Summary
The risk of cancer recurrence after liver surgery mainly depends on tumour biology, but preclinical and clinical evidence suggests that the degree of perioperative liver injury plays a role in creating a favourable microenvironment for tumour cell engraftment or proliferation of dormant micro-metastases. Understanding the contribution of perioperative liver injury to tumour recurrence is imperative, as these pathways are potentially actionable. In this review, we examine the key mechanisms of perioperative liver injury, which comprise mechanical handling and surgical stress, ischaemia-reperfusion injury, and parenchymal loss leading to liver regeneration. We explore how these processes can trigger downstream cascades leading to the activation of the immune system and the pro-inflammatory response, cellular proliferation, angiogenesis, anti-apoptotic signals, and release of circulating tumour cells. Finally, we discuss the novel therapies under investigation to decrease ischaemia-reperfusion injury and increase regeneration after liver surgery, including pharmaceutical agents, inflow modulation, and machine perfusion.
Keywords: hepatocellular carcinoma, cholangiocarcinoma, colorectal liver metastasis, liver resection, liver transplantation, machine perfusion, organ preservation
Key points.
-
•
Tumour recurrence occurs frequently after both liver resection and liver transplantation.
-
•
While tumour biology and patient factors are major determinants of the risk of recurrence, perioperative factors such as mechanical handling, ischaemia-reperfusion injury and liver regeneration also play a role.
-
•
These perioperative factors are responsible for the activation of downstream pathways that promote the release of circulating tumour cells and stimulate cell proliferation in a pro-inflammatory microenvironment that favours tumour engraftment.
-
•
Mitochondrial dysfunction represents the starting point of the perioperative liver injury cascade.
-
•
Novel therapies targeting mitochondria or downstream pathways are under investigation to reduce perioperative liver injury, including pharmaceutical agents, inflow modulation, and machine perfusion.
-
•
Low quality evidence suggests that hypothermic oxygenated machine perfusion (HOPE) and ischaemia-free liver transplantation may reduce post-transplant cancer recurrence by diminishing perioperative liver injury.
Introduction
With a higher number of patients with new and more extensive malignancies accepted for liver transplantation, the field is currently focused on the implementation of novel interventions and surgical techniques to expand the pool of usable donor livers. However, a better understanding of key mechanisms is essential for the successful treatment of such complex patients and to avoid the most feared complications of graft loss, tumour recurrence and patient death.
Tumour recurrence after surgery is a common occurrence for both primary and secondary liver tumours, including hepatocellular carcinoma (HCC),1 cholangiocarcinoma,2 neuroendocrine tumours3 and colorectal liver metastases (CRLMs).4,5 Recurrence occurs in up to 70% of cases after liver resection, with the liver being the most common site.[6], [7], [8], [9] Liver transplantation for oncological indications is also burdened by significant post-transplant recurrence, up to 15% for HCC10 and over 80% for CRLMs.11,12 Unlike for resection, post-transplant recurrence is most commonly extrahepatic.7,13,14
Despite the different tumour recurrence patterns, the mechanisms and risk factors are similar and multifactorial. A major role is certainly played by the tumour per se, with risk factors including size and number of lesions,15,16 tumour markers (alpha-fetoprotein,15 carcinoembryonic antigen,17 carbohydrate antigen 19-9), degree of differentiation16 and vascular invasion.18 However, intraoperative and early postoperative risk factors are increasingly recognized.
For transplantation, these risk factors can be related to donor characteristics and liver quality19,20 (older age, degree of steatosis, cold ischaemia time [CIT],21,22 donation after cardiac death [DCD]), or to recipient risk factors (obesity,23 viral aetiology of underlying liver disease24), and post-transplant immunosuppression.25 For resections, a higher degree of liver manipulation during surgery has been linked to an increase in tumour recurrence and impaired liver regeneration, which involves various multifactorial pathways. For example, patient-based factors (i.e., age, comorbidities,26 previous chemotherapy27), liver parenchyma quality (i.e., cirrhosis,28 fibrosis, cholestasis, and steatosis29,30), and the resection procedure itself (extent of liver resection, blood loss, ischaemic damage) were all described to contribute to delayed liver regeneration and repair.
Both transplantation and resection require a certain degree of postoperative liver regeneration to avoid small for size syndrome and post-hepatectomy liver failure.31 Recently, evidence has emerged that tumour cell engraftment is also triggered by mechanisms linked to the process of liver resection and transplantation itself, with mitochondria being fundamental in both processes and key to immediate liver and patient recovery.
The link between mitochondrial alterations and cancer development has long been recognized.32 While it is still to be determined whether those alterations have a causal effect or are consequences of the carcinogenic process, it is established that mitochondria are essential for the survival, proliferation, and invasiveness of cancer cells through pathways related to energy production, survival in the stressful tumoral microenvironment, and avoidance of cell death. Similarly, hepatectomy and liver transplantation, by causing cellular damage through parenchymal transection, perfusion alterations and ischaemia-reperfusion injury (IRI), trigger a compensatory mitochondrial response in hepatocytes and other liver cells. This response leads to the activation of downstream pro-inflammatory cascades with consequences both at the local and systemic level, though the full spectrum of underlying mechanisms has yet to be completely appreciated.
In this review, we will discuss the main pathways involved in ischaemia-reperfusion injury and liver regeneration, and describe how these processes are ultimately linked to cancer cell engraftment and tumour recurrence. We will also summarize the novel therapies which are under investigation to target these mechanisms, as well as possible future directions for research.
Liver regeneration
The liver has a remarkable capacity for regeneration: depending on parenchymal quality, up to 75% of liver volume can be resected without inducing post-hepatectomy liver failure. This feature is at the basis of liver resection, as well as a requirement to avoid small for size syndrome after liver transplantation with small grafts, splits, and living donor liver transplant(ation) (LDLT). Advanced techniques of hypertrophy induction have been developed to enable hepatectomy in patients who would otherwise have an insufficient post-hepatectomy liver remnant volume, including portal vein embolization (PVE), hepatic vein embolization, two-staged hepatectomy (TSH), and associated liver partition and portal vein ligation for staged-hepatectomy (ALPPS). Recently, methods of auxiliary liver transplantation that exploit liver regeneration have been introduced, such as the RAPID33 and RAVAS34 technique. In these techniques, an auxiliary graft is implanted while maintaining the native liver completely or partially in place. Once the auxiliary graft has hypertrophied to a volume sufficient to sustain the metabolic needs of the body, a completion hepatectomy of the native liver is performed.
Liver regeneration implies an orderly sequence of events which starts 5 min after the start of parenchymal transection and shares many common pathways with wound healing, carcinogenesis, intrahepatic metastatic progression, and response to IRI. Indeed, some IRI is present at the start of liver regeneration.
Unlike at other sites, liver regeneration occurs through differentiation of fully differentiated cells for the most part,35 with the support of oval cells (liver stem cells). As previously mentioned, mitochondria are essential for liver regeneration, which requires an enormous amount of energy production to support both liver hypertrophy and liver function.36,37 Mitochondrial bioenergetics appear to be directly correlated with postoperative liver function after hepatectomy as well as post-hepatectomy volumetric increase.38
Factors that impair liver regeneration are similar to those increasing susceptibility to IRI (e.g. age, comorbidities, quality of the parenchyma), as well as factors related to the extent of resection and blood loss. The degree of IRI itself also influences the capacity to regenerate (i.e., the higher the IRI, the more difficult regeneration is going to be).
Liver regeneration can be divided into three phases: initiation (up to 5 h), proliferation (from 5 to 144 h), and termination.39 Liver volume is usually restored in about a week in mice35 and between 2 to 12 weeks in humans.35
Initiation
After resection, liver volume diminishes while the portal venous flow remains unaltered. This leads to a sudden increase in portal venous pressure, which triggers initiation of liver regeneration. The increase in portal venous pressure causes shear stress and mechanical tension, as well as an increased concentration of growth factors, cytokines and liposaccharide (LPS) from the portal circulation. Shear stress activates urokinase plasminogen activator,40 which upregulates matrix metalloproteinases (MMPs), causing matrix remodelling and activating hepatocyte growth factor (HGF).35,41
During the first hour of the initiation phase, the Wnt/β-catenin pathway is activated.42,43 β-catenin translocates to the cellular nucleus of hepatocytes, where it induces the transcription of Wnt target genes, responsible for cell proliferation.44 Simultaneously, the Notch pathway is stimulated, leading to the nuclear translocation of NICD (Notch intracellular domain).45 The Wnt/β-catenin and Notch pathways are implicated in the epithelial-to-mesenchymal transition (EMT),46 a process required for liver regeneration.
