Abstract
The spirochete that causes tick-borne relapsing fever, Borrelia hermsii, was isolated in pure culture during 1995 and 1996 from three acutely ill human patients infected in southern British Columbia, Canada. The geographic area of exposure is a known focus of this disease dating back to 1930 when the first case was recognized in a human. Analyses of plasmid DNA, protein profiles, and reactivity with a species-specific monoclonal antibody identified the new isolates of spirochetes as B. hermsii, all of which were most similar to an isolate of this spirochete from northern California described previously. These are the first reported isolates of B. hermsii from Canada.
The first human cases of tick-borne relapsing fever recognized in Canada occurred in the Okanagan Valley of southern British Columbia from 1930 to 1932 (16). Six patients became infected after camping on the shore of Arrow Lake during the summer, establishing the area of West Kootenay as an area of endemicity for relapsing fever. While sporadic cases have no doubt occurred in this region over the years, no isolations of the causative agent or definitive identifications of the spirochete causing the infections are known to us. Because the tick Ornithodoros hermsi has been collected from this region (8, 9) and is the known vector of Borrelia hermsii not far to the south in eastern Washington and northern Idaho (7), the presumption has been that tick-borne relapsing fever in British Columbia is caused by B. hermsii (2, 4, 22). For many years, the lack of a productive culture medium for the isolation and propagation of borreliae prevented the development of techniques for the easy identification of species of Borrelia. This problem was solved by Kelly (12) in 1971, and with later modifications (3, 23), we now have a medium that has allowed many species of Borrelia to be cultured and analyzed for a variety of genetic and phenotypic characteristics.
Very few cases of relapsing fever have been documented from British Columbia since those first reported in 1933 (16). Spiller (22) described two cases from the Okanagan Valley in 1984, and between 1984 and 1995, Banerjee and coworkers (1, 2) have reported on numerous cases from several regions of southern British Columbia. A recent retrospective analysis of case reports identified 14 cases of relapsing fever in southern British Columbia from 1980 to 1995 (7). In 1995 and 1996, three people contracted an acute febrile illness consistent with tick-borne relapsing fever while spending time in the Okanagan Valley. In this report we describe these patients, characterize the spirochetes isolated from each patient’s blood, and identify them as B. hermsii.
MATERIALS AND METHODS
Borrelia strains and cultivation.
Three new isolates of B. hermsii were established from the blood of patients who acquired the infections in the Okanagan Valley. These isolates are designated OKA-1, OKA-2, and OKA-3, respectively. OKA-1 was isolated from blood collected on 11 October 1995 from an adult female (patient 1). OKA-2 was isolated from blood collected on 26 June 1996 from an adult male (patient 2). OKA-3 was isolated from blood collected on 11 September 1996 from an adult male (patient 3). B. hermsii HS1 (ATCC 35209) serotype 33 (serotype C) originated from O. hermsi collected near Spokane, Wash. (24). B. hermsii DAH was isolated from the blood of a relapsing fever patient in eastern Washington. B. hermsii YOR was isolated from the blood of a relapsing fever patient in California (13). Borrelia parkeri, Borrelia turicatae, and Borrelia anserina were isolated from Ornithodoros parkeri, Ornithodoros turicata, and a domestic chicken, respectively, and are part of the bacterial reference collection of the Rocky Mountain Laboratories. Borrelia burgdorferi B31 was isolated from an Ixodes scapularis tick collected on Shelter Island, N.Y. (5). Escherichia coli pTA-1 harbors a recombinant plasmid containing the glpQ gene of B. hermsii (20). This gene expresses an immunoreactive protein that is reactive with antibodies produced during relapsing fever infections but not Lyme disease (20).
The three new isolates were established in pure culture only after first inoculating laboratory mice (Mus musculus) with the spirochetemic blood from the human patients, because all attempts to isolate the organisms in culture medium directly from the patients’ blood failed. CD1 mice of either sex (ages 6 to 8 weeks) were inoculated intraperitoneally with 0.25 ml of human blood in EDTA and were examined daily for the presence of spirochetes in the peripheral blood. For this, the tail of the mouse was knicked and a drop of blood was smeared on a microscope slide, air dried, stained with Giemsa, and examined by bright-field microscopy at ×970 magnification under oil immersion. On the second or third day of the spirochetemia, 0.25 ml of the infected blood was collected by cardiac puncture and was passaged in a new mouse, the “amplifying” mouse, by intraperitoneal inoculation; this generally produced an even higher level of spirochetemia in 2 days. Infected blood samples from the amplifying mice were collected by cardiac puncture, 100 μl was inoculated into a tube containing 9 ml of BSK-H medium (Sigma Chemical Co., Melville, N.Y.), and the tube was incubated at 34°C. Spirochetes were harvested and examined after two to four passages.
