Abstract
Background & Aims
Liver stiffness measurement (LSM) and spleen stiffness measurement (SSM) have been shown to be useful tools for assessing the risk of fibrosis and portal hypertension, respectively. However, data on the accuracy of LSM and SSM measured by point-shear wave elastography (pSWE) in patients affected by primary sclerosing cholangitis (PSC) are still lacking. Thus, we aimed to prospectively assess their performance in a cohort of patients with PSC.
Methods
We determined the correlation between LSM assessed by a pSWE technique (ElastPQ) and by FibroScan-transient elastography (F-TE). Furthermore, we used receiver-operating characteristic curves and area under the curves (AUROC) to evaluate the performance of LSM by ElastPQ for the staging of fibrosis, using F-TE as a reference standard, and the performance of LSM and SSM by ElastPQ in predicting the presence of oesophageal varices (OVs).
Results
One hundred and fifty-two patients with PSC (93 males [61.2%], mean age 46 ± 16 years) were prospectively recruited. ElastPQ and F-TE LSMs were available for all patients, while ElastPQ SSM was available in 109 (72%) patients of whom 35 underwent upper gastrointestinal endoscopy within 1 year of the ultrasound assessment. ElastPQ LSM showed an excellent correlation with F-TE (p <0.001, Spearman’s 0.93; Lin’s 0.86) and a good diagnostic accuracy for fibrosis staging along all stages of liver fibrosis (AUROCs 0.96, 0.97, 0.97 and 0.99 for fibrosis stages F≥1, F≥2, F≥3 and F=4, respectively), using F-TE as a surrogate of histological fibrosis. ElastPQ SSM showed a good diagnostic performance in predicting the presence of OVs at endoscopy.
Conclusions
LSM and SSM by ElastPQ can be used as accurate tools for liver fibrosis risk assessment and fibrosis staging, as well as for predicting the presence of OVs in the work-up of patients with PSC.
Impact and implications
Liver and spleen stiffness measurement (LSM and SSM, respectively) by ElastPQ point-shear wave elastography in patients with primary sclerosing cholangitis represent reliable and reproducible tools for non-invasively staging the severity of liver disease and stratifying patients according to their risk of developing liver-related outcomes. In particular, LSM shows good accuracy for staging liver fibrosis and therefore detecting those patients at high risk of having compensated advanced chronic liver disease who require close monitoring. SSM seems to be promising to detect the risk of portal hypertension and therefore of oesophageal varices, enabling the triaging of patients who really need to undergo a screening endoscopy.
Keywords: Autoimmune cholangiopathies, chronic cholestatic liver diseases, compensated advanced chronic liver disease, risk stratification, portal hypertension, upper gastrointestinal varices, non-invasive tests
Graphical abstract
Highlights
-
•
ElastPQ LSM is a reliable non-invasive method for fibrosis staging in PSC.
-
•
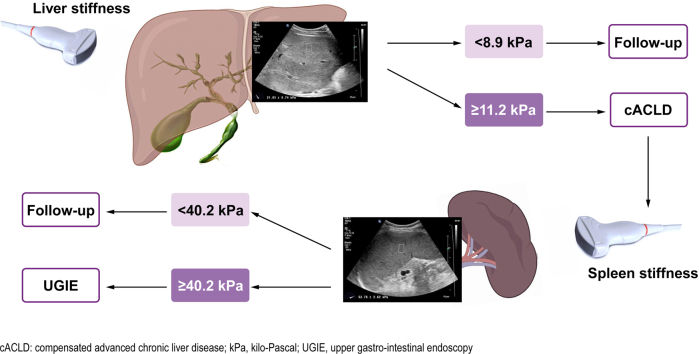
The best cut-offs for ruling-in and ruling-out cACLD in PSC are ≥11.3 and <8.9 kPa, respectively.
-
•
ElastPQ SSM is a promising tool for detecting the presence of oesophageal varices.
-
•
ElastPQ SSM ≥40.2 kPa seems to be more accurate than other non-invasive tests in ruling-in the presence of oesophageal varices.
-
•
The use of ElastPQ SSM could reduce the number of unnecessary upper gastrointestinal endoscopies.
Introduction
Primary sclerosing cholangitis (PSC) is a chronic, progressive cholestatic liver disease characterized by inflammatory and fibrogenic processes affecting both intra- and extrahepatic bile ducts, which can lead to biliary strictures complicated by cholangitis, cirrhosis, liver failure and cholangiocarcinoma.1,2
The aetiology and pathogenesis of PSC are still unknown, although genetic susceptibility factors seem to be involved.3 So far, no medical treatment has proven effective in altering the course of the disease. Excluding the occurrence of hepatobiliary cancer, the prognosis is related to fibrosis progression towards cirrhosis and its complications.4
Histology remains the gold standard to assess and stage liver fibrosis in chronic liver disease. However, in patients with PSC, liver biopsy is not a standard requirement in clinical practice.1 In addition to its invasiveness, high cost, patient discomfort and complications, liver histopathology does not always reflect the actual severity of fibrosis, due to sampling error and inter-observer variability.5,6
On the other hand, hepatic venous pressure gradient (HVPG), which represents the current gold standard to evaluate the presence and severity of portal hypertension (PH), is not widely available and has important limitations such as invasiveness, cost and complications. Overall, upper gastrointestinal endoscopy (UGIE) remains the best method to detect the presence of gastro-oesophageal varices, but it is also an invasive and expensive approach.7
Accordingly, over the past twenty years, major efforts have been made to develop non-invasive methods to stage chronic liver disease with particular focus on surrogate markers of liver fibrosis. In this context, liver shear wave elastography has emerged as a reliable method for the risk assessment of fibrotic transformation in liver tissue and for fibrosis staging, for which it has been extensively validated in chronic viral hepatitis and non-alcoholic steatohepatitis (NASH).[8], [9], [10], [11], [12] Moreover, liver stiffness measurement (LSM) assessed by FibroScan-transient elastography (F-TE) has been shown to correlate with the degree of PH and has been proposed for the detection of clinically significant portal hypertension (CSPH) and to prioritize the need for UGIE in patients with cirrhosis.13,14
The performance and utility of F-TE in detecting liver fibrosis has more recently been evaluated in PSC and primary biliary cholangitis.[15], [16], [17] In this clinical context, several studies have reported that F-TE LSM not only correlates with fibrosis stage, but also predicts clinical progression and outcomes.[18], [19], [20], [21], [22], [23] Further studies have also shown that longitudinal changes in LSM from a baseline measurement can be a good predictor of disease progression in PSC.20
Spleen size has been demonstrated to be a good predictor of clinical outcomes and useful for stratifying patients with PSC according to their risk of disease progression and complications.4 Spleen stiffness measurement (SSM) seems to be a promising tool for predicting the presence of oesophageal varices (OVs) in patients with compensated advanced chronic liver disease (cACLD) due to viral hepatitis. Data about the utility of SSM in patients affected by PSC are still scarce and inconclusive.24
In the last 15 years, new sophisticated elastographic techniques, such as point-shear wave elastography (pSWE) and 2D-/3D-SWE, have been developed and introduced into clinical practice,25 but data on their performance in PSC are lacking.26,27
We therefore aimed to evaluate the diagnostic performance of LSM measured with a pSWE technique as a non-invasive method to assess the risk of fibrotic transformation in liver tissue and for fibrosis staging, using the currently validated F-TE as a reference standard, in a large cohort of patients affected by PSC. Furthermore, we evaluated the performance of LSM and SSM measured by pSWE in assessing the risk of the presence of OVs at endoscopy.