The increased concentration of LPS activates Kupffer cells through Toll-like receptor 4 (TLR4), C3a and C35 receptors. Similarly to what happens in response to IRI, this leads to NF-κB nuclear translocation with subsequent secretion of tumour necrosis factor-α (TNF-α) and interleukin (IL)-6.47,48 TNF-α further sustains the activation of the NF-κB pathway and the production of IL-6. IL-6 binds to the IL-6 receptor on hepatocytes and induces STAT3 (signal transducer and activator of transcription-3), which is responsible for hepatocyte proliferation through cyclin induction.47,49
Proliferation
The proliferation phase is characterized by cell proliferation in the first 72 h, followed by angiogenesis. Several growth factors are involved in the proliferation phase, including HGF,50 epidermal growth factor (EGF),51,52 and vascular endothelial growth factor (VEGF).53 HGF and EGF, together with other signalling molecules, activate the IL-6/JAK/STAT3, Ras/MAPK, and PI3-K/PDK1/Akt pathways, which contribute to cell proliferation and cell growth.54 VEGF is involved in angiogenesis and reconstitution of the sinusoids after cell proliferation.55
Termination
Regeneration is terminated when the liver reaches it pre-hepatectomy size, or a 2.5% liver mass to total body mass ratio is restored.56 Any further proliferation is resolved by apoptosis. The main known factors responsible for termination are TGF-β,[57], [58], [59] activin60,61 and the Hippo/Yap/Taz pathway,62 which act in a similar manner to limit hepatocyte proliferation.63
Ischaemia-reperfusion injury
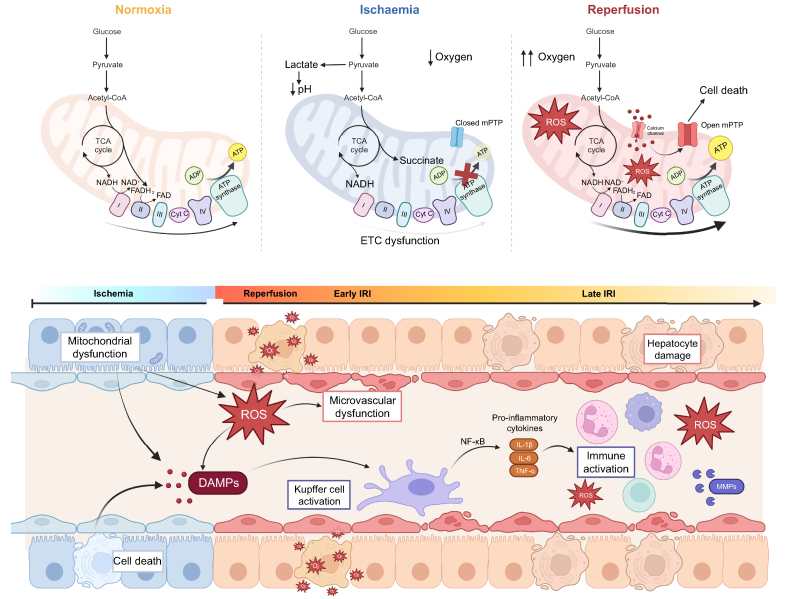
The full spectrum of IRI occurs whenever blood flow is suddenly restored after a period of ischaemia. During ischaemia, a lack of oxygen causes the mitochondrial electron transport chain to stop producing ATP, causing ATP depletion, acidosis and cell oedema.64 The components of the electron transport chain get saturated with electrons, as oxygen is not available for oxidative phosphorylation, and tricarboxylic acid (TCA) intermediates, including NADH and succinate, accumulate. This impaired mitochondrial respiration causes the well-described calcium release into the cytosol,65 which triggers the opening of the mitochondrial permeability transition pore and initiates cell death. When the liver circulation is re-established, the sudden availability of high succinate levels in hypoxic tissue leads to a massive production of reactive oxygen species (ROS) at complex I, which is mainly based on complex II dysfunction. Apoptosis/necrosis, endothelial dysfunction, and inflammation are the direct consequence (Fig. 1).66 Excessive oxidative stress therefore causes nuclear DNA damage, protein oxidation, and further mitochondrial permeability transition pore opening, leading to cell death67 and the subsequent release of damage-associated molecular patterns (DAMPs). DAMPs further push the establishment of an inflammatory microenvironment via activation of the inflammasome,66 the release of inflammatory cytokines and complement proteins, and immune cell activation.68,69 Both, the innate and the adaptive immune response play a major role in IRI pathways and link liver quality to acute liver rejection.70,71
Fig. 1.
Ischaemia-reperfusion injury.
(A) Mitochondrial metabolism during normoxia. During normoxia, the mitochondria exert their physiological functions, in which byproducts of the TCA cycle enter the ETC to be used as substrates for oxidative phosphorylation and the conversion of oxygen to ATP. (B) Mitochondrial metabolism during hypoxia. During hypoxia, the TCA continues operating as usual, while the ETC malfunctions due to the lack of oxygen. NADH and succinate accumulate, an important concept for reperfusion. (C) Mitochondrial metabolism during reperfusion. At reperfusion, the influx of oxygen overwhelms the capacity of the dysfunctional ETC to metabolize it, leading to production or ROS and opening of the mPTP. The open mPTP stimulates cell death. (D) Main mechanisms involved in IRI. Ischaemia is characterized by mitochondrial dysfunction: the cells are not able to function as they do in normoxic conditions, and cells begin to die. After reperfusion, the release of ROS leads to microvascular dysfunction, release of damage-associated molecular patterns, and Kupffer cell activation. In the late part of IRI, the immune system is activated with influx of neutrophils, macrophages and T cells, further exacerbating ROS and hepatocyte damage. ETC, electron transport chain; IRI, ischaemia-reperfusion injury; mPTP, mitochondrial permeability transition pore; ROS, reactive oxygen species; TCA, tricarboxylic acid.
Hepatic ischaemia-reperfusion injury - mechanism
In liver transplantation, IRI is an inevitable consequence of conventional organ procurement. For deceased donors, this process occurs during donor evaluation in the intensive care unit, where loss of brain function is often accompanied by some haemodynamic instability with associated periods of hypotension and hypoxia. The procurement itself involves a period of CIT for donation after brain death (DBD) or a period of warm ischaemia time (WIT) followed by CIT in DCD. While LDLT is commonly regarded as an almost ischaemia-free procedure, a certain IRI can also be observed in this setting due to parenchymal damage during liver transection, elective use of the Pringle manoeuvre, liver manipulation, and CIT during graft back-table preparation. The graft experiences further warm ischaemia during implantation, followed by reperfusion.
IRI occurs not only during transplantation, but during liver resection as well. As we have described for LDLT, liver surgery involves hypoxic and ischaemic damage to the liver parenchyma during vascular occlusion, manipulation, and transection.
Hepatic IRI can be divided into an acute phase, lasting approximately 3 h after reperfusion, and a late phase lasting up to 48 h after reperfusion. The acute phase is characterized by mitochondrial dysfunction causing oxidative stress in hepatocytes, Kupffer cells, and sinusoidal epithelial cells. Oxidative stress induces the release of HMGB1 (high-mobility group box 1),72 a DAMP that mediates Kupffer cell activation through a TLR4-mediated pathway. Activated Kupffer cells produce more ROS, which induce the degradation of IκB, the molecule that inhibits the translocation of NF-κB from the cytosol to the cellular nucleus.73,74 NF-κB is an important mediator of hepatic IRI.[75], [76], [77] Once freed, NF-κB enters the nucleus and upregulates the synthesis of mediators of the pro-inflammatory cytokine cascade, including IL-1β, IL-6, and TNF-α.74,78 Pro-inflammatory cytokines act on adhesion molecules and complement proteins to attract neutrophils and CD4+ T cells into the liver, which are the main mediators of the late phase of IRI. Neutrophils increase liver damage through secretion of ROS, MMPs, and proteases[79], [80], [81] causing hepatocyte necrosis. This liver damage can eventually evolve into chronic inflammation, causing long-term consequences such as liver fibrosis and, as we will further explore in the following sections, cancer recurrence.
Box 1. Critical commentary on cancer recurrence and intraoperative factors.

Hepatic ischaemia-reperfusion injury – clinical implications
IRI is not merely a time-dependent process, but its severity is also related to the characteristics of the liver graft and the recipient. Grafts from extended criteria donors are at an increased risk of more severe IRI after liver transplantation.82 Steatotic liver grafts exhibit comparatively impaired regenerative responses and reduced tolerance to IRI,83 which translates into higher rates of primary non function and early allograft dysfunction.84,85 DCD liver grafts have a higher rate of microthrombi formation86 and cell damage related to WIT.87 The age of the donor is also a risk factor for more severe IRI, with age >70 years being associated with smaller volumes, reduced overall flow,88 and less mitochondria.89 Small for size grafts90,91 are also a risk factor, a relevant consideration in LDLT. Intuitively, the combination of any of these risk factors further exacerbates IRI severity, as demonstrated by reports that prolonged CIT is especially deleterious in steatotic livers.92
Recipient risk factors for more severe IRI are mainly related to cirrhosis93 and subsequent altered coagulation profiles,94 which lead to a pro-thrombotic state with low platelets, altered platelet aggregation and altered production of coagulation factors.95
Similar considerations can be applied to the quality of the liver parenchyma during liver resection, where patients who are older and/or have a higher degree of steatosis or cirrhosis have less tolerance for vascular occlusion and IRI. IRI is also associated with impaired liver regeneration through mechanisms that will be explored in the following section.
Tumour recurrence after liver resection and transplantation
Tumour recurrence after liver surgery is likely linked to the mobilization of circulating tumour cells (CTCs), activation of dormant micrometastases, or a combination of the two. Both resection and transplantation cause major alterations in the local microenvironment and at the systemic level, with activation of pathways that favour the engraftment of residual tumour cells.
The link between cancer recurrence and perioperative acute liver injury is now well accepted. The main processes that tie these two events are mechanical handling, response to IRI, and regeneration (Fig. 2). Mechanical handling and squeezing of the liver during surgery may lead to the release of cancer cells into the systemic circulation. IRI, causing mitochondrial dysfunction, can activate many processes that characterize “oncogenic mitochondria”, together with an inflammatory microenvironment that promotes tumour cell mobilization and engraftment. Finally, in liver regeneration, the drivers of hepatocyte differentiation and hyperplasia may also become drivers of cancer recurrence, as well as promoting factors for the reactivation of dormant stem cells. Each method of transplantation and resection is related to a different degree of any of these three processes (Table 1).
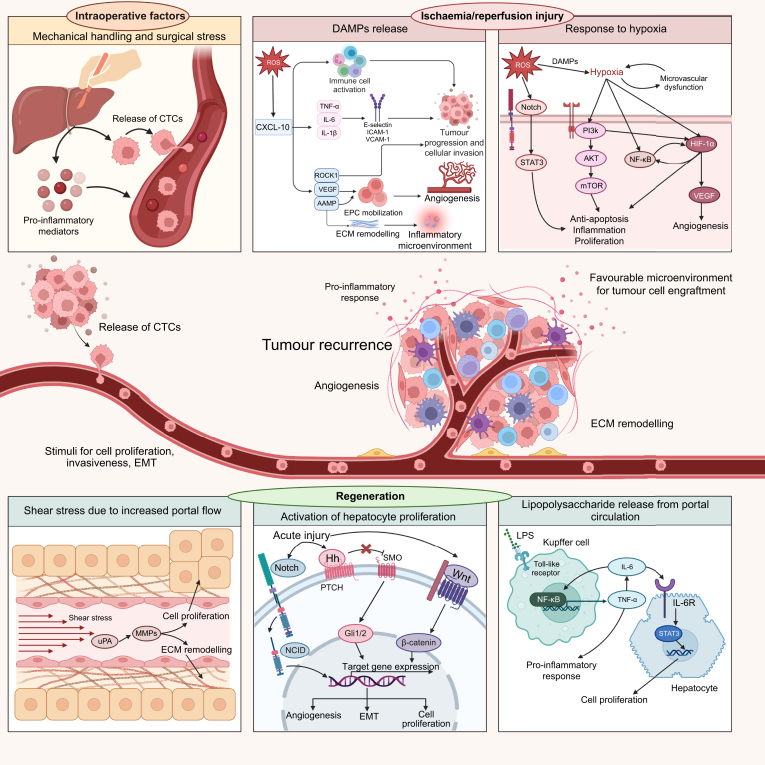
Fig. 2.
The link between intraoperative injury, ischaemia-reperfusion injury, and regeneration.
