Abstract
An aberrant right subclavian artery, the most common anatomic variant of the aortic arch, occurs in 0.5% of the population. Symptoms generally result from compression of the esophagus and/or trachea as the aberrant vessel passes posteriorly in the mediastinum. Treatment includes revascularization of the right subclavian artery from the right common carotid artery using a cervical approach combined with occlusion of the origin of the aberrant vessel from the thoracic aorta. We describe a hybrid treatment approach for a symptomatic aberrant right subclavian artery using cervical revascularization and branched thoracic stent graft coverage of the origin of the aberrant vessel.
Keywords: Aberrant right subclavian artery, Branched thoracic endograft
An aberrant right subclavian artery (ARSCA), also known as arteria lusoria, is the most common congenital anomaly involving the aortic arch.1 This condition has an estimated incidence of 0.5% and occurs two to three times more frequently in females.1,2 Embryologically, an ARSCA develops when the right fourth branch of the proximal descending aorta crosses the midline posteriorly to the esophagus in 80% of cases, between the trachea and esophagus in 15%, and anterior to the trachea in 5%.3,4 The symptoms associated with an ARSCA typically result from tracheoesophageal compression by the aberrant vessel, manifesting as dysphagia, cough, stridor, and/or thoracic pain.5,6 Nevertheless, most patients are generally asymptomatic throughout life, and the condition is often found incidentally during imaging for other clinical reasons.7
The ARSCA was initially described in 1735 by Hunauld8 from autopsy dissection. In 1794, Bayford9 documented the first case of an ARSCA in a 62-year-old woman who died after years of chronic dysphagia. In 1946, Gross10 reported the first open surgical repair of ARSCA using a left thoracotomy approach with ligation of the aberrant vessel. This “classic” method was eventually found to be associated with the development of ischemia and subclavian steal.11 Subsequent investigators later described alternative open revascularization techniques, including right thoracotomy, median sternotomy, and ARSCA reimplantation to either the ascending aorta or the right common carotid artery.12 The open surgical approach remained the first-line treatment for some time; however, modern advancements in endovascular technologies have since permitted more minimally invasive techniques.
Surgical intervention for ARSCA is indicated for all patients with symptoms or aneurysmal involvement. The goal of surgery is to relieve the symptoms associated with the aberrant vessel, in addition to preserving circulation to the right upper extremity. Surgical management typically involves revascularization of the right subclavian artery through a cervical approach, with occlusion of the ARSCA at its origin from the thoracic aorta.5 We present a hybrid approach to the management of a symptomatic ARSCA using endovascular stent graft coverage of the thoracic aorta, including the aberrant artery origin. The patient provided written informed consent for the report of her clinical case details and imaging studies.
Case report
The patient is a 46-year-old woman employed as a truck driver who had a minor car accident after which her ARSCA was discovered incidentally on a computed tomography scan of the chest. She was referred for vascular surgery evaluation. During that consultation, she reported a long history of dysphagia. Barium swallow test results subsequently confirmed compression of her esophagus consistent with the ARSCA and the diagnosis of dysphagia lusoria. Subsequent computed tomography angiography (CTA) of the chest, abdomen, and pelvis verified the anatomy of the arch vessels, with an otherwise normal aorta and iliac system (Fig 1).
Fig 1.
Preoperative computed tomography angiogram. A, Axial image demonstrating aberrant right subclavian artery (red arrow) and compressed esophagus (blue arrow) between the artery and trachea. B, Three-dimensional reconstruction demonstrating the relationship between the aberrant right subclavian artery and remaining brachiocephalic branches.
Surgical repair was then performed using a hybrid approach. Femoral and left brachial artery access was used to exclude the origin of the ARSCA from the proximal descending thoracic aorta using a branched thoracic endovascular aortic stent graft (W.L. Gore & Associates). The Gore TBE (thoracic branch endoprosthesis) device was chosen because of the proximity of the left subclavian artery to the origin of the ARSCA, which would require coverage of the left subclavian artery to achieve an adequate proximal seal. Concurrently, the right subclavian artery was transposed to the cervical portion of the right common carotid artery (Fig 2). The patient had an uneventful recovery and was discharged home on the first postoperative day. She experienced complete resolution of her dysphagia and returned to work 3 weeks after surgery. Follow-up CTA obtained 3 months after surgery demonstrated widely patent arch vessels, including the transposed right subclavian artery and stented left subclavian artery and exclusion of the aberrant artery origin. Most importantly, the ARSCA coursing behind the esophagus was now completely decompressed (Fig 3).
