Abstract
Background & Aims
Glucagon-like peptide (GLP)-2 may exert antifibrotic effects on hepatic stellate cells (HSCs). Thus, we aimed to test whether application of the GLP-2 analogue teduglutide has hepatoprotective and antifibrotic effects in the Mdr2/Abcb4-/- mouse model of sclerosing cholangitis displaying hepatic inflammation and fibrosis.
Methods
Mdr2-/- mice were injected daily for 4 weeks with teduglutide followed by gene expression profiling (bulk liver; isolated HSCs) and immunohistochemistry. Activated HSCs (LX2 cells) and immortalized human hepatocytes and human intestinal organoids were treated with GLP-2. mRNA profiling by reverse transcription polymerase chain reaction and electrophoretic mobility shift assay using cytosolic and nuclear protein extracts was performed.
Results
Hepatic inflammation, fibrosis, and reactive cholangiocyte phenotype were improved in GLP-2-treated Mdr2-/- mice. Primary HSCs isolated from Mdr2-/- mice and LX2 cells exposed to GLP-2 in vitro displayed significantly increased mRNA expression levels of NR4a1/Nur77 (P < .05). Electrophoretic mobility shift assay revealed an increased nuclear NR4a1 binding after GLP-2 treatment in LX2 cells. Moreover, GLP-2 alleviated the Tgfβ-mediated reduction of NR4a1 nuclear binding activity. In vivo, GLP-2 treatment of Mdr2-/- mice resulted in increased intrahepatic levels of muricholic acids (accordingly Cyp2c70 mRNA expression was significantly increased), and in reduced mRNA levels of Cyp7a1 and FXR. Serum Fgf15 levels were increased in Mdr2-/- mice treated with GLP-2. Accordingly, GLP-2 treatment of human intestinal organoids activated their FXR-FGF19 signaling axis.
Conclusions
GLP-2 treatment increased NR4a1/Nur77 activation in HSCs, subsequently attenuating their activation. GLP-2 promoted intestinal Fxr-Fgf15/19 signaling resulting in reduced Cyp7a1 and increased Cyp2c70 expression in the liver, contributing to hepatoprotective and antifibrotic effects of GLP-2 in the Mdr2-/- mouse model.
Keywords: Fibrosis, Bile Acid Homeostasis, Nuclear Binding, FGF15/19
Graphical abstract
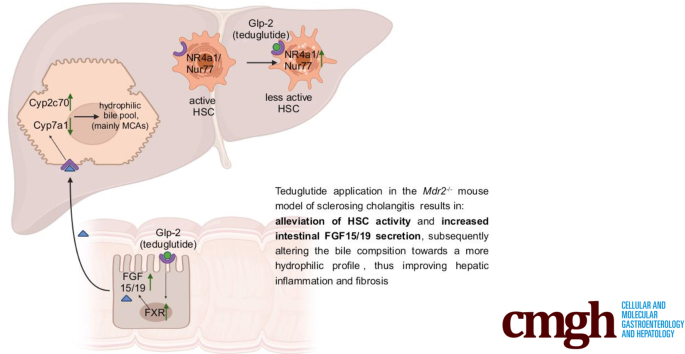
Summary.
In the Mdr2-/- mouse model of sclerosing cholangitis GLP-2 was identified to activate NR4a1 in hepatic stellate cells, thus ameliorating their activation. Furthermore, GLP-2 increased intestinal FXR signaling. Together these features result in improved hepatic inflammation and fibrosis.
As a result of impaired biliary phospholipid secretion and subsequent increase of free nonmicellar bound (potentially toxic) biliary bile acid (BA) concentration, the Mdr2/Abcb4-/- mouse model of sclerosing cholangitis develops pericholangitis, ductular proliferation, reactive cholangiocyte phenotype, and onion skin–type periductal fibrosis,1 reflecting central morphologic features of primary sclerosing cholangitis (PSC).2,3 Activation of hepatic stellate cells (HSC) has been shown to be the primary source to promote chronic cholestatic liver fibrosis.4,5 Therefore, HSCs may be a potential pharmacologic target for new therapy strategies to combat cholangiopathies, such as PSC. Recently, mRNA sequencing identified the presence of the glucagon-like peptide (GLP)-2 receptor in HSC,6,7 and possibly hepatocytes.8 Moreover, it has been demonstrated that loss of GLP-2 receptor signaling in HSCs led to their activation in steatohepatitis,6 indicating a novel hepatic function of GLP-2 beyond its established role in the intestine.9 Furthermore, nuclear receptor subfamily 4 group a member 1 (NR4a1)/Nur77 has been identified as key regulator of hepatic fibrosis and its suppression is heavily involved in the activation process of HSCs.10 Activated HSCs have been also shown to secrete proinflammatory chemokines and cytokines11 inducing hepatic inflammation11 and reactive cholangiocyte phenotype.12 Therefore, the GLP-2 analogue teduglutide, known for its proproliferative effect in the gut, thus reducing the requirement for parenteral nutrition in patients with short-bowel syndrome,13 could be an interesting pharmacologic approach to counteract liver injury. In the present study we investigated whether treatment with the GLP-2 analogue teduglutide improves liver injury in the Mdr2/Abcb4-/- mouse model of sclerosing cholangitis possibly via activation of NR4a1/Nur77 in HSCs.
