Highlights
-
•
PSI and SN showed similar results in margins, bone cut accuracy, local recurrence and functional reconstruction scores.
-
•
A planned bone margin from tumour of 5 mm was safe for bone resections.
-
•
Soft tissue margin cannot be planned.
-
•
Long osteotomies, homogenous bone topology and restricted working spaces reduced accuracy of both techniques.
-
•
In urgent cases, SN is more indicated to avoid PSI production time, while PSI has the advantage of less intraoperative using time.
-
•
They deemed similar technical intraoperative complications rate and demanding learning curve.
Keywords: 3D-Cutting guides, Patient specific instrumentation, Bone tumors, Margins, Surgical navigation, Sarcoma, Osteosarcoma
Abstract
Patient specific instrumentation (PSI) and intraoperative surgical navigation (SN) can significantly help in achieving wide oncological margins while sparing bone stock in bone tumour resections. This is a systematic review aimed to compare the two techniques on oncological and functional results, preoperative time for surgical planning, surgical intraoperative time, intraoperative technical complications and learning curve. The protocol was registered in PROSPERO database (CRD42023422065). 1613 papers were identified and 81 matched criteria for PRISMA inclusion and eligibility. PSI and SN showed similar results in margins (0–19% positive margins rate), bone cut accuracy (0.3–4 mm of error from the planned), local recurrence and functional reconstruction scores (MSTS 81–97%) for both long bones and pelvis, achieving better results compared to free hand resections. A planned bone margin from tumour of at least 5 mm was safe for bone resections, but soft tissue margin couldn’t be planned when the tumour invaded soft tissues. Moreover, long osteotomies, homogenous bone topology and restricted working spaces reduced accuracy of both techniques, but SN can provide a second check. In urgent cases, SN is more indicated to avoid PSI planning and production time (2–4 weeks), while PSI has the advantage of less intraoperative using time (1–5 min vs 15–65 min). Finally, they deemed similar technical intraoperative complications rate and demanding learning curve. Overall, both techniques present advantages and drawbacks. They must be considered for the optimal choice based on the specific case. In the future, robotic-assisted resections and augmented reality might solve the downsides of PSI and SN becoming the main actors of bone tumour surgery.
1. Introduction
1.1. Background
Resections with negative margins might be challenging to obtain, especially when anatomy is complex as in pelvis or in juxta-articular regions [1].
Recently developed technologies allowed the introduction of two surgical techniques that can significantly help in achieving negative surgical margins while preserving as much function as possible: patient specific instrumentation (PSI) and intraoperative surgical navigation (SN) (Fig. 1 a, b, c −2) [2], [3], [4], [5], [6], [7]. PSI are guides created through 3D printing using CT or MRI performed pre-operatively, and then applied during the surgical procedure to precisely guide the bony cuts. SN is an intraoperative technology using sensors that allow to see, on a computer screen, where the cut should be performed, enabling a precise resection based on a CT and/or MRI preoperative plan, with or without intraoperative CT scan (Fig. 3 a, b) [2], [5], [8].Fig. 2..
Fig. 1.
a, 1b, 1c: PSI planning of osteotomy plan and cutting guide positioning. a. MRI planning of the cut with safety margins (on the left) and 3D planning of the resection with osteosarcoma in red (on the right). b. PSI 3D osteotomies planning in a proximal tibia, with tumour in red. c. 3D planning of the cutting guides for a proximal tibia tumour resection (medial view on the left and anterior view on the right). No soft tissue planning has been made with potential intraoperative drawbacks for jig’s proper fit. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
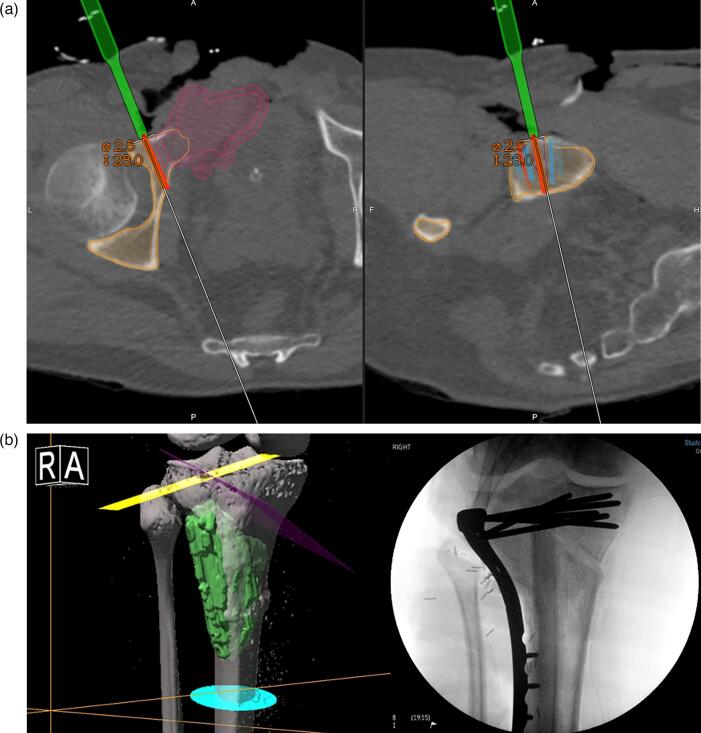
a, 3b. SN intraoperative CT, planning and reconstruction a. Intraoperative CT reconstruction of the SN system. On the left, axial view: in pink, the tumour in the right pubic ramus close to the acetabulum. The outer pink line is the 5 mm planned safety margin. In red, the osteotomy line. In green, the drill. In white, the direction of the osteotomy. On the right, sagittal view of the planned osteotomy. The surgeons are therefore able to see where they are going to perform the navigated osteotomy using the CT reconstruction. b. SN osteotomies planning (on the left) and postoperative radiological result after allograft reconstruction (on the right). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Intraoperative use of the PSI Proximal tibia resection (medial view) of an osteosarcoma using PSI. The jig has been fixed to the bone using positioning pins as planned. Jig positioning has been difficult due to the presence of unplanned soft tissues in the posterior part of the tibia (in particular the posterior cruciate ligament).
1.2. Rationale
Both PSI and SN are deemed to be suitable in obtaining negative resection margins for bone sarcomas, and both provide the possibility to precisely reconstruct the defect [2], [5], [7], [9]. However, it is still not clear if one is superior compared to the other in terms of oncological and functional outcomes. Therefore, the aim of the present paper is to systematically review the literature to compare the two techniques focusing on results provided on oncological margins and functional assessment. Features as preoperative time of surgical planning, surgical intraoperative time, intraoperative complications, and learning curve are also investigated.
2. Methods
2.1. Protocol and registration
This systematic review was conducted in accordance with the PRISMA guidelines [10] as well as the Cochrane Collaboration Guidelines [11]. The protocol was registered in the PROSPERO database (CRD42023422065) on the 28th of May 2023.