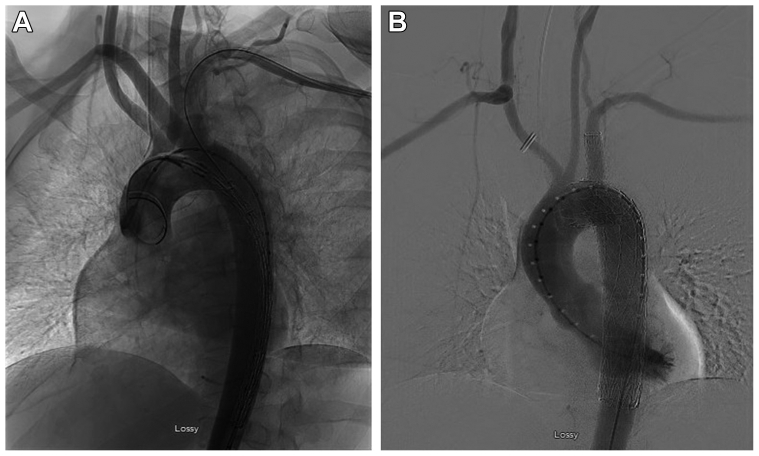
Fig 2.
Intraoperative angiography before (A) and after (B) hybrid repair with right subclavian-to-carotid artery transposition and placement of a branched thoracic endovascular stent graft.
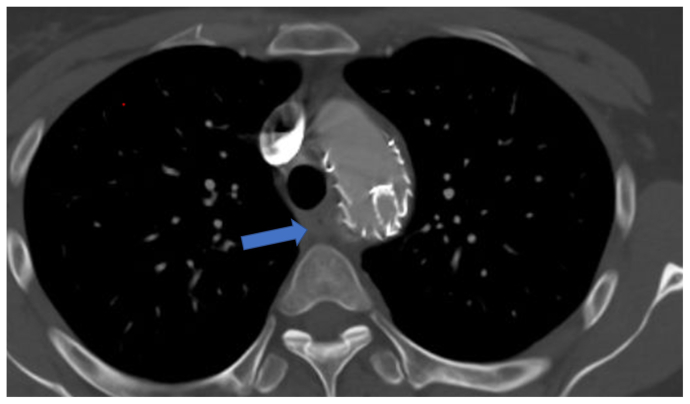
Fig 3.
Axial view of postoperative computed tomography angiogram demonstrating resolution of esophageal compression (blue arrow).
Discussion
In the present case, we opted for a hybrid approach, which enabled excellent management of the ARSCA. The decision to use a branched thoracic stent graft was based on the patient's anatomy and the proximity of the left subclavian artery to the origin of the ARSCA of only 15 mm. To obtain an appropriate seal in the thoracic aorta and exclude the origin of the ARSCA would require coverage of the left subclavian artery origin. The branched thoracic endovascular stent graft (W.L. Gore & Associates) is uniquely designed to achieve this objective and remain within the Food and Drug Administration–approved instructions for use of the device. This was followed by successful transposition of the right subclavian artery to the right common carotid artery. The patient did not experience any procedure-related complications using this hybrid approach and was able to achieve immediate relief of her dysphagia. Follow-up CTA at 3 months postoperatively demonstrated the efficacy of endovascular stent graft treatment in occluding the aberrant vessel origin.
Given that many cases of ARSCA are found incidentally, the use of diagnostic techniques to confirm aberrant vessel pathology cannot be underestimated. For years, the barium swallow examination of the esophagus served as a preliminary diagnostic tool for this condition. This approach allows for visualization of the distinctive compression defect located at the level of the third and fourth thoracic vertebrae. Richardson et al13 previously described a 90% diagnostic accuracy rate using barium esophagrams. The additional use of esophageal manometry can aid in revealing a high-pressure segment within the esophagus; however, these findings are often are considered nonspecific.14 At present, CTA is the most common imaging modality used to confirm the ARSCA diagnosis, as in the present case. Magnetic resonance imaging can also be used to visualize the aberrant vessel in patients who cannot tolerate intravenous contrast material.
The presence of aneurysmal involvement with an ARSCA represents a major source of morbidity and mortality, which must be considered during the diagnostic process. Two centuries after Hunauld8 initially described an ARSCA, Kommerell15 made the first clinical diagnosis of dysphagia lusoria secondary to esophageal compression by an ARSCA with a coexisting aortic diverticulum. Kommerell diverticulum (KD) has since been found to occur in 60% to 82% of ARSCA cases.15,16 KD develops as an aneurysmal enlargement of the descending aorta at the aberrant vessel origin, with a 19% to 53% risk of dissection or rupture.17, 18, 19 Our patient did not have concomitant KD, although the origin of her ARSCA was ectatic. The presence of a KD would not have altered our operative approach, because the TBE device would have excluded its origin.