The contribution of perioperative liver injury to tumour recurrence involves the release of tumour cells into an environment that favours tumour engraftment, similarly to the seed and soil theory. Mechanical handling of the liver promotes the release of CTCs (the “seed”) and pro-inflammatory mediators into the bloodstream. Ischaemia-reperfusion injury causes necrosis and oxidative stress, leading to the release of DAMPs, cytokines, adhesion molecules, and exacerbation of hypoxic conditions. The downstream pathways activated as a consequence are responsible for tumour progression, angiogenesis, and creation of a pro-inflammatory microenvironment that favours tumour engraftment (the “soil”). Finally, regenerative pathways are triggered, stimulating cell proliferation, epithelial-to-mesenchymal transition, remodelling of the ECM, and angiogenesis, i.e. mechanisms linked to tumour growth and invasiveness. CTCs, circulating tumour cells; DAMPs, damage-associated molecular patterns; ECM, extracellular matrix; EPCs, endothelial progenitor cells; ROS, reactive oxygen species.
Table 1.
Level of perioperative liver injury seen with different resection and liver transplant techniques.
| Mechanical handling | Ischemia/reperfusion injury | Regeneration | |
|---|---|---|---|
| Liver transplantation | |||
| DBD – whole | ++ | + to +++† | + |
| DBD – split | ++ | ++ to +++ | ++ |
| DCD - whole | ++ | ++ to +++ | + |
| DCD - split | ++ | +++ | (+) |
| LDLT | ++ | + | ++ |
| Auxiliary | ++ | ++ | ++ |
| Liver resection | |||
| Minor liver resection | + | + | + |
| Major liver resection (one stage procedure) | +++ | ++ | +++ |
| Two-stage hepatectomy∗ ± PVE | +++ | ++ | ++ |
| ALPPS | +++ | ++ | +++ |
| Combine techniques | |||
| RAPID | +++ | ++ | ++ to +++ |
+ = minor; ++ = moderate; +++ = major.
ALPPS, associated liver partition and portal vein ligation for staged-hepatectomy; DBD, donation after brain death; DCD, donation after cardiac death; LDLT, living donor liver transplantation; LT, liver transplantation; PVE, portal vein embolization; RAPID, resection and partial liver segment 2–3 transplantation with delayed total hepatectomy (residual hepatectomy when transplanted segment 2-3 have grown sufficiently = the second stage); TSH, two-stage hepatectomy.
Traditional approach: portal vein ligation and partial liver resection (i.e., wedge resection, segmentectomy or left lateral sectionectomy resection).
In whole DBD grafts, the amount of IRI greatly depends on donor-associated risk factors. High-risk donors (i.e., donor on extracorporeal life support, steatotic liver, need for high pressor support, repeated episodes of hypotension before or during procurement) will trigger more IRI compared to low-risk donors (i.e., young and healthy donors, traumatic cause of death, clinically and medically stable before procurement).
Cancer recurrence and intraoperative factors (Box 1)
The surgical technique has an impact on the pro-inflammatory microenvironment that develops in the perioperative period. The intraoperative handling and “squeezing of the liver” can facilitate the mobilization of tumour cells, turning them into CTCs, as well as increasing the release of catecholamines, prostaglandins, and other inflammatory mediators.96 Catecholamines and prostaglandins have been linked to metastasis development. Norepinephrine and pro-inflammatory cytokines (IL-6, TNF-α) can stimulate VEGF-mediated angiogenesis.97 The increase in pro-inflammatory mediators and growth factors triggers cellular proliferation in an environment which favours motility and invasiveness. In a study comparing anaesthesia alone to laparotomy, appendectomy, and liver resection in a mouse model of colorectal cancer (CRC), concomitant lung metastases increased proportionally to the degree of surgical stress.98 In a retrospective analysis of 106 patients undergoing liver transplantation for HCC,99 59 patients were treated with PGE-1 (prostaglandin E1) to diminish surgical stress and IRI; 3- and 5-year recurrence-free survival was significantly higher in the PGE-1-treated group vs. the untreated group (87.9% and 85.7%, vs. 65.3% and 63.1%, respectively, p = 0.003). Further prospective clinical studies are required to establish this beneficial effect.
Techniques to minimize liver handling during resection have been developed, including the anterior approach to major hepatectomy. Unlike the conventional technique, which requires complete mobilization of the right hepatic lobe for a right hepatectomy, the anterior approach involves early inflow control, the completion of parenchymal transection, and outflow control before liver lobe mobilization.100 The anterior approach reduced liver handling-induced mobilization of tumour cells and has been associated with better survival outcomes, especially disease-free survival, after resection.100,101 This relates to the concept that surgeon’s experience may also be associated with cancer recurrence.102 A technically “cleaner” operation performed by an experienced surgeon may cause less tissue trauma, oedema, blood loss, release of inflammatory mediators and CTCs, all factors that play a role in postoperative cancer recurrence.103
The surgical act is not the only intraoperative event with the potential to promote cancer recurrence. Other factors, such as anaesthetic drugs, hypothermia, and transfusion of blood products also increase the inflammatory response.103 They increase secretion of catecholamines and cytokines, promote platelet activation, and contribute to the immunosuppressed status that is expected after surgery. These changes increase the chances that released CTCs will be able to escape immunological recognition and encounter a favourable microenvironment for homing and metastasis.
Cancer recurrence and regeneration - mechanism
The link between regeneration and recurrence is an intuitive one: regeneration involves cellular proliferation in an inflammatory microenvironment, the ideal conditions for tumour proliferation, and many pathways involved in liver regeneration play a role in cancer development, progression, and recurrence.
The interplay between regeneration and cancer recurrence occurs through regulation of cellular proliferation, EMT, angiogenesis, breakdown and remodelling of the extracellular matrix (ECM), and activation of CTCs (Fig. 3).
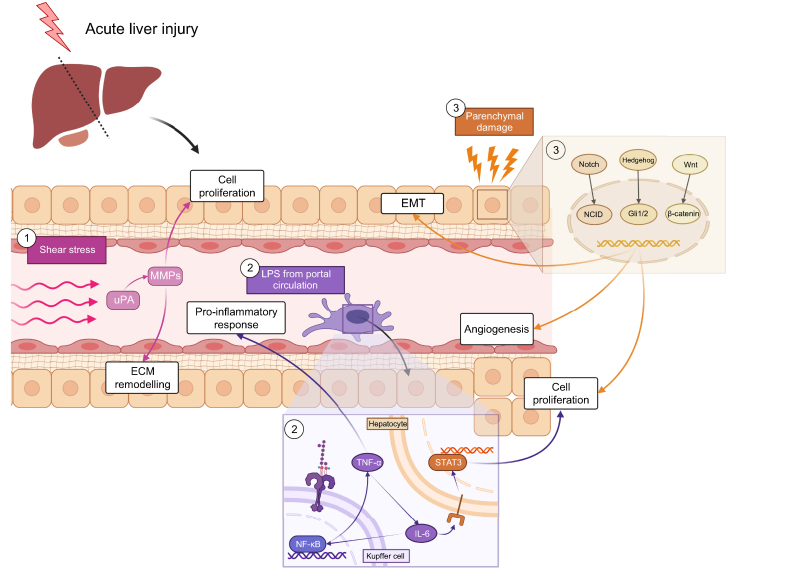
Fig. 3.
The link between liver regeneration and tumour recurrence.
Parenchymal loss and transplantation of a small-for-size graft are major triggers of liver regeneration. Liver regeneration is activated by the increase in portal flow per unit, which causes shear stress and an increase in the concentration of LPS. Shear stress-activated uroplasminogen and matrix metalloproteinases, responsible for cell proliferation through secretion of growth factors and remodelling of the ECM. The increase in LPS stimulates the nuclear localization of NF-κB, which can then exert its effect on the transcription of target genes. These genes modulate the secretion of pro-inflammatory cytokines such as TNF-α and IL-6, which is also responsible for STAT3 activation in hepatocytes, leading to their proliferation. Injury also affects hepatocytes, activating three pathways (Notch, Hedgehog, Wnt/β-catenin) which favour EMT, angiogenesis and cell proliferation. ECM, extracellular matrix; EMT, epithelial-to-mesenchymal transition; LPS, lipopolysaccharide.
The Wnt/β-catenin, Notch, and Hedgehog pathways,46,104 as well as the LPS-mediated release of pro-inflammatory cytokines (IL-6, IL1- β, TNF-α) are responsible for cellular proliferation and EMT.105 Notch signalling has an established role in HCC106 and metastatic CRC development;104 it promotes secretion of growth cytokines responsible for cell proliferation, EMT, and angiogenesis. Hedgehog is another regulator of cell proliferation and EMT, which is highly expressed in HCC.107 Finally, β-catenin is upregulated in up to 35% of HCCs.46 Mutations in APC (adenomatous polyposis coli), which occur in the majority of cases of CRC, cause irreversible activation of this pathway.108,109 β-catenin also regulates the Hippo/Yap/Taz pathway through Yap activation.110 The oncogenic role of Yap has been linked to HCC,111 CRC,112 and cholangiocarcinoma.113
Hippo/Yap/Taz is not the only pathway regulating termination that is involved in carcinogenesis. TGF-β can play both a pro- and an anti-tumorigenic role depending on the timing and the characteristics of the microenvironment. It exhibits anti-carcinogenic effects in the early stages of tumour development. This is followed by a shift, the mechanism of which is poorly understood, in which TGF-β becomes a key player in carcinogenesis and metastatic development.114,115 This phenomenon is well studied in metastatic CRC: the overexpression of TGF-β causes EMT, acquisition of stem cell-like features in cancer cells, and stromal transformation into a pro-carcinogenic microenvironment, driving metastatic development.116,117 Metastatic and invasive CRC cancer cells have higher levels of TGF-β compared to cancer cells in the primary tumour,118 and higher postoperative levels of TGF-β have been shown to correlate with higher risk of recurrence after curative resection.117
EMT and subsequent MET (mesenchymal-to-epithelial transition) after cancer homing are pivotal for metastatic development. CTCs can be both epithelial or mesenchymal cells, or exhibit features of both,119,120 as influenced by the Wnt/β-catenin, TGF-β, and Hedgehog pathways. Growth factors, such as HGF and EGF, also play a role in EMT.121
HGF and EGF are growth factors that sustain the proliferative phase of regeneration. EGF has been linked to proliferation of CRC metastases, and its receptor, EGFR, is a known therapeutic target. Cetuximab and panitumumab are anti-EGFR monoclonal antibodies used in metastatic CRC either in combination with chemotherapy or as monotherapy.122,123 HGF is a major driver of hepatocyte proliferation during liver regeneration. Postoperative HGF elevation is directly correlated with growth of the liver remnant after hepatectomy, ALPPS, and LDLT.[124], [125], [126] Higher postoperative plasmatic concentrations of HGF are also associated with higher recurrence of metastatic CRC,127 and overexpression of the HGF receptor c-Met has been linked to worsened prognosis and more aggressive features.128,129 HGF is also responsible for activating the STAT3 pathway,130 whose activation is increased post-hepatectomy and promotes cancer recurrence, as well as enhancing the secretion and activity of MMPs.131,132
MMPs, together with urokinase plasminogen activator and other proteases, are responsible for ECM remodelling,133 a crucial process in liver regeneration, that promotes both migration and adhesion of cells during cellular proliferation. Likewise, ECM breakdown and remodelling may promote the shedding of tumour cells into the circulation, with subsequent homing either in the liver remnant or in distant organs. Of note, MMPs are also important mediators of tissue injury after IRI133 by promoting apoptosis, endothelial dysfunction, leukocyte migration into the liver, and sustaining the secretion of pro-inflammatory molecules. MMPs also play a role in angiogenesis, together with Notch134,135 and VEGF.136 VEGF receptors are the targets of several anti-angiogenic therapeutics used in advanced HCC,136 including the tyrosine kinase inhibitors sorafenib, regorafenib, sunitinib and lenvatinib, and the monoclonal antibody ramucirumab. Cabozantinib, a second-line tyrosine kinase inhibitor targets both VEGFR2 and c-Met (the HGF receptor).137 Anti-angiogenic therapies are also used in both HCC138 and metastatic CRC,139 with the most common and well-studied agent being the monoclonal antibody bevacizumab.
