Abstract
Rationale & Objective
Limited data exist on patient perspectives of the implications of kidney biopsies. We explored patients' perspectives alongside those of clinicians to better understand how kidney biopsies affect patients' viewpoints and the clinical utility of biopsies.
Study Design
Prospective Cohort Study.
Setting & Participants
Patient participants and clinicians in the Kidney Precision Medicine Project, a prospective cohort study of patients who undergo a research protocol biopsy, at 9 recruitment sites across the United States. Surveys were completed at enrollment before biopsy and additional timepoints after biopsy (participants: 28 days, 6 months; clinicians: 2 weeks).
Analytical Approach
Kappa statistics assessed prebiopsy etiology concordance between clinicians and participants. Participant perspectives after biopsy were analyzed using a thematic approach. Clinician ratings of clinical management value were compared to prebiopsy ratings with Wilcoxon matched-pairs signed-rank tests and paired t tests.
Results
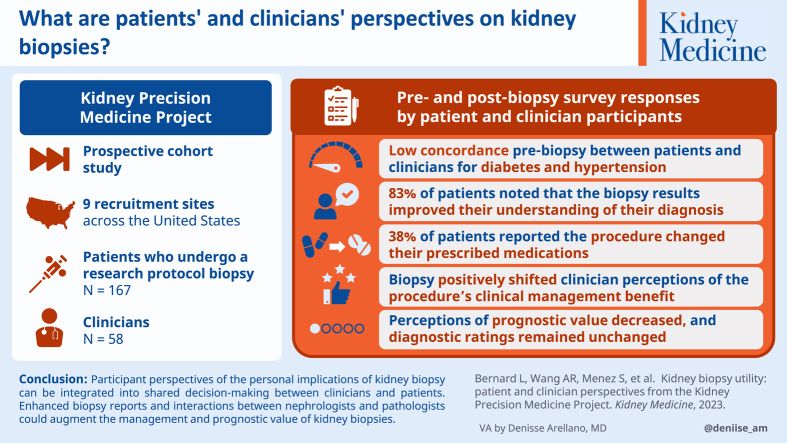
A total of 167 participants undergoing biopsy (124 participants with chronic kidney disease [CKD], 43 participants with acute kidney injury [AKI]) and 58 clinicians were included in this study. CKD participants and clinicians had low etiology concordance for the 2 leading causes of CKD: diabetes (k = 0.358) and hypertension (k = 0.081). At 28 days postbiopsy, 46 (84%) participants reported that the biopsy affected their understanding of their diagnosis, and 21 (38%) participants reported that the results of the biopsy affected their medications. Participants also shared biopsy impressions in free-text responses, including impacts on lifestyle and concurrent condition management. The biopsy positively shifted clinician perceptions of the procedure’s clinical management benefits, while perceptions of prognostic value decreased and diagnostic ratings remained unchanged.
Limitations
Our study did not have demographic data of clinicians and could not provide insight into postbiopsy experiences for participants who did not respond to follow-up surveys.
Conclusions
Participant perspectives of the personal implications of kidney biopsy can be integrated into shared decision-making between clinicians and patients. Enhanced biopsy reports and interactions between nephrologists and pathologists could augment the management and prognostic value of kidney biopsies.
Plain-Language Summary
The utility of kidney biopsy is debated among clinicians, and patients’ perspectives are even less explored. To address these gaps, we synthesized perspectives from clinicians and patient participants of the Kidney Precision Medicine Project (KPMP). Both before and after biopsy, clinicians were surveyed on how the procedure affected their clinical management, diagnosis, and prognosis. After biopsy, participants shared how the procedure affected their diagnosis, medication, and lifestyle changes. Clinicians and patients shared an appreciation for the biopsy’s impact on medical management but diverged in their takeaways on diagnosis and prognosis. These findings highlight the need for greater collaboration between patients and clinicians, particularly as they navigate shared decision-making when considering kidney biopsy.
Index Words: Kidney biopsy, patient feedback, patient-physician communication, procedure benefit
Graphical abstract
Kidney histology obtained by biopsy can be used to determine etiology and prognosis of chronic kidney disease (CKD) and acute kidney injury (AKI). The diagnostic utility of kidney biopsies is recognized in certain patient populations. For example, glomerular diseases such as lupus nephritis are diagnosed and treated based on histological analyses combined with clinical features and laboratory testing. However, the utility of biopsy is less clear for many other kidney diseases. Patients with suspected diabetes-induced or hypertension-induced CKD are typically diagnosed based on clinical criteria, even though histological analysis is required for confirmation and other potentially treatable diagnoses may be missed.1 Similarly, kidney biopsies are rarely pursued to distinguish AKI phenotypes such as acute tubular injury or prerenal azotemia, despite differing structural underpinnings, risks of misdiagnosis, and potential for improper treatment.2
Adding to the unclear utility of biopsy in select cases from clinicians, patient perspectives on the utility of kidney biopsies are largely unexplored. To our knowledge, only 1 prior study has evaluated patients’ attitudes around kidney biopsy, focusing on motivation for study participation and self-reported postbiopsy complications (eg, hematuria and pain).3 However, no study has assessed patient perspectives on the personal value of kidney biopsies – namely their impact on patients’ understanding of disease or caring for comorbid conditions – nor perceptions of the implications of biopsy on medical management. Integrating patient perspectives alongside those of clinicians can help assess the risk-benefit balance of kidney biopsies.