Patients and methods
This is a cross-sectional prospective study conducted at a Hepatology tertiary centre, the Royal Free Hospital, London, UK. Patients were consecutively recruited in the dedicated Autoimmune/Cholestatic Liver Disease Clinic between November 2015 and March 2018.
A confirmed diagnosis of PSC according to the validated EASL and AASLD guidelines,28,29 age ≥18 years and the capacity to sign informed consent were the main inclusion criteria.
Secondary causes of sclerosing cholangitis, other concomitant liver disease, alanine aminotransferase (ALT) values ≥5× the upper limit of normal,25,30 a dominant stricture,28 decompensated cirrhosis, morbid obesity (BMI ≥40 kg/m2), heart failure, cardiac pacemaker, pulmonary hypertension and pregnancy, as well as liver transplant, were the main exclusion criteria.
On the enrolment date, patients underwent a baseline clinical assessment, routine blood collection, liver and spleen stiffness assessment and spleen size measurement. Cirrhosis was diagnosed according to morphological changes detected on CT/MRI scan and ultrasound imaging.
Clinical scores of liver disease severity and prognosis (MELD, Child-Pugh, revised Mayo risk score and Oxford-Amsterdam score)[31], [32], [33], [34] and liver stiffness × spleen diameter-to-platelet ratio risk score (LSPS)35 were calculated based on blood results using their specific algorithms.
UGIE for varices screening was performed in patients with established cirrhosis as part of routine clinical practice. High-risk varices were defined as grade ≥2 OVs, any size varix with a red colour sign or any size of varix in patients with Child-Pugh class C cirrhosis.14,24 Only endoscopies performed within 12 months from the elastographic assessment were considered in the analysis.
Liver shear wave elastography was carried out according to the current EFSUMB guidelines.25
LSM-TE was performed using FibroScan® 502 Touch software 10 version C1.5 (Echosens, France) following the recommended protocol.25 The type of probe (M or XL) was chosen according to the automated suggestion of the machine. The test was considered valid when at least 10 successful acquisitions with an IQR/median ratio ≤30% were obtained. F-TE was used as a surrogate of histological fibrosis and used as the reference method for fibrosis staging, adopting the cut-offs validated against liver biopsy in PSC: 7.4 kPa, 8.6 kPa, 9.6 kPa, and 14.4 kPa for ≥F1, ≥F2, ≥F3 and F4, respectively.20
LSM with the acoustic radiation force impulse (ARFI) technique was performed using the Affiniti 70G ultrasound system (Philips Healthcare, The Netherlands) with a convex C5-1 broadband probe and the latest ElastPQ® software version following the recommended protocol.25
The preferred target area of liver parenchyma was in segment V or VIII.36 After obtaining an adequate B-mode liver image, the region of interest was targeted by placing the 0.5 × 1 cm box-cursor on the liver parenchyma, avoiding large vessels, with the upper edge of the box placed 1.5–2.0 cm away from the Glisson’s capsule.37 Ten consecutive measurements were acquired. The final value was displayed as median in kPa.
The spleen cranio-caudal length was measured with a left intercostal approach using a convex C5-1 broadband probe. A maximum value of 12 cm was used as a cut-off for a normal spleen length.
For SSM-ElastPQ, the transducer was placed in the left intercostal space, with the patient lying in dorsal decubitus and the left arm in maximal abduction. The region of interest was positioned 1–2 cm below the splenic capsule, corresponding to the middle third, and 10 consecutive measurements were acquired. The final value was displayed as median in kPa.
All patients were fasting for at least 3 h before undergoing the ultrasound scan/elastography.25 All ElastPQ measurements were performed by a single expert operator (DR), who had a 5 years’ experience of this technique.
The study was conducted in accordance with the Declaration of Helsinki and its latest amendments and approved by the local Ethics Committee (ref. NC01.14, 9190/20 March 2014). Signed informed consent was obtained from all the patients before taking part to the study.
Statistical analysis
Kolmogorov-Smirnov and Shapiro-Wilk test of normality were used to assess the distribution of quantitative variables. Quantitative variables normally distributed were expressed as mean ± standard deviation (SD), otherwise the median and IQR were reported. Qualitative variables were expressed as counts and percentages. The correlation between quantitative variables was assessed by Pearson’s and Spearman’s tests, according to the distribution of the data. The concordance between quantitative variables was investigated with Lin’s concordance correlation coefficient, which ranges from 0 to +1. Agreement was classified as poor (0.00–0.20), fair (0.21–0.40), moderate (0.41–0.60), good (0.61–0.80) or excellent (0.81–1.00).38 Comparison of correlation coefficients was performed using a z-test on Fisher z-transformed correlation coefficients. The agreement between two quantitative variables was also evaluated by the Bland-Altman plot analysis, with 95% limits of agreement defined as the mean difference ± 1.96 SD of the differences.39
The diagnostic performance of the pSWE technique for staging liver fibrosis was assessed using receiver-operating characteristic (ROC) curves and area under the ROC (AUROC) curve analysis to calculate sensitivity (Se), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR) and negative likelihood ratio (NLR). The optimal cut-off values were chosen to maximise the sum of Se and Sp.
Similarly, ROC curves were used to assess the performance of SSM in detecting the presence of OVs at endoscopy.
Binary logistic regression analyses were carried out to identify potential predictors of the presence of OVs. Potential multicollinearity between variables was checked by Spearman’s rank correlation coefficient. All tests were two-sided and statistical significance was set at p <0.05. The data analysis was performed with SPSS (version 24, IBM, New York, NY, USA), MedCalc (Software for Windows, Version 14.8.1, Ostend, Belgium) and Jamovi 2.3.21.
Results
Baseline population characteristic
One hundred and fifty-two patients with PSC (93 males [61.2%], mean age 46 ± 16 years) were prospectively recruited in the study. The flowchart and the main clinical and elastographic characteristics of the studied population are reported in Fig. 1 and Table 1, respectively. All patients with cirrhosis were in a compensated stage at the time of enrolment. Only three patients had high-risk varices at endoscopy which were treated with endoscopic variceal ligation (EVL) as primary prophylaxis treatment. Three patients had a previous variceal bleeding and started on non-selective beta-blocker (NSBB) after EVL without recurrence of bleeding afterwards. LSM performed with both ElastPQ and F-TE were available for all patients, while SSM was available in 109 (72%) patients. Thirty-five patients had an available SSM and an UGIE performed within 1 year of the elastography assessment.
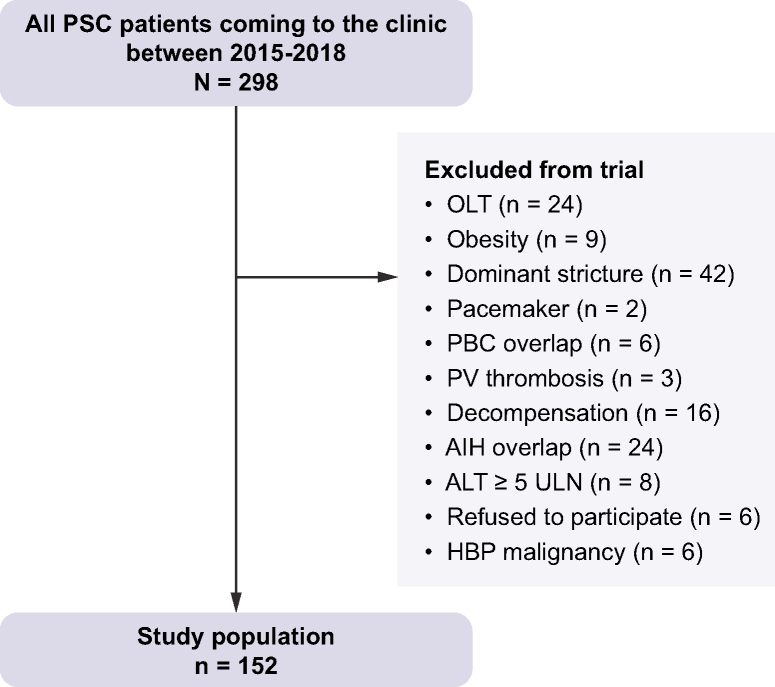
Fig. 1.