In summary, liver regeneration requires extensive cellular activation and proliferation in a microenvironment that favours cancer development and metastases thanks to ECM remodelling, angiogenesis, and inflammation.
Cancer recurrence and regeneration – clinical implications (Box 2)
Box 2. Critical commentary on tumour recurrence and liver regeneration.

Animal models have clearly demonstrated the relationship between post-hepatectomy regeneration and tumour growth. In mouse models of CRC with extrahepatic metastases, the post-hepatectomy growth of extrahepatic lesions exceeded that of the liver remnant.140,141 This effect was observed after major resections, which required regeneration to maintain liver function, but not after minor resections, when the need for subsequent regeneration was limited.141,142
The accelerated growth of cancer tissue compared to the liver has implications for all procedures that require liver hypertrophy with the bulk of the tumour still in place, such as TSH, PVE, ALPPS and auxiliary liver transplantation. These techniques operate on the fine balance between adequate liver hypertrophy and risk of dropout due to tumour progression. The timeframe needed for liver remnant hypertrophy is different with each technique, with TSH requiring up to 3 months, PVE between 4 and 6 weeks, and ALPPS 2 to 3 weeks. This difference in timing is reflected by dropout rates. The dropout rate due to tumour progression is between 28-38%143,144 after the first stage of TSH and around 30% after PVE,145 while it is much lower after the first stage of ALPPS.146 The latter, in particular, is mostly driven by postoperative complications rather than tumour progression.147,148
Whether this interim tumour growth has long-term prognostic implications once the second stage is performed is still to be determined. Growth of liver metastases and even development of new lesions after PVE have been documented both in animal models149 and humans.150 Despite inconsistent reports on the long-term outcomes of PVE, a recent meta-analysis has shown that, once definitive resection is performed, PVE has no detrimental effect on intrahepatic recurrence or overall survival.151
Similar questions can be posed after partial and auxiliary liver transplantation for oncological indications. Patients with cancer are considered ideal candidates for these grafts, as they usually have a preserved liver function and can support the necessary regeneration process better than patients undergoing transplantation for end-stage liver disease. LDLT is often performed with small-for-size grafts, and always requires at least some level of liver regeneration. The evidence on whether HCC recurrence is higher after LDLT compared to deceased donor transplantation is inconclusive, with conflicting reports.152,153 This likely reflects the multifactorial nature of cancer recurrence as well as the limitations of comparing two interventions that are not necessarily offered to the same patient populations.154 The contribution of small-for-size liver grafts to cancer recurrence is controversial, with some retrospective analyses reporting higher recurrence,155 while others report no difference.156,157 The experience on auxiliary transplantation is still scarce, but the risk of tumour progression between the two stages will be a matter of interest as this technique gains traction.
Underlying mechanisms linking cancer recurrence and ischaemia-reperfusion injury
Tumorigenesis and IRI share several downstream pathways and a similar microenvironment, thus it is unsurprising that IRI increases the risk of cancer recurrence. The degree of IRI is clearly linked to the duration of hypoxia and vascular occlusion, but there are other exacerbating factors related to the quality of the liver parenchyma, e.g. age of the patient or donor and the degree of steatosis.20 Higher IRI levels are associated with more tumour recurrence and a more invasive phenotype of cancer cells.
The main drivers of IRI are cell death and mitochondrial dysfunction, with subsequent production of ROS and oxidative stress. As shown in Fig. 4, oxidative stress leads to release of DAMPs, secretion of inflammatory cytokines, and subsequent microvascular dysfunction.
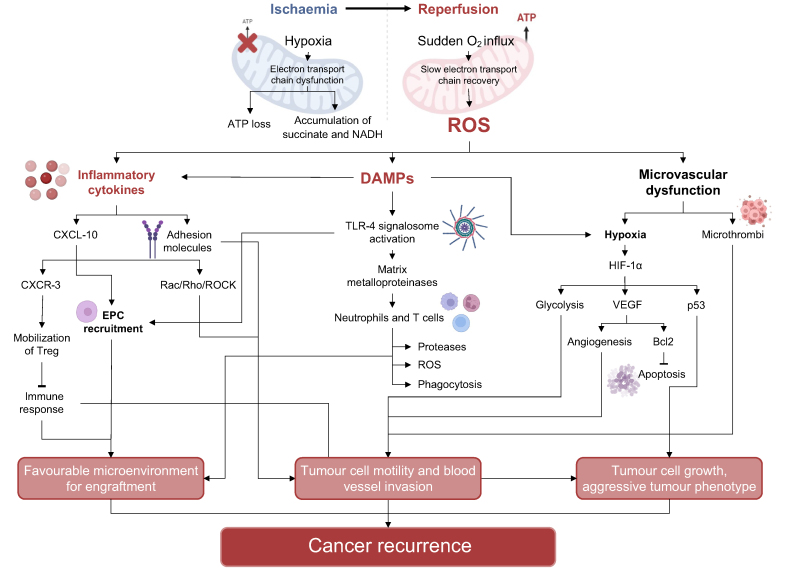
Fig. 4.
The link between ischaemia-reperfusion injury and tumour recurrence.
During ischaemia, dysfunction in the ETC caused by lack of oxygen leads to ATP loss and accumulation of TCA cycle products, such as succinate and NADH. Subsequent reperfusion involves the sudden influx of high quantities of oxygen; the ETC cannot recover fast enough to deal with this oxygen influx, causing the production of a massive amount of ROS. Oxidative stress triggers the release of DAMPs and inflammatory cytokines, and microvascular dysfunction. DAMPs and inflammatory cytokines are responsible for recruitment of endothelial progenitor cells, pathways stimulating cell differentiation, and matrix remodelling through metalloproteinases. They also attract neutrophils and T cells, further stimulating ROS and proteases and contributing to the development of a favourable microenvironment for engraftment. Microvascular dysfunction, ROS and DAMPs further exacerbate hypoxia, leading to the activation of HIF-1α, which stimulates angiogenesis, anti-apoptotic signals, cell growth and glycolysis. As a consequence, tumour cells receive stimuli to proliferate and invade the local and systemic circulation with an aggressive phenotype. DAMPs, damage-associated molecular patterns; EPCs, endothelial progenitor cells; ETC, electron transport chain; ROS, reactive oxygen species.
DAMPs activate C-X-C motif chemokine ligand 10 (CXCL10) through a TLR4-mediated mechanism.158 CXCL10 is a major driver of the pro-inflammatory response to IRI with implications for cancer recurrence. It attracts macrophages, monocytes, regulatory B cells and regulatory T cells to the liver, which have been shown to promote tumour progression and cancer cell invasion.[159], [160], [161] CXCL10 regulates the secretion of inflammatory cytokines, including TNF-α, IL-6 and IL-1β.158 These inflammatory cytokines increase the expression of adhesion molecules (e.g. E-selectin, ICAM-1, VCAM-1), which act as mediators of tumour growth and engraftment. CXCL10 also has a direct effect on the aggressive phenotype of tumour cells, increasing cell motility and invasiveness90 by upregulating ROCK1 (Rho-associated protein kinase-1), VEGF and MMP. Overexpression of ROCK, which regulates cellular motility, proliferation, and apoptosis, leads to infiltrative tumour growth and metastasis.162 VEGF and MMP play a role in recruiting endothelial progenitor cells163,164 from the bone marrow, stimulating neoangiogenesis and creating favourable conditions for recurrence. Furthermore, the expression of MMPs, particularly MMP-9, is pivotal for ECM degradation and remodelling,165 a key step in the recruitment and transmigration of neutrophils and other immune cells into the liver after IRI. Neutrophils and immune cells further aggravate liver damage and ROS production, contributing to a favourable microenvironment for cancer proliferation and recurrence. It must be noted, however, that recent evidence shows that the role of neutrophils in liver transplant IRI is not merely pro-inflammatory.80 Specific subpopulations of neutrophils have been demonstrated to promote clearance of inflammation, revascularization and tissue repair.166
The hypoxic conditions during IRI cause endothelial cell swelling and unbalanced vasoconstriction, leading to microvascular dysfunction. Microvascular dysfunction, together with DAMP release, exacerbates tissue hypoxia, leading to hypoxia-inducible factor-1α (HIF-1α) activation. After nuclear translocation, HIF-1α upregulates the expression of genes related to angiogenesis, glycolytic switch, cell proliferation, and apoptosis,167,168 which can stimulate tumour growth and metastatic potential. The role of HIF-1α in the regulation of hypoxia-induced apoptosis and cell death is controversial. During hypoxia, HIF-1α promotes hypoxia-induced apoptosis via caspase activation and mitochondrial cytochrome c release;169 this happens through different pathways, including p53 stabilization and induction of proteins, such as BNIP3 (Bcl-2 interacting protein 3), that downregulate the anti-apoptotic Bcl-2 (B-cell lymphoma 2). However, HIF-1α may also have anti-apoptotic effects, e.g. by promoting the transcription of genes that inhibit apoptosis, such as IAP2 (inhibitor of apoptosis protein 2). The target genes upregulated by HIF-1α in any given cell seem to depend on cell type and on the microenvironment. HIF-1α also plays a role in the regulation of mitochondrial metabolism. At low oxygen levels, HIF-1α decreases the activity of the TCA cycle,170 thus decreasing oxygen consumption, and stimulates glycolysis and autophagy, mechanisms required for cell survival in hypoxic conditions and often present in cancer cells. The presence of HIF-1α in several cancers has been linked to clonal selection of hypoxia-resistant cancer cells,171 and its expression is associated with resistance to treatment. Increased levels of HIF-1α have been linked to poorer prognosis in HCC172 and post-surgical recurrence in CRLMs.173 Indeed, hypoxia-induced HIF-1α promotes EMT in CRC cells.174 In addition to HIF-1α expression, hypoxia may also prevent apoptosis through induction of the PI3K/Akt signalling pathway, which regulates cell survival and proliferation,175 as well as NF-κB176 and STAT3.177 Notch, whose instigation is mediated by ROS, also stimulates STAT3 activation in a mechanism common to both IRI and regeneration, promoting hepatocyte proliferation.178
Cancer recurrence and ischaemia-reperfusion injury – clinical and preclinical evidence and implications (Box 3)
Box 3. Critical commentary on cancer recurrence and ischaemia-reperfusion injury.