In this study, we synthesize prebiopsy and postbiopsy survey responses of patient participants and clinicians from the Kidney Precision Medicine Project (KPMP) with regard to their views on the use of kidney biopsy to inform diagnosis, prognosis, and clinical management. This multicenter prospective cohort study is designed to interrogate kidney tissue at the structural and molecular levels and generate a new kidney atlas by performing protocol kidney biopsies on adults with diabetes-induced or hypertension-induced CKD or AKI compared with healthy reference tissue.4 In this study, we evaluate the implications of kidney biopsy for both participants and clinicians. Further, we aim to identify areas where these perspectives may diverge.
Methods
Study Population and Design
KPMP is a prospective cohort study of patients who undergo protocol kidney biopsies for research. KPMP aims to identify new ways to treat CKD and AKI through extensive integration of data from clinical phenotypes, tissue interrogation, and biofluid analyses.4 The primary goal of KPMP is to promote patient-driven perspectives and priorities to ultimately advance care.5,6 Patient partners have led the KPMP efforts at every step, including serving on governing committees, advising on research priorities, and authoring major publications.7,8 Patient partners are central to KPMP and guide the consortium’s efforts.
KPMP participant recruitment began in September 2019 at 9 recruitment sites (Johns Hopkins University, Yale University, Columbia University, University of Pittsburgh, University of Texas Southwestern, Cleveland Clinic Foundation, Brigham and Women’s Hospital, Boston Medical Center, and Joslin Diabetes Center/Beth Israel Deaconess Medical Center). Each site was responsible for recruiting patients with either CKD or AKI. Eligibility criteria include rigorous safety criteria developed to minimize potential biopsy risks to participants.9,10 Prospective participants were identified through tools based on electronic health records or local CKD registries. Study candidates were first cleared for approach by their clinicians (via in-person discussion, electronic communication, and telephone calls). Study clinicians and research coordinators then met with prospective participants to discuss the study in detail. Participants provided written informed consent before any study activities. After kidney biopsy, study nephrologists communicated biopsy results to patients in a timely manner via their preferred contact medium (assessed at enrollment: in-person, phone, or electronic communication). Institutional review boards (IRBs) at each recruitment site have a reliance agreement with a single IRB (IRB 201902013, Washington University of St Louis) that has reviewed and approved the study. This study included all KPMP percutaneous biopsy participants up to January 2023.
Assessment of Clinician Perspectives
Before kidney biopsy was performed for each participant, the clinical investigator was asked to complete a survey that queried the most likely cause of the kidney disease (Table S1). Clinicians at CKD recruitment sites were asked, “What renal insults do you think contributed to the participant’s CKD?” Clinicians were given 14 response options and allowed to multiselect options. An analogous question was asked of clinicians at AKI recruitment sites. Clinical investigators also rated the likelihood that the biopsy would provide value for diagnosis, prognosis, or clinical management on a 5-point Likert scale (1 - extremely unlikely, 2 - somewhat unlikely, 3 - neither likely nor unlikely, 4 - somewhat likely, and 5 - extremely likely) (Table S2). Within 2 weeks after biopsy, clinicians returned results to patients and completed a follow-up survey regarding how the procedure affected these same attributes (1 - very much not affected, 2 - somewhat not affected, 3 - neither affected nor not affected, 4 - somewhat affected, and 5 - very much affected).
Assessment of Participant Perspectives
Participants were asked to provide a detailed health history at their baseline assessments. Within this assessment, CKD participants were asked, “If you have been told you have or had chronic kidney disease, what did the doctor tell you was the cause or causes of your kidney disease?” CKD participants were given 17 response options with the opportunity to select multiple items (Table S1). An analogous question was asked to AKI participants. After the kidney biopsy, participants were to complete 28-day and 6-month experience surveys. The survey had 4 iterations, with clinical questions of interest administered in version 2/3 (V1 beginning 10/2019; V2/V3: 6/2020; V4: 2/2022). Surveys were delivered and returned by email. For participants without email access, paper surveys were provided at the closest in-person visit. Survey questions of clinical importance were designed in collaboration with patient partners on the KPMP Community Engagement Committee and were available in English or Spanish (Table S3).
Assessment of Clinical Measures
Baseline clinical measures were collected during the enrollment visit. In the CKD group, enrollment could occur up to 6 weeks before biopsy. Laboratory data was obtained from chart review, and the reporting limit was within the past year. On enrollment, baseline estimated glomerular filtration rate (eGFR) was calculated using the 2021 CKD-EPI equation based on serum creatinine. Medications taken from 30-days before enrollment were taken as baseline use.
Statistical Analysis
Histograms were created for clinician value ratings before and after biopsy. Clinician ratings were comparatively assessed using Wilcoxon matched-pairs signed-rank tests for distributions and paired t tests for means. Kappa statistics were calculated to determine the concordance between clinician-reported and participant-reported diagnoses. The χ2 tests were used to explore whether demographic and background characteristics differed between the CKD and AKI groups. Analyses were completed using Stata version 17 (StataCorp, College Station, Texas).