PAGE.
Whole-cell lysates of spirochetes were prepared as described previously (19). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) with Laemmli buffer (14) and a vertical gel electrophoresis system (Bethesda Research Laboratories-GIBCO, Gaithersburg, Md.) were used to separate proteins following the instructions of the manufacturer. Proteins were stained with Coomassie brilliant blue.
Western blot analysis.
Whole-cell lysates were electrophoresed in one-dimensional acrylamide gels and were blotted onto nitrocellulose membranes with the buffer of Towbin et al. (25) and a Trans-Blot Cell (Bio-Rad Laboratories) following the instructions of the manufacturer. The membranes were blocked overnight at room temperature with TSE-Tween (50 mM Tris [pH 7.4], 150 mM NaCl, 5 mM EDTA, 0.05% Tween 20) and were subsequently incubated either with monoclonal antibody H9826, specific for B. hermsii (18), or with convalescent-phase serum samples (diluted 1:100) from the three patients. Bound antibodies were detected by 125I-labeled protein A autoradiography (19).
DNA purification and analysis.
Total DNA was purified from 500 ml of stationary-phase BSK-H cultures of spirochetes (21). DNA samples were examined by agarose gel electrophoresis with a Mini-Sub DNA Cell (Bio-Rad Laboratories). DNA samples were electrophoresed in 0.3% agarose gels with TBE buffer (90 mM Tris, 90 mM boric acid, 20 mM EDTA) to resolve the plasmids. Gels were run with ethidium bromide at 50 V for 5 min and then at 12 V for 16 h, and the DNA was visualized by UV transillumination.
RESULTS
Case histories.
Patient 1, an adult female, was exposed to ticks in her cottage near Okanagan Lake in South Okanagan, British Columbia, Canada, in late August 1995. Patient 2, an adult male and the husband of the first patient, was also exposed to ticks during his stay in the same cottage during June 1996. Patient 3, an adult male, was exposed to ticks while staying in a cottage on the other side of Okanagan Lake in early September 1996. Each of the patients had been bitten by unknown “insects” during their many nights of sleeping in their cottages, although none of them recalled specifically being bitten by ticks. Subsequently, each patient manifested repeated episodes of high temperature (39.6 to 41°C), night sweats, dizziness, nausea, and loss of appetite. The first patient suffered five episodes before the spirochetes were detected in blood smears examined in early October 1995. The other two patients infected in 1996 were tested for B. hermsii antibodies and for spirochetes in blood smears as soon as the patients had repeated symptoms. All patients were successfully treated with antibiotics.
Analysis of spirochetes.
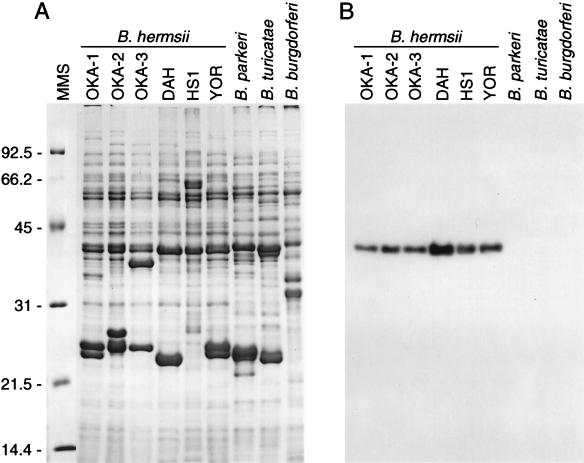
From each of the patients with an illness consistent with tick-borne relapsing fever, spirochetes were detected in their peripheral blood and were subsequently cultivated in vitro. The new isolates had protein profiles that were consistent with those of B. hermsii and other species of relapsing fever spirochetes (Fig. 1A). Their phenotypes included flagellin with a relative migration on polyacrylamide gels of approximately 39 kDa and several prominently stained proteins in the mid-20-kDa range. The latter proteins were presumed to be members of the group of small variable major proteins (Vmps). Reactivity with monoclonal antibody H9826 (Fig. 1B) demonstrated the presence of flagellin and confirmed the identities of the new isolates as B. hermsii.