Evidence that IRI stimulates tumour growth and recurrence comes from animal models, as well as from post-hepatectomy and post-transplantation observations in humans.
Preclinical evidence
In animal models, IRI exerts an effect on both primary and secondary tumours. In a study by Orci et al.,179 the increase in LPS as a consequence of reperfusion after vascular clamping was responsible for higher HCC burden through a TLR4-mediated pathway, in a mechanism shared by both IRI and liver regeneration, and activated during both liver resection with the Pringle manoeuvre and liver transplantation. Oldani et al.180 showed that HCC growth was higher after transplantation with an ischaemic graft, while this effect was reversed if the graft was reperfused prior to implantation. The duration of ischaemia correlated with HCC growth. In a study by the same group,181 HCC recurrence was worse after IRI in mice with severe steatosis compared to controls, and expression of inflammatory genes encoding for IL-6, TNF-α, HIF-1α and E-selectin was upregulated. Similarly, Yang et al.182 found that IRI induces HCC recurrence in fatty livers by inducing EMT and MMP activation through a PI3K/AKT/NF-κB-mediated pathway. These findings highlight the pivotal role of parenchymal quality on IRI tolerance.
Similar evidence has been found for CRLMs. van der Bilt et al.183 reported the accelerated growth of CRLMs after vascular clamping, which was associated with areas of hepatic necrosis in an inflammatory microenvironment. This effect, observed with prolonged vascular clamping, was not present with intermittent vascular clamping. The same group184 found that IRI-accelerated CRLM growth was exacerbated by increasing ischaemia time, and further increased with age and steatosis. Different groups have reported that targeting byproducts of the cascades initiated by IRI, such as MMP-9,185 TNF-α,186,187 E-selectin,188 and circulating progenitor cells,163 lowers the risk of CRLM growth and recurrence.
Similar mechanisms to those identified in animal models have also been found in cases of post-hepatectomy and post-transplantation recurrence in patients.
Clinical evidence – liver resection
The main source of IRI in liver resection is vascular clamping. Vascular clamping through the Pringle manoeuvre refers to the selective occlusion of hepatic inflow from the liver hilum. The Pringle manoeuvre is commonly performed in liver surgery to reduce blood loss during parenchymal transection, in a fine balance between blood loss and IRI. Several authors have reported that an intermittent Pringle manoeuvre (i.e. short periods of occlusion with periods of reperfusion in between) does not increase the risk of recurrence of HCC[189], [190], [191] or CRLMs.191,192 In a post hoc analysis of two randomized-controlled trials193 on liver resection for HCC with vs. without an intermittent Pringle manoeuvre (88 patients per arm), better overall survival was observed after an intermittent Pringle manoeuvre in patients with cirrhosis. Continuous, prolonged vascular clamping has instead been associated with worsened prognosis. In a retrospective analysis of 2,368 patients with HCC,194 a Pringle manoeuvre lasting >15 min was associated with decreased overall and recurrence-free survival compared to one lasting <15 min or no Pringle manoeuvre. Nijkamp et al.195 found that time to recurrence of CRLMs after resection was significantly shorter in patients with severe intraoperative ischaemia (i.e. continuous clamping for >20 min, or more than 3 cycles of ≥15 min) compared to patients with no or minor ischaemia. Yamashita et al.,196 in a retrospective analysis of 202 patients undergoing resection for CRLMs, reported worsened recurrence-free survival and cancer-specific survival in patients with higher remnant liver ischaemia. Similarly, in an analysis of 328 patients undergoing resection for HCC,197 severe remnant liver ischaemia was associated with early recurrence within 6 and 12 months, and severe remnant ischaemia was a risk factor for diminished overall and recurrence-free survival.
Clinical evidence – liver transplantation
The liver graft experiences ischaemic damage at different timepoints during procurement, storage, and implantation, with subsequent reperfusion injury once blood flow is restored inside the recipient. During the past decade, several retrospective studies have explored the correlation between the degree of IRI and post-transplant recurrence.
Factors influencing IRI and response to IRI in liver transplant are type of donor, quality of the graft, and comorbidities of the recipient. Regarding quality of the graft, we know for preclinical models that steatotic grafts have a lower tolerance for IRI. Severe steatosis of the graft has been associated with increased risk of tumour recurrence.20,198 The impact of donor age on post-transplant recurrence is controversial,199,200 but large analyses of the United Network for Organ Sharing (UNOS) database have found an association between older age and recurrence. Similarly, both increased body mass index23,201 and older age202 of the recipient have been associated with increased HCC recurrence and poorer oncological outcomes.
While donor and recipient characteristics certainly play a role, the major driver of IRI is ischaemic time. Ischaemic damage varies greatly between donor types, with LDLT being associated with lower ischaemic times, DCDs being associated with the worst ischaemic damage due to WIT and procurement dynamics, and DBDs being somewhere in between.
As we will further describe in the next section, some authors have associated LDLT with increased HCC recurrence, however, this is thought to be related to the regenerative process that follows transplantation with a partial graft and a possible lack of patient selection due to reduced time on the waitlist and lower risk of waitlist dropout. No evidence on the role of ischaemia time in LDLT is currently available, nor would it be easy to dissect the contribution of IRI vs. regeneration.
Evidence from retrospective studies shows that ischaemia time leads to increased HCC recurrence after transplant with DBD grafts. Kornberg et al.203 found that CIT, WIT, and total ischaemia time were all prolonged in patients who developed HCC recurrence. WIT >50 min was independently associated with HCC recurrence at multivariate analysis, together with FDG (fluorodeoxyglucose)-avid HCC, alpha-fetoprotein levels of >400 IU/ml, and Milan-out status. Similar results were reported by Nagai et al.21 In their analysis on 391 patients undergoing transplant for HCC, CIT of >10 h and WIT of >50 min were associated with increased recurrence, particularly within 1 year post-transplant. On multivariate analysis, both CIT and WIT remained significantly associated with recurrence, as did alpha-fetoprotein >200 ng/ml, Milan-out status, poor differentiation, and micro- and macrovascular invasion. Both Kornberg et al. and Nagai et al. identified a correlation between peak aspartate aminotransferase (AST) and prolonged ischaemia time. In a study by Grat et al., AST was used as a surrogate for IRI, and patients with a peak AST ≥1,896 U/L were found to have significantly lower recurrence-free survival. This correlation was more pronounced in patients who were classified as Milan-in, less so in patients who were Milan-out but Up-to-7-in, and not observed in patients beyond Up-to-7 criteria. This is in contrast with the findings by Kornberg et al. and Nagai et al., who found the strongest correlation in patients with tumour-related prognostic features, FDG-PET avidity and microvascular invasion, respectively.
DCD grafts are considered extended criteria grafts that are associated with a higher risk of impaired outcomes compared to DBD donor livers; however, post-transplant outcomes with optimal DCD grafts (i.e., young donors, short warm and cold ischaemia times) are comparable, if not superior to results seen with extended criteria DBDs.204 The most recent analysis of the UNOS database comparing DBD and DCD liver transplants in HCC recipients205 found no difference in HCC recurrence. While no survival difference was observed between DCD and DBD recipients with low risk for HCC recurrence, liver transplantation with DCD grafts was associated with lower recipient survival rates in patients at high risk of HCC recurrence.205 This further highlights the interplay between IRI levels, perioperative factors, and tumour biology. A previous analysis of the UNOS database20 had found that the risk of HCC recurrence was increased in DCD grafts with prolonged WIT. Smaller single-centre studies[206], [207], [208] have shown that good quality DCD grafts can lead to similar recurrence rates as seen with DBD grafts.
Therapeutic options (Box 4)
Box 4. Critical commentary on therapeutic options to mitigate perioperative factors for recurrence.

Pharmacological interventions
Several therapeutic agents have been shown, in animal models, to reduce IRI, augment regeneration, or both.209 The most common mechanisms of action are either exerted on mitochondria, which represent the beginning of the IRI/regeneration cascade, or on downstream pathways, mainly regulation of the pro-inflammatory response and reduction of apoptosis. Despite promising results in the preclinical setting, translation into clinical practice has been limited. Some clinical evidence exists on the benefits of S-adenosyl-L-methionine210 and prostacyclin,211 but larger and more robust studies would be needed to support their use in standard practice.
Stem cells & extracellular vesicles
Mesenchymal stem cells (MSCs) and stem cell-derived extracellular vesicles (EVs) are novel strategies being investigated to modulate liver injury. The delivery of MSCs and MSC-derived EVs can inhibit inflammation, reduce apoptosis, necrosis and oxidative stress, and increase hepatocyte proliferation.212 Animal studies with mouse, rat and porcine models of IRI after hepatectomy and liver transplantation have shown that MSCs and MSC-derived EVs can improve liver regeneration and postoperative liver function,213 reduce markers of IRI214 and cellular death, and enhance ATP regeneration.215 Clinical evidence on cirrhosis demonstrates improved liver function after treatment with MSCs.216
Ischaemic conditioning
Ischaemic conditioning (IC) employs short periods of ischaemia followed by reperfusion to prime the target tissues, ameliorate their overall response and protect them from IRI. IC can be performed before (ischaemic pre-conditioning [IPC]), during (ischemic peri-conditioning), or after prolonged ischaemia (ischaemic post-conditioning). It can then be applied directly to the target organ (e.g. the Pringle manoeuvre during hepatic resection) or indirectly (remote conditioning, e.g. putting a tourniquet around the patient’s arm). The results of IC on IRI in preclinical cardiac and hepatic models have been encouraging. However, evidence from human studies is inconclusive, and most trials do not report long-term outcomes. Recent meta-analyses of RCTs failed to identify convincing evidence that remote IPC reduces IRI during liver resection217 or transplantation,218 while direct IPC has been shown to lower postoperative AST levels,218 a possible surrogate of IRI, after transplantation. Well-designed studies with long-term endpoints are needed to confirm these preliminary results.
