Summary
Non-alcoholic fatty liver disease (NAFLD) is a major cause of liver disease worldwide, affecting up to 30% of adults. Progression to non-alcoholic steatohepatitis (NASH) is a key risk factor for cirrhosis, hepatocellular carcinoma and cardiovascular events. Alterations in reproductive hormones are linked to the development and/or progression of NAFLD/NASH in women. Women with polycystic ovary syndrome and those with oestrogen deficiency are at increased risk of NAFLD/NASH, with higher mortality rates in older women compared to men of similar ages. NAFLD/NASH is currently the leading indication for liver transplantation in women without hepatocellular carcinoma. Therefore, a better understanding of NAFLD in women is needed to improve outcomes. In this review, we discuss the hormonal and non-hormonal factors that contribute to NAFLD development and progression in women. Furthermore, we highlight areas of focus for clinical practice and for future research.
Keywords: Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Women, Estrogens, Androgens, Menopause
Key points.
-
•
Women with NAFLD have different outcomes compared to men with NAFLD.
-
•
In women, age of menarche, menopausal status, body fat distribution, reproductive hormones, sarcopenia and certain conditions (including Turner syndrome and polycystic ovary syndrome) influence the development and progression of NAFLD.
-
•
Oestrogen deficiency is associated with increased lipogenesis, fatty acid oxidation and progression of NAFLD whereas androgen excess increases the risk of NAFLD development.
-
•
In the absence of licensed treatments for NAFLD, cardiovascular and metabolic risk reduction remain the mainstay of NAFLD management. However, sex-specific guidelines are lacking.
-
•
Evidence-based data on the influence of sex on biomarker sensitivity and sex-specific prediction models are needed to facilitate implementation of personalised treatment strategies in women with NAFLD.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a chronic liver disease characterised by increased hepatic fat content (≥5%), which is diagnosed after exclusion of well-established causes of hepatic steatosis such as alcohol, steatogenic drugs and inherited errors of metabolism.1 Hepatic triglyceride accumulation by itself is not hepatotoxic.2 However, pathogenic processes such as adipose tissue dysfunction,3 gut microbiome dysbiosis,4 fructose-induced mitochondrial dysfunction and endoplasmic reticulum stress5 may drive hepatic steatosis to hepatic inflammation and hepatocellular ballooning (non-alcoholic steatohepatitis or NASH), leading to fibrosis and eventually cirrhosis.6 Liver fibrosis represents the main predictor of liver and non-liver-related adverse clinical outcomes. Hepatocellular carcinoma (HCC) can occur in patients with cirrhosis and in patients without cirrhosis.
Globally, the prevalence of NAFLD is 30%,7 which is projected to rise to 56%,8 paralleling the increasing incidence of obesity and type 2 diabetes. In adults, up to a third of patients with NAFLD develop NASH over a period of ∼7 years,9 and around 40% of the individuals who have histologically proven NASH progress to fibrosis.10 The prevalence of NAFLD is higher in men than in pre-menopausal women below the age of 50 years.9 However, in women, the prevalence of NAFLD increases after menopause, with a rising trend observed after the age of 50 years, which peaks at 60 to 69 years, before declining in those aged ≥70 years.10
Recently, a panel of international experts proposed the redefinition of NAFLD to metabolic dysfunction-associated fatty liver disease (MAFLD) based on the presence of hepatic steatosis and metabolic risk factors (overweight/obesity, type 2 diabetes and/or metabolic dysfunction).11 The term MAFLD may include patients with concomitant causes of liver diseases and it may exclude those with steatosis but without the full spectrum of metabolic risk factors.12 However, some studies suggest women with NAFLD may be less likely to be meet the criteria for diagnosis of MAFLD than men with NAFLD,13 which could have a detrimental effect on outcomes in women. Hence, we have elected to use the NAFLD nomenclature in this review.
Women aged ≥50 years with NAFLD are 1.2 times more likely to develop NASH compared to age-matched men and are more likely to progress to advanced fibrosis,14 with preliminary transcriptomic and plasma profiling studies suggesting that NAFLD may follow a distinct biological trajectory in women aged ≥50 years.15,16 Liver fibrosis stage is associated with increased mortality from 0.32 deaths per 100 person-years at stages F0 to F2 to 1.76 deaths per 100 person-years at stage F4.17 Predicting the presence of fibrosis with blood-based non-invasive markers, which may perform differently according to sex, may require dedicated cut-offs for women.18 Notably, women tend to have lower serum liver enzyme activities than age-matched men.18 Nevertheless, there is no evidence that non-invasive markers of fibrosis, such as the FIB-4 and NAFLD fibrosis scores, which rely heavily on the measurement of transaminase activities, may perform differently in women. Interestingly, a recently developed non-invasive marker, called the AGILE 3+ score, has demonstrated how integrating sex with other clinical parameters may improve the risk stratification of patients with NAFLD.19 In addition, HCC occurs less frequently in women than men, in both patients with and without cirrhosis,20 suggesting that dedicated surveillance strategies may need to be explored.
NASH is the leading cause of end-stage liver disease requiring transplantation in women who do not have HCC.21 In women undergoing liver transplantation, long-term survival is higher than in men.22 However, women are more likely to die whilst on the waiting list for liver transplantation due to NASH, partly due to underestimation of mortality in women using current stratification scores (i.e. the MELD [model of end-stage liver disease] score). A sex- and sodium-adjusted MELD score for liver transplant allocation has recently been proposed,23 which may help to ensure more equitable access to liver transplantation.
Women with NAFLD have increased mortality rates from cardiovascular disease (CVD) compared to women without NAFLD.24 This excess risk of CVD is also higher in women compared to age-matched men with NAFLD (e.g. 10% in a 40-year old woman with NAFLD vs. 8% in a 40-year old man with NAFLD).25 The excess CVD risk increases with age, and is exaggerated after menopause (e.g. in people with NAFLD aged 60 years, the CVD risk in women is 18% vs. 9% in men).25
In this review, we summarise the factors that contribute to the development and progression of NAFLD in women and in specific populations. We aim to raise awareness of NAFLD in women and highlight areas for future research to address gaps in our understanding of its pathophysiology that will hopefully lead to improvements in the clinical management of this complex condition.
Search strategy and selection criteria
A literature search was performed to identify studies investigating NAFLD/NASH in women, published up to November 2022. Original research and review articles were identified through searches in the PubMed database, Scopus database, Ovid Medline, and Ovid EMBASE, with the search limited to articles published in the English language. We included basic science studies, randomized-controlled trials, reviews, original prospective studies, cross-sectional studies, retrospective studies and best practice guidelines using different combinations of the following search terms: “fatty liver” OR “non-alcoholic fatty liver disease” OR “NAFLD” OR “steatohepatitis” OR “NASH” OR “liver fibrosis” OR “liver disease” OR “liver cancer” AND “women” OR “gender” OR “female” OR “sex difference” OR “reproductive age” OR “premenopausal women” OR “postmenopausal women”. For effects of hormones on NAFLD, we used the search terms: “androgens” OR “estrogens” OR “oestrogens” OR “testosterone” OR “sex hormones” OR “sexual dimorphism” OR “menopause” OR “hormone replacement therapy” AND “NAFLD” OR “NASH” OR “steatohepatitis” OR “liver fibrosis”. For effects of NAFLD in specific population groups, we used a combination of search terms including “NAFLD in Polycystic Ovary Syndrome”, “NAFLD in Turner syndrome”.
Reproductive hormones and NAFLD
Oestrogens
Oestrogens play important roles in regulating lipogenesis and fatty acid oxidation. Ovariectomised female rats have a 51% increase in hepatic lipogenesis and a 34% reduction in fatty acid oxidation26 due to decreased synthesis of peroxisome proliferator-activated receptor α (PPARα, a regulator of fatty acid oxidation) and upregulation of the genes encoding sterol regulatory element-binding protein 1 (SREBP-1, a nuclear transcription factor that promotes lipid synthesis).26 Additionally, stearoyl coenzyme A desaturase 1 (the rate-limiting enzyme in triglyceride synthesis) is upregulated following ovariectomy.27
The metabolic actions of oestrogens are typically attributed to classical oestrogen receptor-α (ERα encoded by ESR1) signalling.28 Both male and female Esr1 knockout mice exhibit upregulation of lipogenic (SREBP-1 and fatty acid synthase [FASN]) and adipogenic (PPARγ and lipoprotein lipase) genes, a process that is reversed by ERα agonist treatment.26,27 Mice lacking G-protein coupled oestrogen receptor (GPER) and mice with liver Esr1-knockout (LERKO) exhibit similar metabolic phenotypes including higher body weight and increased visceral adiposity.29,30 Female, but not male, Gper-knockout mice fed a high-fat diet display lower levels of high-density lipoprotein (HDL)-cholesterol and greater liver fat accumulation compared to controls.30 This suggests that both ERα and GPER pathways are important for hepatic and whole-body lipid homeostasis and contribute to sexual dimorphism in NAFLD.
Oestrogens also influence reverse cholesterol export, i.e. the process by which peripheral cholesterol is returned to the liver.31 In LERKO mice, hepatic low-density lipoprotein receptors are reduced by ∼18 to 22%31 and hepatic expression of PDZK1 protein (which plays a role in HDL cholesterol uptake) is reduced by 22% and 33% in male and female mice, respectively.31 Loss of ERα reduces cholesterol efflux from foam cells into HDL particles in female and male LERKO mice.31 Thus, oestrogen deficiency disrupts the molecular machinery involved in hepatic lipogenesis and adipogenesis. Consistent with these findings, progression from pre-to post-menopause is independently associated with an increase in total cholesterol and low-density lipoprotein cholesterol in women aged between 47 and 55 years.32 This may contribute to the higher prevalence of NAFLD in post-menopausal women.