FIG. 1.
Sodium dodecyl sulfate-PAGE of Borrelia whole-cell lysates (A) and immunoblot analysis with monoclonal antibody H9826 (B). The 12.5% gel was stained with Coomassie brilliant blue, and molecular mass standards (MMS) are shown on the left (in kilodaltons). B. hermsii flagellin, which binds to antibody H9826, was detected by 125I-labeled protein A radiography.
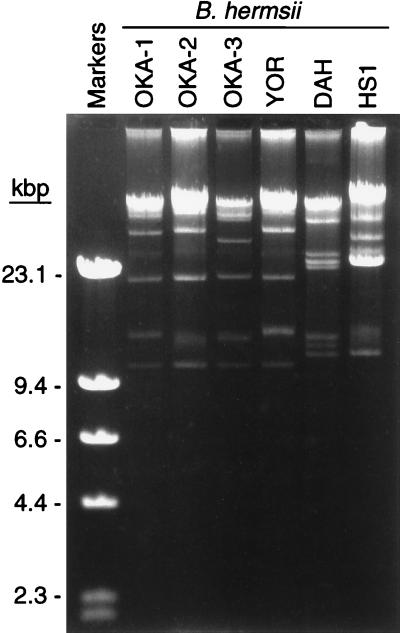
The plasmid profiles of the new isolates were nearly identical to each other (Fig. 2) and were similar to that of an isolate (YOR) that originated from a human with an infection contracted in Siskiyou County in northern California (17). The other isolates, DAH and HS1, which originated from eastern Washington, had plasmid profiles representative of those of another subgroup of B. hermsii (17).
FIG. 2.
Agarose gel electrophoresis of Borrelia DNA demonstrating the similar plasmid profiles of the British Columbia isolates of B. hermsii compared to that of the YOR isolate from northern California. Size standards are shown on the left.
Serology.
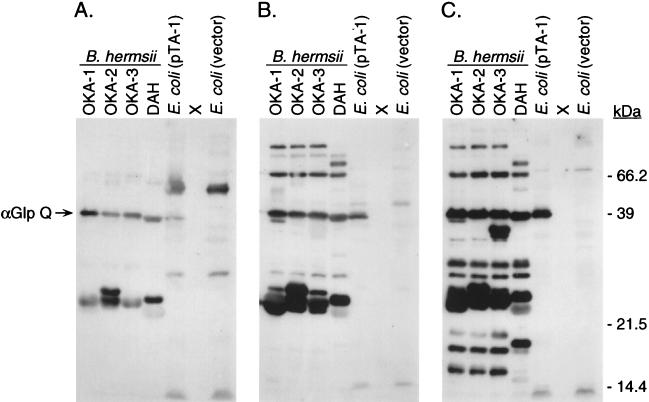
Convalescent-phase serum samples from three patients were tested by immunoblot analysis with the B. hermsii lysates as well as the E. coli lysate containing the B. hermsii GlpQ protein (Fig. 3). Each patient had antibodies reactive to the native and recombinant 39-kDa GlpQ and the mid-20-kDa Vmps. However, antibody reactivity to additional antigens increased with time between the time of onset of illness and the time when the serum sample was collected on days 10, 22, and 33 after the onset of the first febrile episode. For serum from patient 3 collected on day 10, antibody reactivity was primarily observed to GlpQ and the Vmps (Fig. 3A). For serum from patient 1 collected 33 days after the onset of fever and five febrile episodes, antibodies bound to an additional nine antigens (Fig. 3C). Finally, each serum sample was equally reactive with all of the lysates used, demonstrating no increased reactivity with the isolate originating from the same patient.
FIG. 3.
Immunoblots with serum samples from the three relapsing fever patients from southern British Columbia tested with lysates of B. hermsii originating from the same patients. (A) Serum from patient 3 collected 10 days after onset; (B) serum from patient 2 collected 22 days after onset; (C) serum from patient 1 collected 33 days after onset. All samples were tested at a 1:100 dilution, and bound antibody was detected by 125I-labeled protein A radiography. Antibody to GlpQ, 39 kDa, is indicated on the left, and estimated molecular masses are given on the right.