Flowchart of the studied population.
AIH, autoimmune hepatitis; ALT, alanine aminotransferase; HPB, hepato-pancreato-biliary; OLT, orthotopic liver transplant; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; PV, portal vein; ULN, upper limit of normal.
Table 1.
Clinical, biochemical, fibrosis scores and sonographic characteristics of the studied population.
| Characteristics | |
|---|---|
| Number of patients | 152 |
| Age, years, mean ± SD | 47 ± 16 |
| Male sex, n (%) | 93 (61.2) |
| Age at PSC diagnosis, years, mean ± SD | 40 ± 16 |
| PSC duration, months, median (IQR) | 49 (97) |
| Large duct PSC/small duct PSC, n (%) | 133/19 (87.5/12.5) |
| AIH overlap syndrome, n (%) | 6 (3.9) |
| Disease localisation, n (%) | |
| Intrahepatic | 64 (43.2) |
| Extrahepatic | 1 (0.7) |
| Intra- and extrahepatic | 83 (56.1) |
| Hepato-biliary cancer, n (%) | 9 (5.9) |
| HCC | 2 (1.3) |
| CCA | 5 (3.3) |
| GB carcinoma | 1 (0.7) |
| Inflammatory bowel disease, n (%) | 108 (70.6) |
| Ulcerative colitis | 92 (60.5) |
| Crohn’s disease | 14 (9.2) |
| Indeterminate | 2 (1.3) |
| Bowel malignancy, n (%) | 9 (5.9) |
| Dysplasia (low grade) | 1 (0.7) |
| DALM | 3 (2) |
| Dysplasia (high grade) | 1 (0.7) |
| Colon carcinoma | 4 (2.6) |
| Cirrhosis at imaging, n (%) | 45 (29.6) |
| History of ascites, n (%) | 13 (8.6) |
| History of HE, n (%) | 0 (0) |
| History of variceal bleeding, n (%) | 3 (2) |
| UGI endoscopies overall, n (%) | 70 (45.7) |
| UGI endoscopies within 12 months, n (%) | 46 (30) |
| Oesophageal varices, n (%) | 18/46 (39.1) |
| High-risk oesophageal varices, n (%) | 2/18 (11.1) |
| Gastric varices, n (%) | 2/18 (11.1) |
| Portal hypertensive gastropathy, n (%) | 13/46 (28.3) |
| Child-Pugh class, n (%) | |
| A | 32/46 (69.5) |
| B | 12/46 (26.1) |
| C | 2/46 (4.3) |
| MELD score, median (IQR) | 7.5 (3) |
| PSC Mayo risk score, median (IQR) | −0.31 (1.35) |
| Biochemistry at the time of stiffness assessment | |
| Total bilirubin (mg/dl), median (IQR) | 0.7 (0.9) |
| Albumin (g/dl), median (IQR) | 4.4 (0.6) |
| AST (IU/L), median (IQR) | 45 (62) |
| ALT (IU/L), median (IQR) | 53 (81) |
| ALP (IU/L), median (IQR) | 165 (247) |
| PLTs, mean ± SD | 250 ± 105 |
| Serum creatinine (mg/dl), mean ± SD | 0.8 ± 0.2 |
| Serum Na, mean ± SD | 141 ± 2 |
| INR, median (IQR) | 1.0 (0.1) |
| Liver stiffness measurement by F-TE | |
| Probe M/XL, n (%) | 150/2 (99/1) |
| F-TE (kPa), median (IQR) | 8.4 (14) |
| ElastPQ liver stiffness (kPa), median (IQR) | 9.01 (12.69) |
| ElastPQ spleen stiffness (kPa), median (IQR) | 28.78 (14.52) |
| Failure, n (%) | 19 (14.7) |
| Fibrosis stage assessed with F-TE as reference standard, n (%) | |
| F0 | 67 (44.1) |
| F1 | 10 (6.6) |
| F2 | 9 (5.9) |
| F3 | 19 (12.5) |
| F4 | 47 (30.9) |
| Spleen size, cm, mean ± SD | 11.9 ± 3.4 |
| LSPS, median (IQR) | 0.47 (1.05) |
AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CCA, cholangiocarcinoma; DALM, dysplasia-associated lesion or mass; F-TE, transient elastography performed by FibroScan; GB, gallbladder; HCC, hepatocellular carcinoma; HE, hepatic encephalopathy; INR, international normalized ratio; LSPS, liver stiffness x spleen diameter-to-platelet ratio score; MELD, model of end-stage liver disease; PLTs, platelets; PSC, primary sclerosing cholangitis; UGI, upper gastrointestinal endoscopy.
Comparison between liver ElastPQ and F-TE
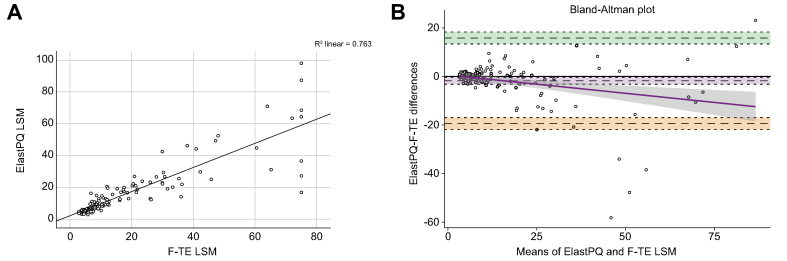
ElastPQ LSM showed an excellent correlation with F-TE (p <0.001, Spearman’s correlation coefficient 0.93; Lin’s correlation coefficient 0.86, 95% CI 0.82–0.90) (Fig. 2A). The Bland-Altman plot agreement analysis showed lack of agreement for F-TE LSM above 15 kPa (mean of differences −1.79, 95% CI −3.2 to 0.35, lower limit −19.4 upper limit 15.8, SD 8.98, p = 0.015) (Fig. 2B).
Fig. 2.
Correlation between FibroScan-transient elastography and ElastPQ stiffness values.
(A) Linear correlation (scatterplot analysis used). The values of ElastPQ LSM appear on the vertical axis, and the values of the F-TE LSM appear on the horizontal axis. Each individual in the data appears as a point on the graph and the continuous black line represents the best line fit. (B) Bland-Altman plot agreement analysis. The continuous black line is the perfect concordance whereas the dotted black line represents the mean of differences (−1.76). The dashed grey lines define the 95% limits of agreement (−19.4 to 15.8), with the green and orange shadings representing the confidence interval (mean of the difference [±2SD], 13.3 to 18.3, −16.9 to −21.9, respectively). The continuous purple line is the proportional bias and the grey shadowing defines the proportional bias line confidence interval. Outliers had a past medical history of cholangitis and decompensation. LSM, liver stiffness measurement; F-TE, FibroScan-transient elastography.