Results
Study Population Characteristics
The KPMP kidney biopsy cohort for this study included 167 participants (124 participants with CKD and 43 participants with AKI) aged 22-82 years and 58 clinicians. Across the 167 participants, 99 (59%) were White, 52 (31%) Black, 32 (19%) Hispanic, and 70 (42%) were female (Table 1). Participants with CKD often reported a history of hypertension (n=114, 92%), diabetes (n=102, 82%), or hypercholesterolemia (n=85, 69%). Conversely, a smaller percentage of participants with AKI reported a history of hypertension (n=17, 40%), diabetes (n=14, 33%), or hypercholesterolemia (n=9, 21%). A majority of participants in the CKD group reported some college or higher education (n=89, 72%), while fewer participants with AKI reported the same level of education (n=18, 43%; P = 0.001). both participants with CKD and AKI most commonly reported full-time employment, though significant differences were observed in the proportions who were retired (n=43, 35% participants with CKD vs n=4, 9% participants with AKI; P = 0.001) and unemployed (n=13, 10% participants with CKD vs n=13, 30% participants with AKI; P = 0.002). Numerically, more participants with CKD participated in prior research studies compared with participants with AKI (28% vs 15%; P = 0.21). A total of 5 participants in the CKD group reported a prior kidney biopsy. Some participants reported family histories of hypertension (n=17, 11%) or diabetes (n=14, 9%).
Table 1.
Baseline Characteristics of KPMP Participants
| Characteristics | Overall (N=167) | CKD (N=124) | AKI (N=43) |
|---|---|---|---|
| Age, y | 55.8 (13.4) | 58.2 (12.2) | 48.7 (14.3) |
| Female sex | 70 (41.9%) | 57 (46.0%) | 13 (30.2%) |
| Race | |||
| White | 99 (59.3%) | 73 (58.9%) | 26 (60.5%) |
| Black | 52 (31.1%) | 36 (29.0%) | 16 (37.2%) |
| Asian | 7 (4.2%) | 6 (4.8%) | 1 (2.3%) |
| Other | 3 (1.8%) | 3 (2.4%) | 0 (0%) |
| Declined/don’t know | 7 (4.2%) | 7 (5.7%) | 0 (0%) |
| Hispanic ethnicity | 32 (19.2%) | 23 (18.6%) | 9 (20.9%) |
| Health history | |||
| History of kidney disease or kidney failure | 110 (65.9%) | 100 (80.7%) | 10 (23.3%) |
| Diabetes | 116 (69.5%) | 102 (82.3%) | 14 (32.6%) |
| Reported duration of diabetes, ya | 19.6 (12.0) | 20.1 (12.1) | 14.6 (10.6) |
| Diabetic retinopathy | 51 (44.0%) | 46 (45.1%) | 5 (35.7%) |
| Hypertension | 131 (78.4%) | 114 (91.9%) | 17 (39.5%) |
| Reported duration of hypertension, ya | 15.8 (12.3) | 16.0 (12.2) | 13.2 (13.6) |
| Hypercholesterolemia | 94 (56.6%) | 85 (69.1%) | 9 (20.9%) |
| Heart failure | 14 (8.4%) | 10 (8.1%) | 4 (9.3%) |
| Prior kidney biopsyb | 5 (4.6%) | 5 (5.0%) | 0 (0%) |
| Use of angiotensin-converting enzyme/Angiotensin II receptor blockers | 109 (65.2%) | 100 (80.6%) | 9 (20.9%) |
| Baseline eGFRcr, mL/min/1.73 m2 | 52.7 (26.6) | 55.5 (23.3) | 43.1 (34.1) |
| Hemoglobin A1C (%)c | N/A | 8.26 (1.2) | N/A |
| Systolic blood pressure, mm Hg | 136.5 (19.8) | 136.3 (20.1) | 137.7 (18.5) |
| Diastolic blood pressure, mm Hg | 78.0 (10.9) | 77.3 (11.0) | 81.7 (9.38) |
| Educationb | |||
| <High school | 26 (15.7%) | 16 (12.9%) | 10 (23.8%) |
| High school graduate | 29 (17.5%) | 18 (14.5%) | 11 (26.2%) |
| Some college education or more | 107 (64.5%) | 89 (71.8%) | 18 (42.9%) |
| Declined or don’t know | 4 (2.4%) | 1 (0.8%) | 3 (7.1%) |
| Employment | |||
| Full-time | 59 (35.3%) | 46 (37.1%) | 13 (30.2%) |
| Part-time | 12 (7.2%) | 7 (5.7%) | 5 (11.6%) |
| Retired | 47 (28.1%) | 43 (34.7%) | 4 (9.3%) |
| Unemployed | 26 (15.6%) | 13 (10.5%) | 13 (30.2%) |
| Permanently disabled | 15 (9.0%) | 10 (8.1%) | 5 (11.6%) |
| Otherd | 8 (4.8%) | 6 (4.8%) | 2 (4.7%) |
| Research participationb | |||
| Participates in other studies | 24 (24.5%) | 20 (27.8%) | 4 (15.4%) |
| Family historyb | |||
| Kidney disease | 3 (2.0%) | 3 (2.6%) | 0 (0%) |
| Kidney stones | 4 (2.7%) | 4 (3.5%) | 0 (0%) |
| Hypertension | 17 (11.3%) | 14 (12.1%) | 3 (8.8%) |
| Diabetes | 14 (9.0%) | 12 (10.1%) | 2 (5.6%) |
Note: Values reported as n (%) or mean (SD).