Results
GLP-2 Treatment Improves Hepatic Inflammation and Fibrosis in Mdr2-/- Mice
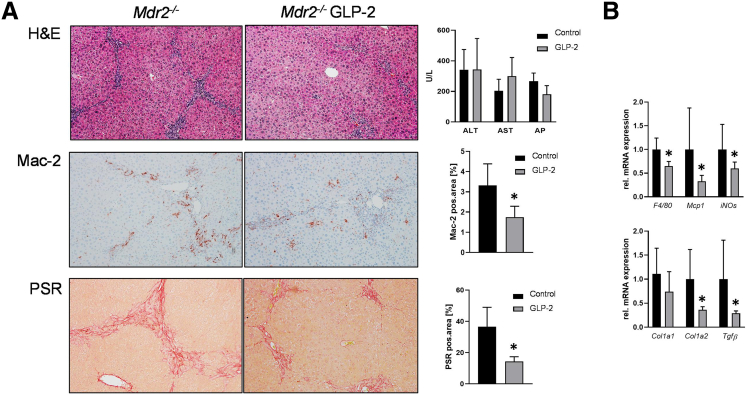
To investigate whether the GLP-2 analogue teduglutide has a positive effect on the liver phenotype of Mdr2-/- mice, GLP-2 was administered via intraperitoneal injection for a time period of 4 weeks. Although serum levels of liver transaminases alanine aminotransferase and aspartate aminotransferase and alkaline phosphatase remained unchanged between untreated control and GLP-2-injected Mdr2-/- mice, liver phenotype of Mdr2-/- mice (eg, pericholangitis) improved in presence of GLP-2 (Figure 1A). Accordingly, hepatic inflammation and hepatic fibrosis were ameliorated in GLP-2-treated animals, reflected by significantly lower numbers of MAC-2-positive cells (MAC-2 immunohistochemistry) and markedly lower amount of collagen (Sirius red staining) in the livers of these mice (Figure 1A). In line with these findings, gene expression profiling revealed a clear reduction of mRNA levels of inflammatory markers F4/80, Mcp1, and iNOs and of the fibrotic markers Col1a1, Col1a2, and Tgfβ (Figure 1B).
Figure 1.
GLP-2 treatment improves hepatic inflammation and fibrosis in Mdr2-/-mice. (A) Representative hematoxylin-eosin images (×10 magnification) with markedly improved liver histology in Mdr2-/- mice treated with GLP-2. Serum biochemistry reflects unchanged levels of transaminases (alanine aminotransferase, aspartate aminotransferase) and alkaline phosphatase. Representative Mac-2 and Sirius red (SR) pictures (×10 magnification) show reduced hepatic inflammation and fibrosis in Mdr2-/- mice treated with GLP-2. (B) Real-time polymerase chain reaction was used to assess the mRNA expression of inflammatory and fibrotic markers F4/80, Mcp1, iNOs, Col1a1, Col1a2, and Tgfβ, which were significantly reduced in Mdr2-/- mice treated with GLP-2. mRNA expression values were normalized against 36b4 levels and are shown relative to the expression level in Mdr2-/- control subjects. ∗Significant difference from Mdr2-/- control mice; P < .05. Computational analysis of histologic pictures was done via Image J 1.51j8. ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase.
GLP-2 Treatment Improves Reactive Cholangiocyte Phenotype in Mdr2-/- Mice
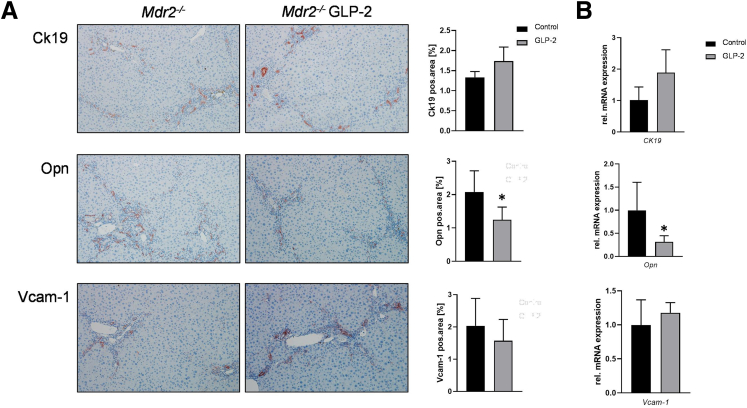
Because the development of hepatic inflammation and fibrosis under cholestatic conditions is linked to a reactive cholangiocyte phenotype,14 respective markers were investigated by immunohistochemistry (Figure 2A) and real-time polymerase chain reaction (Figure 2B). Although Ck19 (marker for cholangiocyte proliferation) staining and mRNA levels tended to be increased, osteopontin was significantly decreased in Mdr2-/- mice treated with GLP-2. Of note, Vcam-1 remained unchanged at mRNA and at protein level (Figure 2A and B). Together these data indicate that despite a trend for increased ductular proliferation, treatment with the GLP-2 analogue teduglutide reduced secretion of proinflammatory markers, such as osteopontin and Vcam-1, from cholangiocytes in the Mdr2-/- mouse model of sclerosing cholangitis.
Figure 2.
GLP-2 treatment improves reactive cholangiocyte phenotype in Mdr2-/-mice. (A) Representative Ck19 images (×10 magnification) show tendentially increased cholangiocyte proliferation in GLP-2-treated Mdr2-/- animals, whereas representative osteopontin (Opn) images (×10 magnification) reflect reduced Opn secretion from cholangiocytes. Vcam-1 images (×10 magnification) remained unchanged among the groups. (B) Real-time polymerase chain reaction was used to assess the mRNA expression of Ck19, Opn, and Vcam-1. Whereas Ck19 expression tended to be increased, Opn levels were reduced and Vcam-1 remained unchanged in Mdr2-/- mice treated with GLP-2. mRNA expression values were normalized against 36b4 levels and are shown relative to the expression level in Mdr2-/- control animals. ∗Significant difference from Mdr2-/- control mice; P < .05. Computational analysis of histologic pictures was done via Image J 1.51j8.
GLP-2 Treatment Increases the Transcription of NR4a1/Nur77 in Primary Hepatic Stellate Cells
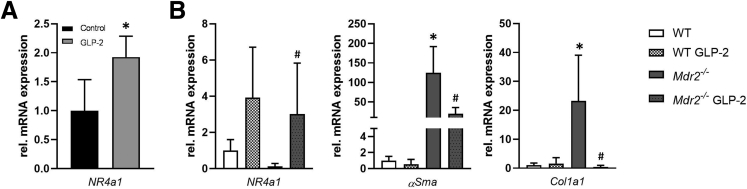
Because GLP-2 activates NR4a1/Nur77 in HSCs, thereby counteracting their activation,15 we subsequently investigated NR4a1 mRNA expression in whole liver lysates (Figure 3A) and in primary HSCs isolated from wild-type and Mdr2-/- mice subjected to GLP-2 treatment (Figure 3B). NR4a1 transcription was increased in whole liver lysates and in isolated HSCs from GLP-2-treated animals. In line with this, αSma and Col1a1 expression was reduced in HSCs in Mdr2-/- mice subjected to GLP-2 treatment (Figure 3B).