To date, no standard operative strategy exists for this condition. Rather, surgeons should consider which option among the open, endovascular, or hybrid techniques will provide the most optimal approach to manipulate and transpose the aberrant vessel. A recent systematic review by Loschi et al20 reported low 30-day mortality rates after open (3.5%), hybrid (6.8%), and endovascular (3.9%) repair for ARSCA with concomitant KD. The risk of stroke was low for both open (4.9%) and hybrid (4.1%) repair, with endovascular repair associated with a slightly higher risk (9.8%). Such findings suggest all three surgical modalities are relatively safe, effective, and capable of producing appropriate outcomes in the immediate- and mid-term postoperative period.
Other investigators have also reported encouraging results with hybrid techniques, although some methods were found to result in significant complications. Baker et al21 described the successful use of thoracic endovascular aortic repair (TEVAR) with graft exclusion of the ARSCA, followed by revascularization. Tinelli et al22 reported similar results using TEVAR. However, this approach typically requires coverage of the left subclavian artery orifice, followed by bilateral subclavian-to-carotid transposition. We were able to avoid this by using a branched thoracic endovascular aortic stent graft to exclude the aberrant vessel origin and also preserve the left subclavian artery. Moreover, some investigators suggest the TEVAR approach might unnecessarily increase morbidity via the risk of developing an endoleak, arterial–esophageal fistula, and/or upper extremity claudication symptoms.23,24
Leon et al5 demonstrated successful management of an ARSCA with a right supraclavicular approach to achieve aberrant vessel ligation, followed by carotid artery transposition and endovascular occlusion of the residual stump. The use of a vascular “plug” in a hybrid ARSCA intervention has been previously described.23,25 Although rare, it is possible for migration of the plug device to occur. This can result in recurrent dysphagia or even necessitate additional thoracotomy to extract the plug.23,25
Conclusions
The operative management for symptomatic ARSCA should be tailored for each patient according to the anatomic variations, case-specific factors, and surgeon experience. In this case report, we demonstrate successful hybrid repair using open cervical artery revascularization and branched endovascular stenting techniques. This technique provides a minimally invasive hybrid approach that achieves symptom resolution, preserves right subclavian artery perfusion, and results in complete exclusion of the aberrant vessel origin.
Disclosures
None.
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Polguj M., Chrzanowski L., Kasprzak J.D., Stefańczyk L., Topol M., Majos A. The aberrant right subclavian artery (arteria lusoria): the morphological and clinical aspects of one of the most important variations--a systematic study of 141 reports. Sci World J. 2014;2014 doi: 10.1155/2014/292734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuliani L., Di Toro A., Urtis M., et al. Prevalence and complications of aberrant subclavian artery in patients with Heritable and nonheritable arteriopathies. J Am Coll Cardiol. 2023;81:979–991. doi: 10.1016/j.jacc.2023.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Yang C., Shu C., Li M., Li Q., Kopp R. Aberrant subclavian artery pathologies and Kommerell's diverticulum: a review and analysis of published endovascular/hybrid treatment options. J Endovasc Ther. 2012;19:373–382. doi: 10.1583/11-3673MR.1. [DOI] [PubMed] [Google Scholar]
- 4.Kieffer E., Bahnini A., Koskas F. Aberrant subclavian artery: surgical treatment in thirty-three adult patients. J Vasc Surg. 1994;19:100–111. doi: 10.1016/s0741-5214(94)70125-3. [DOI] [PubMed] [Google Scholar]
- 5.Leon M., Garibaldi M., Virgen F., Ramírez-Cerda C., Cohen-Mussali S. Hybrid treatment of aberrant right subclavian artery causing dysphagia lusoria by subclavian to carotid transposition and endovascular plug. Vasc Specialist Int. 2020;36:258–262. doi: 10.5758/vsi.200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atay Y., Engin C., Posacioglu H., et al. Surgical approaches to the aberrant right subclavian artery. Tex Heart Inst J. 2006;33:477–481. [PMC free article] [PubMed] [Google Scholar]