Correlation between liver ElastPQ, prognostic scores and serum markers of inflammation and cholestasis
LSM ElastPQ was significantly correlated with MELD (Spearman’s rho 0.46, p <0.001), PSC Mayo risk score (Spearman’s rho 0.65, p = 0.003) and Oxford-Amsterdam score (Spearman’s rho 0.57, p <0.001). The highest correlation coefficient for Mayo risk score was significantly better than the correlation coefficient for MELD (p = 0.014). No statistically significant difference was found between the correlation coefficients of PSC Mayo risk and Oxford-Amsterdam scores (p = 0.29). A positive and significant correlation was found between LSM ElastPQ and serum bilirubin (Spearman’s rho 0.61, p <0.001), alkaline phosphatase (ALP) (Spearman’s rho 0.47, p <0.001), ALT (Spearman’s rho 0.47, p <0.001) and AST (Spearman’s rho 0.64, p <0.001) levels.
There was no statistically significant difference between the correlations of ElastPQ and those of F-TE with the prognostic scores and serum markers of inflammation and cholestasis (Table S1).
Performance of liver ElastPQ in assessing liver fibrosis stages
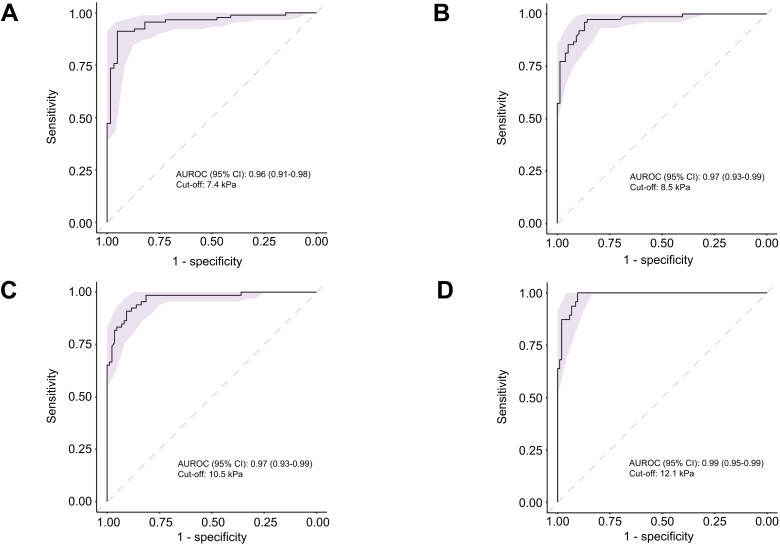
AUROC curves (95% CI) of the median value of liver ElastPQ obtained for each stage of fibrosis (Fig. 3) were 0.96 (0.91–0.98), 0.97 (0.93–0.99), 0.97 (0.93–0.99), 0.99 (0.95–0.99) for fibrosis stage F≥1, F≥2, F≥3 and F=4, respectively. Optimal cut-off values were 7.4 kPa (Se 91%, Sp 92%), 8.5 kPa (Se 95%, Sp 88%), 10.5 kPa (Se 89%, Sp 94%) and 12.1 kPa (Se 100%, Sp 89%) for mild, moderate, severe fibrosis and cirrhosis, respectively. The best cut-off values for ruling-in, with at least 95% specificity, and ruling-out, with at least 95% sensitivity, cACLD (F≥3) were ≥11.3 and <8.9 kPa, respectively (Table 2).
Fig. 3.
AUROC curves of the performance of liver ElastPQ for identifying different fibrosis stages.
Performance of liver ElastPQ in detecting (A) mild fibrosis (F ≥1), (B) moderate fibrosis (F ≥2), (C) severe fibrosis (F ≥3) and (D) cirrhosis (F = 4). The light purple shadowings represent the 95% CI. AUROC, area under receiver-operating characteristic. CI, confident interval.
Table 2.
Performance of liver ElastPQ in differentiating liver fibrosis stages according to F-TE.
| Stage | N | Cut-off (kPa) | Se (95% CI) | Sp (95% CI) | PPV (95% CI) | NPV (95% CI) | PLR (95% CI) | NLR (95% CI) | AUROC (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| ≥F1 | 91 | 7.4 | 0.91 (0.83–0.96) | 0.92 (0.82–0.97) | 0.94 (0.89–0.99) | 0.88 (0.80–0.96) | 11.4 (4.8–25.8) | 0.10 (0.05–0.19) | 0.96 (0.91–0.98) |
| ≥F2 | 75 | 8.5 | 0.95 (0.87–0.99) | 0.88 (0.79–0.95) | 0.89 (0.81–0.94) | 0.94 (0.87–0.98) | 7.9 (4.4–15) | 0.06 (0.02–0.16) | 0.97 (0.93–0.99) |
| ≥F3 | 66 | 10.5 | 0.89 (0.79–0.96) | 0.94 (0.87–0.98) | 0.92 (0.83–0.97) | 0.92 (0.85–0.96) | 14.8 (6.5–36.1) | 0.12 (0.06–0.23) | 0.97 (0.93–0.99) |
| F4 | 47 | 12.1 | 1.0 (0.93–1) | 0.89 (0.80–0.93) | 0.80 (0.69–0.86) | 1.0 | 9.1 (4.9–13.4) | 0 | 0.99 (0.95–0.99) |
| Rule-in ≥F3 | 66 | ≥11.3 | 83 | 95 | 93 | 88 | 17.2 | 0.18 | |
| Rule-out ≥F3 | 66 | <8.9 | 95 | 84 | 83 | 96 | 5.94 | 0.06 |
ROC curve analysis and contingency table analysis used.
AUROC, area under receiver-operating characteristic; CI, confidence interval; F-TE, transient elastography performed by FibroScan; NLR, negative likelihood ratio; NPV negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value; Se, sensitivity; Sp, specificity.
The misclassification of ElastPQ in staging liver fibrosis is reported in Table 3. A difference of LSM ≥2 kPa between F-TE and ElastPQ was found in 33 patients (22%). On the univariate analysis, variables significantly associated with a LSM difference ≥2 kPa were the Mayo risk score, total bilirubin, ALT, AST, ALP, platelet count and the presence of cirrhosis. On the binary logistic regression analysis, the diagnosis of cirrhosis (as defined by F-TE values) was the only independent predictor (odds ratio [OR] 9.87, 95% CI 3.35–29.10, p <0.001) (Table S2). The results did not change with a LSM difference ≥5 and ≥10 kPa (data not shown).
Table 3.
Misclassification of ElastPQ in staging liver fibrosis.
| Fibrosis stage (F-TE) | ElastPQ FN | ElastPQ FP |
|---|---|---|
| ≥F1 | 8/91 (9%) | 5/61 (8%) |
| ≥F2 | 4/75 (5%) | 9/77 (12%) |
| ≥F3 | 7/66 (11%) | 5/86 (6%) |
| F4 | 0/47 (0%) | 12/105 (11%) |
| Total misclassified | 19/152 (13%) | 31/152 (20%) |
Contingency table analysis used.
FN, false negative; FP, false positive; F-TE, transient elastography performed by FibroScan.
Performance of liver and spleen ElastPQ in detecting the presence of OVs
The factors potentially associated with the presence of OVs were investigated in the subgroup of 35 patients who underwent an UGIE within 12 months from the date of the elastographic assessment. Eighteen patients had OVs, with only two patients having varices at high risk of bleeding. The differences in clinical, biochemical and elastographic parameters between the groups of patients with and without OVs are shown in Table S3. In particular, median values of F-TE, ElastPQ LSM, ElastPQ SSM and LSPS were significantly higher in patients with OVs compared to those without (p <0.001).