Figure 3.
NR4a1/Nur77 mRNA expression is significantly increased in primary hepatic stellate cells because of GLP-2 treatment. (A) Real-time polymerase chain reaction was used to assess the mRNA expression of NR4a1/Nur77 in whole liver homogenate. mRNA expression values were normalized against 36b4 levels and are shown relative to expression level in Mdr2-/- control subjects. ∗Significant difference from Mdr2-/- control mice; P < .05 (B) Real-time polymerase chain reaction was used to assess the mRNA expression of NR4a1/Nur77 αSma and Col1a1 in primary hepatic stellate cells isolated from wild-type (WT) and Mdr2-/- mice with and without GLP-2 treatment. GLP-2 treatment significantly increased expression levels of Nr4a1/Nur77. αSma and Col1a1 mRNA levels were significantly reduced in primary hepatic stellate cells isolated from Mdr2-/- mice treated with GLP-2 compared with untreated Mdr2-/- mice. mRNA expression values were normalized against 36b4 levels and are shown relative to the expression level in WT control animals. #Significant difference from Mdr2-/- control mice; P < .05.
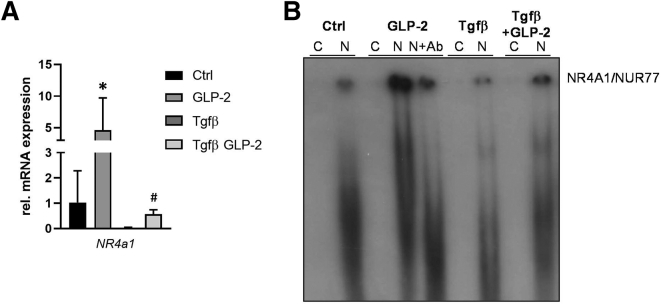
GLP-2 Treatment Restores Tgfβ-Related NR4a1/Nur77 Suppression in Human Hepatic Stellate Cells In Vitro
To investigate whether GLP-2 is also effective in counteracting HSC activation in humans, LX-2 cells were activated with Tgfβ (Figure 4A). As predicted, GLP-2 increased NR4a1 mRNA expression, whereas Tgfβ treatment led to a significant reduction of NR4a1 expression (Figure 4A). GLP-2 treatment was able to counteract the Tgfβ-related NR4a1 suppression and restored normal levels of the transcription factor (Figure 4A). To assess the direct interaction of GLP-2 and NR4a1, an electrophoretic mobility shift assay was performed (Figure 4B). In contrast to Tgfβ, GLP-2 treatment was able to increase nuclear binding of NR4a1. Importantly, GLP-2 treatment was able to counteract Tgfβ-related NR4a1 suppression.
Figure 4.
GLP-2 treatment increases NR4a1/Nur77 expression and nuclear binding in human hepatic stellate cells in vitro. (A) Real-time polymerase chain reaction was used to assess the mRNA expression of NR4a1/Nur77 in the human hepatic stellate cell line LX-2. Tgfβ-induced suppression of NR4a1/Nur77 could be counteracted by GLP-2 treatment. mRNA expression values were normalized against 36b4 levels and are shown relative to expression level in untreated control cells. ∗Significant difference from untreated control (Ctrl) cells. #Significant difference from Tgfβ-treated cells. P < .05. (B) Representative electrophoretic mobility shift assay demonstrated that GLP-2 treatment increases the nuclear binding of NR4A1/NUR77, whereas Tgfβ challenge led to a reduction of the NR4A1/NUR77 nuclear binding. Of note, GLP-2 treatment was able to counteract the Tgfβ-related reduction of the NR4A1/NUR77 nuclear binding. Ab, antibody; C, cytoplasmatic protein fraction; N, nuclear protein fraction.
GLP-2 Treatment Alters Hepatic Bile Acid Metabolism
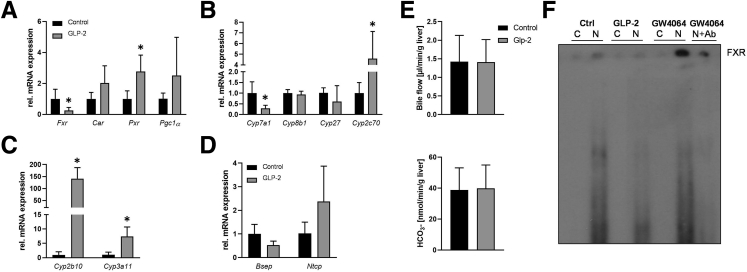
Next, we explored whether GLP-2 also impacts on BA homeostasis and signaling in addition to its antifibrotic function in HSCs. Despite reduced hepatic Fxr expression (Figure 5A), Cyp7a1 mRNA levels were significantly repressed by GLP-2. Cyp8b1 and Cyp27 remained unchanged but Cyp2c70 was increased upon GLP-2 challenge (Figure 5B). In line with this, increased expression of Cyp2c70 increased the abundance of muricholic acids in Mdr2-/- mice subjected to GLP-2 treatment (Table 1). Expression of the nuclear receptor Pxr was markedly increased in the presence of GLP-2, whereas expression of Car and its coactivator Pgc1α was unchanged (Figure 5A). The gene expression of the constitutive androstane receptor and pregnane-X-receptor downstream targets Cyp2b10 and Cyp3a11 was increased in Mdr2-/- mice treated with GLP-2 (Figure 5C). Farnesoid X receptor (FXR) targets, such as Bsep and Ntcp, tended to be reduced and increased, respectively (Figure 5D). However, hepatobiliary bile flow and biliary HCO3- output remained unchanged among the groups (Figure 5E).
Figure 5.