Abbreviations: CKD, chronic kidney disease; AKI, acute kidney injury; eGRF, estimated glomerular filtration rate.
Sample reporting duration of diabetes n=110 (CKD, n=99; AKI, n=11). Sample reporting duration of hypertension n=112 (CKD, n=102; AKI, n=10).
Percentage denominators are lower than the overall participant sample because not all participants answered these questions (prior biopsy, n=110; education, n=166; research participation, n=98; family histories, n=147-155).
This measurement was averaged from 2 tests within the year before biopsy (n=42/87 participants in diabetes-induced CKD group). A1C was only reported for diabetes-induced CKD participants.
CKD: Full-time homemaker (n=2), temporarily laid off/on strike (n=1), medical leave (n=1), student (n=1), N/A or declined (n=1); AKI: N/A or declined (n=2).
Mean enrollment eGFR differed between groups: 55.5 mL/min/1.73m2 for CKD group and 43.1 mL/min/1.73m2 for AKI group. For the CKD group, staging distribution was as follows: stage 1, n=13 (10%); stage 2, n=25 (20%); stage 3a, n=39 (31%); stage 3b, n=42 (34%); and stage 4, n=5 (4%). A total of 100 (81%) participants with CKD were on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Regarding actionable postbiopsy complications, there were 2 (1.3%) embolization procedures. No blood transfusions were required in this case or any others in the study cohort.
Prebiopsy Disease Etiology
In the CKD group, 83 clinician-patient pairs answered questions regarding disease cause. Participants with CKD reported a mean of 1.43 causes, while clinicians selected a mean of 1.74 causes. The 2 most commonly reported causes, diabetes and hypertension, had low concordance between clinicians and patient participants with k = 0.358 and k = 0.081, respectively (Figure S1). Participants participating in these pairings had long-standing diabetes and/or hypertension, with reported mean times between prior diagnoses of diabetes or hypertension to CKD of 15 and 12 years, respectively. In cases where hypertension or diabetes was selected, participants selected both in 27% (17/64) of cases, while clinicians selected both in 45% (35/78) of the cases.
In the AKI group, clinicians completed the preclinical assessment questionnaire for 43 participants. However, only 5 (12%) participants answered questions regarding the cause of their AKI, precluding a concordance analysis with clinicians, who reported a mean 1.00 for cause(s) of AKI.
Participant Perspectives on Biopsy
Across all survey iterations, 111 (88 from CKD group and 23 from AKI group) participants completed the 28-day survey and 87 (69 from CKD group and 18 from AKI group) participants completed the 6-month survey. A total of 55 (48 from CKD group and 7 from AKI group) participants completed the 28-day survey and 60 (48 from CKD group and 12 from AKI group) participants completed the 6-month survey versions 2 and 3, which included clinically-relevant questions (eg, medications and diagnosis). Of the 48 participants with CKD who completed the 28-day survey (versions 2 or 3), 40 (83%) reported that the biopsy results improved their understanding of their diagnosis, 18 (38%) reported that the procedure changed the medications they were prescribed, and 46 (96%) were receptive to another biopsy (Table 2). The majority of participants with CKD were willing to have research kidney tissue collected during a future clinical biopsy (n=42, 93%), and a high percentage (n=35, 76%) were willing to have another biopsy for “research purposes” only. Similar results were observed among respondents with AKI. Among the 60 participants who completed the 6-month survey, similar responses were also observed regarding the implications of the biopsy. Both surveys provided participants with the opportunity to provide free-text responses. The majority (26/30, 87%) of free-text respondents were in the CKD group. Themes identified across participant responses included impacts of the biopsy on disease understanding, clinical management, and perceptions of research participation (Table 3). Demographics of value respondents and nonrespondents were similar in the 28-day and 6-month surveys (Table S4). For the 28-day survey versions 2 and 3, a greater proportion of respondents were of White race, had completed at least some college education, and were retired compared with nonrespondents. For the 6-month survey versions 2 and 3, a greater proportion of respondents were of White race compared with nonrespondents.
Table 2.
Participant-Reported Measures of Biopsy Valuea
| 28-day Survey |
6-mo Survey |
|||||
|---|---|---|---|---|---|---|
| Overall (N=55) | CKD (N=48) | AKI (N=7) | Overall (N=60) | CKD (N=48) | AKI N=12) |
|
| Biopsy affected understanding of diagnosis | 46 (84%) | 40 (83%) | 6 (86%) | 47 (78%) | 37 (77%) | 10 (83%) |
| Biopsy affected medication | 21 (38%) | 18 (38%) | 3 (43%) | 19 (32%) | 15 (31%) | 4 (33%) |
| Willing to get another biopsy | 53 (96%) | 46 (96%) | 7 (100%) | 54 (90%) | 43 (90%) | 11 (92%) |
| With research tissue collected | 48/52 (92%) | 42/45 (93%) | 6/7 (86%) | 48/52 (92%) | 39/41 (95%) | 9/11 (82%) |
| Only for research purposes | 40/53 (75%) | 35/46 (76%) | 5/7 (71%) | 34/54 (63%) | 29/44 (66%) | 5/10 (50%) |
Abbreviations: CKD, chronic kidney disease; AKI, acute kidney injury.