On univariate analysis, variables significantly associated with the presence of OVs were total bilirubin, platelet count, albumin and international normalized ratio, spleen longitudinal diameter, LSM with both F-TE and ElastPQ, SSM, the composite scores LSPS, Child-Pugh, MELD and Mayo risk score (Table 4). Due to the small number of events, covariates to be retained in the multivariate analysis were chosen based on their significance at the univariate analysis and clinical judgement. ElastPQ SSM was the only independent predictor of the presence of OVs on multivariate analysis (OR 1.14; CI 1.02–1.27; p = 0.021), regardless of whether the Mayo risk score, MELD or Child-Pugh score were included in the model as indicators of liver disease severity (Table 4, Fig. S1). Similarly, the results did not change when either F-TE or ElastPQ were included in the multivariate analysis as a measure of liver stiffness. Finally, in the multivariate analysis model, SSM remained the only statistically significant variable, even after including the LPSP score (OR 1.12; CI 1.01–1.26; p = 0.034).
Table 4.
Univariate and multivariate logistic regression analysis of factors potentially associated with the presence of OVs in patients with PSC with an UGIE within 12 months.
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Male sex | 2.308 | 0.492–10.818 | 0.289 | |||
| Age, years | 0.976 | 0.932–1.022 | 0.297 | |||
| PSC Mayo RS | 3.672 | 1.694–7.962 | 0.001 | 1.339 | 0.332–5.404 | 0.682 |
| Child-Pugh score | 3.868 | 1.780–8.402 | 0.001 | 1.660 | 0.373–7.393 | 0.506 |
| MELD score | 1.634 | 1.215–2.198 | 0.001 | 1.396 | 0.641–3.039 | 0.401 |
| Spleen LD, cm | 1.264 | 1.040–1.535 | 0.018 | 1.180 | 0.735–1.894 | 0.493 |
| Total bilirubin, mg/dl | 1.901 | 1.185–3.048 | 0.008 | 1.026 | 0.946–1.114 | 0.536 |
| ALT (IU/L) | 1.004 | 0.944–1.013 | 0.468 | |||
| AST (IU/L) | 1.012 | 0.999–1.025 | 0.063 | |||
| ALP (IU/L) | 0.999 | 0.996–1.001 | 0.412 | |||
| Platelets/mmc | 0.994 | 0.988–1.000 | 0.043 | 0.996 | 0.987–1.005 | 0.369 |
| Albumin g/dl | 0.090 | 0.020–0.397 | 0.001 | 0.889 | 0.601–1.314 | 0.555 |
| F-TE LSM, kPa | 1.075 | 1.032–1.121 | 0.001 | 0.997 | 0.937–1.060 | 0.920 |
| ElastPQ LSM, kPa | 1.073 | 1.024–1.124 | 0.003 | 0.916 | 0.813–1.032 | 0.149 |
| ElastPQ SSM, kPa | 1.123 | 1.037–1.216 | 0.004 | 1.137 | 1.020–1.268 | 0.021 |
| LSPS | 1.741 | 1.199–2.527 | 0.004 | 1.606 | 0.974–2.648 | 0.063 |
Binary logistic regression analysis used; p value ≤0.05 represents the significance level.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; F-TE, liver elastography performed with FibroScan; LD, longitudinal diameter; LSM, liver stiffness measurement; LSPS, liver stiffness x spleen diameter-to-platelet ratio score; MELD, model of end-stage liver disease; OR, odds ratio; OVs, oesophageal varices; PSC, primary sclerosing cholangitis; RS, risk score; SSM, spleen stiffness measurement; UGIE, upper gastro-intestinal endoscopy.
The ability of F-TE, ElastPQ LSM and ElastPQ SSM to predict the presence of OVs was evaluated and compared. Spleen ElastPQ showed the best ROC curve which, however, did not significantly differ from those obtained for Liver ElastPQ, F-TE and LSPS. AUROCs (95% CI) were: 0.87 (0.76–0.99) for ElastPQ SSM, 0.83 (0.70–0.97) for ElastPQ LSM, 0.82 (0.68–0.96) for F-TE and 0.86 (0.74–0.98) for LSPS. The best cut-off values for detecting OVs were 40.2 kPa, 21.1 kPa, 18.5 kPa and 1.96 for ElastPQ SSM, ElastPQ LSM, F-TE LSM and LSPS, respectively (Table 5).
Table 5.
Performance of liver stiffness (F-TE and ElastPQ), ElastPQ spleen stiffness and LSPS in predicting the presence of oesophageal varices in PSC.
| Test | OVs/n | Se (95% CI) | Sp (95%CI) | PPV (95%CI) | NPV (95%CI) | PLR (95%CI) | NLR (95%CI) | Cut-off value (kPa) | AUROC (95% CI) | FN | FP | Tot misclassified |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F-TE LSM | 13/35 | 0.92 (0.64–1) | 0.62 (0.41–0.83) | 0.60 (0.46–0.73) | 0.93 (0.67–0.99) | 2.4 (1.4–4.5) | 0.13 (0.02–0.82) | 18.5 | 0.82 (0.68–0.96) | 1 | 8 | 9 |
| ElastPQ LSM | 13/35 | 0.92 (0.64–1) | 0.69 (0.45–0.86) | 0.63 (0.48–0.76) | 0.94 (0.69–0.99) | 3 (1.5–5.5) | 0.12 (0.02–0.76) | 21.1 | 0.83 (0.70–0.97) | 1 | 7 | 8 |
| ElastPQ SSM | 13/35 | 0.92 (0.64–1) | 0.77 (0.55–0.92) | 0.71 (0.52–0.84) | 0.94 (0.72–0.99) | 4 (1.9–8.9) | 0.10 (0.01–0.66) | 40.2 | 0.87 (0.76–0.99) | 1 | 5 | 6 |
| LSPS | 13/34 | 0.85 (0.55–0.98) | 0.67 (0.43–0.85) | 0.61 (0.45–0.75) | 0.86 (0.65–0.96) | 2.6 (1.3–4.9) | 0.22 (0.06–0.86) | 1.96 | 0.86 (0.74–0.98) | 2 | 7 | 9 |
ROC curve analysis and contingency table analysis used.
AUROC, area under receiver-operating characteristic; CI, confidence interval; FN, false negative; FP, false positive; F-TE, liver elastography performed with FibroScan; LSM, liver stiffness measurement; LSPS, liver stiffness x spleen diameter-to-platelet ratio score; n, number of patients with endoscopy; NLR, negative likelihood ratio; NPV negative predictive value; OVs, oesophageal varices; PLR, positive likelihood ratio; PPV, positive predictive value; PSC, primary sclerosing cholangitis; Se, sensitivity; Sp, specificity; SSM, spleen stiffness measurement.
The overall performance of SSM to detect OVs did not change after excluding the three patients on NSBBs as secondary prophylaxis (after a first episode of variceal bleeding treated with EVL) from the analysis.
Discussion
The results of the present study demonstrate that ElastPQ pSWE is an accurate non-invasive tool for evaluating the risk of fibrotic transformation in the liver, for fibrosis staging, and for detecting the risk of having OVs in patients affected by PSC. LSM assessed with ElastPQ showed an excellent correlation with F-TE, which is an established non-invasive method for fibrosis staging in PSC.20 Moreover, ElastPQ LSM correlated well with the validated prognostic scores currently used for staging liver disease severity, and thus appears to be a reliable tool for stratifying patients with PSC according to their risk of developing clinical outcomes. Furthermore, SSM assessed by ElastPQ proved to be a useful tool for the non-invasive prediction of the presence of OVs.