Interactions between oestrogens and glucagon may be important in the pathogenesis of NAFLD. Glucagon promotes hepatic lipolysis and suppresses de novo lipogenesis. Glucagon levels have been observed to be inversely associated with NAFLD progression.33 Attenuation of glucagon receptor signalling is also proposed to increase the risk of NAFLD.34 Furthermore, in NAFLD, expression of the glucagon receptor gene and the function of the glucagon protein may be impaired, resulting in glucagon resistance.33,35 In vitro studies have shown that physiological levels of oestrogen can inhibit glucagon secretion by binding to the GPR30 oestrogen receptor,36 while oestradiol-mediated inhibition of glucagon release is attenuated by deletion of GPR30 receptors.37 Ovariectomy has also been shown to increase circulating glucagon in rodents36,38 and glucagon levels are suppressed by oestradiol treatment.36,39 These data suggest oestrogen deficiency could have beneficial effects in NAFLD via increased glucagon levels. However, oestrogen deficiency has detrimental effects as described above. Therefore, the roles of oestrogen (and oestrogen deficiency) in the development and progression of NAFLD require further study.
Androgens
Prenatal exposure of female rodents to androgens disrupts the balance between enzymes involved in lipogenesis (SREBP, PPAR and ChREBP [carbohydrate-responsive element-binding protein]) and lipolysis.40 In young adult ewes, prenatal exposure to androgens downregulates hepatic PEPCK and causes hepatic insulin resistance.41 Upregulation of expression of other hepatic metabolic genes including mitogen activated protein kinase 4 (a pro-inflammatory protein involved in ceramide signalling), UDP-glucose ceramide glucosyltransferase (involved in ceramide metabolism) and acyl-coenzyme A dehydrogenase (involved in lipid metabolism) also occurs, further exacerbating liver damage.41
The effects of androgens in animal models could be mediated by changes in body adiposity/composition exacerbated by a high-fat diet42 and/or via changes in transcriptional activity of gluconeogenic genes.43 Postnatal exposure of female rodents to dihydrotestosterone (DHT) induces hepatic steatosis, insulin resistance and recapitulates the reproductive phenotype of polycystic ovary syndrome (PCOS).44 In normal weight female mice, low-dose DHT upregulates SCAP (SREBP cleavage activating protein) and SREBP-1, which promotes FASN and acetyl-CoA carboxylase expression, resulting in hepatic steatosis.45 In DHT-exposed female rats, NASH may develop via activation of NF-κB signalling, enhanced expression of pro-inflammatory cytokines (IL-6, IL-1β, and TNFα) and an increase in pro-apoptotic markers.46 Cumulatively, prenatal or postnatal androgen exposure appears to increase the risk of NAFLD development and progression by increasing lipogenesis and pro-inflammatory mediators.
Factors contributing to the development and progression of NAFLD in women
Age of menarche
Earlier onset of menstruation (i.e. age of menarche <12 years) has been associated with increased risk of cardiometabolic disease in post-menopausal women.47 In the CARDIA study, earlier menarche by 1 year conferred a 10% increased risk of NAFLD (diagnosed using CT scans) in adulthood independent of socio-economic factors and baseline BMI.47 Early menarche is often preceded by rapid accumulation of fat during childhood, a physically less active lifestyle and/or behavioural factors that could also increase the risk of the metabolic syndrome.48 Therefore, other factors such as obesity, insulin resistance or a hyperandrogenic phenotype (such as in PCOS)49 may interact with early menarche to confer an additional risk of developing NAFLD.
Menopausal status
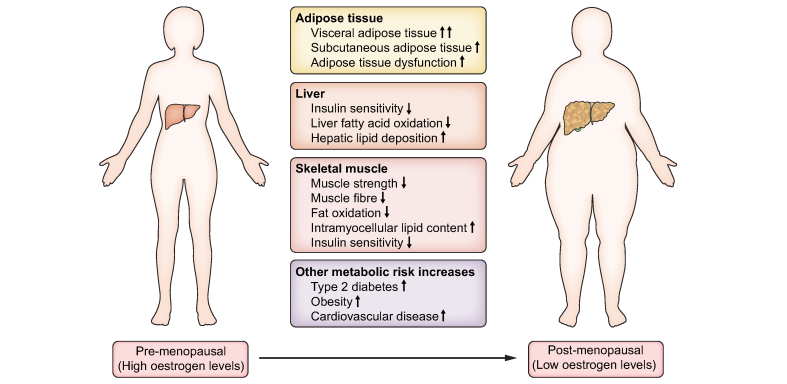
Oestradiol, being the most abundant circulating female reproductive hormone, plays important roles in the regulation of lipid and glucose metabolism in hepatic and adipose tissues. In pre-menopausal women, oestradiol is predominantly secreted by the ovaries.50 However, after menopause,50 ovarian oestrogen secretion ceases and circulating oestradiol levels decline to a mean value of ∼10 pmol/L51, but low quantities are still produced by non-ovarian tissues.50,52 The decline of circulating oestradiol during natural menopause is associated with increased risk of NAFLD, type 2 diabetes, central adiposity and hypertriglyceridemia (Fig. 1).53
Fig. 1.
Changes occur in adipose tissue, liver and skeletal muscle during the menopause that have detrimental metabolic effects.
These may contribute to the increased prevalence of metabolic conditions in post-menopausal women.
In a cross-sectional study involving 541 people with biopsy-proven NASH,54 advanced fibrosis was more prevalent in post-menopausal women (27.6%) compared to men (22.2%) and pre-menopausal women (14.4%).54 Women over the age of 50 years have increased odds of advanced fibrosis (odds ratio 1.8, 95% CI 1.2-2.7) even after adjustment for covariates (enrolling site, ethnicity and degrees of portal inflammation).54 The risk of severe fibrosis remained elevated in lean post-menopausal women with NAFLD compared to lean pre-menopausal women with NAFLD (odds ratio 2.17, 95% CI 1.1-4.5).55 This suggests that menopause is associated with severe fibrosis that is, in part, independent of age or body fat composition.
Women who have undergone oophorectomy have an increased risk of NAFLD compared to pre-menopausal women who have not undergone oophorectomy.56 In fact, a stronger association was observed in women who underwent oophorectomy before the age of 45 years.56 Similarly, women with premature menopause prior to the age of 40 years have a 90% increased risk of severe fibrosis on histology compared to women who went through menopause after 40 years.57 Conceivably, the duration of oestradiol deficiency contributes significantly to the risk of post-menopausal hepatic fibrosis.
Hormone replacement therapy
The role of hormone replacement therapy (HRT) in preventing the development and/or progression of NAFLD remains unclear. A randomised double-blind study comparing women with type 2 diabetes on oral HRT (1 mg oestradiol plus 0.5 mg norethisterone) to those on placebo for 6 months showed that women on HRT (n = 19) had reduced circulating concentrations of liver enzymes compared to the placebo group (n = 23).58 A South American study reported that post-menopausal women on HRT (dose and type of hormones not specified) for at least 6 months (n = 14) had lower waist circumference, lower HOMA-IR index, lower ferritin levels (a surrogate marker of parenchymal inflammation) and lower gamma-glutamyltransferase when compared with women not taking HRT (n = 79).59 However, improvement in liver biochemistry may not reflect improvement in liver histology. Thus, the same group of researchers assessed the frequency of NAFLD diagnosed by abdominal ultrasound and reported a lower frequency of NAFLD in women taking HRT (14/53, 26.4%) compared with women not taking HRT (79/198, 39.9%) irrespective of the type of HRT, duration of use and route of administration.60
However, other studies did not report a reduction in the risk of NAFLD61 or severe hepatic fibrosis amongst post-menopausal women taking HRT.54 One study demonstrated an increased risk of severe lobular inflammation with HRT use in post-menopausal women and oral contraceptive use in pre-menopausal women.62 Details of the types, routes of administration and doses of oestrogens (and progestins), and their differential effects on the risk of severe inflammation, were not reported. Future studies are indicated to investigate the impact of synthetic oestrogens and progestins on the natural history of NAFLD and/or NASH in post-menopausal women.
Selective oestrogen receptor modulators
Selective oestrogen receptor modulators (e.g. tamoxifen) are agents that elicit tissue-specific oestrogen receptor agonist or antagonist activity. Women treated with tamoxifen have a higher prevalence of NAFLD and an increased risk of progression to NASH and advanced fibrosis.63 The mechanisms by which tamoxifen influences NAFLD risk remain unclear. In vitro, genes involved in lipogenesis and fatty acid synthesis (e.g. SREBP-1c, FASN, stearoyl coenzyme A desaturase 1 and acetyl coenzyme A carboxylase) are upregulated after treating HepG2 cells with tamoxifen.64 Obese female Wistar rats who were fed a high-fat diet for 15 weeks and then given tamoxifen for 2 weeks were observed to have increased hepatic lipid synthesis and decreased triglyceride export.65 This was associated with a marked downregulation of sirtuin 1 (SIRT1) and upregulation of p-FoxO1/LXRα-SREBP1c signalling, leading to increased hepatic steatosis.65 Administration of a SIRT1 agonist inhibited the promotion of tamoxifen-induced lipid synthesis, suggesting that SIRT1 is a regulator of tamoxifen-induced fatty liver disease.65
In addition, tamoxifen-treated ovariectomized C57BL6/J female mice are protected from HFD-induced steatosis via selective activation of ERα-activating factor 1 (ERα-AF1).66 This contradicts findings from a previous study indicating that protective metabolic actions of oestradiol are mediated mostly via ERα-AF2.66 It is likely that there is redundancy in the ERα-AF1 and ERα-AF2 systems or that the effects of tamoxifen may differ depending on the tissue type.66 More mechanistic studies are needed to elucidate the influence of selective oestrogen receptor modulators on NAFLD. More importantly, targeting liver ERα-AF1 or SIRT1 are potential future strategies to mitigate against the development and progression of NAFLD.