For our study’s survey cohorts, we required participants to have answered the first 3 questions (ie, if the biopsy affected diagnosis, medication, and willingness for future biopsy), which were present in versions 2/3 of the 28-day and 6-month surveys. For the 28-day survey overall, 111 (66%) participants completed the survey, 52 (21%) are yet to complete the survey, and 4 (2%) participants left the study. For the 6-month survey overall, 87 (52%) participants completed the survey, 51 (30%) are yet to complete the survey, 5 (3%) participants left the study, and 24 (14%) participants did not reach the 6-month window.
Table 3.
Participant Free-Text Responsesa
| Biopsy Takeaway | Comments |
|---|---|
| On diagnosis and education |
“[Biopsy] brought awareness of my disease to the forefront. I just brushed it off before and didn’t realize the seriousness of my situation.” “I can take better control of my kidneys and better my education on how to take care of this disease.” “[The biopsy] raised awareness of how fragile are my kidneys, and that I had to change my life (diet, exercise, weight loss).” |
| On clinical management |
“I’m working to keep my blood pressure down.” “I know that I have to keep my diabetes in control. Everyday I try to eat properly for my kidneys.” |
| On research and study participation |
“I have a new appreciation for volunteering in a number of areas.” “Knowing that I could maybe help someone in the future makes me pay more attention to my kidney problem and education on how to handle it.” “Research will provide some innovative ways to treat CKD with medicine and knowledge about the progression of the disease. I look forward to understanding even more about CKD in the future.” |
Patients were asked, “How has participating in the KPMP changed your life?” and provided a textbox to record their response within the 28-day and 6-month surveys.
Clinician Before and After Biopsy Value Ratings
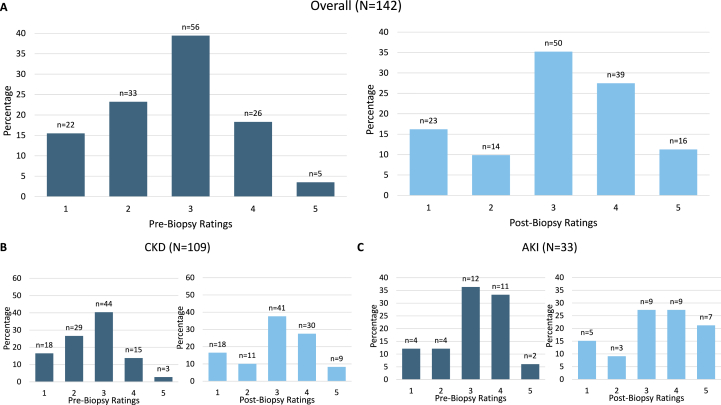
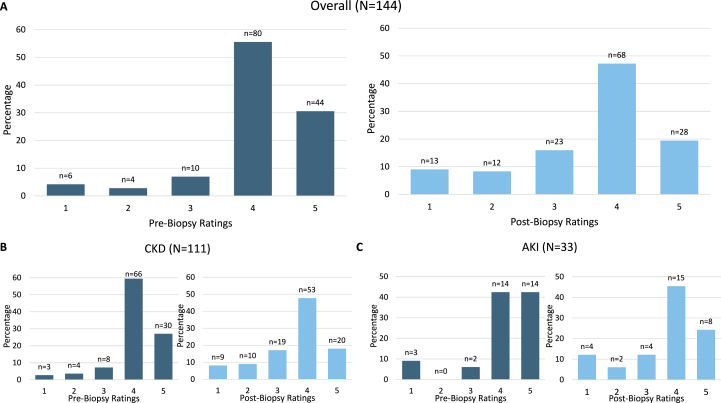
Before biopsy, clinicians often rated the biopsy as unlikely to change clinical management or to identify a cause different than clinical impression (in CKD cases, mean = 2.78 and 2.36, respectively). Clinicians surveyed provided their highest affirmative ratings on a Likert scale when asked if the biopsy would yield prognostic information (overall mean = 4.02). Ratings of the biopsy’s impact on clinical management demonstrated significant shifts after biopsy: 22% to 38% of cases were rated somewhat or very much affected, with a significant CKD subgroup shift observed (17% to 35% of cases) (Figure 1). Rating of the biopsy’s ability to affect the clinical diagnosis did not significantly change (Figure S2). After biopsy, the distribution of prognosis impact ratings significantly shifted, with 25 (17%) cases where clinicians rated very much unaffected or somewhat unaffected compared with 10 (7%) before biopsy (Figure 2). The average rating of effect on prognosis also fell in both CKD (3.61) and AKI (3.63) groups (Table S5). However, clinicians rated two-thirds (n=95) of cases somewhat or very much affected prognosis discussions.
Figure 1.