GLP-2 treatment interferes with bile acid homeostasis in Mdr2-/-mice. Real-time polymerase chain reaction was used to assess the mRNA expression of (A) Fxr, Car, Pxr, and Pgc1α, (B) Cyp7a1, Cyp8b1, Cyp27, and Cyp2c70, (C) Cyp2b10 and Cyp3a11, and (D) Bsep and Ntcp in the liver. mRNA levels of Fxr were significantly lowered, whereas Car, Pxr, and Pgc1α were increased. Cyp7a1 was significantly lowered because of GLP-2 treatment in Mdr2-/- mice, whereas Cyp8b1 and Cyp27 remained unchanged and Cyp2c70 was increased. Gene expression of detoxification enzymes Cyp2b10 and Cyp3a11 was significantly increased because of GLP-2. Although Bsep expression levels tended to be lowered, Ntcp showed a tendential increase. mRNA expression values were normalized against 36b4 levels and are shown relative to the expression level in Mdr2-/- control subjects. ∗Significant difference from Mdr2-/- control mice; P < .05. (E) Hepatobiliary bile flow and bicarbonate (HCO3-) output were assessed. Neither bile flow nor HCO3- output was changed because of GLP-2 treatment. (F) Representative electrophoretic mobility shift assay demonstrated that GLP-2 treatment suppresses the nuclear binding of FXR, whereas GW4064 (FXR agonist) challenge led to an increase of FXR nuclear binding. Ab, antibody; C, cytoplasmatic protein fraction; N, nuclear protein fraction.
Table 1.
Total and Individual Intrahepatic Bile Acid Levels
| pmol/mg liver | Mdr2-/- Ctrl | Mdr2-/- GLP-2 |
|---|---|---|
| ToMCA | 23.9 ± 4.2 | 81.93 ± 52.2 |
| TαMCA | 5.5 ± 1.4 | 4.2 ± 2.4 |
| TβMCA | 67.2 ± 21.3 | 158.4 ± 21.3a |
| TCA | 106.0 ± 15.3 | 211.3 ± 108.9 |
| TUDCA | 1.0 ± 0.2 | 1.1 ± 0.6 |
| TCDCA | 5.5 ± 0.59 | 8.5 ± 3.2 |
| TDCA | 1.7 ± 0.4 | 1.4 ± 1.0 |
| oMCA | 5.4 ± 2.2 | 16.4 ± 4.76a |
| αMCA | 1.3 ± 0.9 | 1.8 ± 1.0 |
| βMCA | 19.3 ± 5.2 | 47.5 ± 12.0a |
| Total | 240.1 ± 27.8 | 527.5 ± 312.6 |
αMCA, alpha muricholic acid; βMCA, beta muricholic acid; oMCA, omega muricholic acid; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; TDCA, taurodeoxycholic acid; TαMCA, tauro-alpha muricholic acid; TβMCA, tauro-beta muricholic acid; ToMCA, tauro-omega muricholic acid; TUDCA, tauroursodeoxycholic acid.
Significantly different from Mdr2-/- Ctrl. P ≤ .05 was considered significant.
GLP-2 Treatment Suppresses Hepatic FXR Nuclear Binding in Human Hepatocytes In Vitro
To examine the mechanisms of reduced Fxr mRNA expression observed in GLP-2-treated Mdr2-/- mice and whether reduced expression results in reduced FXR function and subsequent signaling, electrophoretic mobility shift assay was performed in immortalized human hepatocytes cells treated with GLP-2 and GW4064 (FXR agonist, positive control) (Figure 5F). GW4064 treatment increased the nuclear binding of FXR, whereas GLP-2 led to a reduction of FXR nuclear binding (Figure 5F).
GLP-2 Treatment Increases Intestinal Fgf15 Expression
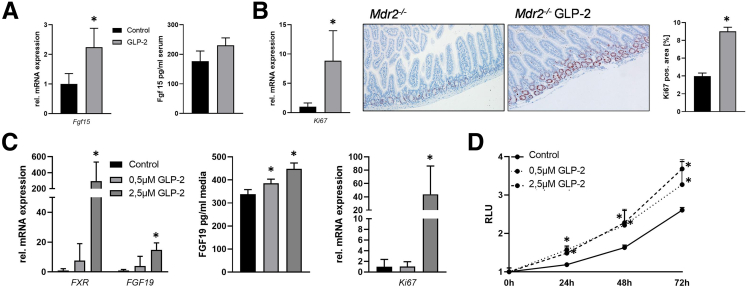
Because in Mdr2-/- mice treated with GLP-2 Fxr and Cyp7a1 mRNA expression was reduced, we next explored intestinal Fgf15 gene expression. In line with low hepatic Cyp7a1 levels, intestinal Fgf15 expression was increased on GLP-2 treatment (Figure 6A). Accordingly, serum levels of Fgf15 were tendentially increased in Mdr2-/- mice treated with GLP-2 (Figure 6A). In consistence with GLP-2 effects known from literature, the proliferation marker Ki67 was increased at mRNA and protein level (Figure 6B), suggesting that increased proliferation might be linked to increased Fgf15 secretion.
Figure 6.
GLP-2 treatment activates intestinal FGF15/19 expression. Real-time polymerase chain reaction was used to assess the intestinal mRNA expression of (A) Fgf15 and (B) Ki67. Fgf15 enzyme-linked immunosorbent assay (ELISA) was used to investigate Fgf15 levels in systemic blood of Mdr2-/- mice on GLP-2 treatment (A). Intestinal Fgf15 mRNA and protein expression as well as Ki67 mRNA levels were elevated in Mdr2-/- mice on GLP-2 treatment. Accordingly, Ki67 immunohistochemistry (×10 magnification) (B) showed an increase in Ki67-positive cells numbers in the intestine of Mdr2-/- mice treated with GLP-2. mRNA expression values were normalized against 18sRNA levels and are shown relative to the expression level in Mdr2-/- control animals. ∗Significant difference from Mdr2-/- control mice; P < .05. (C) Real-time polymerase chain reaction was used to assess the mRNA expression of FXR, FGF19, and KI67 in human-derived intestinal organoids treated with GLP-2. FGF19 ELISA was used to assess FGF19 protein concentration in cell culture supernatant. Gene expression of the aforementioned genes was significantly increased by GLP-2 treatment. mRNA expression values were normalized against 18sRNA levels and are shown relative to expression level in untreated control subjects. FGF19 levels were significantly increased in supernatant of organoids treated with 0.5 μM and 2.5 μM GLP-2. ∗Significant difference from untreated control cells; P < .05. (D) CellTiter-Glo Luminescent Cell Viability Assay was performed to assess cell proliferation in human-derived intestinal organoids. GLP-2 significantly increased cell proliferation independent of the used concentrations. ∗Significant difference from untreated control cells; P < .05.