Turner syndrome
Turner syndrome (TS) is a sex-chromosome disorder in females caused by an abnormal or absent X chromosome.67 Women with TS have a 4.4-fold increased risk of type 2 diabetes,68 and a 5.5-fold increased risk of developing cirrhosis.68 Histological evidence of nodular hyperplasia, NAFLD and cirrhosis have been described in women with TS.69 Elevated liver enzymes were found in ∼50% of women with TS (n = 125).70 Of the 21 women who had Fibroscans, liver stiffness measurements suggestive of fibrosis were reported in 38%70 and liver architectural changes were found in the 11 women who consented to a biopsy.70 Compared to age-matched eugonadal women or oestradiol-treated women with premature ovarian insufficiency, women with TS have higher waist circumference, elevated BMI, and increased IL-6 and triglyceride levels.71 Women with TS also have increased intrahepatocellular lipid content, which is correlated to duration of oestrogen deficiency.72 Although larger studies are needed to explore the relationship between oestradiol and metabolic risk, these data suggest a role for oestrogen deficiency in promoting hepatic steatosis and insulin resistance in this context.
It is difficult to disentangle the contributions of gonadal hormones from those of sex chromosomes in patients with TS. In the FCG (four core genotype) model (in which sex chromosomes are unrelated to gonadal sex), mice with one X chromosome had reduced body weight compared to XX mice.73 By contrast, women with one X chromosome have higher body weight and increased risks of developing metabolic disease than women with two X chromosomes.74 Although low levels of sex hormones contribute to the increased risk of developing metabolic disease, imprinting of X-linked genes may also contribute to metabolic dysregulation in TS.75 Depending on the parental origin of the X chromosome, imprinting of maternally transmitted X-linked genes in patients with TS has been shown to prevent visceral fat accumulation whereas imprinting of paternally transmitted X-linked genes promoted higher triglyceride and lipid levels.75 The rarity of sex chromosome aneuploidies presents challenges in determining the relative contributions of reduced numbers of sex chromosomes and hypogonadism in the development of NAFLD in women with TS. However, the FCG mouse model may help advance our understanding of these two contributing factors.
Polycystic ovary syndrome (PCOS)
PCOS affects up to 13% of women of reproductive age and is characterised by ovulatory dysfunction, hyperandrogenism and/or polycystic ovarian morphology.76 Women with PCOS have an increased prevalence of NAFLD compared to age-, BMI- and waist circumference-matched women without PCOS.77 This excess risk is also present in lean women (BMI <25 kg/m2) with PCOS.78 A concerning finding is the higher prevalence of biopsy-proven NASH in women with PCOS younger than 40 years.79
Hyperandrogenism is associated with increased NAFLD risk in women with PCOS. In a retrospective study involving 63,210 women with PCOS, serum testosterone levels >3.0 nmol/L were associated with an increased risk of NAFLD (hazard ratio 2.30, 95% CI 1.16–4.53).78 Liver fat is greater in hyperandrogenic women with PCOS compared to normo-androgenic women with PCOS after correcting for visceral adiposity and BMI.80 Consistent with these findings, a cross-sectional study of 400 Chinese women with PCOS concluded that the risk of NAFLD increases with free androgen index, which is a surrogate measure of androgen bioavailability.81 Notably, excess androgens are associated with an increased risk of developing NAFLD in women, independently of obesity and insulin resistance.81 Women with hyperandrogenic PCOS also had higher circulating levels of glycerophospholipids and lysoglycerophospholipids which are potential biomarkers of NASH.82 Intra-adipose androgen generation by the enzyme aldo-ketoreductase type 1C3 was increased in the subcutaneous adipose tissue (SAT) of women with PCOS, resulting in lipotoxicity and predisposing women with hyperandrogenic PCOS to liver injury.83 Although a causative role for androgens has not been proven, these association studies suggest a potential use for anti-androgens in treating women with PCOS and NAFLD.
Body fat distribution
Sex-specific body fat distribution influences an individual’s predisposition to cardiometabolic complications independently of body weight or body fat percentage.84 Compared to age- and BMI-matched men, pre-menopausal women typically have greater SAT mass in the abdominal85 and femoral-gluteal areas.86 By contrast, men have a higher percentage of visceral adipose tissue (VAT), 10-20% in men vs. 5-8% in women.87 Given the higher percentage of VAT in men, they have a greater ability to deposit meal-derived free fatty acids (FFAs) into VAT, which results in higher liver fat disposal (Fig. 2).88 Excess FFAs released into the bloodstream predispose individuals to lipotoxicity and increased lipid uptake by the liver, pancreas and/or muscle.89 This overflow of FFAs to the liver could lead to increased cellular levels of ceramides, long-chain fatty acyl-coenzyme A and pro-inflammatory processes causing chronic low-grade inflammation.89,90 Unsurprisingly, people with increased VAT mass are more insulin resistant, have impaired glucose metabolism and are more likely to develop NAFLD.91 Indeed, a prospective study showed a rising incidence of NAFLD based on ultrasound and CT imaging with increasing quintiles of VAT (10.2%, 17.1%, 18.1%, 25.2% and 34.4%, respectively) in both men and women after a median follow-up of 4.4 years.92 In this study, after adjusting for cholesterol and triglyceride levels individuals with the highest quintile of VAT were more likely to develop NAFLD (hazard ratio 2.23, 95% CI 1.28–3.89) compared to individuals with the lowest quintile of VAT.
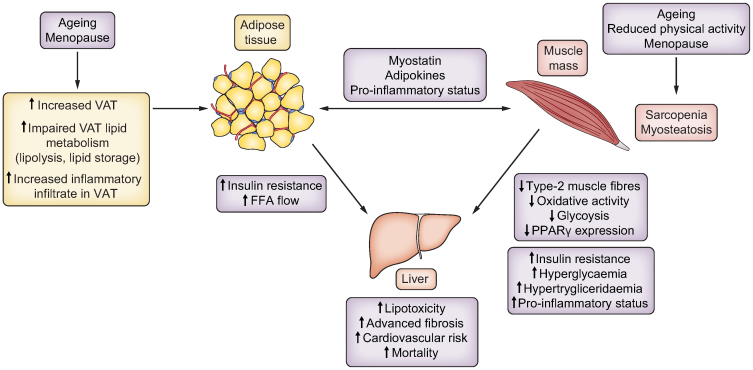
Fig. 2.
Interactions between adipose tissue, muscle and liver contribute to the development and progression of NAFLD in women.
Adipokines and myokines (such as myostatin) mediate adipose tissue-muscle interactions. Ageing and the menopause (i.e. oestrogen deficiency) increase VAT depots and reduce muscle mass and quality. Expanded VAT depots increase FFA delivery to the liver, which has detrimental effects. These alterations in body composition contribute to insulin resistance, hyperglycaemia and/or hyperlipidaemia, with consequent development and progression of NAFLD. FFA, free fatty acid; NAFLD, non-alcoholic fatty liver disease; VAT, visceral adipose tissue.
Prior to menopause, women accrue more fat in the SAT, which protects them from the negative consequences of the metabolic syndrome.93 As women transition through menopause, both SAT and VAT increase but VAT expands more at the onset of menopause and then plateaus at a higher set-point after menopause.93
During menopause, changes in SAT and VAT metabolism also result in alterations in body fat distribution.94 Although pre-menopausal and post-menopausal women retain similar sensitivity and responsiveness to sympathetic activation by beta-adrenergic agonists, adipose tissue basal lipolysis rate is reduced and lipoprotein lipase activity (which promotes hydrolysis of circulating triglycerides to FFAs) is enhanced in the gluteal and abdominal adipose tissues of post-menopausal women.95 Compared to pre-menopausal women, expression of FASN is reduced in the SAT of post-menopausal women by 61%,94 whereas PPARγ expression is increased in VAT by 83%.94 The increased PPARγ expression in VAT may reflect a compensatory attempt to curtail the need for increased lipid storage, as VAT accumulation correlates with features of insulin resistance.94 Interestingly, thiazolidinediones (PPARγ agonists used to treat type 2 diabetes), may promote a redistribution of SAT and a lower expression of transcriptional genes for VAT, suggesting an effect on adipose tissue depot-specific regulation.96 However, their unfavourable safety profile (e.g. increased risks of atypical humeral fracture and bladder cancer) limits their use in clinical practice. Changes in adipose tissue metabolism, coupled with preferential fat accumulation in VAT during menopause predispose women to increased cardiometabolic risk (Fig. 2).97
Oestrogen levels correlate positively with percentage of SAT and negatively with visceral fat accumulation in pre-menopausal women.97 Oestrogen treatment decreases insulin resistance by ∼50% and decreases abdominal visceral adiposity in post-menopausal women and ovariectomized female animal models.98,99 Oestrogen also reverses the increase in hepatic triglyceride content caused by diet-induced obesity in LERKO mice.100 Evidently, oestrogen plays a role in insulin sensitivity and glucose homeostasis in women, in addition to promoting fat accumulation in SAT and modifying the risk of NAFLD progression.
Muscle quality and quantity
Sarcopenia is defined as generalised progressive loss of skeletal muscle mass, muscle function and muscle strength. Meta-analyses have shown that the risks of NAFLD and NASH are increased by 1.5- to 2.5-fold among individuals with sarcopenia.101,102 Furthermore, among individuals with NAFLD, sarcopenia is independently associated with hepatic fibrosis after adjusting for obesity and insulin resistance (odds ratio 2.59, 95% CI 1.22-5.48).103 Coexistence of sarcopenia and NAFLD doubles mortality risk, independently of fibrosis stage.104 It remains unclear if NAFLD directly contributes to sarcopenia or sarcopenia causes NAFLD.