Before vs After Biopsy Clinician Ratings of Biopsy’s Impact on Clinical Management.a,b Abbreviations: CKD, chronic kidney disease; AKI, acute kidney injury. aWilcoxon signed-rank tests: Overall P < 0.001; CKD P < 0.001; AKI P = 0.30; bBefore biopsy, the Likert scale is: (1) extremely unlikely, (2) somewhat unlikely, (3) neither likely nor unlikely, (4) somewhat likely, and (5) extremely likely. After biopsy the Likert scale is: (1) very much not affected, (2) somewhat not affected, (3) neither affected nor not affected, (4) somewhat affected, and (5) very much affected.
Figure 2.
Before vs After Biopsy Clinician Ratings of Prognosis Impact.a,b,c Abbreviations: CKD, chronic kidney disease; AKI, acute kidney injury. aBefore biopsy, clinicians were asked to rate the likelihood the biopsy would provide prognostic information. After biopsy, clinicians were asked how the results affected discussions with patients regarding their prognosis; bWilcoxon signed-rank tests for prognosis impact: Overall P < 0.001; CKD P < 0.001; AKI P = 0.02; cBefore biopsy, the Likert scale is: (1) extremely unlikely, (2) somewhat unlikely, (3) neither likely nor unlikely, (4) somewhat likely, and (5) extremely likely. After biopsy the Likert scale is: (1) very much not affected, (2) somewhat not affected, (3) neither affected nor not affected, (4) somewhat affected, and (5) very much affected.
Discussion
In this study, we found that clinicians and patient participants demonstrated low concordance regarding kidney disease etiology(ies) before kidney biopsy. Participants reported a variety of biopsy takeaways, including positive impacts on medical management and interest in future biopsy. Clinicians reported that kidney biopsy informed their postprocedure clinical management more than expected but affected clinical prognosis less. Although clinicians noted no impact of kidney biopsy on diagnosis, participants reported that the procedure improved their disease understanding. Our findings demonstrate gaps in clinician-patient communication before and after biopsy and speak to the multifaceted role of kidney biopsy in clinical care of patients.
Participants with CKD had high disease awareness but low concordance with clinicians regarding their disease cause before biopsy. In community-dwelling adults, approximately 20% of those with CKD were aware they had the disease.11 We observed higher CKD awareness (n=100, 81%) in the KPMP than in community-dwelling cohorts possibly because of greater interactions with health systems or the high proportion of participants with CKD with formal education. However, they demonstrated low concordance (diabetes k = 0.358 and hypertension k = 0.081) with providers when reporting the etiology of their disease. These findings might be partially explained by the common co-occurrence of diabetes and hypertension.12 Higher levels of prebiopsy kidney disease awareness may affect the understanding of postbiopsy counseling, medication consumed, and willingness to attend health care visits.
Although we were unable to assess prebiopsy disease understanding in the AKI group because of the small sample size (n= 5, 12%), we observed several contextual factors that differed from the CKD group. AKI affects up to 23% of hospitalized patients,13 and each incremental stage in AKI severity has been associated with 4.4-fold higher odds of developing advanced CKD.14 However, patients with AKI are often unaware that they have reduced kidney function, because the focus is often on another diagnosis leading to hospitalization. KPMP recruitment sites often report difficulties creating and maintaining relationships with participants with AKI. At enrollment, the AKI group’s employment status differed from the CKD group (eg, 30% unemployed vs 10% in CKD group, 9% retired vs 35% in CKD group), as did education status (eg, 43% with some college education or more vs 72% in CKD group). These demographic factors may have affected kidney disease awareness and etiology reporting, as well as postbiopsy behaviors (eg, retired participants may have more time to attend follow-up visits).
Our postbiopsy investigation indicates that both participants with CKD and AKI report a myriad of takeaways from undergoing research-oriented kidney biopsies. Namely, participants note that the procedure improved understanding of their kidney disease (n=46, 84%) and medications consumed (n=21, 38%). A total of 26 CKD survey respondents elaborated further in text that participation in KPMP motivated changes in their lifestyle (eg, diet and exercise), gave stronger adherence to managing comorbidities (eg, monitoring blood pressure or blood sugar), or provided appreciation for volunteering. In contrast, few participants with AKI reported impacts of biopsy on their understanding of kidney disease. Lower levels of engagement both before and after biopsy suggest that clinicians should consider approaches to emphasize the long-term implications of AKI. Altogether, these participant perspectives on kidney biopsy extend beyond clinical metrics to encompass daily life, knowledge exchange, and interest in research.