2.2. Search strategy
PubMed and EMBASE databases were queried independently by two authors (AB and CDB) from January 2010 to November 2022 for papers on Patient Surgical Instrumentation (PSI) and Surgical Navigation (SN) for bone tumor resection. Authors specifically searched for papers dealing with oncological margins, restoration of function, preoperative time of surgical planning, surgical intraoperative time, intraoperative complications, and learning curve. 3D- Cutting guides AND bone tumours AND margins, patient specific instrumentation AND sarcoma, patient specific instrumentation AND bone tumors, surgical navigation AND margins were keywords used in Boolean search. Titles and Abstracts have been independently screened to identify studies that potentially met inclusion criteria. Subsequently full-text screening has been independently assessed for inclusion. Disagreements/conflicts regarding study eligibility have been reviewed by an independent assessment of a third authors (DMD) and subsequent discussed with the other two authors for definitive decision. Authors have been initially blinded to eligibility decisions, but blinding has not occurred after the final decision and for articles requiring discussion. In addition to database-searching cross-referencing has been also used to identify eligible publications.
2.3. Study selection
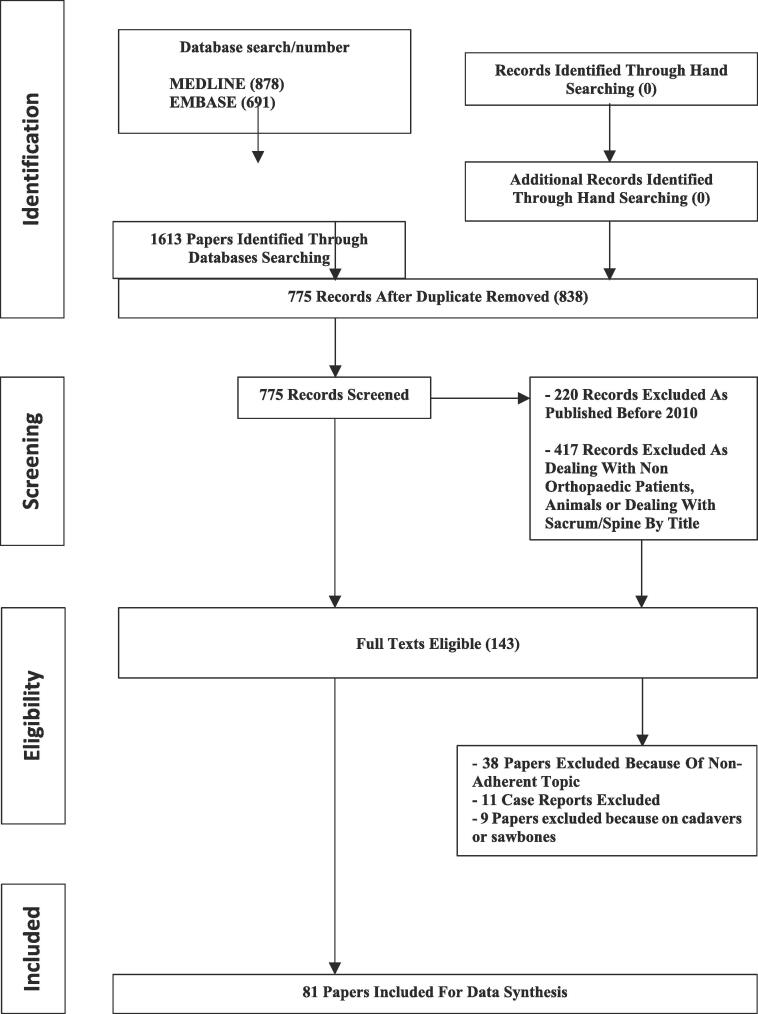
Papers dealing with PSI and SN for orthopaedic bone tumour resection published from January 2010 to November 2022 have been searched. 1613 papers were initially identified and 81 of them matched criteria for inclusion and eligibility. PRISMA flowchart of search and paper inclusion is reported in Fig. 4. Due to different anatomy and potential different results in terms of oncological margins and functional outcomes, we divided our analysis based on the location of the tumour between long bones and pelvis.
Fig. 4.
PRISMA flowchart of search and studies selection.
2.4. Data extraction
Standardized pre-built data extraction forms have been utilized. Data extraction has been performed independently by two authors (AB and CDB) and any discrepancy has been resolved through discussion, with the involvement of a third author (DMD) where necessary. Data extraction included but is not limited to: study details, study aim, study design, patient characteristics & outcomes, results and utility of the patient specific instrumentation’s or surgical navigation’s tools.
2.5. Risk of bias assessment
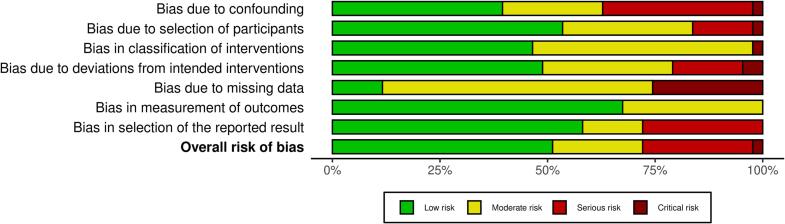
Risk of bias assessments has been conducted independently by two authors (AB and CDC) using RoB 2 (Version 2 of the Cochrane risk-of-bias tool for randomised trials) for Randomised-Controlled Trials and ROBINS-I for the risk of bias in non-randomised studies of interventions. The result of risk of bias assessment is reported in Fig. 5 a, b.
Fig. 5.
A, 5b. ROBINS-I risk of bias assessment a. ROBINS-I summary plot of risk of bias assessment. b. ROBINS-I traffic lights plot of risk of bias assessment.
2.6. Strategy for data synthesis
This systematic review provides a synthesis regarding the use of patient specific instrumentation and surgical navigation for bone tumor resection. Quantitative synthesis has been performed when enough eligible studies and adequate homogeneity in patients and treatments were provided, and outcome measures was present.
2.7. Subgroup analysis
Subgroup analysis has been conducted to examine the effects of patient-specific instrumentation or surgical navigation resection in two distinct subgroups: subgroup 1 consisted of patients undergoing interventions on long bones, and subgroup 2 comprised patients undergoing interventions on the pelvis. This analysis has been conducted solely for the assessment of the main outcomes (oncological margins and function of the reconstruction) and didn’t include the evaluation of secondary outcomes. In subgroup 1, patients who receive patient-specific instrumentation or surgical navigation resection for long bone interventions were compared to each other and, when possible, to those who underwent standard instrumentation or conventional resection methods. The primary objective was to investigate the impact of these advanced techniques on the main outcomes of interest. Similarly, subgroup 2 involved patients who underwent patient-specific instrumentation or surgical navigation resection specifically for pelvic interventions. A comparison was made between these subgroups and, when possible, with patients who underwent standard instrumentation or conventional resection approaches. The primary goal was to explore the influence of these advanced techniques on the main outcomes of interest. The subgroup analysis aimed to provide additional insights into the differential effects of patient-specific instrumentation or surgical navigation resection in patients undergoing interventions on long bones and pelvis. By considering these subgroups separately, we aimed to enhance the precision and accuracy of our findings, thereby contributing to a more comprehensive understanding of the impact of these advanced techniques on the main outcomes.