Skeletal muscle is a major site of insulin-stimulated glucose uptake.105 Ageing results in loss of muscle mass and reduction in type 2 (fast-twitch) muscle fibres (by ∼10 to 14% per decade).105 Fast-twitch muscles depend on glycolysis for energy production,106 and the gradual reduction in fast-twitch muscle during ageing results in reduced dependence on cytosolic glycolytic processes for glucose disposal.105 Mitochondrial bioenergetics are also altered with ageing. Reduced expression of gene regulators, such as PGC-1α (PPARγ coactivator-1α) in aged skeletal muscles suppresses AMP-activated protein kinase, SIRT1 and p38 mitogen-activated protein kinase.107 Suppression of SIRT1 limits oxidative capacity and lipid metabolism leading to hyperlipidaemia, dysregulated glucose metabolism, hyperinsulinemia and insulin resistance.108
Ectopic fat accumulation in the muscles (myosteatosis) can be a consequence of insulin resistance and perpetuate NAFLD. Severe myosteatosis is associated with a 2- to 3-fold increased risk of early NASH in patients with NAFLD.109 In a recent study, the fat content in psoas skeletal muscle (measured by a parameter known as skeletal muscle fat index) was observed to be higher in individuals with NASH and advanced fibrosis (≥F3) than in those with NASH and early stages of fibrosis (F1 to F2).110 Myosteatosis promotes endoplasmic reticulum stress, which in turn impairs mitochondrial function.111 Furthermore, myosteatosis contributes to reduced skeletal muscle protein synthesis stimulated by anabolic hormones (insulin, oestradiol and testosterone).111 Oestradiol reduction during menopause further promotes proteolysis, reduction in lean mass, and increased fat mass.111
Mechanisms underlying the manifestation of sarcopenia are likely to be multifactorial. Although low oestradiol levels may play a role in the decline in muscle mass in women aged ≥50 years old, evidence elucidating the contribution of menopause to sarcopenia remains unclear. Some studies have reported an accelerated decline in muscle mass in women during menopausal transition.[137], [138] Samson et al. observed a decline in isometric knee extensor strength (IKES) and handgrip strength (HGS) of 40.2% and 28%, respectively, in elderly women aged 55 to 80 years old, whereas the decrease in IKES and HGS was 10.3% and 8.2%, respectively, in women aged 20 to 55 years old.113 By contrast, the decline in IKES and HGS was 23% and 17.4%, respectively, in men aged 55 to 80 years old but 24% and 19.6%, respectively, in men aged 20 to 55 years old.113 A 20% reduction in maximum voluntary force of the adductor pollicis (by ∼20%) has also been seen around the time of menopause in women followed by little change after that, whereas in men (n = 176), muscle force was maintained before weakness started at age 60.114 In the same study, women receiving HRT had attenuated loss of muscle force, suggesting a possible role of oestrogens in preventing loss of muscle strength and weakness.114 However, other studies did not find any differences in the rate of decline of height-adjusted appendicular skeletal muscle mass between males and females before the age of 60.115
The fluctuation of oestradiol during the menstrual cycle (oestrus cycle in rodents) also does not seem to affect the muscle strength, fatiguability or power performance of young female athletes (n = 29)116 or rodents.117 Evidence to support the impact of menopause on muscle strength and muscle mass independent of ageing are equivocal and further research is needed to specify the contribution of menopause to sarcopenia. Nevertheless, sarcopenia and NAFLD remain closely linked, with each entity increasing the risk of the other and potentially being exacerbated by the menopause (Fig. 2).
Areas of focus in clinical practice
Diagnosis
Despite the high prevalence of NAFLD, diagnostic and management approaches in clinical practice are variable. This is partly due to the low rate of recognition of NAFLD among non-hepatology specialists112 and delayed referral of patients at risk of advanced liver disease to specialists for evaluation and care.112 Even more worryingly, data collected from 102 countries revealed that at least 31% of the countries surveyed do not have any national guidance, strategies or action plans in place to address the increasing prevalence of NAFLD.113
Due to the lack of data on cost-effectiveness and value of non-invasive liver tests, screening for NAFLD in the general population is currently not recommended.[114], [115], [116], [117], [118] American and Asia-Pacific guidelines advise adopting a high index of suspicion to investigate for presence of NAFLD in high-risk individuals.116,118,119 European and Latin-American guidelines offer more specific recommendations and suggest screening in patients with persistently elevated liver enzymes, metabolic syndrome, type 2 diabetes and/or obesity (BMI ≥30 kg/m2).6,114 Risk prediction tools, such as the FIB-4 score, NAFLD fibrosis score or enhanced liver fibrosis score, and transient elastography are recommended as the next step in identifying patients at risk of advanced fibrosis and cirrhosis, as these patients should be referred to a hepatologist for specialist management.120 However, these prediction tools do not consider the effects of sex, ethnic heritage and hormonal status on liver-related outcomes. Reassuringly, sex does not influence the likelihood of unreliable liver stiffness measurements using vibration-controlled transient elastography.121
Lifestyle interventions
Current management is focused on optimising associated co-morbidities including diabetes, hypertension, hyperlipidaemia, and reducing cardiovascular risk by encouraging smoking cessation and prescribing lipid-lowering medication. Data from Korea suggest that women (but not men) with NAFLD have an increased risk of cardiovascular and liver-related mortality.122 By contrast, data from America indicate that men with NAFLD have an increased risk of death from cancer and cardiovascular causes compared to women.123,124 Therefore, more data are required before recommending sex-specific risk factor reduction.
Lifestyle modification remains the initial step in the management of NAFLD. Physical activity exceeding 150 min/week decreases serum aminotransferase levels.[116], [117], [118],125 Reducing calories by 750-1,000 kcal/day improves insulin resistance and hepatic steatosis.[116], [117], [118],125 Weight loss of at least 5% of body weight reduces hepatic steatosis but greater weight loss of ≥7%-10% improves NASH.[116], [117], [118],125 However, in women, a ≥7-10% weight loss is associated with a lower probability of NASH resolution, highlighting a need for sex-specific weight loss targets.126 Additionally, the optimal amount of weight loss required to produce beneficial effects in NAFLD in post-menopausal women is not known. Furthermore, weight loss interventions that preserve or increase muscle mass127 may have added benefits.
Therapeutics
There are currently no licensed medications for the treatment of NAFLD. Vitamin E and pioglitazone have been recommended in some guidelines.[116], [117], [118] Vitamin E has been demonstrated to have beneficial effects on liver transaminases, hepatic steatosis, lobular inflammation and hepatocellular ballooning.128 However, sex-specific outcomes were not reported in this meta-analysis,128 nor in the individual studies included in the meta-analysis.129,130 Furthermore, long-term high-dose vitamin E use may increase the risk of heart failure131 and prostate cancer.138 Therefore, sex-specific analyses of treatment responses and adverse events are required as the risk-benefit ratio of vitamin E use in NAFLD may differ between men and women.
Pioglitazone, a PPARγ activator, improves insulin sensitivity and attenuates inflammation and fibrosis in patients with and without diabetes with biopsy-proven NASH, but weight gain, fluid retention and increased risk of bone fractures are commonly occurring adverse effects that limit its use.[116], [117], [118] Interestingly, women with NAFLD and pre-diabetes or type 2 diabetes treated with pioglitazone have greater reductions in liver fat content than men with similar co-morbidities.132 This may be due to a greater reduction in insulin resistance by pioglitazone in women compared to men.132 Until further data are available, both vitamin E and pioglitazone are not recommended for patients without biopsy-proven NASH.[116], [117], [118]
Reproductive hormones impact the risk of NAFLD development and progression in women. However, current evidence is insufficient to recommend HRT as a treatment for NAFLD in post-menopausal women. In a small study that included men and women with NAFLD, combination treatment with spironolactone (which has anti-androgenic effects) and vitamin E reduced hepatic fat scores after 52 weeks of treatment.133 Sub-analyses by sex were not reported in this study. In women with PCOS, spironolactone use has been shown to improve insulin resistance and lipid levels.134 Whether the anti-androgen effect of spironolactone would modify the risk of developing NASH in women with PCOS remains to be explored. As current management options for NAFLD are limited, patients should be offered the opportunity to participate in research as they may benefit from early access to emerging therapies.
Future directions and conclusions
While several medications have failed to demonstrate an improvement in clinical trials endpoints, there are still promising agents in the pipeline for the treatment of NAFLD.135 In addition, reproductive hormone receptor agonists involved in hepatic steatosis, inflammation and/or fibrosis, such as the kisspeptin receptor136, are being developed as potential therapeutic agents. Data from large-scale studies like DAISY-PCOS (Dissecting Androgen excess and metabolic dysfunction – an Integrated Systems approach to PCOS) may advance our understanding of the influence of androgens on NAFLD and offer tailored management strategies in women.
In conclusion, management of women with NAFLD should take into consideration their risk profiles, hormonal status, age and metabolic factors (Fig. 3). Evidence-based data on the influence of sex on biomarker sensitivity and/or sex-specific prediction models are needed. A better understanding of the influence of reproductive hormones on NAFLD and reporting of sex-based responses to therapeutic interventions could lead to the development of beneficial personalised management approaches in women.