KPMP researchers previously noted that study participants may be motivated by altruism and the opportunity to benefit the communities from which they may identify.9 Moreover, a number of KPMP participants cite family histories of kidney disease, diabetes, and/or hypertension, perhaps increasing their interest in research. Recently, KPMP opened a participant portal to allow them to view their biopsy tissue.4 In consideration of their KPMP experience, participants reported positive perceptions of the kidney biopsy overall. Although most study participants had never undergone a biopsy before, the vast majority were receptive to another biopsy (n=53, 96%). A total of 40 (75%) of these participants were willing to consider another biopsy for “research purposes” only, demonstrating a greater degree of support for research protocol biopsies than participants from a prior clinical biopsy cohort (n=23, 20%).3
Insights on clinicians’ perspectives differ from KPMP participant findings. After receiving biopsy results, clinicians rated the procedure’s impact on clinical management as greater than expected before biopsy. This observation was driven largely by CKD cases, even though diabetes-induced and hypertension-induced CKD diagnoses are usually based on clinical evaluation without biopsy.15,16 However, clinicians reported after biopsy that the procedure yielded less prognostic information than expected, though the question differed slightly before versus after biopsy. Standard biopsy reports do not include clinical risk prediction; therefore, prognostic discussions may not include this information. Strong prediction models for disease progression, response to treatment, and remission of proteinuric glomerular diseases have recently been developed using tissue descriptors from kidney biopsies.17 Nonspecific tissue findings can confer critical information regarding CKD prognosis (eg, tubulointerstitial fibrosis is associated with decline in eGFR).18 Altogether, greater interactions between nephrologists and pathologists can accelerate the integration of such findings into more effective collaborations between patients and clinicians, particularly as both parties navigate shared decision-making (SDM) discussions.19 Integrating patient takeaways from kidney biopsy into prebiopsy SDM discussions enables clinicians to better elucidate potential harms and benefits, thus enhancing clinician-patient communication. Likewise, by creating summaries of digestible biopsy reports or plain-language results, clinicians provide viable tools that demystify patients’ options for diagnosis and treatment.
Study strengths included having both clinician and participant perspectives on the kidney biopsy captured via quantitative and qualitative methods. KPMP created these surveys with patient partners to ensure that the surveys were informative and relevant to them. Comparison of clinician prebiopsy and postbiopsy ratings showed an increase for clinical management of CKD and AKI. Nevertheless, the study was limited by the lack of data on clinicians’ demographics and years of experience. Just as patients’ demographics can influence their disease understanding or comfort speaking candidly with physicians,20, 21, 22 clinicians’ demographics may affect their practice patterns and communication styles.23,24 In addition, there is likely an element of nonresponse bias in the participant 28-day and 6-month follow-up surveys. However, demographic characteristics were similar among our survey respondents and nonrespondents. Finally, there may be inherent bias in some clinically-relevant responses to the participant survey, because patients willing to contribute to research may have greater inclination to seek value in procedures.
In conclusion, this study identified several areas where clinicians can align more closely with patients regarding the value of a kidney biopsy. Patient participants found a favorable influence of the tissue diagnosis on clinical management and self-care, though the results may be affected by response bias. Nephrologists can integrate these perspectives into patient discussions for SDM when considering kidney biopsy. Enhanced biopsy reports and interactions between nephrologists and pathologists could also augment the management and prognostic value of kidney biopsies.
Article Information
Kidney Precision Medicine Project Collaborators
A list of the collaborators is provided in Item S1.
Authors’ Full Names and Academic Degrees
Lauren Bernard, MHS, Ashley R. Wang, BA, Steven Menez, MD, MHS, Joel M. Henderson, MD PhD, Ashveena Dighe, MS, MPH, Glenda V. Roberts, BA, Christine Stutzke, BA, Katherine R. Tuttle, MD, and R. Tyler Miller, MD, on behalf of the Kidney Precision Medicine Project.
Authors’ Contributions
Research idea, study design, statistical plan/analysis: LB; data interpretation: LB, ARW, JMH, AD, GR, CS, SM, RTM, KRT; supervision/mentorship: SM, RTM, KRT. Each author contributed important intellectual content and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately resolved.
Support
The Kidney Precision Medicine Project is funded by the following grants from the National Institute of Diabetes and Digestive and Kidney Diseases: U2C DK114886, UH3DK114861, UH3DK114866, UH3DK114870, UH3DK114908, UH3DK114915, UH3DK114926, UH3DK114907, UH3DK114920, UH3DK114923, UH3DK114933, and UH3DK114937. SM is supported by 5K23DK128538.
Financial Disclosure
JMH reports research funding from Q32 Bio, Pfizer, and Visterra. SM is supported by NIH research grant K23DK128538 and receives additional research funding from the George M. O’Brien Kidney Center at Yale University and RenalytixAI. KRT is supported by NIH research grants R01MD014712, U2CDK114886, UL1TR002319, U54DK083912, U01DK100846, OT2HL161847, and UM1AI109568 and CDC contract 75D301-21-P-12254; and reports consultancy for Eli Lilly, Boehringer Ingelheim, and AstraZeneca; consultancy and grants from Bayer; consultancy and speaker for Novo Nordisk; and grants from Travere outside the submitted work. The remaining authors declare that they have no relevant financial interests.
Acknowledgments
The authors would like to thank the entire Kidney Precision Medicine Project’s consortium, especially the participants for their vital contributions to this project.
Peer Review
Received March 16, 2023, as a submission to the expedited consideration track with 2 external peer reviews. Direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form June 4, 2023.
Footnotes
Complete author and article information provided before references.
Figure S1. Prebiopsy clinician-patient concordance for chronic kidney disease etiology (n=83).
Figure S2. Distribution of prebiopsy and postbiopsy clinician ratings of biopsy’s ability to affect diagnosis.
Item S1. List of Kidney Precision Medicine Project (KPMP) collaborators.