3. Results
3.1. Oncological margins
3.1.1. Long bones
16 studies provided information on oncological margins after resection using PSI and SN; only 2 studies focused on PSI, compared to 14 focusing on SN. Resections resulted in negative margins in all PSI and SN studies considered [3], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. At follow up, the study of Staats et al. on SN reported one local recurrence (4%) [22], lower than the 9% reported by Albergo et al [13], [22]. No studies reported local recurrence in long bones for PSI. From these results the two techniques can be considered equivalent in providing negative margins in long bones tumour resections; however, the rate of local recurrence was reported only by a small number of studies.
With regards to cutting accuracy, the vast majority of the studies reported similar results between PSI and SN, ranging from 0.3 mm to 4 mm of accuracy compared to the planned, with most studies showing a mean cutting error less than 2 mm [12], [19], [20], [22], [23], [24], [25], [26]. The highest mean cutting error (7 mm) has been reported in the SN study of Lim et al. on proximal humerus resection [21]. Overall, the two techniques deemed to have similar accuracy in long bones tumour resections.
3.1.2. Pelvis
24 studies analyzed PSI and SN with regards to oncological margins in pelvic resections, 9 on PSI and 18 on SN, with 3 studies comparing the two. All studies comparing PSI or SN against free hand resection demonstrated better margins and lower recurrence rates (28–35% is the bone recurrence rate for a free hand resection in pelvis vs 18–23% of SN and 0% of PSI) [8], [37]. Negative margins have been presented by the majority of papers both for PSI and SN, but compared to long bones, there are more studies showing intralesional resections ranging from 4% to 19%, [13], [27], [28], [29], [30]. The two techniques deemed to be equivalent in providing negative margins also in pelvic resections, as well as superior to the free hand resection that reaches a rate of positive margin up to 50% [28].
Despite similar results on negative margins, the rate of local recurrence is higher in SN studies, ranging from 13% to 23%, while no PSI study reported bone recurrence [6], [27], [29]. However, neither PSI nor SN provided any benefit on soft tissue margin and recurrence when the tumour invaded soft tissues as well: Bosma et al. showed no difference for soft tissue margin comparing SN to free hand technique (R0 in 50% vs 54%), stating that soft tissue margins appeared to not be influenced by navigation resection [28]. This is similar to the experience showed by Gouin et al., who showed a positive margin on iliac vessels after PSI resection due to impossibility of planning a soft tissue margin [31]. Albergo et al. showed that on 57 patients with pelvic tumours, SN provided 100% negative bone margins, but 2 soft tissue positive margins and 22% of local recurrence [13]. Similarly, Nandra et al. in 23 patients with pelvic tumour SN resections showed negative margins in all cases with no bone recurrences, but 35.1% of soft tissue recurrence at a mean long-term follow up of 59 months; thus, the Authors encourage wide resection margins over compromising resections for preserving function [32]. Fujiwara et al. found 8% of positive soft tissue margin using SN [29]. Therefore, both PSI and SN are equivalent in not providing any benefit on soft tissue margin, but PSI seemed to have less local recurrences compared to SN.
The majority of studies reported similar accuracy between PSI and SN in pelvic tumour resections; the mean cutting error ranged from 0.8 mm to 4 mm, being usually less than 2 mm [8], [33], [34], [35], [36], [37].
In the study of De Paolis et al. on PSI, the guide positioning error was 8 mm in 20% of the patients, often related to the length of the osteotomy as a longer cut can suffer more of potential errors of saw inclination on the entry point, compared to a shorter cut; despite no oncological or reconstruction drawback reported in the study, this result outlines the critical importance of avoiding the use of PSI in large pelvic tumours, as stated by the Authors [38].
The importance of bone topology is critical for SN as well. Stoll et al. showed a mean measurement registration error of 12 ± 6.52 mm compared to planned, significantly more than the “system reported error” (SRE) of 0.68 ± 0.15 mm; this was due to the technical accuracy of the navigation system and to the limit intrinsic to the manual registration of bony landmarks during surgery [39]. Therefore, length of the osteotomy, restricting working space and bone topology are critical issues both for PSI and SN.
3.2. Reconstruction
3.2.1. Long bones
11 studies focused on functional results after PSI (6 studies) or SN (5 studies) resection in long bones. 5 studies considered prosthetic reconstruction and 6 biological ones, with 3 of them in adults and the remaining 3 in children. The Musculoskeletal Tumour Society Scoring System was the most used scoring system (8 studies), followed by the Society of Limb Salvage Score (1 study) [40].
In adults, Wong et al. showed excellent MSTS scores with PSI on 8 patients: 29 (97%) at 41 months of follow up [23]. High scores have been stated by Ding et al. too, with 8 excellent and 4 good outcomes in 12 patients with around knee SN tumour resection and endoprosthetic reconstruction using Society of Limb Salvage Score [41]. Functional results of 80% on MSTS scale have been reached by Ippolito et al. for unicondylar PSI resection and reconstruction with custom made unicondylar knee hemiarthroplasty in their 4 patients case series [42]. Considering shoulder salvage surgery followed by prosthetic reconstruction, in 7 patients who underwent SN proximal humerus tumour resection, Li et al. reported a MSTS of 92%, being one of the few studies assessing function after upper limb SN resection [21]. The same Author reported an MSTS of 89% after juxta-articular tumour resection in other 6 patients with osteosarcoma in humerus (3 patients), tibia (2 patients) and femur (1 patient) [19]. Therefore, neither PSI or SN resections seem to provide better functional performance compared to each other, both yielding to high functional scores when followed by prosthetic reconstruction.
Just one study focusing on biological reconstruction in adults provided MSTS score: in 5 patients with around knee multiplanar osteotomies for chondrosarcoma resection, Aponte-Tinao et al. showed 97% of MSTS score [25]. However, other studies considered adult biological reconstruction focusing on non-union rates: in the review of Gasparro et al. non-union rate reached 25% for osteotomy in long bones in a series of 6 patients and allograft reconstruction after PSI resection [16]. The rate of non-union reported by Aponte-Tinao et al. is instead of 6% in a series of 69 patients with long bones tumour resection with SN and massive allograft reconstruction [24]. However, due to the low number of studies considering adult biological reconstruction it is not possible to state if one technique can be considered superior to the other, either for functional score and non-union rates.