GLP-2 Treatment Increases FXR-FGF19 Signaling Axis in Human Intestinal Organoids
To verify whether our findings in murine intestines translate to humans, human-derived intestinal organoids (expressing GLP-2r at a Ct value of 33.4 in average) were treated with GLP-2 over 24 hours. Gene expression profiling revealed a significant increase in FXR and FGF19 mRNA expression and fibroblast growth factor (FGF)19 levels in cell culture supernatant because of GLP-2 treatment (Figure 6C), indicating a direct effect of GLP-2 on the FXR-FGF19 signaling axis. In addition, GLP-2 treatment increases also proliferation of human intestinal organoids shown by increased Ki67 mRNA levels and by the proliferation assay (Figure 6C and D).
Discussion
In this study we demonstrated that the GLP-2 analogue teduglutide improves hepatic inflammation and fibrosis in the Mdr2-/- mouse model of sclerosing cholangitis. Our observations demonstrate that GLP-2 treatment may be a novel therapeutic approach to counteract hepatic inflammation and fibrosis in the context of cholestasis beyond its role as proproliferative agent serving primarily in the gut. Herein we provide evidence that GLP-2 receptor signaling found to be present in HSCs6,7 improves liver injury via increasing NR4a1 nuclear binding and subsequent antifibrotic function in HSCs. Moreover, GLP-2 exerts Cyp7a1 inhibitory effects via activating intestinal FXR-FGF15/19 signaling axis. Presence of GLP-2 in the system leads also to increased expression of Cyp2c70, thus promoting muricholic acid synthesis, thereby restoring a less toxic BA homeostasis.
NR4a1 was identified as a key target to control fibrogenesis10 and loss of GLP-2 signaling results in activation of HSCs.6 Our findings in whole liver and in primary HSCs isolated from Mdr2-/- mice subjected to GLP-2 treatment discovered antifibrotic function of the GLP-2 analogue teduglutide caused by activation of NR4a1 expression. Furthermore, electrophoretic mobility shift assay performed in the human HSC cell line LX-2, activated with Tgfβ revealed that GLP-2 treatment was able to restore Tgfβ-related reduction of NR4a1 nuclear binding further underlining the antifibrotic function of teduglutide in HSCs.
In hepatocytes, presence of GLP-2 led to changes in hepatic BA homeostasis resulting in a less toxic bile pool mainly consisting of muricholic acids. In line with increased muricholic acid levels, known to serve as FXR antagonist,16 hepatic FXR expression found in GLP-2-treated animals was reduced. In addition to the potential muricholic acid–related reduction we observed that treatment with GLP-2 reduced FXR nuclear binding in vitro. This finding may be of particular relevance to translate our findings into the human situation because levels of muricholic acids in human are neglectable. Of note, also the discrepancy that FXR expression is increased in piglets subjected to GLP-217 could be attributed to the fact that muricholic acids are mainly present in mice. Despite reduced FXR activity, Cyp7a1 (normally repressed by FXR) was also reduced because of GLP-2 treatment. This phenomenon might be explained by increased intestinal Fgf15/19 expression caused by GLP-2 treatment. Accordingly, FXR and FGF15/19 mRNA expression and FGF15/19 levels in mouse serum and cell culture supernatant were increased in GLP-2-treated Mdr2-/- mice and human-derived intestinal organoids, respectively. These finding argue for a direct GLP-2-related activation of the FXR-FGF19 signaling axis. Furthermore, increased proliferation was also observed, possibly also contributing to increased FGF19 expression/secretion because of increased mass of enterocytes based on GLP-2 treatment.18
Reduced intrahepatic FXR signaling could also explain the trend toward elevated intrahepatic total BA levels because it regulates hepatic BA uptake (Na+-taurocholate cotransporting polypeptide) and export (bile salt export pump). Reduced expression of Bsep, the main gatekeeper for hepatic BA export, could also be relevant for the finding of unchanged bile flow, which is in line with what has been shown previously.19 Moreover, reduced gallbladder contraction caused by the presence of GLP-2 (as reported previously19) may also be relevant for unchanged levels of hepatobiliary bile flow and increased levels of intrahepatic BAs.
Decreased hepatic FXR signaling in the context of cholestatic liver disease might be seen as critical because FXR agonists are currently exported as therapeutic strategies in PSC20 and have been shown to be an effective second-line treatment in primary biliary cholangitis.20 However, suppression of Cyp7a1 as main therapeutic target of FXR was also achieved by GLP-2 via activation of the FXR–FGF15/19 signaling axis. Thus, a combination of FXR agonists, such as obeticholic acid or cilofexor, and antifibrotic drugs, such as GLP-2 analogues, could be considered as future perspective in counteracting cholestatic liver diseases. Furthermore, the combination of FXR agonists and GLP-2 analogues may also be attractive for patients suffering from PSC accompanied by inflammatory bowel disease, because it has been shown that GLP-2 treatment has anti-inflammatory effects in a mouse model of inflammatory bowel disease.21
Because GLP-2 receptor expression was not found in murine cholangiocytes,22 the observation of reduced cholangiocyte activation might be an indirect effect of GLP-2 treatment. Activated HSCs secrete proinflammatory cytokines and chemokines,11 which were shown to contribute to activation of cholangiocytes,12 thus the GLP-2-related deceleration of HSC activation may, in addition to the beneficial changes in the BA profile, contribute to the reduced reactive cholangiocyte phenotype observed in Mdr2-/- mice subjected to GLP-2 treatment.
In conclusion, we demonstrated that activation of NR4a1 via teduglutide improves hepatic inflammation and fibrosis in the Mdr2-/- mouse model of sclerosing cholangitis. Additionally, teduglutide treatment modified the intrahepatic BA composition toward a more favorable hydrophilic direction, which at least in part, could also contribute to improvement of liver and bile duct injury and fibrosis.