Machine perfusion
The standard graft preservation technique after procurement is static cold storage (SCS), i.e. an ice box. Recently, advances have been made in the field of organ preservation, including different types of machine perfusion. Machine perfusion represents a fascinating field, as it can potentially improve different aspects of the transplant process, from logistics, graft utilization, and viability assessment, to organ reconditioning and remodelling through the delivery of therapeutics during prolonged perfusion, e.g. defatting agents, senolytics, cell-based therapies, and gene modulation.
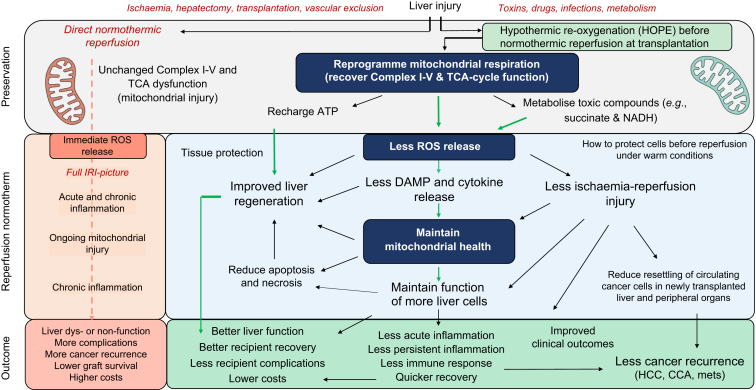
The two main machine perfusion approaches are hypothermic oxygenated perfusion (HOPE) and normothermic machine perfusion (NMP). Preservation with HOPE involves perfusion of the explanted graft, through either the portal vein alone or both the portal vein and the artery (dual HOPE), with a hypothermic solution containing a high concentration of oxygen (>60 kPa). Four RCTs are currently available and have shown that HOPE reduces early allograft dysfunction and leads to less overall and biliary complications, resulting in better post-transplant graft survival and lower retransplantation rates, compared to SCS in extended criteria donor livers.[219], [220], [221] By restoring oxygen in a hypothermic environment, HOPE reverses hypoxia and mitochondrial dysfunction and thus downregulates all the cascades that derive from them. The mitochondrial electron transport chain, in particular Complex I, resumes its activities, using oxygen for oxidative phosphorylation and ATP production. The byproducts of the TCA cycle that accumulate during hypoxia, which are responsible for massive ROS production during reperfusion, get degraded. This has a great effect in dampening IRI and all its consequences, as shown in Fig. 5. HOPE can also promote liver regeneration. By diminishing mitochondrial dysfunction, improving oxygenation through increased perfusion, and reducing inflammation, it allows mitochondria to provide sufficient energy to sustain the demands of regeneration.222,223
Fig. 5.
How HOPE dampens liver injury during transplantation, leading to improved outcomes and reduced cancer recurrence.
In case of direct normothermic reperfusion after static cold storage, the mitochondrial dysfunction caused by ischemia is not reversed, causing the full picture of IRI to develop as previously specified. Conversely, HOPE restores mitochondrial respiration and oxidative phosphorylation, leading to reduced release of reactive oxygen species. Less oxidative stress translated into less activation of downstream pathways and less inflammation, resulting in better postoperative liver function and better overall outcomes. CCA, cholangiocarcinoma; HCC, hepatocellular carcinoma; HOPE, hypothermic oxygenated machine perfusion; ROS, reactive oxygen species.
Unlike HOPE, NMP is performed by perfusing the graft with a red blood cell-based solution at 35.5–37.5 °C. Since the liver metabolism is restored through perfusion with oxygen and nutrients, NMP allows for viability assessment through the measurement of IRI injury levels and functional parameters. A series of investigational parameters (i.e., bile production, lactate clearance, perfusate transaminases, pH, electrolytes, glucose, LDH, bicarbonate) were explored in a number of smaller case series lacking validation. While a standardized set of viability criteria has still not been established, NMP has proven successful in enabling the evaluation of the transplantability of grafts that would have been discarded based on clinical assessment alone.224 NMP has been shown to diminish early allograft dysfunction and ischaemic biliary complications compared to SCS.225,226 While NMP may also exert a dampening effect on IRI, its mechanisms of action are downstream relative to those of HOPE and mostly linked to a reduction in pro-inflammatory cytokines, neutrophil infiltration and cell death during the late IRI phase, when a major part of graft injury has already occurred. No evidence exists so far on the effect of endischaemic NMP on cancer recurrence.
However, the significant reduction of IRI through the concept of “ischaemia-free” liver transplantation (IFLT), presented first by a pioneering group from China, could be of interest.227 For successful IFLT, the perfusion device is connected to the vessels during donor liver procurement, where the graft undergoes NMP with continuous perfusion during procurement, ex situ liver transfer to the recipient operating theatre and transplantation. Once the implantation is completed with revascularization of the entire liver graft, NMP is stopped.228 The same group performed a retrospective propensity score matched analysis,229 wherein 30 patients with HCC underwent IFLT and were compared to 85 patients undergoing liver transplantation after standard SCS. After matching, the two cohorts were similar with regards to demographic characteristics, pre-transplant AFP, tumours within Milan criteria, tumour differentiation, microvascular invasion, and pre-transplant treatments. Surrogates for IRI, such as AST and LDH on postoperative day 1, were diminished in the IFLT cohort. Recurrence-free survival at 1 and 3 years was higher in the IFLT group vs. the SCS group (92.2% and 86.7% vs. 88.1% and 53.6%, respectively, p = 0.048).
Meanwhile, Mueller et al.,230 in a retrospective multicentre analysis, matched 70 DCD grafts transplanted after endischaemic HOPE with 70 DBD and 70 DCD grafts transplanted after SCS. Post-transplant HCC recurrence was significantly lower in the HOPE-treated DCD group than in the unperfused DBD and DCD groups.
To date, HOPE is the only therapeutic modality that has been associated with reduction of IRI, promotion of regeneration, and reduction of post-transplant HCC recurrence. This has led to the design and recent initiation of two key clinical trials. The first is a multicentre RCT in Italy designed to confirm the previously described impact of HOPE on HCC recurrence after liver transplantation. The second is a study on the effect of HOPE in living donor grafts deemed too small with a high corresponding risk of small for size syndrome. This study will elegantly assess the combined effect of HOPE on IRI and regeneration, considering the key role of mitochondria in all liver cells. The results of these two trials are eagerly awaited.
Conclusions & future perspectives
Prevention of tumour recurrence after curative-intent liver surgery remains largely an unmet need. Adequate patient selection, an operation conducted according to oncological principles, and the select use of adjuvant therapy are essential to reduce this risk; however, a deeper understanding of the mechanisms that favour recurrence may reveal novel potentially actionable targets. It is now evident that the degree of perioperative injury has an impact on oncological outcomes. This injury has several components, ranging from the simple act of squeezing the liver parenchyma to the dramatic haemodynamic changes that occur during transplantation. These components have interlacing roles that may exacerbate one another and eventually determine whether the liver will recover its function in the immediate postoperative period. As discussed, the striking activation of downstream pathways caused by perioperative injury not only affects the early postoperative period, but may create the perfect conditions for tumour engraftment while CTCs are released or activated in response to surgical manipulation and stress. Perioperative liver injury can be modulated by targeting different aspects, which may either diminish sources of damage (ischaemia-reperfusion, oxidative stress, immune activation, pro-inflammatory response) or boost liver function and regeneration. Therapeutic strategies that appear particularly promising are those addressing upstream pathways and exerting their effects at multiple levels in the liver injury cascade, such as stem cell therapy and hypothermic machine perfusion. Clinical studies are ongoing, and their results will hopefully shed light on ways to reduce perioperative liver injury and ameliorate short- and long-term outcomes that can be implemented in routine patient care.
Financial support
The present study received no funding.