With regards to pediatric reconstructions, Li et al. in their study using SN reported an MSTS of 26.7 (89%) after a mean follow up time of 25.2 months [20]. In pediatric juxta-articular tumour resection with PSI resection, Li et al. have reported a MSTS of 27.1 (90%) at 40.5 months follow up using biological reconstruction, without any case of non-union [18]. This MSTS result was similar to the 27.6 (92%) reported by Kim et al. in the same kind of patient with PSI [17]. The three studies reported equivalent results for the two techniques in pediatric biological reconstructions; however, data are not enough for a definitive conclusion.
3.2.2. Pelvis
7 studies analyzed functional scores after pelvic resection, 4 on PSI and 3 on SN. 4 studies focused on prosthetic reconstruction (2 for PSI and 2 for SN), 2 PSI studies on allograft reconstruction and 1 SN on resection sparing the acetabulum followed by no reconstruction.
Considering prosthetic reconstruction, the MSTS results of PSI studies is 90% when reconstruction is performed with 3D printed custom made prosthesis [38]. Superior results have been shown in studies using SN: 93% of MSTS score after SN pelvic resection followed by prosthetic reconstruction has been reported by Wong et al. in 21 patients operated between 2006 and 2009 with a mean follow up of 31 months [37]. In periacetabular resections and reconstruction with ice-cone prosthesis, Fujiwara et al. stated that functional scores were better when navigation was used in 33 patients reconstructed (MSTS 73%), compared to non-navigated resections (MSTS 55%) [30]. Once again, due to the low number of studies it is not possible to clearly state if one technique is superior to the other for pelvic prosthetic reconstruction.
When allograft reconstruction is performed after a pelvic resection using PSI, Evrard et al. reported satisfactory bone to bone contact; this is further supported by the study of Cernat et al., where cutting guides were also used for shaping the pelvic bone allograft [34], [43]. In these studies, however no functional score is reported. No studies reported functional scores for SN resection followed by allograft reconstruction. While, for avoiding any reconstruction and sparing the weightbearing acetabulum in periacetabular tumours, Lam et al. showed that SN can provide an MSTS of 28 (93%) after resection, but no studies using PSI have investigated this type of resection [44]. No comparison is therefore possible for allograft reconstruction and joint sparing resection.
3.2.3. Preoperative time of surgical planning, surgical intraoperative time, intraoperative complications, and learning curve
Considering preoperative planning time, 3 to 4 weeks were usually needed for producing PSI guides; however, in urgent cases like chondrosarcomas, where no neoadjuvant therapies are effective, custom guides might be provided in 2 weeks [31], [34]. On the other hand, with the exception of pre-operative planning, for SN there is no need of preoperative waiting time, therefore SN can be considered more appropriate than PSI for urgent cases [24]. Conflicting results have been reported with regards of intra-operative time using PSI. Studies have shown intra-operative time using SN between 15 and 65 min (Aponte-Tinao et al.; Cho et al.) [24], [45].). Similar results stating the superiority of PSI in terms of shorter surgical time compared to SN in pelvis were presented by Gouin et al. [31], reporting a maximum of 5 min time needed for PSI guide positioning [31]. 14 min was instead the time reported by Fujiwara et al. for SN intraoperative use, again far more than the 60–92 s reported by Biscaccianti et al. for PSI use [46]. On the other side, Evrard et al. reported no difference in intraoperative time between PSI and free hand technique [34]. Overall, the intraoperative time using PSI (ranging around 1 to 5 min) is less compared to SN registration time (ranging from 15 to 65 min).
Focusing on intraoperative technical complication using SN, Young et al. reported that SN had to be interrupted in 2 out of 18 pelvic resections due to inaccuracy in registration (registration error > 2 mm) linked to patient body mass index (BMI) and difficulty to expose suitable bony registration landmarks; the Authors reported the importance of a correct preoperative planning, also considering the cartilage cup or apophysis in children or adolescent patients [47]. On the other side, Jeys et al. reported no intraoperative complications for SN pelvic resections [6]. Another technical difficulty was discussed by Ould-Slimane et al., who reported 1 case out of six in which the system needed to be reinstalled. This however did not affect resection accuracy [48]. Compared to other results, the 2015 study of Stoll et al. presents the lowest accuracy results due to intraoperative technical limitation of SN; the Authors state that one of the major factors influencing a suitable registration is the correct manual registration of anatomical landmarks; therefore, a double check is mandatory by the surgeon for assuring the precise registration of the anatomical landmarks; moreover, the reported error by the navigation system (0.65 ± 0.15 mm) was far way less than the resulted one (12.2 ± 6.52 mm), arising concerns by the Authors on the feasibility of SN for sarcoma surgery [39]. Just like SN, PSI can also incur in intraoperative complications: two studies highlighted the technical difficulty in correctly fitting the jig on bones with complex anatomy due to soft tissues, stating that PSI should be therefore used by experienced surgeons, highly skilled in bone exposure [31], [43]. The same concept was suggested by Jud et al. and Staats et al., who stated that it is critical that PSI systems are used by experienced surgeons as a lack of precision in positioning the jig might result in positive margins and/or impossibility to reconstruct as originally planned [22], [49].
Considering the learning curve for SN, Farfalli et al. in 78 patients who underwent bone tumour resection by experienced surgeons, showed that the mean intraoperative time reduced as more procedures were performed (mean intraoperative time of 31 min); however, the registration accuracy (0.9 mm) and the oncological results (negative margins in all 78 patients) didn’t show any improvement over time [50]. Therefore, both PSI and SN proved to be technically burdened by potential intraoperative complications. Learning curve is demanding and depends on the ability to expose the bone to correctly fit the jig when using PSI and for bony landmarks registration when using SN.
4. Discussion
4.1. Oncological margins
In all studies presented both for long bones and pelvis, PSI and SN provided very good results in terms of oncological margins (negative margins ranging from 81% to 100%) and superior to free hand resections (range of negative margins 50% to 100%). There were no studies reporting positive margins either for PSI and SN in long bones. In the pelvis, the majority of studies reported negative margins, but due to the more complex anatomy, margins results were poorer than in long bones both for PSI and SN. For both SN and PSI, positive margins in soft tissues are reported [28], [31]; this highlights the concept that, despite these technologies might improve negative margins on bone, their accuracy on soft tissue margin cannot be planned and therefore surgeons must be aware of this limitation. Especially in complex anatomy such as the pelvis, the rate of negative margins in bone improves when resection is performed using PSI and SN rather than freehand, but when the tumour comes out of the bone and involves soft tissues the use of PSI and SN doesn’t make any difference [13], [29].