Materials and Methods
Animals
Eight-week-old male FVB/N Mdr2/Abcb4−/− mice obtained from Jackson Laboratory (Bar Habor, ME) were injected intraperitoneally with the Glp-2 analogue teduglutide 0.05 mg/kg daily for 4 weeks. Animals were housed in a 12-hour light/dark house facility with water and standard chow diet (SSNIFF, Soest, Germany) ad libitum. The experimental procedures were approved by the Animal Ethics Committee of the Medical University of Vienna and the Federal Ministry of Science, Research and Economy (BMWFW-66.009/0315-WF/V/3b/2014) and performed according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
Hepatic Stellate Cell Isolation
HSCs were isolated from mouse livers as described before for rats with small modifications, adapting the protocol to mice.23 Briefly, livers of anesthetized mice were perfused first with 20 mL preperfusion buffer (HBSS, Thermo Fisher Scientific, Waltham, MA; + 1% heparin, Gilvasan Pharma), followed by 40 mL perfusion buffer A (HBSS + 0.001% DNAse + 0.015% collagenase A + 0.15% pronase, all Merck, Darmstadt, Germany). Digested livers were excised, minced, and further digested in 20 mL buffer B (HBSS + 0.0005% DNAse + 0.01% collagenase A + 0.04% pronase) in vitro at 37°C for 5 minutes. For cirrhotic livers, 30 % extra collagenase A was added to both buffers. The suspension was passed through a 100 μm cell strainer into 20 mL ice cold HBSS. After centrifugation, the nonparenchymal cells of 3 mice were pooled and subjected to a density gradient centrifugation using 11.5% OptiPrep (Merck) to purify HSCs, which were plated overnight into 6-well plates, before lysing them in TriFast (VWR, Radnor, PA) for RNA extraction.
Routine Serum Biochemistry and Histology
Serum biochemistry and histologic stainings (hematoxylin-eosin, Sirius red) were performed as described previously.24
Immunohistochemistry
Detection of hepatic MAC-2 and osteopontin was performed as described previously.25,26
Hepatic Bile Acid Analysis
Hepatic BA profiles were acquired using ultraperformance liquid chromatography tandem mass spectrometry as described previously.27,28
Messenger RNA Analysis and Polymerase Chain Reaction
RNA isolation from liver and primary HSCs, complementary DNA synthesis, and real-time polymerase chain reactions were performed as described previously.29 Oligonucleotide sequences are available on request.
Measurement of Bile Flow
Bile flow and hepatobiliary bicarbonate (HCO3-) measurements were performed as described previously.1 In brief, the common bile duct was ligated and the gallbladder was cannulated. After an equilibration period, bile was collected in preweighted tubes for 20 minutes. Bile flow was determined gravimetrically and normalized to liver weight. Biliary bicarbonate concentrations were measured in a routine laboratory and normalized to bile flow.
Cell Culture
LX-2 cells, an immortalized HSC line,30 kindly provided by Professor S.L. Friedman (Mount Sinai School of Medicine, New York, NY) were cultured with Dulbecco's modified Eagle medium (Life Technologies) supplemented with 5% non-heat-inactivated fetal bovine serum and 1% penicillin/streptomycin solution (EuroClone). Cells were treated with 2.5μM GLP-2 and Tgfβ for 36 hours. Immortalized human hepatocytes31 were cultured with Dulbecco's modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin/streptomycin solution (EuroClone). Cells were treated with 2.5 μM Glp-2 and GW4064 for o36 hours.
Electrophoretic Mobility Shift Assay
Cytosolic and nuclear extracts from LX-2 cells treated with 2.5 μM GLP-2 and Tgfβ and from immortalized human hepatocytes treated with 2.5 μM GLP-2 and GW5064 were obtained after lysis with 1% Nonidet P40 and specific buffer (25 mM 4-[2-hydroxyethyl]-1-piperazine ethanesulfonic acid [pH 7.9], 50 mM NaCl, 5% glycerol, and 0.5 mM dithiothreitol) containing protease inhibitors. Double-stranded NR4a1 response element were labeled with P32adenosine triphosphate, purified using the QIAquick Nucleotide Removal kit (Qiagen, Germany), and incubated with a binding buffer (10 mM tris-hydroxymethyl aminomethane [pH 8.0], 40 mM KCl, 0.05% Nonidet P40, 6% glycerol). Four micrograms of either cytosolic or nuclear extract were incubated with labeled probes and buffer for 10 minutes at room temperature and loaded on 4% acrylamide/bis-acrylamide gel. After the run, gel was dried for 1 hour at 60°C and transferred into a developing cassette (Biomax, Kodak) for overnight film exposure at –80°C.
Organoid Isolation from Human Biopsies and Organoid Cultures
Ileal biopsies from unaffected areas of the small intestine were taken in the operating room during small intestinal resections of patients with Crohn’s disease after informed consent and institutional review board approval (EK# 1915/2021) at the Division of Visceral Surgery, Department of General Surgery, Medical University of Vienna. On sample collection, they were immediately transported to the laboratory for processing. The biopsy was cut into pieces and incubated by rotating in chelating solution at 4°C for 30 minutes. Afterwards, glands were squeezed out of the tissues pieces by applying pressure on a glass slide placed on top. Glands were then collected for cell digestion 5 minutes in trypsin.32,33 Intestinal crypts were resuspended in Cultrex Basement Membrane Extract (R&D Systems) and seeded in 40–50 μL droplets into multiwell plates. Organoid culture medium containing growth factors and supplements was gently added onto Basement Membrane Extract drops and incubated at 37°C, 5% CO2, 95% air, and 95 % humidity.32,34 For the first 3 days of establishing new cultures, fungin (InvivoGen) was added to prevent fungal contamination during culture initiation and RhoK inhibitor is added to increase survival of cells and to promote expansion of stem cells in culture.32 Ileal organoids were passaged 1 time a week using a 0.05 % trypsin/EDTA solution33 (TrypLE Express, Gibco). During every passage, RhoK inhibitor was added but not required when medium was exchanged.32 The human intestinal organoids were treated with 0.5 μM and 2.5 μM GLP-2 for 24 hours. RNA was isolated using the Sigma-Aldrich RNA isolation kit (RTN350-1KT) according to the manufacturer’s protocol. Another batch of organoids treated with Glp-2 was used for the CellTiter-Glo Luminescent Cell Viability Assay (Promega, #G7570). The assay was performed according to the manufacturer’s protocol.