Table S1. Questions Asked for Disease Etiology to Patients and Clinicians
Table S2. Questions Asked Prebiopsyand Postbiopsy to Clinicians
Table S3. Questions Asked PostBiopsy to Patients in Table 2
Table S4. Demographics of Respondents and Nonrespondents for the 28-day and 6-month Participant Surveys
Table S5. Clinical Measures of Biopsy Value
Supplementary Material
Figure S1-S2. Item S1, Table S1-S5
References
- 1.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49:S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Waikar S.S., McMahon G.M. Expanding the role for kidney biopsies in acute kidney injury. Semin Nephrol. 2018;38:12–20. doi: 10.1016/j.semnephrol.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moledina D.G., Cheung B., Kukova L., et al. A survey of patient attitudes toward participation in biopsy-based kidney research. Kidney Int Rep. 2018;3:412–416. doi: 10.1016/j.ekir.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boer IH de, Alpers C.E., Azeloglu E.U., et al. Rationale and design of the Kidney Precision Medicine Project. Kidney Int. 2021;99:498–510. doi: 10.1016/j.kint.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuttle K.R., Knight R., Appelbaum P.S., et al. Integrating patient priorities with science by community engagement in the Kidney Precision Medicine Project. Clin J Am Soc Nephrol. 2021;16:660–668. doi: 10.2215/CJN.10270620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuttle K.R., Bebiak J., Brown K., et al. Patient perspectives and involvement in precision medicine research. Kidney Int. 2021;99:511–514. doi: 10.1016/j.kint.2020.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Menon R., Bomback A.S., Lake B.B., et al. Integrated single-cell sequencing and histopathological analyses reveal diverse injury and repair responses in a participant with acute kidney injury: a clinical-molecular-pathologic correlation. Kidney Int. 2022;101:1116–1125. doi: 10.1016/j.kint.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel J., Torrealba J.R., Poggio E.D., et al. Molecular signatures of diabetic kidney disease hiding in a patient with hypertension-related kidney disease: a clinical pathologic molecular correlation. Clin J Am Soc Nephrol. 2022;17:594–601. doi: 10.2215/CJN.10350721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler C.R., Appelbaum P.S., Ascani H., et al. A participant-centered approach to understanding risks and benefits of participation in research informed by the Kidney Precision Medicine Project. Am J Kidney Dis Off J Natl Kidney Found. 2022;80:132–138. doi: 10.1053/j.ajkd.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poggio E.D., McClelland R.L., Blank K.N., et al. Systematic review and meta-analysis of native kidney biopsy complications. Clin J Am Soc Nephrol. 2020;15:1595. doi: 10.2215/CJN.04710420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuot D.S., Wong K.K., Velasquez A., et al. CKD awareness in the general population: performance of CKD-specific questions. Kidney Med. 2019;1:43–50. doi: 10.1016/j.xkme.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bretzel R.G. Comorbidity of diabetes mellitus and hypertension in the clinical setting: a review of prevalence, pathophysiology, and treatment perspectives. Clin Ther. 2007;29:S35–S43. [Google Scholar]
- 13.Wang H.E., Muntner P., Chertow G.M., Warnock D.G. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol. 2012;35:349–355. doi: 10.1159/000337487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu R.K., Hsu C.Y. The role of acute kidney injury in chronic kidney disease. Semin Nephrol. 2016;36:283–292. doi: 10.1016/j.semnephrol.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGrath K., Edi R. Diabetic kidney disease: diagnosis, treatment, and prevention. Am Fam Physician. 2019;99:751–759. [PubMed] [Google Scholar]
- 16.Bidani A.K., Griffin K.A. Pathophysiology of hypertensive renal damage. Hypertension. 2004;44:595–601. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 17.Zee J., Liu Q., Smith A.R., et al. Kidney biopsy features most predictive of clinical outcomes in the spectrum of minimal change disease and focal segmental glomerulosclerosis. J Am Soc Nephrol. 2022;33:1411–1426. doi: 10.1681/ASN.2021101396. (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menn-Josephy H., Lee C.S., Nolin A., et al. Renal interstitial fibrosis: an imperfect predictor of kidney disease progression in some patient cohorts. Am J Nephrol. 2016;44:289–299. doi: 10.1159/000449511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elwyn G., Frosch D., Thompson R., et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361–1367. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha S., Beach M.C. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084–1091. doi: 10.1007/s11606-020-05646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saha S., Komaromy M., Koepsell T.D., Bindman A.B. Patient-physician racial concordance and the perceived quality and use of health care. Arch Intern Med. 1999;159:997–1004. doi: 10.1001/archinte.159.9.997. [DOI] [PubMed] [Google Scholar]
- 22.Shen M.J., Peterson E.B., Costas-Muniz R., et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities. 2018;5:117–140. doi: 10.1007/s40615-017-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger J.T. The influence of physicians’ demographic characteristics and their patients’ demographic characteristics on physician practice: implications for education and research. Acad Med. 2008;83:100–105. doi: 10.1097/ACM.0b013e31815c6713. [DOI] [PubMed] [Google Scholar]
- 24.McKinlay J.B., Lin T., Freund K., Moskowitz M. The unexpected influence of physician attributes on clinical decisions: results of an experiment. J Health Soc Behav. 2002;43:92–106. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1-S2. Item S1, Table S1-S5