Poorer margin results reported in the pelvis might be explained by a number of factors: one is the length of osteotomy in iliac bone, and another is the importance of bone landmarks affecting the precision of the cut. One study reported the lowest accuracy on the longest osteotomy of their study [38]. Actually, an error in the inclination of the saw in the entry point produces an exit point more distant from the planned one depending on the length of the cut. Therefore, long osteotomies are more prone to a cutting error than shorter ones. Although this problem of osteotomy length has been reported only in PSI studies, we believe it might affect SN as well. The issue of topology of the bone and the possibility to expose suitable bony landmarks has been reported as a disadvantage for both PSI and SN [38], [39]. In fact, not having specific bony landmarks for PSI planning and SN registration, as well as having difficulties to expose them due to complex anatomy, represents a factor negatively influencing accuracy of the cut for both techniques [38], [50]. Specifically with regards to complex anatomy and restricted working space, PSI cut might be less accurate because of the difficulty to correctly fit the guide onto the bone due to limited exposure and soft tissue overlying the bone. In the same context, also SN shows disadvantages due to a narrow exposure of bony landmarks, critical to ensure that intra-operative registration is accurate.
As expected by the results on negative margins, despite many papers reporting no local recurrence both for PSI and SN, the rate of local recurrence was higher in the pelvis compared to the extremities (13.0 %–23.0% vs 1.44% − 8.96%). No studies reported local recurrence after PSI resection in the pelvis and the three studies stating local recurrences were on SN; nevertheless, in the three papers, it is not clear if the local recurrence is a bone recurrence or it is due to soft tissue involvement [6], [27], [29]; in fact, a bone recurrence would be product of an error in the planning or in the use of the technique; on the contrary, a soft tissue recurrence would depend on PSI or SN intrinsic limitation of not being able to plan soft tissue margin [13], [32]. Therefore, despite PSI seems to be superior in these finding, we should be cautious in stating that PSI is a technique that provides less local recurrence than SN. However, the rate of local recurrence in the bone using PSI and SN is lower compared to the ones reported for resections without using cutting aids (13.0 %–23.0% vs 28%-35%) [6], [13], [25], [51].
Most of the papers reported a similar cutting accuracy between PSI and SN. Overall, the mean error of the performed osteotomy compared to the planned one is usually < 5 mm both for PSI and SN, with the majority of studies presenting a mean error of less than 2 mm. Only the paper of Stoll et al. presented an accuracy significantly worse, with a mean cutting error of 1 cm for SN in the pelvis [39]. As stated by Zhang et al. a difference between long bones and pelvic resections was found in SN, stating that the safe margin should be 3.95 mm in pelvis and 2.69 mm in long bones [33]. Other studies instead, despite the drawbacks already presented on pelvis’s complex anatomy, showed no difference between the accuracy of PSI and SN comparing pelvis and long bones [26], [29], [33], [36].
Considering all the data reported, we found 5 mm a safe planned margin both for PSI and SN for a bone tumour resection.
We therefore believe that PSI and SN are similarly helpful in negative margins results, accuracy of bone cut and rate of local recurrence, both achieving better outcomes in long bone than in pelvis. Furthermore, both are superior compared to free-hand resections. Length of the osteotomy, absence of suitable bone markers and restricted workspace represent a challenge for both techniques for preoperative planning and intraoperative registration; however, due to the presence of soft tissue overlying the bone impairing the desired fit of the jigs, PSI might be more affected by these features compared to SN, above all in restricted working spaces; moreover, SN has also the possibility to have a second check before cutting, while this is not possible with PSI. Potentially, these drawbacks related to length of osteotomy, restricted working space and bone topology, could be reduced with co-registration of pre-operative scans with intra-operative CT [8]. Moreover, major attention on soft tissue margin must be taken during planning and resection as both techniques can’t plan soft tissue resection and reduce local soft tissue relapse.
4.2. Reconstruction
Both PSI and SN were developed for guaranteeing wide excision of the tumour while providing options for a suitable reconstruction, avoiding un-necessary loss of bone stock. In doing so, they proved to be reliable techniques for sparing bone and therefore giving the possibility of restoring functionality in patients after bone tumour resection. The majority of papers analyzed in this systematic review were focused on margins results and cutting precision. Nevertheless, the data we reported confirmed the suitability of PSI and SN for providing satisfactorily reconstruction options and functional scores. The MSTS score reported for reconstruction after juxta-articular and pelvis standard resections by most studies were around 70% [30], [52], [53], [54]. Both PSI and SN deemed to guarantee high scores in the data reported. Moreover, it has not been shown a significant superiority compared to each other, showing MSTS scores ranging around 80%-90%, regardless the type of reconstruction (biological or prosthetic), with higher scores in long bones (90%) than in pelvis (80%). Lower than the average rate showed by the majority of the studies, Fujiwara et al reported MSTS of 73% after SN periacetabular resection followed by ice-cone prosthesis reconstruction, still higher than the control group who underwent free-hand resection (MSTS 55%) [30]. Thus, due to the possibility of customizing resection using PSI or SN, sparing as much bone as possible, functional results are higher than the ones presented for free-hand resections. Moreover, it is important to highlight that PSI offers the advantage to customize the allograft, which is not possible with SN (Fig. 6) [34], [43]. Overall, PSI and SN showed similar functional results compared to each other, both for biological and prosthetic reconstruction, with higher scores in juxta-articular regions.
Fig. 6.

Intraoperative allograft cut using PSI. Using PSI it is possible to shape the allograft for matching the bone defect when a biological reconstruction is performed. Differently to the cut in the native bone on the patient, this resection is less harmed by cut inaccuracy as no overlying soft tissues are present on the allograft.
4.3. Preoperative time of surgical planning, surgical intraoperative time, intraoperative complications, and learning curve
The main difference between PSI and SN is linked to preoperative planning time and intraoperative timing. The major drawback for PSI compared to SN is the preoperative time needed for PSI jigs planning and production; studies agreed that the usual time needed is about three-four weeks, but in urgent cases PSI could be produced in 2 weeks [31], [34]. There are clinical circumstances in which these times are not acceptable. An example could be a high grade chondrosarcoma in which no neoadjuvant therapies are available. Therefore, in these cases SN would be more suitable than PSI. On the other hand, no study reported complications with PSI because of the changes in tumour size and shape occurred during the preoperative planning time. In the studies presented, this might be linked to their use in tumours with effective neoadjuvant therapies maintaining neoplasms growth under control, avoiding increase in size that might harm the accuracy of the planned cut.
On the contrary, the major drawback of SN compared to PSI is the increased intraoperative time used for registration, which in turn might affect infection rate; most of the papers presented intraoperative time ranging from 15 to 25 min, required for registration of bone landmarks necessary for intraoperative navigation [29] [57] [58]. Compared to that, PSI requires shorter intraoperative time, often needing less than 5 min for jigs positioning.