Existing data on the efficacy of pSWE for the detection of liver fibrosis in PSC are limited to small and/or mixed aetiology series. Furthermore, in most studies the elastographic technique used was a different pSWE technique (Virtual Touch Quantification, Siemens Healthineers).40 A single dedicated study evaluating ElastPQ pSWE in PSC has been published so far. The authors showed that the median value of LSM in a group of patients with PSC was significantly higher than the median LSM value of a healthy control group, indicating that patients with PSC had stiffer livers compared to controls. However, no comparison was made with F-TE as a reference technique.41
Based on the established performance of F-TE in assessing and staging liver fibrosis in PSC,20 in the absence of an adequate number of liver biopsies in our cohort, we used F-TE as a surrogate of liver fibrosis to evaluate the performance of ElastPQ LSM. Liver ElastPQ and F-TE showed an excellent overall agreement. As expected,42 the Bland-Altman plot analyses showed a lower F-TE and ElastPQ agreement for higher values of liver stiffness and the presence of severe fibrosis/cirrhosis turned out to be the only predictor of a LSM difference ≥2 kPa between ElastPQ and F-TE. This finding has already been reported in other studies including patients with chronic liver disease due to different aetiologies and could be due to a different way of generating the shear waves and tracking them.42,43
Liver ElastPQ showed a good diagnostic accuracy for all stages of fibrosis, with a lower misclassification rate for advanced fibrosis/cirrhosis. As reported for all the shear wave elastography techniques in other aetiologies of chronic liver disease, including F-TE,25,44 ElastPQ was more accurate in ruling-out than ruling-in cirrhosis, with a best cut-off of 12.1 kPa. The obtained cut-off values were lower than those obtained with F-TE, in line with data from the literature.43,45,46 The best cut-off values for ruling-in with at least 95% specificity and ruling-out with at least 95% sensitivity cACLD (F≥3) were ≥11.3 and <8.9 kPa, respectively.
Similarly to what has previously been reported for F-TE,47,48 ElastPQ LSM was also significantly associated with established prognostic scores such as MELD, Mayo risk score and Oxford-Amsterdam score, confirming its potential ability to stratify patients with PSC according to their prognostic risk, although further studies are needed to confirm this finding.
Total bilirubin, ALP and transaminase levels were also significantly associated with LSM ElastPQ. Due to the scarce number of liver biopsies, the influence of histological inflammation on liver stiffness could not be assessed. For this reason, patients with transaminases ≥5× the upper limit of normal or with dominant biliary strictures were excluded from the study since it is well known that stiffness can be affected by inflammation and cholestasis.25 Two different studies from Corpechot et al. evaluated F-TE in comparison with histological features in PSC. The authors did not find a correlation between F-TE LSM and histological necro-inflammatory activity, and the stage of liver fibrosis remained the only predictor of liver stiffness.15,20 The correlation between ElastPQ LSM and established markers of disease progression, such as ALP and bilirubin levels, further supports the possible role of this technique as a prognostic biomarker.49,50
One of the consequences of PH is splenomegaly, which is due not only to the congestion of the portal system and reduced blood drainage from the spleen through the splenic vein, but also to the hyperplasia and fibrosis of the splenic tissue.51 For this reason, the degree of splenomegaly might be different according to the aetiology of the underling liver disease. Spleen size is known to be independently associated with the presence of OVs in patients with compensated cirrhosis. Furthermore, spleen size has been included in scores combining platelet count and F-TE LSM, improving their diagnostic accuracy to detect the presence of varices and high-risk varices.35 A study published in 2016 showed that spleen size predicts clinical outcome (hepatic decompensation, liver transplantation and liver-related death) in patients with PSC.23 In line with these data, in our cohort, the spleen diameter was significantly longer in patients with OVs compared to those without OVs. However, published data indicate that spleen size loses its accuracy in detecting CSPH in the more advanced stages of liver disease, reflecting a non-linear relationship with PH.52,53
The availability of shear wave elastography techniques offers a valuable alternative for the non-invasive assessment of CSPH, potentially sparing invasive procedures and enabling real-time/bedside diagnosis.54 LSM and SSM represent very promising tools for assessing the presence of varices. Moreover, data show that SSM measured by F-TE and ARFI techniques may be a superior marker of PH to LSM in cirrhotic patients with viral aetiologies, and an increasing body of evidence suggests this may also be the case for other aetiologies,55 even though data regarding the use of ARFI techniques are still limited.
The superiority of SSM to LSM as a marker of PH cannot be stated for PSC, with the studies performed so far including small and/or mixed aetiology cohorts with a marked under representation of patients with such liver disease.53 Moreover, consistent data regarding ElastPQ SSM are currently lacking.56
Results regarding the diagnostic accuracy of SSM obtained using F-TE in predicting CSPH are disputable and affected by some limitations, such as the high rate of failure in small spleens and a maximal detectable value of 75 kPa30,53,54 using a 50 Hz F-TE probe, which can be overcome by ARFI techniques as well as by the recent availability of a FibroScan tuned on a100 Hz frequency. For these reasons, in cACLD due to viral hepatitis, Baveno VII recommend an SSM <21 kPa and an SSM >50 kPa to rule-out and rule-in CSPH, respectively, but validation of the best cut-off using a 100 Hz specific F-TE probe, as well as using pSWE and 2D-SWE is needed.24
In the present study, ElastPQ SSM was the only independent predictor of the presence of OVs, with an OR of 1.14. Using the best cut-off values of 40.2 kPa, the diagnostic performance (AUROC) of ElastPQ SSM to detect OVs was slightly better than that of ElastPQ LSM, F-TE and LSPS, even though the difference did not reach statistical significance. However, while they showed a similar sensitivity, the specificity was better for ElastPQ SSM than for the other non-invasive techniques. This means that there is no significant difference in the ability of ElastPQ SSM, ElastPQ LSM, F-TE or LSPS to rule-out the presence of OVs. However, ElastPQ SSM seems to be better in ruling-in the presence of OVs, thus reducing the number of unnecessary UGIEs. The accuracy of ElastPQ SSM to detect high-risk varices could not be assessed due to their low number in our cohort. NSBB treatment did not change our results, probably because of the small number of treated patients and more studies are needed in order to see if the portal pressure changes under NSBBs might affect the performance of SSM.
In summary, this study supports the reliability of LSM assessed by ElastPQ for assessing the risk of fibrotic transformation in liver tissue and for staging liver fibrosis and disease severity in PSC, with cut-off values of 11.3 and 8.6 kPa for ruling-in and ruling-out cACLD, respectively. Moreover, SSM measured by ElastPQ showed a promising diagnostic accuracy for ruling-in the risk of the presence of OVs in the same cohort of patients using a cut-off value of 40.2 kPa. However, because of the small number of UGIEs performed within 12 months, the small number of patients with high-risk varices and the wide time interval between the stiffness and the UGIE assessment, it was not possible to reliably assess the performance of SSM and these results require validation in larger populations of patients affected by PSC.
Financial support
No financial support has been provided for the conduct of the research and the preparation of the article.
Authors’ contributions
Davide Roccarina is the author who is acting as the submission's guarantor. Davide Roccarina and Francesca Saffioti designed the study, collected the data, performed the analysis and wrote the manuscript. Matteo Rosselli, Massimo Pinzani and Aileen Marshall reviewed the manuscript and improved the English. Douglas Thorburn contributed to the design of the study, reviewed the manuscript and improved the English. All authors approved the final version of the manuscript.