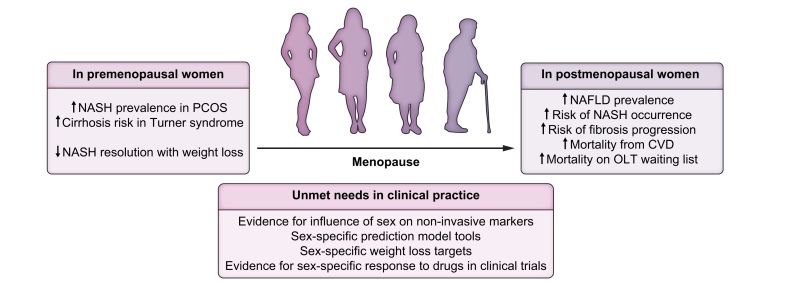
Fig. 3.
Women with certain conditions (PCOS and Turner syndrome) are at an increased risk of NASH and cirrhosis.
In addition, pre-menopausal women are less likely to have resolution of NASH following weight loss. Post-menopausal women have increased prevalence of NAFLD, increased risk of NASH progression and death (due to CVD and liver failure requiring OLT). There are several unmet needs in clinical practice, which if addressed, may improve outcomes. CVD, cardiovascular disease; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PCOS, polycystic ovary syndrome; OLT, orthotopic liver transplantation.
Financial support
CI-E is funded by an Imperial-BRC IPPRF Fellowship (P79696), a Society for Endocrinology Early Career Grant, an Association of Physicians of Great Britain and Ireland Young Investigator Award (P90797) and a Mason Medical Research Foundation Grant (P91847). WSD is funded by an UK National Institute for Health Research (NIHR) Research Professorship (NIHR-RP-2014-05-001) and an NIHR Senior Investigator Award. The Department of Metabolism, Digestion and Reproduction is funded by grants from the MRC, NIHR and is supported by the NIHR Imperial BRC Funding Scheme and the NIHR/Imperial Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the abovementioned funders, the UK National Health Service (NHS), the NIHR, or the UK Department of Health.
Authors’ contributions
Conceptualisation: All authors; Writing - Original Draft: PCE, RF, CI-E; Writing - Review & Editing: All authors.
Conflict of interest
All authors report no potential conflicts of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100835
Supplementary data
The following are the supplementary data to this article.
References
- 1.Pierantonelli I., Svegliati-Baroni G. Nonalcoholic fatty liver disease: basic pathogenetic mechanisms in the progression from NAFLD to NASH. Transplantation. 2019;103 doi: 10.1097/TP.0000000000002480. [DOI] [PubMed] [Google Scholar]
- 2.Parthasarathy G., Revelo X., Malhi H. Pathogenesis of nonalcoholic steatohepatitis: an overview. Hepatol Commun. 2020;4 doi: 10.1002/hep4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duval C., Thissen U., Keshtkar S., Accart B., Stienstra R., Boekschoten M., et al. Adipose tissue dysfunction signals progression of hepatic steatosis towards nonalcoholic steatohepatitis in C57Bl/6 mice. Diabetes. 2010;59:3181. doi: 10.2337/db10-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safari Z., Gérard P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD) Cellular Mol Life Sci. 2019;76(8):1541–1558. doi: 10.1007/s00018-019-03011-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jegatheesan P., De Bandt J.P. Fructose and NAFLD: the multifaceted aspects of fructose metabolism. Nutrients. 2017;9 doi: 10.3390/nu9030230. [DOI] [Google Scholar]
- 6.Arab J.P., Dirchwolf M., Álvares-da-Silva M.R., Barrera F., Benitez C., Castellanos-Fernandez M., et al. Latin American Association for the study of the liver (ALEH) practice guidance for the diagnosis and treatment of non-alcoholic fatty liver disease. Ann Hepatol. 2020;19:674–690. doi: 10.1016/j.aohep.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Younossi Z.M., Golabi P., Paik J.M., Henry A., Van Dongen C., Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335–1347. doi: 10.1097/HEP.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estes C., Anstee Q.M., Arias-Loste M.T., Bantel H., Bellentani S., Caballeria J., et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 9.Sherif Z.A., Saeed A., Ghavimi S., Nouraie S.-M., Laiyemo A.O., Brim H., et al. Global epidemiology of nonalcoholic fatty liver disease and perspectives on US minority populations. Dig Dis Sci. 2016;61(5):1214–1225. doi: 10.1007/s10620-016-4143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64 doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 11.Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 12.Kim D., Konyn P., Sandhu K.K., Dennis B.B., Cheung A.C., Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75:1284–1291. doi: 10.1016/j.jhep.2021.07.035. [DOI] [PubMed] [Google Scholar]
- 13.Yu C., Wang M., Zheng S., Xia M., Yang H., Zhang D., et al. Comparing the diagnostic criteria of MAFLD and NAFLD in the Chinese population: a population-based prospective cohort study. J Clin Transl Hepatol. 2022;10:6–16. doi: 10.14218/JCTH.2021.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balakrishnan M., Patel P., Dunn-Valadez S., Dao C., Khan V., Ali H., et al. Women have lower risk of nonalcoholic fatty liver disease but higher risk of progression vs men: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19:61. doi: 10.1016/j.cgh.2020.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandel J., Dubois-Chevalier J., Gheeraert C., Derudas B., Raverdy V., Thuillier D., et al. Hepatic molecular signatures highlight the sexual dimorphism of nonalcoholic steatohepatitis (NASH) Hepatology. 2021;73:920–936. doi: 10.1002/hep.31312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grzych G., Vonghia L., Bout M.A., Weyler J., Verrijken A., Dirinck E., et al. Plasma BCAA changes in patients with NAFLD are sex dependent. J Clin Endocrinol Metab. 2020;105:2311–2321. doi: 10.1210/clinem/dgaa175. [DOI] [PubMed] [Google Scholar]
- 17.Sanyal A.J., Van Natta M.L., Clark J., Neuschwander-Tetri B.A., Diehl A., Dasarathy S., et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. New Engl J Med. 2021;385:1559–1569. doi: 10.1056/NEJMoa2029349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lonardo A., Ballestri S., Bedogni G., Bellentani S., Tiribelli C. The Fatty liver Index (FLI) 15 years later: a reappraisal. Metab Target Organ Damage. 2021;1:10. [Google Scholar]
- 19.Pennisi G., Enea M., Pandolfo A., Celsa C., Antonucci M., Ciccioli C., et al. AGILE 3+ score for the diagnosis of advanced fibrosis and for predicting liver-related events in NAFLD. Clin Gastroenterol Hepatol. 2023:21. doi: 10.1016/J.CGH.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Pinyopornpanish K., Khoudari G., Saleh M.A., Angkurawaranon C., Pinyopornpanish K., Mansoor E., et al. Hepatocellular carcinoma in nonalcoholic fatty liver disease with or without cirrhosis: a population-based study. BMC Gastroenterol. 2021;21:1–7. doi: 10.1186/s12876-021-01978-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noureddin M., Vipani A., Bresee C., Todo T., Kim I.K., Alkhouri N., et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113:1649–1659. doi: 10.1038/s41395-018-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loy V.M., Joyce C., Bello S., VonRoenn N., Cotler S.J. Gender disparities in liver transplant candidates with nonalcoholic steatohepatitis. Clin Transpl. 2018;32 doi: 10.1111/CTR.13297. [DOI] [PubMed] [Google Scholar]
- 23.Sealock J.M., Ziogas I.A., Zhao Z., Ye F., Alexopoulos S.P., Matsuoka L., et al. Proposing a sex-adjusted sodium-adjusted MELD score for liver transplant allocation. JAMA Surg. 2022;157:618–626. doi: 10.1001/jamasurg.2022.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arshad T., Golabi P., Paik J., Mishra A., Younossi Z.M. Prevalence of nonalcoholic fatty liver disease in the female population. Hepatol Commun. 2019;3:74–83. doi: 10.1002/hep4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen A.M., Therneau T.M., Mara K.C., Larson J.J., Watt K.D., Hayes S.N., et al. Women with nonalcoholic fatty liver disease lose protection against cardiovascular disease: a longitudinal cohort study. Am J Gastroenterol. 2019;114 doi: 10.14309/ajg.0000000000000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paquette A., Chapados N.A., Bergeron R., Lavoie J.M. Fatty acid oxidation is decreased in the liver of ovariectomized rats. Horm Metab Res. 2009;41:511–515. doi: 10.1055/s-0029-1202348. [DOI] [PubMed] [Google Scholar]
- 27.Paquette A., Wang D., Jankowski M., Gutkowska J., Lavoie J.M. Effects of ovariectomy on PPARα, SREBP-1c, and SCD-1 gene expression in the rat liver. Menopause. 2008;15:1169–1175. doi: 10.1097/gme.0b013e31817b8159. [DOI] [PubMed] [Google Scholar]