Looking at intraoperative complications, we found that the main cause for PSI complications was the difficulty to expose the bone for the proper jig fitting, as well as longer osteotomies leading to inaccurate cut; these issues might be worsened in bones with homogenous topology due to increased planning errors for the lacking of bone fiducials [2], [31], [38]; similarly, for SN, the difficulty to expose bone landmarks for registration was considered the most important cause of complication intraoperatively, above all with patients with high BMI and in bones with homogenous topology [39], [47]; however, the possibility to have a second check in SN registration, gives a chance to recognize errors, otherwise not possible to detect when positioning the jig; therefore, in restricted working space, in bones with homogenous topology and for long osteotomies, SN might be more suitable than PSI due to the possibility of a second check.
Finally, studies agreed that both PSI and SN should be used by experienced surgeon for guaranteeing a correct exposure for jig positioning and SN registration, as well as of experience in detecting positioning or registration errors: errors in the cut might signify an intralesional resection or the impossibility to proceed with a custom reconstruction [31], [50]. For the Authors, as the bone exposure for fitting the jig needs to be more accurate than the exposure for the positioning of the registration probe, PSI use might require even a longer learning curve than SN; however, no data are currently available to support this consideration. Overall, both are demanding techniques, potentially presenting technical issues during surgery. Neither of both can be considered superior to the other considering intraoperative complications or learning curve.
We also aimed to report a cost assessment, but no comparison is possible between PSI and SN due to the lack of economic features in the paper we reviewed.
5. Conclusion
In this systematic review focused on bone tumour resections, PSI and SN showed similar results in providing negative margins, accuracy of the cut, rate of local recurrence and suitable functional reconstruction scores both for long bones and pelvis, yielding better results compared to standard resections. A planned margin of at least 5 mm from the tumour appeared to be safe for both, either in both long bones and pelvis, while not providing any benefit on soft tissue margin.
Shorter intraoperative time represented the main advantage of PSI over SN. However, cutting errors related to long osteotomies, planning inaccuracy due to the homogenous topology of bone and restricted working spaces for correctly positioning the jigs seemed to affect PSI more than SN. As well, in urgent cases SN might be more indicated, to avoid long waiting times for PSI planning and production. Finally, for both, technical intraoperative complications can occur and surgical experience to properly expose the bone is mandatory. Overall, providing all advantages of PSI and SN compared to a standard resection, while potentially avoiding the disadvantages presented, robotic assisted surgery and augmented reality could be the evolution of these techniques and the future of bone tumour resection.
Declaration of Competing Interest
This research (and APC) were funded by the Italian Ministry of Health — 5 × 1000 Anno 2020, Redditi 2019" Utilizzo della Rete Trasversale della Ricerca Oncologica Traslazionale dello IOR (RT-ROT) per l’ottimizzazione dello studio di materiale biologico derivante da pazienti con Osteosarcoma.
Contributor Information
Alessandro Bruschi, Email: alessandro.bruschi@ior.it.
Davide Maria Donati, Email: davidemaria.donati@ior.it.
Claudia Di Bella, Email: claudia.dibella@unimelb.edu.au.
References
- 1.Cartiaux O., Docquier P.-L., Paul L., Francq B.G., Cornu O.H., Delloye C., Raucent B., Dehez B., Banse X. Surgical inaccuracy of tumor resection and reconstruction within the pelvis: An experimental study. Acta Orthopaedica. 2008;79(5):695–702. doi: 10.1080/17453670810016731. [DOI] [PubMed] [Google Scholar]
- 2.Cartiaux O., Banse X., Paul L., Francq B.G., Aubin C.-É., Docquier P.-L. Computer-assisted planning and navigation improves cutting accuracy during simulated bone tumor surgery of the pelvis. Computer Aided Surgery. 2013;18(1-2):19–26. doi: 10.3109/10929088.2012.744096. [DOI] [PubMed] [Google Scholar]
- 3.Bellanova L., Paul L., Docquier P.-L. Surgical guides (Patient-Specific instruments) for pediatric tibial bone sarcoma resection and allograft reconstruction. Sarcoma. 2013;2013:1–7. doi: 10.1155/2013/787653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong K.C., Kumta S.M., Sze K.Y., Wong C.M. Use of a patient-specific CAD/CAM surgical jig in extremity bone tumor resection and custom prosthetic reconstruction. Computer Aided Surgery. 2012;17:284–293. doi: 10.3109/10929088.2012.725771. [DOI] [PubMed] [Google Scholar]
- 5.Fehlberg S., Eulenstein S., Lange T., Andreou D., Tunn P.-U. In: Recent Results in Cancer ResearchTreatment of Bone and Soft Tissue Sarcomas. Tunn P.-U., editor. Springer Berlin Heidelberg; Berlin, Heidelberg: 2009. Computer-Assisted pelvic tumor resection: Fields of application, limits, and perspectives; pp. 169–182. [DOI] [PubMed] [Google Scholar]
- 6.Jeys L., Matharu G.S., Nandra R.S., Grimer R.J. Can computer navigation-assisted surgery reduce the risk of an intralesional margin and reduce the rate of local recurrence in patients with a tumour of the pelvis or sacrum? The Bone & Joint Journal. 2013;95-B(10):1417–1424. doi: 10.1302/0301-620X.95B10.31734. [DOI] [PubMed] [Google Scholar]
- 7.Khan F.A., Lipman J.D., Pearle A.D., Boland P.J., Healey J.H. Surgical technique: Computer-generated custom jigs improve accuracy of wide resection of bone tumors. Clinical Orthopaedics & Related Research. 2013;471(6):2007–2016. doi: 10.1007/s11999-012-2769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujiwara T., Kunisada T., Takeda K., Hasei J., Nakata E., Nakahara R., Yoshida A., Ozaki T. intraoperative o-arm-navigated resection in musculoskeletal tumors. Journal of Orthopaedic Science. 2018;23(6):1045–1050. doi: 10.1016/j.jos.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Docquier P.-L., Paul L., Cartiaux O., Delloye C., Banse X. Computer-Assisted resection and reconstruction of pelvic tumor sarcoma. Sarcoma. 2010;2010:1–8. doi: 10.1155/2010/125162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535–b. [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane Available from wwwtrainingcochraneorg/handbook. 2022.