DISCUSSION
Tick-borne relapsing fever was first recognized in North America in 1915 (15) and continues to cause sporadic illness in numerous regions of endemicity (4). While most cases usually occur singly, many outbreaks of multiple cases have been reported during the last 30 years in the western United States (4, 6, 24). In many areas including western Canada, however, we believe that many cases are not recognized or are misdiagnosed, resulting in an underestimate of the impact that this disease has on human health (7). Many patients have only a few relapses or may have no relapses. One patient in the present study experienced five febrile episodes before a correct diagnosis of tick-borne relapsing fever was made. We hope that this report will help increase the awareness of relapsing fever so that prompt detection of spirochetes and treatment with antibiotics can control the infection sooner and in more patients.
When the first cases of tick-borne relapsing fever in British Columbia were reported in 1933, the authors assumed that the tick involved in the transmission of spirochetes was the Rocky Mountain wood tick, Dermacentor andersoni (16). Soon afterward in 1934, however, another report questioned this identity of the vector and suggested that a species of Ornithodoros was a much more likely candidate (10, 16). Yet no specimens of any Ornithodoros species were known from Canada until 1948, when O. hermsi was first collected at Summerland, British Columbia (8), and again during 1949 and 1950 in the same area (9). Since the known presence of O. hermsi in southern British Columbia, the assumption has been that the agent causing tick-borne relapsing fever in this region was B. hermsii, the same species of spirochete maintained and transmitted by O. hermsi in other regions of endemicity to the south. We have confirmed this long-held assumption by isolating and identifying B. hermsii from three patients.
In a recent study of tick-borne relapsing fever in western North America, spirochetes were detected in BSK medium by 48 h after inoculation with blood samples from 10 of 18 patients who had not yet received antibiotic therapy (7). In our experience, however, the presence of live spirochetes during the first several days after inoculation does not guarantee that these bacteria will continue to replicate and become established in continuous passage. Our use of the term isolation, therefore, refers only to the successful cultivation through several weeks or more of continuous passage and not to the detection of live spirochetes only days after inoculation into medium. We have succeeded at isolation only after the spirochetes in the patients’ blood have been amplified through one or more passages in mice, followed by the inoculation of infected mouse blood into multiple tubes of medium.
While animal inoculation and in vitro cultivation provide the potential for isolation of spirochetes, the diagnosis of relapsing fever is based primarily on the direct detection of spirochetes in the peripheral blood of patients. During acute episodes, high numbers of spirochetes may circulate in the blood and can be detected by light or dark-field microscopy of wet preparations made from a drop of whole blood. Often, the first evidence of the presence of spirochetes is the erratic movement of erythrocytes as the bacteria bump or adhere to and pull these cells. Giemsa or Wright stains are used to stain dried thin smears and thick drops of blood for examination by conventional light microscopy, but densities of 104 to 105 organisms per ml of blood need to be present for detection by these methods.
Our preliminary characterization of the British Columbia isolates demonstrates plasmid profiles that are shared with the YOR isolate described previously (17). Other isolates including the type strain, HS1, and strains DAH, MAN, and CON all share similar but distinctly different plasmid profiles (17) and repertoires of variable major protein genes (11). These observations and several other analyses under way at the Rocky Mountain Laboratories demonstrate two primary subgroups of B. hermsii in western North America that occur sympatrically and across wide geographic distances. The nucleotide sequences of several families of genes in these isolates are being determined to assess the phylogenetic relatedness among these subgroups of B. hermsii and their relatedness to other relapsing fever spirochetes.
ACKNOWLEDGMENTS
We thank Merry Schrumpf and Robert Karstens for technical assistance, Jerry Schmidt for animal care, and Robert Evans and Gary Hettrick for photographic assistance.