- 28.Meda C., Barone M., Mitro N., Lolli F., Pedretti S., Caruso D., et al. Hepatic ERα accounts for sex differences in the ability to cope with an excess of dietary lipids. Mol Metab. 2020;32:97–108. doi: 10.1016/j.molmet.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weigt C., Hertrampf T., Kluxen F.M., Flenker U., Hulsemann F., Fritzemeier K.H., et al. Molecular effects of ER alpha- and beta-selective agonists on regulation of energy homeostasis in obese female Wistar rats. Mol Cel Endocrinol. 2013;377:147–158. doi: 10.1016/j.mce.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Meoli L., Isensee J., Zazzu V., Nabzdyk C.S., Soewarto D., Witt H., et al. Sex- and age-dependent effects of Gpr30 genetic deletion on the metabolic and cardiovascular profiles of diet-induced obese mice. Gene. 2014;540:210–216. doi: 10.1016/j.gene.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 31.Zhu L., Shi J., Luu T.N., Neuman J.C., Trefts E., Yu S., et al. Hepatocyte estrogen receptor alpha mediates estrogen action to promote reverse cholesterol transport during Western-type diet feeding. Mol Metab. 2018;8:106–116. doi: 10.1016/j.molmet.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karvinen S., Jergenson M.J., Hyvärinen M., Aukee P., Tammelin T., Sipila S., et al. Menopausal status and physical activity are independently associated with cardiovascular risk factors of healthy middle-aged women: cross-sectional and longitudinal evidence. Front Endocrinol (Lausanne) 2019;10:589. doi: 10.3389/fendo.2019.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y., Lin Z., Wan H., Zhang W., Xia F., Chen Y., et al. Glucagon is associated with NAFLD inflammatory progression in type 2 diabetes, not with NAFLD fibrotic progression. Eur J Gastroenterol Hepatol. 2021;33:E818–E823. doi: 10.1097/MEG.0000000000002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang G., Zhang B.B. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284 doi: 10.1152/AJPENDO.00492.2002. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L., Yao W., Xia J., Wang T., Huang F. Glucagon-induced acetylation of energy-sensing factors in control of hepatic metabolism. Int J Mol Sci. 2019;20 doi: 10.3390/IJMS20081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandour T., Kissebah A.H., Wynn V. Mechanism of oestrogen and progesterone effects on lipid and carbohydrate metabolism: alteration in the insulin: glucagon molar ratio and hepatic enzyme activity. Eur J Clin Invest. 1977;7:181–187. doi: 10.1111/j.1365-2362.1977.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 37.Martensson U.E.A., Salehi S.A., Windahl S., Gomez M.F., Sward K., Daszkiewicz-Nilsson J., et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 38.Faure A., Sutter-Dub M.T., Sutter B.C.J., Assan R. Ovarian-adrenal interactions in regulation of endocrine pancreatic function in the rat. Diabetologia. 1983;24:122–127. doi: 10.1007/BF00297394. [DOI] [PubMed] [Google Scholar]
- 39.Ropero A.B., Pang Y., Alonso-Magdalena P., Thomas P., Nadal Á. Role of ERβ and GPR30 in the endocrine pancreas: a matter of estrogen dose. Steroids. 2012;77:951–958. doi: 10.1016/j.steroids.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Abruzzese G.A., Heber M.F., Ferreira S.R., Velez L.M., Reynoso R., Ferreira S.R., et al. Prenatal hyperandrogenism induces alterations that affect liver lipid metabolism. J Endocrinol. 2016;230:67–79. doi: 10.1530/JOE-15-0471. [DOI] [PubMed] [Google Scholar]
- 41.Hogg K., Wood C., McNeilly A.S., Duncan W.C. The in utero programming effect of increased maternal androgens and a direct fetal intervention on liver and metabolic function in adult sheep. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai H., Jia X., Yu Q., Zhang C., Qiao J., Guan Y., et al. High-fat diet induces significant metabolic disorders in a mouse model of polycystic ovary Syndrome. Biol Reprod. 2014;91:127–128. doi: 10.1095/biolreprod.114.120063. [DOI] [PubMed] [Google Scholar]
- 43.Andrisse S., Childress S., Ma Y., Billings K., Chen Y., Xue P., et al. Low-dose dihydrotestosterone drives metabolic dysfunction via cytosolic and nuclear hepatic androgen receptor mechanisms. Endocrinology. 2017;158:531. doi: 10.1210/en.2016-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caldwell A.S.L., Middleton L.J., Jimenez M., Desai R., McMahon A.C., Allan C.M., et al. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology. 2014;155:3146–3159. doi: 10.1210/en.2014-1196. [DOI] [PubMed] [Google Scholar]
- 45.Seidu T., McWhorter P., Myer J., Alamgir R., Eregha N., Bogle D., et al. DHT causes liver steatosis via transcriptional regulation of SCAP in normal weight female mice. J Endocrinol. 2021;250:49–65. doi: 10.1530/JOE-21-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui P., Hu W., Ma T., Hu M., Tong X., Zhang F., et al. Long-term androgen excess induces insulin resistance and non-alcoholic fatty liver disease in PCOS-like rats. J Steroid Biochem Mol Biol. 2021;208:105829. doi: 10.1016/j.jsbmb.2021.105829. [DOI] [PubMed] [Google Scholar]
- 47.Mueller N.T., Pereira M.A., Demerath E.W., Dreyfus J.G., MacLehose R.F., Carr J.J., et al. Earlier menarche is associated with fatty liver and abdominal ectopic fat in midlife, independent of young adult BMI: the CARDIA study. Obesity. 2015;23:468–474. doi: 10.1002/oby.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laitinen J., Power C., Järvelin M.R. Family social class, maternal body mass index, childhood body mass index, and age at menarche as predictors of adult obesity. Am J Clin Nutr. 2001;74:287–294. doi: 10.1093/ajcn/74.3.287. [DOI] [PubMed] [Google Scholar]
- 49.Cao X., Zhou J., Yuan H., Chen Z. Duration of reproductive lifespan and age at menarche in relation to metabolic syndrome in postmenopausal Chinese women. J Obstet Gynaecol Res. 2016;42:1581–1587. doi: 10.1111/jog.13093. [DOI] [PubMed] [Google Scholar]
- 50.Simpson E.R., Davis S.R. Minireview: aromatase and the regulation of estrogen biosynthesis—some new perspectives. Endocrinology. 2001;142:4589–4594. doi: 10.1210/endo.142.11.8547. [DOI] [PubMed] [Google Scholar]
- 51.Santen R.J., Mirkin S., Bernick B., Constantine G.D. Systemic estradiol levels with low-dose vaginal estrogens. Menopause. 2020;27:361. doi: 10.1097/GME.0000000000001463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simpson E.R., Misso M., Hewitt K.N., Hill R.A., Boon W.C., Jones M.E., et al. Estrogen—the good, the bad, and the unexpected. Endocr Rev. 2005;26:322–330. doi: 10.1210/er.2004-0020. [DOI] [PubMed] [Google Scholar]
- 53.Turola E., Petta S., Vanni E., Milosa L., Valenti L., Critelli R., et al. Ovarian senescence increases liver fibrosis in humans and zebrafish with steatosis. DMM Dis Models Mech. 2015;8:1037–1046. doi: 10.1242/dmm.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang J.D., Abdelmalek M.F., Pang H., Guy C.D., Smith A.D., Diehl A.M., et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:1406–1414. doi: 10.1002/hep.26761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoneda M., Thomas E., Sumida Y., Eguchi Y., Schiff E.R. The influence of menopause on the development of hepatic fibrosis in nonobese women with nonalcoholic fatty liver disease. Hepatology. 2014;60:1792. doi: 10.1002/hep.27097. 1792. [DOI] [PubMed] [Google Scholar]
- 56.Florio A.A., Graubard B.I., Yang B., Thistle J.E., Bradley M.C., McGlynn K.A., et al. Oophorectomy and risk of non-alcoholic fatty liver disease and primary liver cancer in the Clinical Practice Research Datalink. Eur J Epidemiol. 2019;34:871. doi: 10.1007/s10654-019-00526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klair J.S., Yang J.D., Abdelmalek M.F., Guy C.D., Gill R.M., Yates K., et al. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology. 2016;64:85–91. doi: 10.1002/hep.28514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKenzie J., Fisher B.M., Jaap A.J., Stanley A., Paterson K., Sattar N. Effects of HRT on liver enzyme levels in women with type 2 diabetes: a randomized placebo-controlled trial. Clin Endocrinol (Oxf) 2006;65:40–44. doi: 10.1111/j.1365-2265.2006.02543.x. [DOI] [PubMed] [Google Scholar]
- 59.Florentino G., Cotrim H.P., Florentino A., Padilha C., Medeiros-Neto M., Bragagnol G., et al. Hormone replacement therapy in menopausal women: risk factor or protection to nonalcoholic fatty liver disease? Ann Hepatol. 2012;11:147–149. [PubMed] [Google Scholar]
- 60.Florentino GS. de A., Cotrim H.P., Vilar C.P., Florentino AV. de A., Guimarães G.M.A., Barreto V.S.T. Nonalcoholic fatty liver disease in menopausal women. Arq Gastroenterol. 2013;50:180–185. doi: 10.1590/S0004-28032013000200032. [DOI] [PubMed] [Google Scholar]
- 61.Hamaguchi M., Kojima T., Ohbora A., Takeda N., Fukui M., Kato T. Aging is a risk factor of nonalcoholic fatty liver disease in premenopausal women. World J Gastroenterol. 2012;18:237–243. doi: 10.3748/wjg.v18.i3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang J.D., Abdelmalek M.F., Guy C., Gill R.M., Lavine J.E., Yates K., et al. Patient sex, reproductive status, and synthetic hormone use associate with histologic severity of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2017;15:127. doi: 10.1016/j.cgh.2016.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruno S., Maisonneuve P., Castellana P., Rotmensz N., Rossi S., Maggioni M., et al. Incidence and risk factors for non-alcoholic steatohepatitis: prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ. 2005;330:932. doi: 10.1136/bmj.38391.663287.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao F., Xie P., Jiang J., Zhang L., An W., Zhan Y. The effect and mechanism of tamoxifen-induced hepatocyte steatosis in vitro. Int J Mol Sci. 2014;15:4019. doi: 10.3390/ijms15034019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li M., Cai Y., Chen X., Zhang L., Jiang Z., Yu Q. Tamoxifen induced hepatic steatosis in high-fat feeding rats through SIRT1-Foxo1 suppression and LXR-SREBP1c activation. Toxicol Res (Camb) 2022;11:673–682. doi: 10.1093/toxres/tfac043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Handgraaf S., Riant E., Fabre A., Waget A., Burcelin R., Liere P., et al. Prevention of obesity and insulin resistance by estrogens requires ERα activation function-2 (ERαAF-2), whereas eraaf-1 is dispensable. Diabetes. 2013;62:4098–4108. doi: 10.2337/db13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gravholt C.H., Andersen N.H., Conway G.S., Dekkers O.M., Geffner M.E., Klein K.O., et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. 2017;177:G1–G70. doi: 10.1530/EJE-17-0430. [DOI] [PubMed] [Google Scholar]