- 12.Müller D.A., Stutz Y., Vlachopoulos L., et al. The accuracy of Three-Dimensional planned bone tumor resection using Patient-Specific instrument. CMAR. 2020;12:6533–6540. doi: 10.2147/CMAR.S228038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albergo J.I., Farfalli G.L., Ayerza M.A., Ritacco L.E., Aponte-Tinao L.A. Computer-assisted surgery (CAS) in orthopedic oncology. Which were the indications, problems and results in our first consecutive 203 patients? European Journal of Surgical Oncology. 2021;47(2):424–428. doi: 10.1016/j.ejso.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Benady A., Meyer J.S., Ran Y., Mor Y., Gurel R., Rumack N., Golden E., Gortzak Y., Segal O., Merose O., Sternheim A., Dadia S. Intercalary and geographic lower limb tumor resections with the use of 3D printed patient specific instruments- when less is more. Journal of Orthopaedics. 2022;32:36–42. doi: 10.1016/j.jor.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evrard R., Schubert T., Paul L., Docquier P.-L. Quality of resection margin with patient specific instrument for bone tumor resection. Journal of Bone Oncology. 2022;34 doi: 10.1016/j.jbo.2022.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasparro M.A., Gusho C.A., Obioha O.A., Colman M.W., Gitelis S., Blank A.T. 3D-Printed cutting guides for resection of long bone sarcoma and intercalary allograft reconstruction. Orthopedics. 2022;45(1) doi: 10.3928/01477447-20211124-07. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y., Jang W.Y., Park J.W., Park Y.K., Cho H.S., Han I., Kim H.-S. Transepiphyseal resection for osteosarcoma in patients with open physes using MRI assessment: safety and clinical outcomes. The Bone & Joint Journal. 2020;102-B(6):772–778. doi: 10.1302/0301-620X.102B6.BJJ-2019-1141.R2. [DOI] [PubMed] [Google Scholar]
- 18.Li J., Shi L., Chen G. Image navigation assisted joint-saving surgery for treatment of bone sarcoma around knee in skeletally immature patients. Surgical Oncology. 2014;23:132–139. doi: 10.1016/j.suronc.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Li J., Wang Z., Guo Z., Chen G.-J., Yang M., Pei G.-X. Irregular osteotomy in limb salvage for juxta-articular osteosarcoma under computer-assisted navigation. Journal of Surgical Oncology. 2012;106(4):411–416. doi: 10.1002/jso.23105. [DOI] [PubMed] [Google Scholar]
- 20.Li J., Wang Z., Guo Z., Chen G.-J., Yang M., Pei G.-X. Precise resection and biological reconstruction under navigation guidance for young patients with juxta-articular bone sarcoma in lower extremity: Preliminary report. Journal of Pediatric Orthopaedics. 2014;34(1):101–108. doi: 10.1097/BPO.0b013e31829b2f23. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Wang Z, Guo Z, et al (2012) Precise Resection and Biological Reconstruction for Patients with Bone Sarcomas in the Proximal Humerus. J reconstr Microsurg s-0032-1315766. https://doi.org/10.1055/s-0032-1315766. [DOI] [PubMed]
- 22.Staats K., Panotopoulos J., Tiefenboeck T.M., Windhager R., Funovics P.T. Computer navigation-assisted surgery for musculoskeletal tumors: a closer look into the learning curve. European Journal of Orthopaedic Surgery and Traumatology. 2017;27(6):851–858. doi: 10.1007/s00590-017-2004-y. [DOI] [PubMed] [Google Scholar]
- 23.Wong K.C., Kumta S.M. Joint-preserving tumor resection and reconstruction using image-guided computer navigation. Clinical Orthopaedics & Related Research. 2013;471:762–773. doi: 10.1007/s11999-012-2536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aponte-Tinao L., Ritacco L.E., Ayerza M.A., Muscolo L.D., Albergo J.I., Farfall G.L. Does intraoperative navigation assistance improve bone tumor resection and allograft reconstruction results? Clinical Orthopaedics & Related Research. 2015;473(3):796–804. doi: 10.1007/s11999-014-3604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aponte-Tinao L.A., Ritacco L.E., Ayerza M.A., Muscolo D.L., Farfalli G.L. Multiplanar osteotomies guided by navigation in chondrosarcoma of the knee. Orthopedics. 2013;36(3) doi: 10.3928/01477447-20130222-21. [DOI] [PubMed] [Google Scholar]
- 26.Ieguchi M., Hoshi M., Takada J., Hidaka N., Nakamura H. Navigation-assisted surgery for bone and soft tissue tumors with bony extension. Clinical Orthopaedics & Related Research. 2012;470(1):275–283. doi: 10.1007/s11999-011-2094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laitinen M.K., Parry M.C., Albergo J.I., Grimer R.J., Jeys L.M. Is computer navigation when used in the surgery of iliosacral pelvic bone tumours safer for the patient? The Bone & Joint Journal. 2017;99-B(2):261–266. doi: 10.1302/0301-620X.99B2.BJJ-2016-0149.R2. [DOI] [PubMed] [Google Scholar]
- 28.Bosma S.E., Cleven A.H.G., Dijkstra P.D.S. Can navigation improve the ability to achieve tumor-free margins in pelvic and sacral primary bone sarcoma resections? A historically controlled study. Clinical Orthopaedics and Related Research. 2019;477:1548–1559. doi: 10.1097/CORR.0000000000000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujiwara T., Kaneuchi Y., Stevenson J., Parry M., Kurisunkal V., Clark R., Tsuda Y., Laitinen M., Grimer R., Jeys L. Navigation-assisted pelvic resections and reconstructions for periacetabular chondrosarcomas. European Journal of Surgical Oncology. 2021;47(2):416–423. doi: 10.1016/j.ejso.2020.05.025. [DOI] [PubMed] [Google Scholar]
- 30.Fujiwara T., Sree D.V., Stevenson J., Kaneuchi Y., Parry M., Tsuda Y., Le Nail L.-R., Medellin R.M., Grimer R., Jeys L. Acetabular reconstruction with an ice-cream cone prosthesis following resection of pelvic tumors: Does computer navigation improve surgical outcome? Journal of Surgical Oncology. 2020;121(7):1104–1114. doi: 10.1002/jso.25882. [DOI] [PubMed] [Google Scholar]
- 31.Gouin F., Paul L., Odri G.A., Cartiaux O. Computer-Assisted planning and Patient-Specific instruments for bone tumor resection within the pelvis: A series of 11 patients. Sarcoma. 2014;2014:1–9. doi: 10.1155/2014/842709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nandra R., Matharu G., Stevenson J., Parry M., Grimer R., Jeys L. Long-term outcomes after an initial experience of computer-navigated resection of primary pelvic and sacral bone tumours: soft-tissue margins must be adequate to reduce local recurrences. The Bone & Joint Journal. 2019;101-B(4):484–490. doi: 10.1302/0301-620X.101B4.BJJ-2018-0981.R1. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y.u., Zhang Q., Zhong L., Qiu L., Xu L., Sun Y., Niu X., Zhang L.i. New perspectives on surgical accuracy analysis of image-guided bone tumour resection surgery. International Orthopaedics (SICOT) 2020;44(5):987–994. doi: 10.1007/s00264-020-04539-4. [DOI] [PubMed] [Google Scholar]