REFERENCES
- 1.Banerjee S N, Proctor E M. Lyme disease and relapsing fever in B.C. Dis Surveil. 1988;9:63–64. [Google Scholar]
- 2.Banerjee S N, Proctor E M, O’Hanlon D P. Relapsing fever due to Borrelia hermsii in British Columbia. Dis Surveil. 1987;8:155–162. [Google Scholar]
- 3.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer K M, Munford R S, Maupin G O, Pattison C P, Fox M D, Barnes A M, Jones W L, Maynard J E. Tick-borne relapsing fever: an interstate outbreak originating at Grand Canyon National Park. Am J Epidemiol. 1977;105:469–479. doi: 10.1093/oxfordjournals.aje.a112406. [DOI] [PubMed] [Google Scholar]
- 5.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control. Lyme disease—United States, 1987 and 1988. Morbid Mortal Weekly Rep. 1989;38:668–672. [PubMed] [Google Scholar]
- 7.Dworkin M S, Anderson D E, Jr, Schwan T G, Shoemaker P C, Banerjee S N, Kassen B O, Burgdorfer W. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin Infect Dis. 1998;26:122–131. doi: 10.1086/516273. [DOI] [PubMed] [Google Scholar]
- 8.Gregson J D. Notes on the occurrence of Ornithodoros hermsi in British Columbia, and its probable relation to relapsing fever. Argasidae, Ixodoidea. Proc Entomol Soc British Columbia. 1948;45:15–16. [Google Scholar]
- 9.Gregson J D. The Ixodoidea of Canada. Ottawa, Ontario, Canada: Canada Department of Agriculture; 1956. p. 92. [Google Scholar]
- 10.Hearle E. Vectors of relapsing fever in relation to an outbreak of the disease in British Columbia. Can Med Assoc J. 1934;30:494–497. [PMC free article] [PubMed] [Google Scholar]
- 11.Hinnebusch B J, Barbour A G, Restrepo B I, Schwan T G. Population structure of the relapsing fever spirochete Borrelia hermsii as indicated by polymorphism of two multigene families that encode immunogenic outer surface lipoproteins. Infect Immun. 1998;66:432–440. doi: 10.1128/iai.66.2.432-440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly R. Cultivation of Borrelia hermsi. Science. 1971;173:443–444. doi: 10.1126/science.173.3995.443. [DOI] [PubMed] [Google Scholar]
- 13.Kurashige S, Bissett M, Oshiro L. Characterization of a tick isolate of Borrelia burgdorferi that possesses a major low-molecular-weight surface protein. J Clin Microbiol. 1990;28:1362–1366. doi: 10.1128/jcm.28.6.1362-1366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Meador C N. Five cases of relapsing fever originating in Colorado, with positive blood findings in two. Colo Med. 1915;12:365–369. [Google Scholar]
- 16.Palmer J H, Crawford D J M. Relapsing fever in North America, with report of an outbreak in British Columbia. Can Med Assoc J. 1933;28:643–647. [PMC free article] [PubMed] [Google Scholar]
- 17.Schwan T G, Gage K L, Hinnebusch B J. Analysis of relapsing fever spirochetes from the western United States. J Spirochetal Tick-Borne Dis. 1995;2:3–8. [Google Scholar]
- 18.Schwan T G, Gage K L, Karstens R H, Schrumpf M E, Hayes S F, Barbour A G. Identification of the tick-borne relapsing fever spirochete Borrelia hermsii by using a species-specific monoclonal antibody. J Clin Microbiol. 1992;30:790–795. doi: 10.1128/jcm.30.4.790-795.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwan T G, Kime K K, Schrumpf M E, Coe J E, Simpson W J. Antibody response in white-footed mice (Peromyscus leucopus) experimentally infected with the Lyme disease spirochete (Borrelia burgdorferi) Infect Immun. 1989;57:3445–3451. doi: 10.1128/iai.57.11.3445-3451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwan T G, Schrumpf M E, Hinnebusch B J, Anderson D E, Konkel M E. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J Clin Microbiol. 1996;34:2483–2492. doi: 10.1128/jcm.34.10.2483-2492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson W J, Garon C F, Schwan T G. Analysis of supercoiled circular plasmids in infectious and non-infectious Borrelia burgdorferi. Microb Pathog. 1990;8:109–118. doi: 10.1016/0882-4010(90)90075-2. [DOI] [PubMed] [Google Scholar]
- 22.Spiller G W. Tick-borne relapsing fever due to Borrelia hermsii in British Columbia. Can Med Assoc J. 1986;134:46–47. [PMC free article] [PubMed] [Google Scholar]
- 23.Stoenner H G. Biology of Borrelia hermsii in Kelly medium. Appl Microbiol. 1974;28:540–543. doi: 10.1128/am.28.4.540-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson R S, Burgdorfer W, Russell R, Francis B J. Outbreak of tick-borne relapsing fever in Spokane County, Washington. JAMA. 1969;210:1045–1050. [PubMed] [Google Scholar]
- 25.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]