Authors’ contributions
Concept and design, M.M. and A.S.; writing of article, M.M., A.S.., S.Y., B.C., RR., K.A.; review and editing of the article: all coauthors.; supervision, V.M, A.S.; All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100846.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Shah S.A., Cleary S.P., Wei A.C., et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141(3):330–339. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 2.Mazzaferro V., Gorgen A., Roayaie S., Droz dit Busset M., Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72(2):364–377. doi: 10.1016/J.JHEP.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Sposito C., Rossi R.E., Monteleone M., et al. Postrecurrence survival after liver transplantation for liver metastases from neuroendocrine tumors. Transplantation. 2021 Dec 1;105(12):2579-2586. [DOI] [PubMed]
- 4.Tomlinson J.S., Jarnagin W.R., DeMatteo R.P., et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 5.Nordlinger B., Sorbye H., Glimelius B., et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 6.Park H.M., Yun S.P., Lee E.C., et al. Outcomes for patients with recurrent intrahepatic cholangiocarcinoma after surgery. Ann Surg Oncol. 2016;23(13):4392–4400. doi: 10.1245/s10434-016-5454-2. [DOI] [PubMed] [Google Scholar]
- 7.Maspero M., Rossi R.E., Sposito C., Coppa J., Citterio D., Mazzaferro V. Long-term outcome of patients undergoing resection versus transplantation for neuroendocrine liver metastases meeting the Milan Criteria. Am J Transpl. July 2022 doi: 10.1111/AJT.17156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ercolani G., Vetrone G., Grazi G.L., et al. Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann Surg. 2010;252(1):107–114. doi: 10.1097/SLA.0b013e3181e462e6. [DOI] [PubMed] [Google Scholar]
- 9.Hu L.-S., Zhang X.-F., Weiss M., et al. Recurrence patterns and timing courses following curative-intent resection for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2019;26(8):2549–2557. doi: 10.1245/s10434-019-07353-4. [DOI] [PubMed] [Google Scholar]
- 10.Bodzin A.S., Lunsford K.E., Markovic D., Harlander-Locke M.P., Busuttil R.W., Agopian V.G. Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation: impact of treatment modality and recurrence characteristics. Ann Surg. 2017;266(1) doi: 10.1097/SLA.0000000000001894. https://journals.lww.com/annalsofsurgery/Fulltext/2017/07000/Predicting_Mortality_in_Patients_Developing.18.aspx [DOI] [PubMed] [Google Scholar]
- 11.Toso C., Pinto Marques H., Andres A., et al. Liver transplantation for colorectal liver metastasis: survival without recurrence can be achieved. Liver Transpl. 2017;23(8):1073–1076. doi: 10.1002/lt.24791. [DOI] [PubMed] [Google Scholar]
- 12.Solheim JM, Dueland S, Line P-D, Hagness M. Transplantation for nonresectable colorectal liver metastases - long term follow- up of the first prospective pilot study. Ann Surg. 2023 Aug 1;278(2):239-245 [DOI] [PubMed]
- 13.Dueland S., Smedman T.M., Røsok B., et al. Treatment of relapse and survival outcomes after liver transplantation in patients with colorectal liver metastases. Transpl Int. 2021;34(11):2205–2213. doi: 10.1111/tri.13995. [DOI] [PubMed] [Google Scholar]
- 14.Bodzin A.S., Lunsford K.E., Markovic D., Harlander-Locke M.P., Busuttil R.W., Agopian V.G. Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation: impact of treatment modality and recurrence characteristics. Ann Surg. 2017;266(1) doi: 10.1097/SLA.0000000000001894. [DOI] [PubMed] [Google Scholar]
- 15.Mazzaferro V., Sposito C., Zhou J., et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154(1):128–139. doi: 10.1053/j.gastro.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Mavros M.N., Economopoulos K.P., Alexiou V.G., Pawlik T.M. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149(6):565–574. doi: 10.1001/jamasurg.2013.5137. [DOI] [PubMed] [Google Scholar]
- 17.Dueland S., Grut H., Syversveen T., Hagness M., Line P.D. Selection criteria related to long-term survival following liver transplantation for colorectal liver metastasis. Am J Transpl. 2020;20(2):530–537. doi: 10.1111/ajt.15682. [DOI] [PubMed] [Google Scholar]
- 18.Jonas S., Bechstein W.O., Steinmüller T., et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33(5):1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 19.Vagefi P.A., Dodge J.L., Yao F.Y., Roberts J.P. Potential role of the donor in hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl Off Publ Am Assoc Study Liver Dis Int Liver Transpl Soc. 2015;21(2):187–194. doi: 10.1002/lt.24042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orci L.A., Berney T., Majno P.E., et al. Donor characteristics and risk of hepatocellular carcinoma recurrence after liver transplantation. Br J Surg. 2015;102(10):1250–1257. doi: 10.1002/bjs.9868. [DOI] [PubMed] [Google Scholar]
- 21.Nagai S., Yoshida A., Facciuto M., et al. Ischemia time impacts recurrence of hepatocellular carcinoma after liver transplantation. Hepatology. 2015;61(3):895–904. doi: 10.1002/hep.27358. [DOI] [PubMed] [Google Scholar]
- 22.Kornberg A., Witt U., Kornberg J., Friess H., Thrum K. Extended ischemia times promote risk of HCC recurrence in liver transplant patients. Dig Dis Sci. 2015;60(9):2832–2839. doi: 10.1007/S10620-015-3541-Z/FIGURES/2. [DOI] [PubMed] [Google Scholar]
- 23.Mathur A., Franco E.S., Leone J.P., et al. Obesity portends increased morbidity and earlier recurrence following liver transplantation for hepatocellular carcinoma. HPB Off J Int Hepato Pancreato Biliary Assoc. 2013;15(7):504–510. doi: 10.1111/j.1477-2574.2012.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu T.-J., Chan K.-M., Chou H.-S., et al. Liver transplantation in patients with hepatitis B virus-related hepatocellular carcinoma: the influence of viral characteristics on clinical outcome. Ann Surg Oncol. 2013;20(11):3582–3590. doi: 10.1245/s10434-013-3023-5. [DOI] [PubMed] [Google Scholar]
- 25.Vivarelli M., Cucchetti A., Piscaglia F., et al. Analysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: key role of immunosuppression. Liver Transpl Off Publ Am Assoc Study Liver Dis Int Liver Transpl Soc. 2005;11(5):497–503. doi: 10.1002/lt.20391. [DOI] [PubMed] [Google Scholar]
- 26.Gilgenkrantz H., Collin de l’Hortet A. Understanding liver regeneration: from mechanisms to regenerative medicine. Am J Pathol. 2018;188(6):1316–1327. doi: 10.1016/j.ajpath.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Robinson S.M., Wilson C.H., Burt A.D., Manas D.M., White S.A. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19(13):4287–4299. doi: 10.1245/s10434-012-2438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portolani N., Coniglio A., Ghidoni S., et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243(2) doi: 10.1097/01.sla.0000197706.21803.a1. https://journals.lww.com/annalsofsurgery/Fulltext/2006/02000/Early_and_Late_Recurrence_After_Liver_Resection.12.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Truant S., Bouras A.F., Petrovai G., et al. Volumetric gain of the liver after major hepatectomy in obese patients: a case-matched study in 84 patients. Ann Surg. 2013;258(5):696–704. doi: 10.1097/SLA.0b013e3182a61a22. [DOI] [PubMed] [Google Scholar]
- 30.Gomez D., Malik H.Z., Bonney G.K., et al. Steatosis predicts postoperative morbidity following hepatic resection for colorectal metastasis. Br J Surg. 2007;94(11):1395–1402. doi: 10.1002/bjs.5820. [DOI] [PubMed] [Google Scholar]
- 31.Kauffmann R., Fong Y. Post-hepatectomy liver failure. Hepatobiliary Surg Nutr. 2014;3(5):238–246. doi: 10.3978/j.issn.2304-3881.2014.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zong W.-X., Rabinowitz J.D., White E. Mitochondria and cancer. Mol Cel. 2016;61(5):667–676. doi: 10.1016/j.molcel.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nadalin S., Settmacher U., Rauchfuß F., Balci D., Königsrainer A., Line P.D. RAPID procedure for colorectal cancer liver metastasis. Int J Surg. 2020;82:93–96. doi: 10.1016/j.ijsu.2020.03.078. December 2019. [DOI] [PubMed] [Google Scholar]
- 34.Ravaioli M., Fallani G., Cerri M., et al. Two surgical techniques are better than one: RAVAS and RAPID are answers for the same issue. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. 2021;21(2):905–906. doi: 10.1111/ajt.16301. [DOI] [PubMed] [Google Scholar]
- 35.Michalopoulos G.K. Liver regeneration. J Cel Physiol. 2007;213(2):286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerrieri F., Muolo L., Cocco T., et al. Correlation between rat liver regeneration and mitochondrial energy metabolism. Biochim Biophys Acta. 1995;1272(2):95–100. doi: 10.1016/0925-4439(95)00072-c. [DOI] [PubMed] [Google Scholar]
- 37.Mann D.V., Lam W.W.M., Hjelm N.M., et al. Metabolic control patterns in acute phase and regenerating human liver determined in vivo by 31-phosphorus magnetic resonance spectroscopy. Ann Surg. 2002;235(3):408–416. doi: 10.1097/00000658-200203000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexandrino H., Rolo A., Teodoro J.S., et al. Bioenergetic adaptations of the human liver in the ALPPS procedure - how liver regeneration correlates with mitochondrial energy status. HPB Off J Int Hepato Pancreato Biliary Assoc. 2017;19(12):1091–1103. doi: 10.1016/j.hpb.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Mohammed F.F., Khokha R. Thinking outside the cell: proteases regulate hepatocyte division. Trends Cel Biol. 2005;15(10):555–563. doi: 10.1016/j.tcb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Mars W.M., Liu M.L., Kitson R.P., Goldfarb R.H., Gabauer M.K., Michalopoulos G.K. Immediate early detection of urokinase receptor after partial hepatectomy and its implications for initiation of liver regeneration. Hepatology. 1995;21(6):1695–1701. [PubMed] [Google Scholar]
- 41.Pediaditakis P., Lopez-Talavera J.C., Petersen B., Monga S.P.S., Michalopoulos G.K. The processing and utilization of hepatocyte growth factor/scatter factor following partial hepatectomy in the rat. Hepatology. 2001;34(4, Part 1):688–693. doi: 10.1053/jhep.2001.27811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monga S.P., Pediaditakis P., Mule K., Stolz D.B., Michalopoulos G.K. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33(5):1098–1109. doi: 10.1053/jhep.2001.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nemeth M.J., Topol L., Anderson S.M., Yang Y., Bodine D.M. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci U S A. 2007;104(39):15436–15441. doi: 10.1073/pnas.0704747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell J.O., Monga S.P. Wnt/β-Catenin signaling in liver development, homeostasis, and pathobiology. Annu Rev Pathol. 2018;13:351–378. doi: 10.1146/annurev-pathol-020117-044010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Köhler C., Bell A.W., Bowen W.C., Monga S.P., Fleig W., Michalopoulos G.K. Expression of Notch-1 and its ligand Jagged-1 in rat liver during liver regeneration. Hepatology. 2004;39(4):1056–1065. doi: 10.1002/hep.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y., Zheng S., Qi D., et al. Inhibition of notch signaling by a γ-secretase inhibitor attenuates hepatic fibrosis in rats. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0046512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marshall K.M., He S., Zhong Z., Atkinson C., Tomlinson S. Dissecting the complement pathway in hepatic injury and regeneration with a novel protective strategy. J Exp Med. 2014;211(9):1793–1805. doi: 10.1084/jem.20131902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang L.-I., Mars W.M., Michalopoulos G.K. Signals and cells involved in regulating liver regeneration. Cells. 2012;1(4):1261–1292. doi: 10.3390/cells1041261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cressman D.E., Diamond R.H., Taub R. Rapid activation of the Stat3 transcription complex in liver regeneration. Hepatology. 1995;21(5):1443–1449. [PubMed] [Google Scholar]