- 34.Evrard R., Schubert T., Paul L., Docquier P.-L. Resection margins obtained with patient-specific instruments for resecting primary pelvic bone sarcomas: A case-control study. Orthopaedics & Traumatology: Surgery & Research. 2019;105:781–787. doi: 10.1016/j.otsr.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 35.Jentzsch T., Vlachopoulos L., Fürnstahl P., Müller D.A., Fuchs B. Tumor resection at the pelvis using three-dimensional planning and patient-specific instruments: a case series. World J Surg Onc. 2016;14(1) doi: 10.1186/s12957-016-1006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritacco L.E., Milano F.E., Farfalli G.L., Ayerza M.A., Muscolo D.L., Aponte-Tinao L.A. Accuracy of 3-D planning and navigation in bone tumor resection. Orthopedics. 2013;36(7) doi: 10.3928/01477447-20130624-27. [DOI] [PubMed] [Google Scholar]
- 37.Wong K.C., Kumta S.M. Computer-assisted tumor surgery in malignant bone tumors. Clinical Orthopaedics & Related Research. 2013;471:750–761. doi: 10.1007/s11999-012-2557-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Paolis M., Sambri A., Zucchini R., Frisoni T., Spazzoli B., Taddei F., Donati D.M. Custom-made 3D-Printed prosthesis in periacetabular resections through a novel ileo-adductor approach. Orthopedics. 2022;45(2) doi: 10.3928/01477447-20211227-01. [DOI] [PubMed] [Google Scholar]
- 39.Stoll K.E., Miles J.D., White J.K., Punt S.E.W., Conrad E.U., Ching R.P. Assessment of registration accuracy during computer-aided oncologic limb-salvage surgery. Int J CARS. 2015;10(9):1469–1475. doi: 10.1007/s11548-014-1146-1. [DOI] [PubMed] [Google Scholar]
- 40.Enneking W.F., Dunham WILLIAM, Gebhardt M.C., Malawar MARTIN, Pritchard D.J. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clinical Orthopaedics and Related Research. 1993;286:241???246. [PubMed] [Google Scholar]
- 41.Ding H.-W., Yu G.-W., Tu Q., Liu B., Shen J.-J., Wang H., Wang Y.-J. Computer-aided resection and endoprosthesis design for the management of malignant bone tumors around the knee: outcomes of 12 cases. BMC Musculoskeletal Disorders. 2013;14(1) doi: 10.1186/1471-2474-14-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ippolito J.A., Campbell M.L., Siracuse B.L., Benevenia J. Reconstruction with custom unicondylar hemiarthroplasty following tumor resection: A case series and review of the literature. The Journal of Knee Surgery. 2020;33:818–824. doi: 10.1055/s-0039-1688556. [DOI] [PubMed] [Google Scholar]
- 43.Cernat E., Docquier P.-L., Paul L., Banse X., Codorean I.-B. Patient specific instruments for complex tumor Resection-Reconstruction surgery within the pelvis: A series of 4 cases. chr. 2016;111(5):439. doi: 10.21614/chirurgia.111.5.439. [DOI] [PubMed] [Google Scholar]
- 44.Lam Y.-L., Yau R., Ho K.W.Y., Mak K.-L., Fong S.-T., So T.Y.C. Is it possible and safe to perform acetabular-preserving resections for malignant neoplasms of the periacetabular region? Clinical Orthopaedics & Related Research. 2017;475(3):656–665. doi: 10.1007/s11999-016-4792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho H.S., Park I.-H., Jeon I.-H., Kim Y.-G., Han I., Kim H.-S. Direct application of MR images to computer-assisted bone tumor surgery. Journal of Orthopaedic Science. 2011;16(2):190–195. doi: 10.1007/s00776-011-0035-5. [DOI] [PubMed] [Google Scholar]
- 46.Biscaccianti V., Fragnaud H., Hascoët J.-Y., Crenn V., Vidal L. Digital chain for pelvic tumor resection with 3D-printed surgical cutting guides. Frontiers in Bioengineering and Biotechnology. 2022;10 doi: 10.3389/fbioe.2022.991676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young P.S., Bell S.W., Mahendra A. The evolving role of computer-assisted navigation in musculoskeletal oncology. The Bone & Joint Journal. 2015;97-B(2):258–264. doi: 10.1302/0301-620X.97B2.34461. [DOI] [PubMed] [Google Scholar]
- 48.Ould-Slimane M., Thong P., Perez A., Roussignol X., Dujardin F.-H. The role of intraoperative 3D navigation for pelvic bone tumor resection. Orthopaedics & Traumatology: Surgery & Research. 2016;102(6):807–811. doi: 10.1016/j.otsr.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 49.Jud L., Müller D.A., Fürnstahl P., Fucentese S.F., Vlachopoulos L. Joint-preserving tumour resection around the knee with allograft reconstruction using three-dimensional preoperative planning and patient-specific instruments. The Knee. 2019;26(3):787–793. doi: 10.1016/j.knee.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 50.Farfalli G.L., Albergo J.I., Ritacco L.E., Ayerza M.A., Milano F.E., Aponte-Tinao L.A. What is the expected learning curve in computer-assisted navigation for bone tumor resection? Clinical Orthopaedics & Related Research. 2017;475(3):668–675. doi: 10.1007/s11999-016-4761-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mueller J.L., Fu H.L., Mito J.K., Whitley M.J., Chitalia R., Erkanli A., Dodd L., Cardona D.M., Geradts J., Willett R.M., Kirsch D.G., Ramanujam N. A quantitative microscopic approach to predict local recurrence based on In vivo Intraoperative imaging of sarcoma tumor margins: Quantitative In vivo imaging. International Journal of Cancer. 2015;137(10):2403–2412. doi: 10.1002/ijc.29611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang B., Hao Y., Pu F., Jiang W., Shao Z. Computer-aided designed, three dimensional-printed hemipelvic prosthesis for peri-acetabular malignant bone tumour. International Orthopaedics (SICOT) 2018;42(3):687–694. doi: 10.1007/s00264-017-3645-5. [DOI] [PubMed] [Google Scholar]
- 53.Bruschi A., Cevolani L., Spazzoli B., Focaccia M., Pasini S., Frisoni T., Donati D.M. Periacetabular tumour resection under anterosuperior iliac spine allows better alloprosthetic reconstruction than above: Bone contact matters. JCM. 2022;11(15):4499. doi: 10.3390/jcm11154499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donati D., Di Bella C., Frisoni T., Cevolani L., DeGroot H. Alloprosthetic composite is a suitable reconstruction after periacetabular tumor resection. Clinical Orthopaedics & Related Research. 2011;469(5):1450–1458. doi: 10.1007/s11999-011-1799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]