Data availability statement
The research data are available upon reasonable request. To request the data, contact the corresponding author of the article.
Conflict of interest
Davide Roccarina, Matteo Rosselli and Aileen Marshall declare no conflicts of interest that pertain to this work. Francesca Saffioti received a support from Novo Nordisk to attend ESSENCE UK investigator meeting in London. Douglas Thorburn is Chair Liver Advisory Group for NHS Blood and Transplant, and Trustee British Trust. He reports consulting activities fees for Ipsen, Chemomab, Gilead and Pliant and lectures payment from Falk and Advanz. Massimo Pinzani is EASL Treasurer Elect and reports consulting activities fees for Novo-Nordisk, Resolution Therapeutics, Galecto, Boehringer Ingelheim, Engitix Therapeutics and Chemomab.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100873.
Supplementary data
The following are the supplementary data to this article.
References
- 1.European Association for the Study of the Liver Electronic address eee, European association for the study of the L. EASL clinical practice guidelines on sclerosing cholangitis. J Hepatol. 2022;77(3):761–806. doi: 10.1016/j.jhep.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Maggs J.R., Chapman R.W. An update on primary sclerosing cholangitis. Curr Opin Gastroenterol. 2008;24(3):377–383. doi: 10.1097/MOG.0b013e3282f9e239. [DOI] [PubMed] [Google Scholar]
- 3.Karlsen T.H., Schrumpf E., Boberg K.M. Genetic epidemiology of primary sclerosing cholangitis. World J Gastroenterol. 2007;13(41):5421–5431. doi: 10.3748/wjg.v13.i41.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehlken H., Wroblewski R., Corpechot C., Arrive L., Lezius S., Hartl J., et al. Spleen size for the prediction of clinical outcome in patients with primary sclerosing cholangitis. Gut. 2016;65(7):1230–1232. doi: 10.1136/gutjnl-2016-311452. [DOI] [PubMed] [Google Scholar]
- 5.Bedossa P., Dargere D., Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology (Baltimore, Md) 2003;38(6):1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Regev A., Berho M., Jeffers L.J., Milikowski C., Molina E.G., Pyrsopoulos N.T., et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97(10):2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 7.Ravaioli F., Montagnani M., Lisotti A., Festi D., Mazzella G., Azzaroli F. Noninvasive assessment of portal hypertension in advanced chronic liver disease: an update. Gastroenterol Res Pract. 2018;2018 doi: 10.1155/2018/4202091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandrin L., Fourquet B., Hasquenoph J.M., Yon S., Fournier C., Mal F., et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29(12):1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Fraquelli M., Branchi F. The role of transient elastography in patients with hepatitis B viral disease. Dig Liver Dis. 2011;43(Suppl 1):S25–S31. doi: 10.1016/S1590-8658(10)60689-5. [DOI] [PubMed] [Google Scholar]
- 10.Talwalkar J.A., Kurtz D.M., Schoenleber S.J., West C.P., Montori V.M. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5(10):1214–1220. doi: 10.1016/j.cgh.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142(6):1293–1302.e4. doi: 10.1053/j.gastro.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Gaia S., Carenzi S., Barilli A.L., Bugianesi E., Smedile A., Brunello F., et al. Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J Hepatol. 2011;54(1):64–71. doi: 10.1016/j.jhep.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Vizzutti F., Arena U., Romanelli R.G., Rega L., Foschi M., Colagrande S., et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology (Baltimore, Md) 2007;45(5):1290–1297. doi: 10.1002/hep.21665. [DOI] [PubMed] [Google Scholar]
- 14.de Franchis R., Baveno V.I.F. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Corpechot C., El Naggar A., Poujol-Robert A., Ziol M., Wendum D., Chazouilleres O., et al. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology (Baltimore, Md) 2006;43(5):1118–1124. doi: 10.1002/hep.21151. [DOI] [PubMed] [Google Scholar]
- 16.Floreani A., Cazzagon N., Martines D., Cavalletto L., Baldo V., Chemello L. Performance and utility of transient elastography and noninvasive markers of liver fibrosis in primary biliary cirrhosis. Dig Liver Dis. 2011;43(11):887–892. doi: 10.1016/j.dld.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Dominguez E., Mendoza J., Garcia-Buey L., Trapero M., Gisbert J.P., Jones E.A., et al. Transient elastography to assess hepatic fibrosis in primary biliary cirrhosis. Aliment Pharmacol Ther. 2008;27(5):441–447. doi: 10.1111/j.1365-2036.2007.03585.x. [DOI] [PubMed] [Google Scholar]
- 18.Merchante N., Rivero-Juarez A., Tellez F., Merino D., Jose Rios-Villegas M., Marquez-Solero M., et al. Liver stiffness predicts clinical outcome in human immunodeficiency virus/hepatitis C virus-coinfected patients with compensated liver cirrhosis. Hepatology (Baltimore, Md) 2012;56(1):228–238. doi: 10.1002/hep.25616. [DOI] [PubMed] [Google Scholar]
- 19.Corpechot C., Carrat F., Poujol-Robert A., Gaouar F., Wendum D., Chazouilleres O., et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology (Baltimore, Md) 2012;56(1):198–208. doi: 10.1002/hep.25599. [DOI] [PubMed] [Google Scholar]
- 20.Corpechot C., Gaouar F., El Naggar A., Kemgang A., Wendum D., Poupon R., et al. Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology. 2014;146(4):970–979. doi: 10.1053/j.gastro.2013.12.030. quiz e15-6. [DOI] [PubMed] [Google Scholar]
- 21.Corpechot C., Carrat F., Gaouar F., Chau F., Hirschfield G., Gulamhusein A., et al. Liver stiffness measurement by vibration-controlled transient elastography improves outcome prediction in primary biliary cholangitis. J Hepatol. 2022 Dec;77(6):1545–1553. doi: 10.1016/j.jhep.2022.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Wang J.H., Changchien C.S., Hung C.H., Tung W.C., Kee K.M., Chen C.H., et al. Liver stiffness decrease after effective antiviral therapy in patients with chronic hepatitis C: longitudinal study using FibroScan. J Gastroenterol Hepatol. 2010;25(5):964–969. doi: 10.1111/j.1440-1746.2009.06194.x. [DOI] [PubMed] [Google Scholar]
- 23.Wong V.W., Vergniol J., Wong G.L., Foucher J., Chan H.L., Le Bail B., et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology (Baltimore, Md) 2010;51(2):454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 24.de Franchis R., Bosch J., Garcia-Tsao G., Reiberger T., Ripoll C., Baveno V.I.I.F. Baveno VII - renewing consensus in portal hypertension. J Hepatol. 2022;76(4):959–974. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dietrich C.F., Bamber J., Berzigotti A., Bota S., Cantisani V., Castera L., et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version) Ultraschall in der Medizin. 2017;38(4):e16–e47. doi: 10.1055/s-0043-103952. (Stuttgart, Germany: 1980) [DOI] [PubMed] [Google Scholar]
- 26.European Association for the Study of the Liver Electronic address eee, Clinical Practice Guideline P, Chair, representative EGB, Panel m. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75(3):659–689. doi: 10.1016/j.jhep.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Barr R.G., Wilson S.R., Rubens D., Garcia-Tsao G., Ferraioli G. Update to the society of radiologists in ultrasound liver elastography consensus statement. Radiology. 2020;296(2):263–274. doi: 10.1148/radiol.2020192437. [DOI] [PubMed] [Google Scholar]