- 68.Gravholt C.H., Juul S., Naeraa R.W., Hansen J. Morbidity in turner syndrome. J Clin Epidemiol. 1998;51:147–158. doi: 10.1016/s0895-4356(97)00237-0. [DOI] [PubMed] [Google Scholar]
- 69.Roulot D. Liver involvement in Turner syndrome. Liver Int. 2013;33:24–30. doi: 10.1111/liv.12007. [DOI] [PubMed] [Google Scholar]
- 70.Calanchini M., Moolla A., Tomlinson J.W., Cobbold J.F., Grossman A., Fabbri A., et al. Liver biochemical abnormalities in Turner syndrome: a comprehensive characterization of an adult population. Clin Endocrinol (Oxf) 2018;89:667–676. doi: 10.1111/cen.13811. [DOI] [PubMed] [Google Scholar]
- 71.Ostberg J.E., Attar M.J.H., Mohamed-Ali V., Conway G.S. Adipokine dysregulation in turner syndrome: comparison of circulating interleukin-6 and leptin concentrations with measures of adiposity and C-reactive protein. J Clin Endocrinol Metab. 2005;90:2948–2953. doi: 10.1210/jc.2004-1966. [DOI] [PubMed] [Google Scholar]
- 72.Ostberg J.E., Thomas E.L., Hamilton G., Attar M.J.H., Bell J.D., Conway G.S. Excess visceral and hepatic adipose tissue in turner syndrome determined by magnetic resonance imaging: estrogen deficiency associated with hepatic adipose content. J Clin Endocrinol Metab. 2005;90:2631–2635. doi: 10.1210/jc.2004-1939. [DOI] [PubMed] [Google Scholar]
- 73.Link J.C., Chen X., Arnold A.P., Reue K. Metabolic impact of sex chromosomes. Adipocyte. 2013;2:74–79. doi: 10.4161/adip.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bakalov V.K., Cheng C., Zhou J., Bondy C.A. X-chromosome gene dosage and the risk of diabetes in Turner syndrome. J Clin Endocrinol Metab. 2009;94:3289–3296. doi: 10.1210/jc.2009-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van P.L., Bakalov V.K., Zinn A.R., Bondy C.A. Maternal X chromosome, visceral adiposity, and lipid profile. JAMA. 2006;295:1373–1374. doi: 10.1001/jama.295.12.1373. [DOI] [PubMed] [Google Scholar]
- 76.Teede H.J., Misso M.L., Costello M.F., Dokras A., Laven J., Moran L., et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364. doi: 10.1016/j.fertnstert.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rađenović S.S., Pupovac M., Andjić M., Bila J., Sreckovic S., Gudovic A., et al. Prevalence, risk factors, and pathophysiology of nonalcoholic fatty liver disease (NAFLD) in women with polycystic ovary syndrome (PCOS) Biomedicines. 2022;10 doi: 10.3390/BIOMEDICINES10010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumarendran B., O’Reilly M.W., Manolopoulos K.N., Toulis K.A., Gokhale K.M., Sitch A.J., et al. Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: a longitudinal study based on a United Kingdom primary care database. Plos Med. 2018;15 doi: 10.1371/journal.pmed.1002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brzozowska M.M., Ostapowicz G., Weltman M.D. An association between non-alcoholic fatty liver disease and polycystic ovarian syndrome. J Gastroenterol Hepatol. 2009;24:243–247. doi: 10.1111/j.1440-1746.2008.05740.x. [DOI] [PubMed] [Google Scholar]
- 80.Jones H., Sprung V.S., Pugh C.J.A., Daousi C., Irwin A., Aziz N., et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97:3709–3716. doi: 10.1210/jc.2012-1382. [DOI] [PubMed] [Google Scholar]
- 81.Cai J., Wu C.H., Zhang Y., Wang Y.Y., Xu W.D., Lin T.C., et al. High-free androgen index is associated with increased risk of non-Alcoholic fatty liver disease in women with polycystic ovary syndrome, independent of obesity and insulin resistance. Int J Obes. 2017;41:1341–1347. doi: 10.1038/ijo.2017.116. [DOI] [PubMed] [Google Scholar]
- 82.Anjani K., Lhomme M., Sokolovska N., Poitou C., Aron-Wisnewsky J., Bouillot J.-L., et al. Circulating phospholipid profiling identifies portal contribution to NASH signature in obesity. J Hepatol. 2015;62:905–912. doi: 10.1016/j.jhep.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 83.O’Reilly M.W., Kempegowda P., Walsh M., Taylor A.E., Manolopoulos K.N., Allwood J.W., et al. AKR1C3-Mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:3327–3339. doi: 10.1210/jc.2017-00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kissebah A.H., Vydelingum N., Murray R., Evans D.J., Kalkhoff R.K., Adams P.W. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54:254–260. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 85.Ibrahim M.M. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 86.Yim J.E., Heshka S., Albu J.B., Heymsfield S., Gallagher D. Femoral-gluteal subcutaneous and intermuscular adipose tissues have independent and opposing relationships with CVD risk. J Appl Physiol (1985) 2008;104:700–707. doi: 10.1152/japplphysiol.01035.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karastergiou K., Smith S.R., Greenberg A.S., Fried S.K. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3:1–12. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spalding K.L., Bernard S., Näslund E., Salehpour M., Possnert G., Appelsved L., et al. Impact of fat mass and distribution on lipid turnover in human adipose tissue. Nat Commun. 2017;8 doi: 10.1038/NCOMMS15253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gastaldelli A., Cusi K. From NASH to diabetes and from diabetes to NASH: mechanisms and treatment options. JHEP Rep. 2019;1:312. doi: 10.1016/j.jhepr.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hocking S.L., Wu L.E., Guilhaus M., Chisholm D.J., James D.E. Intrinsic depot-specific differences in the secretome of adipose tissue, preadipocytes, and adipose tissue–derived microvascular endothelial cells. Diabetes. 2010;59:3008–3016. doi: 10.2337/db10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ballestri S., Nascimbeni F., Baldelli E., Marrazzo A., Romagnoli D., Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. 2017;34(6):1291–1326. doi: 10.1007/s12325-017-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim D., Chung G.E., Kwak M.S., Seo H.B., Kang J.H., Kim W., et al. Body fat distribution and risk of incident and regressed nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14:132–138.e4. doi: 10.1016/j.cgh.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 93.Lovejoy J.C., Champagne C.M., De Jonge L., Xie H., Smith S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes. 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abildgaard J., Ploug T., Al-Saoudi E., Wagner T., Thomsen C., Ewertsen C., et al. Changes in abdominal subcutaneous adipose tissue phenotype following menopause is associated with increased visceral fat mass. Sci Rep. 2021;11:14750. doi: 10.1038/s41598-021-94189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ferrara C.M., Lynch N.A., Nicklas B.J., Ryan A.S., Berman D.M. Differences in adipose tissue metabolism between postmenopausal and perimenopausal women. J Clin Endocrinol Metab. 2002;87:4166–4170. doi: 10.1210/jc.2001-012034. [DOI] [PubMed] [Google Scholar]
- 96.Walker G.E., Marzullo P., Verti B., Guzzaloni G., Maestrini S., Zurleni F., et al. Subcutaneous abdominal adipose tissue subcompartments: potential role in rosiglitazone effects. Obesity (Silver Spring) 2008;16:1983–1991. doi: 10.1038/oby.2008.326. [DOI] [PubMed] [Google Scholar]
- 97.Puder J.J., Monaco S.E., Sen Gupta S., Wang J., Ferin M., Warren M.P. Estrogen and exercise may be related to body fat distribution and leptin in young women. Fertil Steril. 2006;86:694–699. doi: 10.1016/j.fertnstert.2006.02.085. [DOI] [PubMed] [Google Scholar]
- 98.Riant E., Waget A., Cogo H., Arnal J.F., Burcelin R., Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150:2109–2117. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- 99.Nabulsi A.A., Folsom A.R., White A., Patsch W., Heiss G., Wu K.K., et al. Association of hormone-replacement therapy with various cardiovascular risk factors in postmenopausal women. N Engl J Med. 1993;328:1069–1075. doi: 10.1056/NEJM199304153281501. [DOI] [PubMed] [Google Scholar]
- 100.Dakin R.S., Walker B.R., Seckl J.R., Hadoke P.W.F., Drake A.J. Estrogens protect male mice from obesity complications and influence glucocorticoid metabolism. Int J Obes. 2015;39(10):1539–1547. doi: 10.1038/ijo.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee Y.H.O., Kim S.U. Sarcopenia: an emerging risk factor for non-alcoholic fatty liver disease. Hepatol Int. 2020;14:5–7. doi: 10.1007/s12072-019-09999-4. [DOI] [PubMed] [Google Scholar]