- 7.Molz G., Burri B. Aberrant subclavian artery (arteria lusoria): sex differences in the prevalence of various forms of the malformation. Evaluation of 1378 observations. Virchows Arch A Pathol Anat Histol. 1978;380:303–315. doi: 10.1007/BF00431315. [DOI] [PubMed] [Google Scholar]
- 8.Hunauld P. Examen de quelques parties d’un singe. Hist Acad R Sci. 1735;2:516–523. [Google Scholar]
- 9.Bayford D. An account of a singular case of obstructed deglutition. Memoirs Med Soc London. 1794;2:275–286. [Google Scholar]
- 10.Gross R.E. Surgical treatment for dysphagia lusoria. Ann Surg. 1946;124:532–534. [PubMed] [Google Scholar]
- 11.Mok C.K., Cheung K.L., Kong S.M., Ong G.B. Translocating the aberrant right subclavian artery in dysphagia lusoria. Br J Surg. 1979;66:113–116. doi: 10.1002/bjs.1800660210. [DOI] [PubMed] [Google Scholar]
- 12.Bailey C.P., Hirose T., Alba J. Re-establishment of the continuity of the anomalous right subclavian artery after operation for dysphagia lusoria. Angiology. 1965;16:509–513. doi: 10.1177/000331976501600901. [DOI] [PubMed] [Google Scholar]
- 13.Richardson J.V., Doty D.B., Rossi N.P., Ehrenhaft J.L. Operation for aortic arch anomalies. Ann Thorac Surg. 1981;31:426–432. doi: 10.1016/s0003-4975(10)60994-0. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen P., Gideon R.M., Castell D.O. Dysphagia lusoria in the adult: associated esophageal manometric findings and diagnostic use of scanning techniques. Am J Gastroenterol. 1994;89:620–623. [PubMed] [Google Scholar]
- 15.Kommerell B. Verlagerung des Oesophagus durch eine abnorm verlaufende A. subclavia dextra (A. lusoria) Fortschr Rontgenstr. 1936;54:590–595. [Google Scholar]
- 16.Plotkin A., Ng B., Han S.M., et al. Association of aberrant subclavian arteries with aortic pathology and proposed classification system. J Vasc Surg. 2020;72:1534–1543. doi: 10.1016/j.jvs.2020.01.042. [DOI] [PubMed] [Google Scholar]
- 17.Idrees J., Keshavamurthy S., Subramanian S., Clair D.G., Svensson L.G., Roselli E.E. Hybrid repair of Kommerell diverticulum. J Thorac Cardiovasc Surg. 2014;147:973–976. doi: 10.1016/j.jtcvs.2013.02.063. [published correction appears in J Thorac Cardiovasc Surg. 2016 Jul;152(1):291. Idrees, Jahanzaib J [corrected to Idrees, Jay J]] [DOI] [PubMed] [Google Scholar]
- 18.Austin E.H., Wolfe W.G. Aneurysm of aberrant subclavian artery with a review of the literature. J Vasc Surg. 1985;2:571–577. doi: 10.1067/mva.1985.avs0020571. [DOI] [PubMed] [Google Scholar]
- 19.Weiss S., Haligur D., Jungi S., et al. Symptomatic or aneurysmal aberrant subclavian arteries: results of surgical and hybrid repair. Interact Cardiovasc Thorac Surg. 2019;29:344–351. doi: 10.1093/icvts/ivz095. [DOI] [PubMed] [Google Scholar]
- 20.Loschi D., Santoro A., Rinaldi E., Bilman V., Chiesa R., Melissano G. A systematic review of open, hybrid, and endovascular repair of aberrant subclavian artery and Kommerell's diverticulum treatment. J Vasc Surg. 2023;77:642–649.e4. doi: 10.1016/j.jvs.2022.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Baker A.C., Atkins B.Z., Clouse W.D., Noll R., Sampson J., Williams T. Repair of aberrant right subclavian artery entirely via a supraclavicular approach. Ann Vasc Surg. 2014;28:489.e1–489.e4. doi: 10.1016/j.avsg.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Tinelli G., Ferrer C., Giudice R., et al. Long-term results of hybrid repair techniques for Kommerell's diverticulum. J Vasc Surg. 2020;72:1213–1221. doi: 10.1016/j.jvs.2019.11.052. [DOI] [PubMed] [Google Scholar]
- 23.Maleux G., Rega F., Heye S., Troost E., Budts W. Asymptomatic migration of a first-generation AMPLATZER vascular plug into the abdominal aorta: conservative management may be an option. J Vasc Interv Radiol. 2011;22:569–570. doi: 10.1016/j.jvir.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 24.van Bogerijen G.H., Patel H.J., Eliason J.L., et al. Evolution in the management of aberrant subclavian arteries and related Kommerell diverticulum. Ann Thorac Surg. 2015;100:47–53. doi: 10.1016/j.athoracsur.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 25.Soo Hoo A.J., Rokkas C.K., Rossi P.J. Migration of endovascular plug in hybrid repair of dysphagia lusoria. J Vasc Surg Cases Innov Tech. 2018;4:140–143. doi: 10.1016/j.jvscit.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]