- 28.European Association for the Study of the L. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51(2):237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Chapman R., Fevery J., Kalloo A., Nagorney D.M., Boberg K.M., Shneider B., et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology (Baltimore, Md) 2010;51(2):660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 30.European Association for Study of L. Asociacion Latinoamericana para el Estudio del H. EASL-ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Kim W.R., Therneau T.M., Wiesner R.H., Poterucha J.J., Benson J.T., Malinchoc M., et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clinic Proc. 2000;75(7):688–694. doi: 10.4065/75.7.688. [DOI] [PubMed] [Google Scholar]
- 32.Mells G.F., Pells G., Newton J.L., Bathgate A.J., Burroughs A.K., Heneghan M.A., et al. Impact of primary biliary cirrhosis on perceived quality of life: the UK-PBC national study. Hepatology (Baltimore, Md) 2013;58(1):273–283. doi: 10.1002/hep.26365. [DOI] [PubMed] [Google Scholar]
- 33.de Vries E.M., Wang J., Williamson K.D., Leeflang M.M., Boonstra K., Weersma R.K., et al. A novel prognostic model for transplant-free survival in primary sclerosing cholangitis. Gut. 2018;67(10):1864–1869. doi: 10.1136/gutjnl-2016-313681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malinchoc M., Kamath P.S., Gordon F.D., Peine C.J., Rank J., ter Borg P.C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology (Baltimore, Md) 2000;31(4):864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 35.Berzigotti A., Seijo S., Arena U., Abraldes J.G., Vizzutti F., Garcia-Pagan J.C., et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144(1):102–111.e1. doi: 10.1053/j.gastro.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Samir A.E., Dhyani M., Vij A., Bhan A.K., Halpern E.F., Mendez-Navarro J., et al. Shear-wave elastography for the estimation of liver fibrosis in chronic liver disease: determining accuracy and ideal site for measurement. Radiology. 2015;274(3):888–896. doi: 10.1148/radiol.14140839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon J.H., Lee J.M., Joo I., Lee E.S., Sohn J.Y., Jang S.K., et al. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273(3):772–782. doi: 10.1148/radiol.14132000. [DOI] [PubMed] [Google Scholar]
- 38.Lin L.I. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45(1):255–268. [PubMed] [Google Scholar]
- 39.Bland J.M., Altman D.G. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 40.Goertz R.S., Gassmann L., Strobel D., Wildner D., Schellhaas B., Neurath M.F., et al. Acoustic radiation force impulse (ARFI) elastography in autoimmune and cholestatic liver diseases. Ann Hepatol. 2018;18(1):23–29. doi: 10.5604/01.3001.0012.7858. [DOI] [PubMed] [Google Scholar]
- 41.Mjelle A.B., Mulabecirovic A., Hausken T., Havre R.F., Gilja O.H., Vesterhus M. Ultrasound and point shear wave elastography in livers of patients with primary sclerosing cholangitis. Ultrasound Med Biol. 2016;42(9):2146–2155. doi: 10.1016/j.ultrasmedbio.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Ferraioli G., De Silvestri A., Lissandrin R., Maiocchi L., Tinelli C., Filice C., et al. Evaluation of inter-system variability in liver stiffness measurements. Ultraschall in der Medizin. 2019;40(1):64–75. doi: 10.1055/s-0043-124184. (Stuttgart, Germany: 1980) [DOI] [PubMed] [Google Scholar]
- 43.Fraquelli M., Baccarin A., Casazza G., Conti C.B., Giunta M., Massironi S., et al. Liver stiffness measurement reliability and main determinants of point shear-wave elastography in patients with chronic liver disease. Aliment Pharmacol Ther. 2016;44(4):356–365. doi: 10.1111/apt.13711. [DOI] [PubMed] [Google Scholar]
- 44.Ferraioli G., Wong V.W., Castera L., Berzigotti A., Sporea I., Dietrich C.F., et al. Liver ultrasound elastography: an update to the world federation for ultrasound in medicine and biology guidelines and recommendations. Ultrasound Med Biol. 2018;44(12):2419–2440. doi: 10.1016/j.ultrasmedbio.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Conti F., Serra C., Vukotic R., Fiorini E., Felicani C., Mazzotta E., et al. Accuracy of elastography point quantification and steatosis influence on assessing liver fibrosis in patients with chronic hepatitis C. Liver Int : official J Int Assoc Study Liver. 2017;37(2):187–195. doi: 10.1111/liv.13197. [DOI] [PubMed] [Google Scholar]
- 46.Friedrich-Rust M., Nierhoff J., Lupsor M., Sporea I., Fierbinteanu-Braticevici C., Strobel D., et al. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J viral Hepat. 2012;19(2):e212–e219. doi: 10.1111/j.1365-2893.2011.01537.x. [DOI] [PubMed] [Google Scholar]
- 47.Robic M.A., Procopet B., Metivier S., Peron J.M., Selves J., Vinel J.P., et al. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol. 2011;55(5):1017–1024. doi: 10.1016/j.jhep.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 48.Berzigotti A. Transient elastography and prognosis of cirrhosis. Hepatology (Baltimore, Md) 2012;55(5):1629–1631. doi: 10.1002/hep.25620. [DOI] [PubMed] [Google Scholar]
- 49.Lammers W.J., van Buuren H.R., Hirschfield G.M., Janssen H.L., Invernizzi P., Mason A.L., et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147(6):1338–1349. doi: 10.1053/j.gastro.2014.08.029. e5; quiz e15. [DOI] [PubMed] [Google Scholar]
- 50.Ponsioen C.Y., Chapman R.W., Chazouilleres O., Hirschfield G.M., Karlsen T.H., Lohse A.W., et al. Surrogate endpoints for clinical trials in primary sclerosing cholangitis: review and results from an International PSC Study Group consensus process. Hepatology (Baltimore, Md) 2016;63(4):1357–1367. doi: 10.1002/hep.28256. [DOI] [PubMed] [Google Scholar]
- 51.Bolognesi M., Merkel C., Sacerdoti D., Nava V., Gatta A. Role of spleen enlargement in cirrhosis with portal hypertension. Digestive and liver disease. official J Ital Soc Gastroenterol Ital Assoc Study Liver. 2002;34(2):144–150. doi: 10.1016/s1590-8658(02)80246-8. [DOI] [PubMed] [Google Scholar]
- 52.Sharma P., Kirnake V., Tyagi P., Bansal N., Singla V., Kumar A., et al. Spleen stiffness in patients with cirrhosis in predicting esophageal varices. Am J Gastroenterol. 2013;108(7):1101–1107. doi: 10.1038/ajg.2013.119. [DOI] [PubMed] [Google Scholar]
- 53.Roccarina D., Rosselli M., Genesca J., Tsochatzis E.A. Elastography methods for the non-invasive assessment of portal hypertension. Expert Rev Gastroenterol Hepatol. 2018;12(2):155–164. doi: 10.1080/17474124.2017.1374852. [DOI] [PubMed] [Google Scholar]
- 54.Procopet B., Berzigotti A. Diagnosis of cirrhosis and portal hypertension: imaging, non-invasive markers of fibrosis and liver biopsy. Gastroenterol Rep (Oxf) 2017;5(2):79–89. doi: 10.1093/gastro/gox012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reiberger T. The value of liver and spleen stiffness for evaluation of portal hypertension in compensated cirrhosis. Hepatol Commun. 2022;6(5):950–964. doi: 10.1002/hep4.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berzigotti A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. 2017;67(2):399–411. doi: 10.1016/j.jhep.2017.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The research data are available upon reasonable request. To request the data, contact the corresponding author of the article.