- 102.Wijarnpreecha K., Panjawatanan P., Thongprayoon C., Jaruvongvanich V., Ungprasert P. Sarcopenia and risk of nonalcoholic fatty liver disease: a meta-analysis. Saudi J Gastroenterol. 2018;24:12–17. doi: 10.4103/sjg.SJG_237_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koo B.K., Kim D., Joo S.K., Kim J.H., Chang M.S., Kim B.G., et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123–131. doi: 10.1016/j.jhep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 104.Moon J.H., Koo B.K., Kim W. Non-alcoholic fatty liver disease and sarcopenia additively increase mortality: a Korean nationwide survey. J Cachexia Sarcopenia Muscle. 2021;12:964–972. doi: 10.1002/jcsm.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dao T., Green A.E., Kim Y.A., Bae S.-J., Ha K.-T., Gariani K., et al. Sarcopenia and muscle aging: a brief overview. Endocrinol Metab. 2020;35:716. doi: 10.3803/EnM.2020.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pedersen B.K., Febbraio M.A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 107.Rygiel K.A., Picard M., Turnbull D.M. The ageing neuromuscular system and sarcopenia: a mitochondrial perspective. J Physiol. 2016;594:4499–4512. doi: 10.1113/JP271212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Majeed Y., Halabi N., Madani A.Y., Engelke R., Bhagwat A.M., Abdesselem H., et al. SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Sci Rep. 2021:11. doi: 10.1038/S41598-021-87759-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hsieh Y.-C., Joo S.K., Koo B.K., Lin H.-C., Lee D.H., Chang M.S., et al. Myosteatosis, but not sarcopenia, predisposes NAFLD subjects to early steatohepatitis and fibrosis progression. Clin Gastroenterol Hepatol. 2022 doi: 10.1016/J.CGH.2022.01.020. published online Jan 31. [DOI] [PubMed] [Google Scholar]
- 110.Nachit M., Kwanten W.J., Thissen J.P., De Beeck B.O., Van Gaal L., Vonghia L., et al. Muscle fat content is strongly associated with NASH: a longitudinal study in patients with morbid obesity. J Hepatol. 2021;75:292–301. doi: 10.1016/j.jhep.2021.02.037. [DOI] [PubMed] [Google Scholar]
- 111.Zambon Azevedo V., Silaghi C.A., Maurel T., Silaghi H., Ratziu V., Pais R. Impact of sarcopenia on the severity of the liver damage in patients with non-alcoholic fatty liver disease. Front Nutr. 2022;8 doi: 10.3389/FNUT.2021.774030/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wieland A.C., Quallick M., Truesdale A., Mettler P., Bambha K.M. Identifying practice gaps to optimize medical care for patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2013;58:2809–2816. doi: 10.1007/s10620-013-2740-8. [DOI] [PubMed] [Google Scholar]
- 113.Lazarus J.V., Mark H.E., Villota-Rivas M., Palayew A., Carrieri P., Colombo M., et al. The global NAFLD policy review and preparedness index: are countries ready to address this silent public health challenge? J Hepatol. 2022;76:771–780. doi: 10.1016/j.jhep.2021.10.025. [DOI] [PubMed] [Google Scholar]
- 114.Clinical practice guidelines EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. 2016. [DOI] [PubMed] [Google Scholar]
- 115.Aller R., Fernández-Rodríguez C., lo Iacono O., Banares R., Abad J., Carrion J.A., et al. Consensus document. Management of non-alcoholic fatty liver disease (NAFLD). Clinical practice guideline. Gastroenterología y Hepatología (English Edition) 2018;41:328–349. doi: 10.1016/j.gastrohep.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 116.Chitturi S., Wong V.W.S., Chan W.K., Wong G.L-H., Wong S.K.-H., Sollano J., et al. The Asia–Pacific working party on non-alcoholic fatty liver disease guidelines 2017—Part 2: management and special groups. J Gastroenterol Hepatol (Australia) 2018;33:86–98. doi: 10.1111/jgh.13856. [DOI] [PubMed] [Google Scholar]
- 117.National Institute for Health and Care Excellence . NICE guidelines; 2016. Non-alcoholic fatty liver disease (NAFLD): assessment and management NICE guideline; pp. 1–17. [PubMed] [Google Scholar]
- 118.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., et al. 2017. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. [DOI] [PubMed] [Google Scholar]
- 119.Clinical Assessment and Management of Nonalcoholic Fatty Liver Disease | AASLD. https://www.aasld.org/practice-guidelines/clinical-assessment-and-management-nonalcoholic-fatty-liver-disease (accessed April 3, 2023). [DOI] [PMC free article] [PubMed]
- 120.Dietrich C.G., Rau M., Geier A. Screening for nonalcoholic fatty liver disease-when, who and how? World J Gastroenterol. 2021;27:5803. doi: 10.3748/wjg.v27.i35.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vuppalanchi R., Siddiqui M.S., Van Natta M.L., Hallinan E., Brandman D., Kowdley K., et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology. 2018;67:134–144. doi: 10.1002/hep.29489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hwang Y.C., Ahn H.Y., Park S.W., Park C.Y. Nonalcoholic fatty liver disease associates with increased overall mortality and death from cancer, cardiovascular disease, and liver disease in women but not men. Clin Gastroenterol Hepatol. 2018;16:1131–1137.e5. doi: 10.1016/j.cgh.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 123.Paik J.M., Henry L., De Avila L., Younossi E., Racila A., Younossi Z.M. Mortality related to nonalcoholic fatty liver disease is increasing in the United States. Hepatol Commun. 2019;3:1459–1471. doi: 10.1002/hep4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Golabi P., Paik J.M., Eberly K., de Avila L., Alqahtani S.A., Younossi Z.M. Causes of death in patients with Non-alcoholic Fatty Liver Disease (NAFLD), alcoholic liver disease and chronic viral Hepatitis B and C. Ann Hepatol. 2022;27 doi: 10.1016/j.aohep.2021.100556. [DOI] [PubMed] [Google Scholar]
- 125.Raza S., Rajak S., Upadhyay A., Tewari A., Sinha R.A. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front Biosci (Landmark Ed. 2021;26:206. doi: 10.2741/4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L., et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378.e5. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 127.Heymsfield S.B., Coleman L.A., Miller R., Rooks D.S., Laurent D., Petricoul O., et al. Effect of bimagrumab vs placebo on body fat mass among adults with type 2 diabetes and obesity: a phase 2 randomized clinical trial. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.33457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Amanullah I., Khan Y.H., Anwar I., Gulzar A., Mallhi T.H., Raja A.A. Effect of vitamin E in non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomised controlled trials. Postgrad Med J. 2019;95:601–611. doi: 10.1136/postgradmedj-2018-136364. [DOI] [PubMed] [Google Scholar]
- 129.Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M., et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. New Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Harrison S.A., Torgerson S., Hayashi P., Ward J., Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 131.Lonn E., Bosch J., Yusuf S., Sheridan P., Pogue J., Arnold J.M.O., et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 132.Yan H., Wu W., Chang X., Xia M., Ma S., Wang L., et al. Gender differences in the efficacy of pioglitazone treatment in nonalcoholic fatty liver disease patients with abnormal glucose metabolism. Biol Sex Differ. 2021;12:1–8. doi: 10.1186/s13293-020-00344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Polyzos S.A., Kountouras J., Mantzoros C.S., Polymerou V., Katsinelos P. Effects of combined low-dose spironolactone plus vitamin E vs vitamin E monotherapy on insulin resistance, non-invasive indices of steatosis and fibrosis, and adipokine levels in non-alcoholic fatty liver disease: a randomized controlled trial. Diabetes Obes Metab. 2017;19:1805–1809. doi: 10.1111/dom.12989. [DOI] [PubMed] [Google Scholar]
- 134.Zulian E., Sartorato P., Benedini S., Baro G., Armanini D., Mantero F., et al. Spironolactone in the treatment of polycystic ovary syndrome: effects on clinical features, insulin sensitivity and lipid profile. J Endocrinological Invest. 2005;28(3):49–53. doi: 10.1007/BF03345529. [DOI] [PubMed] [Google Scholar]
- 135.Newsome P.N., Buchholtz K., Cusi K., Linder M., Okanoue T., Ratziu V., et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–1124. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 136.Guzman S., Dragan M., Kwon H., de Oliveira V., Rao S., Bhatt V., et al. Targeting hepatic kisspeptin receptor ameliorates nonalcoholic fatty liver disease in a mouse model. J Clin Invest. 2022:132. doi: 10.1172/JCI145889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Samson M.M., Meeuwsen I.B.A.E., Crowe A., Dessens J.A.G., Duursma S.A., Verhaar H.J.J. Relationships between physical performance measures, age, height and body weight in healthy adults. Age Ageing. 2000;29:235–242. doi: 10.1093/ageing/29.3.235. [DOI] [PubMed] [Google Scholar]
- 138.Phillips S.K., Rook K.M., Siddle N.C., Bruce S.A., Woledge R.C. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond) 1993;84:95–98. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- 139.Kyle U.G., Genton L., Hans D., Karsegard L., Slosman D.O., Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr. 2001;55:663–672. doi: 10.1038/sj.ejcn.1601198. [DOI] [PubMed] [Google Scholar]
- 140.Dasa M.S., Kristoffersen M., Ersvær E., Bovim L.P., Bjorkhaug L., Moe-Nilssen R., et al. The female menstrual cycles effect on strength and power parameters in high-level female team athletes. Front Physiol. 2021;12 doi: 10.3389/FPHYS.2021.600668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rosa-Caldwell M.E., Mortreux M., Kaiser U.B., Sung D-M., Bouxsein M.L., Dunlap K.R., et al. The oestrous cycle and skeletal muscle atrophy: investigations in rodent models of muscle loss. Exp Physiol. 2021;106:2472–2488. doi: 10.1113/EP089962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Klein E.A., Thompson I.M., Tangen C.M., Crowley J.J., Lucia M.S., Goodman P.J., et al. Vitamin E and the risk of prostate cancer: the selenium and vitamin E cancer prevention trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.