FGF15/19 Enzyme-Linked Immunosorbent Assay from Mouse Serum and Cell Culture Supernatant
FGF15 enzyme-linked immunosorbent assay was performed from serum obtained from venous blood of the animals. Enzyme-linked immunosorbent assay was performed according to manufacturer’s protocol (FineTest, EM0286). FGF19 enzyme-linked immunosorbent assay was performed from cell culture supernatant taken after 24-hour treatment of human intestinal organoids with 0.5 μM and 2.5 μM GLP-2. FGF19 enzyme-linked immunosorbent assay from Sigma-Aldrich (RAB0540-1KT) was performed according to the manufacturer’s protocol.
Statistical Analysis
Results were evaluated using GraphPad Prism 8.4.1. Statistical analysis was performed using 2-way analysis of variance. Data were reported as means of 5–7 animals per group ± standard deviation. In vitro experiments were performed 2 times with 3 biologic replicates (and 2 technical replicates). A P ≤ .05 was considered significant.
Acknowledgements
We dedicate this paper to the memory of our wonderful friend and colleague Prof Hanns-Ulrich Marschall (†), who always inspired us with his enthusiasm for science and his deep knowledge of bile acid metabolism.
CRediT Authorship Contributions
Claudia Daniela Fuchs, PhD (Conceptualization: Equal; Data curation: Lead; Writing – original draft: Lead)
Thierry Claudel, PhD (Data curation: Supporting; Methodology: Supporting; Writing – review & editing: Supporting)
Veronika Mlitz, PhD (Data curation: Supporting; Methodology: Supporting; Writing – review & editing: Supporting)
Alessandra Riva, PhD (Data curation: Supporting; Writing – review & editing: Supporting)
Moritz Menz, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Ksenia Brusilovskaya, MSc (Data curation: Supporting; Methodology: Supporting; Writing – review & editing: Supporting)
Felix Haller (Data curation: Supporting; Methodology: Supporting; Writing – review & editing: Supporting)
Maximilian Baumgartner, MD, PhD (Data curation: Supporting; Writing – review & editing: Supporting)
Philipp Königshofer, PhD (Data curation: Supporting; Methodology: Supporting; Writing – review & editing: Supporting)
Lukas W. Unger, MD (Data curation: Supporting; Writing – review & editing: Supporting)
Wilhelm Sjöland, MD (Data curation: Supporting; Methodology: Supporting; Writing – review & editing: Supporting)
Hubert Scharnagl, PhD (Data curation: Supporting; Methodology: Supporting; Writing – review & editing: Supporting)
Tatjana Stojakovic, MD (Data curation: Supporting; Methodology: Supporting; Writing – review & editing: Supporting)
Georg Busslinger, PhD (Writing – review & editing: Supporting)
Thomas Reiberger, MD (Writing – review & editing: Supporting)
Hanns-Ulrich Marschall, MD (Data curation: Supporting; Methodology: Supporting; Writing – review & editing: Supporting)
Michael Trauner, MD (Conceptualization: Equal; Funding acquisition: Lead; Supervision: Lead; Writing – review & editing: Lead)
Footnotes
Conflicts of interest These authors disclose the following: Claudia D. Fuchs received travel grants from Gilead, Roche, Falk, Merck, Vifor, AbbVie, and Boehringer Ingelheim. Thomas Reiberger received grant support from AbbVie, Boehringer-Ingelheim, Gilead, Gore, Intercept, MSD, Myr Pharmaceuticals, Philips Healthcare, Pliant, and Siemens; speaking honoraria from AbbVie, Gilead, Gore, Intercept, Roche, and MSD; consulting/advisory board fees from AbbVie, Bayer, Boehringer-Ingelheim, Gilead, Intercept, MSD, and Siemens; and travel support from AbbVie, Boehringer-Ingelheim, Gilead, and Roche. Thomas Reiberger, Ksenia Brusilovskaya, and Philipp Königshofer were cosupported by the Austrian Federal Ministry for Digital and Economic Affairs, the National Foundation for Research, Technology and Development, Boehringer Ingelheim, and the Christian Doppler Research Association. Hanns-Ulrich Marschall served as consultant for Albireo, Calliditas, Mirum, Zealand, and Intercept; received travel grants from Falk; and received lecture fees from Albireo and Intercept. Michael Trauner received speaker fees from BMS, Falk Foundation, Gilead, Intercept, Jannsen, Madrigal, MSD, and Roche; advised for AbbVie, Albireo, BiomX, Boehringer Ingelheim, Falk Pharma GmbH, Genfit, Gilead, Hightide, Intercept, Janssen, MSD, Novartis, Phenex, Pliant, Regulus, Siemens, and Shire; received travel grants from AbbVie, Falk, Gilead Intercept, and Jannsen; received research grants from Albireo, Alnylam, Cymabay, Falk, Gilead, Intercept, MSD, Takeda, and UltraGenyx; and is coinventor of patents on the medical use of norUDCA filed by the Medical Universities of Graz and Vienna. The remaining authors disclose no conflicts.
Funding Michael Trauner is supported by the Austrian Science Fund (FWF) (grant number F7310).
