Tsien C., Garber A., Narayanan A., Shah S.N., Barnes D., Eghtesad B., et al. Post-liver transplantation sarcopenia in cirrhosis: a prospective evaluation. J Gastroenterol Hepatol. 2014;29:1250–1257. doi: 10.1111/jgh.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaido T., Tamai Y., Hamaguchi Y., Okumura S., Kobayashi A., Shirai H., et al. Effects of pretransplant sarcopenia and sequential changes in sarcopenic parameters after living donor liver transplantation. Nutrition. 2017;33:195–198. doi: 10.1016/j.nut.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Plank L.D., Metzger D.J., McCall J.L., Barclay K.L., Gane E.J., Streat S.J., et al. Sequential changes in the metabolic response to orthotopic liver transplantation during the first year after surgery. Ann Surg. 2001;234:245–255. doi: 10.1097/00000658-200108000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moya-Nájera D., Moya-Herraiz Á., Compte-Torrero L., Hervás D., Borreani S., Calatayud J., et al. Combined resistance and endurance training at a moderate-to-high intensity improves physical condition and quality of life in liver transplant patients. Liver Transpl. 2017;23:1273–1281. doi: 10.1002/lt.24827. [DOI] [PubMed] [Google Scholar]
- 16.Tandon P., Montano-Loza A.J., editors. Frailty and sarcopenia in cirrhosis: the Basics, the Challenges, and the future. Springer; New York: 2019. [Google Scholar]
- 17.Mitchell W.K., Williams J., Atherton P., Larvin M., Lund J., Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.