References
- 1.Fickert P., Fuchsbichler A., Wagner M., et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Fickert P., Pollheimer M.J., Beuers U., et al. Characterization of animal models for primary sclerosing cholangitis (PSC) J Hepatol. 2014;60:1290–1303. doi: 10.1016/j.jhep.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazaridis K.N., Strazzabosco M., Larusso N.F. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Nishio T., Hu R., Koyama Y., et al. Activated hepatic stellate cells and portal fibroblasts contribute to cholestatic liver fibrosis in MDR2 knockout mice. J Hepatol. 2019;71:573–585. doi: 10.1016/j.jhep.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Mederacke I., Hsu C.C., Troeger J.S., et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs S., Yusta B., Baggio L.L., Varin E.M., Matthews D., Drucker D.J. Loss of Glp2r signaling activates hepatic stellate cells and exacerbates diet-induced steatohepatitis in mice. JCI Insight. 2020;5 doi: 10.1172/jci.insight.136907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halpern K.B., Shenhav R., Matcovitch-Natan O., et al. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature. 2017;542:352–356. doi: 10.1038/nature21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Jamal N., Erdual E., Neunlist M., et al. Glugacon-like peptide-2: broad receptor expression, limited therapeutic effect on intestinal inflammation and novel role in liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2014;307:G274–285. doi: 10.1152/ajpgi.00389.2012. [DOI] [PubMed] [Google Scholar]
- 9.Drucker D.J. Glucagon-like peptide 2. J Clin Endocrinol Metab. 2001;86:1759–1764. doi: 10.1210/jcem.86.4.7386. [DOI] [PubMed] [Google Scholar]
- 10.Palumbo-Zerr K., Zerr P., Distler A., et al. Orphan nuclear receptor NR4A1 regulates transforming growth factor-beta signaling and fibrosis. Nat Med. 2015;21:150–158. doi: 10.1038/nm.3777. [DOI] [PubMed] [Google Scholar]
- 11.Marra F. Hepatic stellate cells and the regulation of liver inflammation. J Hepatol. 1999;31:1120–1130. doi: 10.1016/s0168-8278(99)80327-4. [DOI] [PubMed] [Google Scholar]
- 12.Pinto C., Giordano D.M., Maroni L., Marzioni M. Role of inflammation and proinflammatory cytokines in cholangiocyte pathophysiology. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1270–1278. doi: 10.1016/j.bbadis.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Jeppesen P.B., Gilroy R., Pertkiewicz M., Allard J.P., Messing B., O'Keefe S.J. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902–914. doi: 10.1136/gut.2010.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guicciardi M.E., Trussoni C.E., LaRusso N.F., Gores G.J. The spectrum of reactive cholangiocytes in primary sclerosing cholangitis. Hepatology. 2020;71:741–748. doi: 10.1002/hep.31067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Q., Xu J., Ge Y., Shi Y., Wang F., Zhu M. NR4A1 inhibits the epithelial-mesenchymal transition of hepatic stellate cells: involvement of TGF-beta-Smad2/3/4-ZEB signaling. Open Life Sci. 2022;17:447–454. doi: 10.1515/biol-2022-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayin S.I., Wahlstrom A., Felin J., et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Lim D.W., Wales P.W., Mi S., et al. Glucagon-like peptide-2 alters bile acid metabolism in parenteral nutrition–associated liver disease. JPEN J Parenter Enteral Nutr. 2016;40:22–35. doi: 10.1177/0148607115595596. [DOI] [PubMed] [Google Scholar]
- 18.Naimi R.M., Hvistendahl M.K., Poulsen S.S., et al. Effects of glepaglutide, a long-acting glucagon-like peptide-2 analog, on intestinal morphology and perfusion in patients with short bowel syndrome: findings from a randomized phase 2 trial. JPEN J Parenter Enteral Nutr. 2023;47:140–150. doi: 10.1002/jpen.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yusta B., Matthews D., Flock G.B., et al. Glucagon-like peptide-2 promotes gallbladder refilling via a TGR5-independent, GLP-2R-dependent pathway. Mol Metab. 2017;6:503–511. doi: 10.1016/j.molmet.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trauner M., Fuchs C.D. Novel therapeutic targets for cholestatic and fatty liver disease. Gut. 2022;71:194–209. doi: 10.1136/gutjnl-2021-324305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu J., Liu J., Huang T., et al. The protective and anti-inflammatory effects of a modified glucagon-like peptide-2 dimer in inflammatory bowel disease. Biochem Pharmacol. 2018;155:425–433. doi: 10.1016/j.bcp.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Yusta B., Matthews D., Koehler J.A., Pujadas G., Kaur K.D., Drucker D.J. Localization of glucagon-like peptide-2 receptor expression in the mouse. Endocrinology. 2019;160:1950–1963. doi: 10.1210/en.2019-00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Iglesias A., Ortega-Ribera M., Guixe-Muntet S., Gracia-Sancho J. 4 in 1: Antibody-free protocol for isolating the main hepatic cells from healthy and cirrhotic single rat livers. J Cell Mol Med. 2019;23:877–886. doi: 10.1111/jcmm.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baghdasaryan A., Fuchs C.D., Osterreicher C.H., et al. Inhibition of intestinal bile acid absorption improves cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis. J Hepatol. 2016;64:674–681. doi: 10.1016/j.jhep.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 25.Baghdasaryan A., Claudel T., Kosters A., et al. Curcumin improves sclerosing cholangitis in Mdr2-/- mice by inhibition of cholangiocyte inflammatory response and portal myofibroblast proliferation. Gut. 2010;59:521–530. doi: 10.1136/gut.2009.186528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fickert P., Stoger U., Fuchsbichler A., et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–536. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tremaroli V., Karlsson F., Werling M., et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22:228–238. doi: 10.1016/j.cmet.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haange S.B., Till A., Bergh P.O., et al. Ring Trial on quantitative assessment of bile acids reveals a method- and analyte-specific accuracy and reproducibility. Metabolites. 2022;12:583. doi: 10.3390/metabo12070583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner M., Fickert P., Zollner G., et al. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825–838. doi: 10.1016/s0016-5085(03)01068-0. [DOI] [PubMed] [Google Scholar]
- 30.Xu L., Hui A.Y., Albanis E., et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schippers I.J., Moshage H., Roelofsen H., et al. Immortalized human hepatocytes as a tool for the study of hepatocytic (de-)differentiation. Cell Biol Toxicol. 1997;13:375–386. doi: 10.1023/a:1007404028681. [DOI] [PubMed] [Google Scholar]
- 32.Busslinger G.A., Weusten B.L.A., Bogte A., Begthel H., Brosens L.A.A., Clevers H. Human gastrointestinal epithelia of the esophagus, stomach, and duodenum resolved at single-cell resolution. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108819. [DOI] [PubMed] [Google Scholar]
- 33.Bartfeld S., Clevers H. Organoids as model for infectious diseases: culture of human and murine stomach organoids and microinjection of Helicobacter pylori. J Vis Exp. 2015;105 doi: 10.3791/53359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pleguezuelos-Manzano C., Puschhof J., van den Brink S., Geurts V., Beumer J., Clevers H. Establishment and culture of human intestinal organoids derived from adult stem cells. Curr Protoc Immunol. 2020;130:e106. doi: 10.1002/cpim.106. [DOI] [PMC free article] [PubMed] [Google Scholar]