- 50.Kono S., Nagaike M., Matsumoto K., Nakamura T. Marked induction of hepatocyte growth factor mRNA in intact kidney and spleen in response to injury of distant organs. Biochem Biophys Res Commun. 1992;186(2):991–998. doi: 10.1016/0006-291X(92)90844-B. [DOI] [PubMed] [Google Scholar]
- 51.Olsen P.S., Boesby S., Kirkegaard P., et al. Influence of epidermal growth factor on liver regeneration after partial hepatectomy in rats. Hepatology. 1988;8(5) doi: 10.1002/hep.1840080503. https://journals.lww.com/hep/Fulltext/1988/09000/Influence_of_epidermal_growth_factor_on_liver.2.aspx [DOI] [PubMed] [Google Scholar]
- 52.Michalopoulos G.K. Principles of liver regeneration and growth homeostasis. Compr Physiol. 2013;3(1):485–513. doi: 10.1002/cphy.c120014. [DOI] [PubMed] [Google Scholar]
- 53.Bockhorn M., Goralski M., Prokofiev D., et al. VEGF is important for early liver regeneration after partial hepatectomy. J Surg Res. 2007;138(2):291–299. doi: 10.1016/j.jss.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 54.Ozaki M. Cellular and molecular mechanisms of liver regeneration: proliferation, growth, death and protection of hepatocytes. Semin Cel Dev Biol. 2020;100:62–73. doi: 10.1016/j.semcdb.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Kato T., Ito Y., Hosono K., et al. Vascular endothelial growth factor receptor-1 signaling promotes liver repair through restoration of liver microvasculature after acetaminophen hepatotoxicity. Toxicol Sci. 2011;120(1):218–229. doi: 10.1093/toxsci/kfq366. [DOI] [PubMed] [Google Scholar]
- 56.Tao Y., Wang M., Chen E., Tang H. Liver regeneration: analysis of the main relevant signaling molecules. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/4256352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romero-Gallo J., Sozmen E.G., Chytil A., et al. Inactivation of TGF-beta signaling in hepatocytes results in an increased proliferative response after partial hepatectomy. Oncogene. 2005;24(18):3028–3041. doi: 10.1038/sj.onc.1208475. [DOI] [PubMed] [Google Scholar]
- 58.Zimmermann A. Regulation of liver regeneration. Nephrol Dial Transpl. 2004;19(suppl_4):iv6–iv10. doi: 10.1093/ndt/gfh1034. [DOI] [PubMed] [Google Scholar]
- 59.Karkampouna S., Ten Dijke P., Dooley S., Julio M.K. TGFβ signaling in liver regeneration. Curr Pharm Des. 2012;18(27):4103–4113. doi: 10.2174/138161212802430521. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen L.N., Furuya M.H., Wolfraim L.A., et al. Transforming growth factor-beta differentially regulates oval cell and hepatocyte proliferation. Hepatology. 2007;45(1):31–41. doi: 10.1002/hep.21466. [DOI] [PubMed] [Google Scholar]
- 61.Deneme M.A., Ok E., Akcan A., Akyildiz H., Soyuer I., Muhtaroglu S. Single dose of anti-transforming growth factor-beta1 monoclonal antibody enhances liver regeneration after partial hepatectomy in biliary-obstructed rats. J Surg Res. 2006;136(2):280–287. doi: 10.1016/j.jss.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 62.Derynck R., Zhang Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 63.Taki-Eldin A., Zhou L., Xie H.Y., Zheng S.S. Liver regeneration after liver transplantation. Eur Surg Res. 2012;48:139–153. doi: 10.1159/000337865. [DOI] [PubMed] [Google Scholar]
- 64.Wu M.-Y., Yiang G.-T., Liao W.-T., et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem Int J Exp Cel Physiol Biochem Pharmacol. 2018;46(4):1650–1667. doi: 10.1159/000489241. [DOI] [PubMed] [Google Scholar]
- 65.Görlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiménez-Castro M.B., Cornide-Petronio M.E., Gracia-Sancho J., Peralta C. Inflammasome-mediated inflammation in liver ischemia-reperfusion injury. Cells. 2019;8(10) doi: 10.3390/cells8101131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Videla L.A., Fernández V. Biochemical aspects of cellular oxidative stress. Arch Biol Med Exp (Santiago) 1988;21(1):85–92. [PubMed] [Google Scholar]
- 68.Czigany Z., Lurje I., Schmelzle M., et al. Ischemia-reperfusion injury in marginal liver grafts and the role of hypothermic machine perfusion: molecular mechanisms and clinical implications. J Clin Med. 2020;9(3) doi: 10.3390/jcm9030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szabo G., Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12(7):387–400. doi: 10.1038/nrgastro.2015.94. [DOI] [PubMed] [Google Scholar]
- 70.Zhai Y., Busuttil R.W., Kupiec-Weglinski J.W. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. 2011;11(8):1563–1569. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y., Wu J., Jiang B., et al. Relationship between ischemia/reperfusion injury and acute rejection of allogeneic liver transplant in rats. Transpl Proc. 2014;46(1):50–55. doi: 10.1016/j.transproceed.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 72.Tsung A., Klune J.R., Zhang X., et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204(12):2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baeuerle P.A., Baltimore D. NF-κB: ten years after. Cell. 1996;87(1):13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 74.Ghosh S., May M.J., Kopp E.B. NF-(kappa) B and REL proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 75.Wullaert A., van Loo G., Heyninck K., Beyaert R. Hepatic tumor necrosis factor signaling and nuclear factor-kappaB: effects on liver homeostasis and beyond. Endocr Rev. 2007;28(4):365–386. doi: 10.1210/er.2006-0031. [DOI] [PubMed] [Google Scholar]
- 76.Tsoulfas G., Geller D.A. NF-κB in transplantation: friend or foe? Transpl Infect Dis Basic Sci. 2001;3(4):212–219. doi: 10.1034/j.1399-3062.2001.30405.x. [DOI] [PubMed] [Google Scholar]
- 77.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 78.Llacuna L., Marí M., Lluis J.M., García-Ruiz C., Fernández-Checa J.C., Morales A. Reactive oxygen species mediate liver injury through parenchymal nuclear factor-kappaB inactivation in prolonged ischemia/reperfusion. Am J Pathol. 2009;174(5):1776–1785. doi: 10.2353/ajpath.2009.080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Konishi T., Lentsch A.B. Hepatic ischemia/reperfusion: mechanisms of tissue injury, repair, and regeneration. Gene Expr. 2017;17(4):277–287. doi: 10.3727/105221617X15042750874156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakamura K., Kageyama S., Kupiec-Weglinski J.W. The evolving role of neutrophils in liver transplant ischemia-reperfusion injury. Curr Transpl Rep. 2019;6(1):78–89. doi: 10.1007/s40472-019-0230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 82.Durand F., Renz J.F., Alkofer B., et al. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transpl Off Publ Am Assoc Study Liver Dis Int Liver Transpl Soc. 2008;14(12):1694–1707. doi: 10.1002/lt.21668. [DOI] [PubMed] [Google Scholar]
- 83.McCormack L., Dutkowski P., El-Badry A.M., Clavien P.-A. Liver transplantation using fatty livers: always feasible? J Hepatol. 2011;54(5):1055–1062. doi: 10.1016/j.jhep.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 84.Spitzer A.L., Lao O.B., Dick A.A.S., et al. The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. Liver Transpl Off Publ Am Assoc Study Liver Dis Int Liver Transpl Soc. 2010;16(7):874–884. doi: 10.1002/lt.22085. [DOI] [PubMed] [Google Scholar]
- 85.Gehrau R.C., Mas V.R., Dumur C.I., et al. Donor hepatic steatosis induce exacerbated ischemia-reperfusion injury through activation of innate immune response molecular pathways. Transplantation. 2015;99(12):2523–2533. doi: 10.1097/TP.0000000000000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu J., Sayed B.A., Casas-Ferreira A.M., et al. The impact of ischemia/reperfusion injury on liver allografts from deceased after cardiac death versus deceased after brain death donors. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0148815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blok J.J., Detry O., Putter H., et al. Longterm results of liver transplantation from donation after circulatory death. Liver Transpl Off Publ Am Assoc Study Liver Dis Int Liver Transpl Soc. 2016;22(8):1107–1114. doi: 10.1002/lt.24449. [DOI] [PubMed] [Google Scholar]
- 88.Wynne H.A., Cope L.H., Mutch E., Rawlins M.D., Woodhouse K.W., James O.F. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology. 1989;9(2):297–301. doi: 10.1002/hep.1840090222. [DOI] [PubMed] [Google Scholar]
- 89.Okaya T., Blanchard J., Schuster R., et al. Age-dependent responses to hepatic ischemia/reperfusion injury. Shock. 2005;24(5):421–427. doi: 10.1097/01.shk.0000181282.14050.11. [DOI] [PubMed] [Google Scholar]
- 90.Man K., Co Shih K., Ng K.T.P., et al. Molecular signature linked to acute phase injury and tumor invasiveness in small-for-size liver grafts. Ann Surg. 2010;251(6):1154–1161. doi: 10.1097/SLA.0B013E3181D96E3D. [DOI] [PubMed] [Google Scholar]
- 91.Man K., Lo C.M., Xiao J.W., et al. The significance of acute phase small-for-size graft injury on tumor growth and invasiveness after liver transplantation. Ann Surg. 2008;247(6):1049–1057. doi: 10.1097/SLA.0B013E31816FFAB6XXX. [DOI] [PubMed] [Google Scholar]
- 92.Baidya R., Crawford D.H.G., Gautheron J., Wang H., Bridle K.R. Necroptosis in hepatosteatotic ischaemia-reperfusion injury. Int J Mol Sci. 2020;21(16) doi: 10.3390/ijms21165931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yerdel M.A., Gunson B., Mirza D., et al. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation. 2000;69(9):1873–1881. doi: 10.1097/00007890-200005150-00023. [DOI] [PubMed] [Google Scholar]
- 94.Tripodi A., Mannucci P.M. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365(2):147–156. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 95.Uemura M., Fujimura Y., Matsumoto M., et al. Comprehensive analysis of ADAMTS13 in patients with liver cirrhosis. Thromb Haemost. 2008;99(6):1019–1029. doi: 10.1160/TH08-01-0006. [DOI] [PubMed] [Google Scholar]
- 96.Yu J.-J., Xiao W., Dong S.-L., et al. Effect of surgical liver resection on circulating tumor cells in patients with hepatocellular carcinoma. BMC Cancer. 2018;18(1):835. doi: 10.1186/s12885-018-4744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fredriksson J.M., Lindquist J.M., Bronnikov G.E., Nedergaard J. Norepinephrine induces vascular endothelial growth factor gene expression in brown adipocytes through a β-adrenoreceptor/cAMP/protein kinase A pathway involving Src but independently of Erk1/2. J Biol Chem. 2000;275(18):13802–13811. doi: 10.1074/jbc.275.18.13802. [DOI] [PubMed] [Google Scholar]
- 98.Tsuchiya Y., Sawada S., Yoshioka I., et al. Increased surgical stress promotes tumor metastasis. Surgery. 2003;133(5):547–555. doi: 10.1067/msy.2003.141. [DOI] [PubMed] [Google Scholar]
- 99.Kornberg A., Witt U., Kornberg J., Friess H., Thrum K. Treating ischaemia-reperfusion injury with prostaglandin E1 reduces the risk of early hepatocellular carcinoma recurrence following liver transplantation. Aliment Pharmacol Ther. 2015;42(9):1101–1110. doi: 10.1111/apt.13380. [DOI] [PubMed] [Google Scholar]
- 100.Liu C.L., Fan S.T., Cheung S.T., Lo C.M., Ng I.O., Wong J. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg. 2006;244(2):194–203. doi: 10.1097/01.sla.0000225095.18754.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang J.-X., Li J.-J., Weng R.-H., Liang Z.-M., Jiang N. Anterior vs conventional approach right hepatic resection for large hepatocellular carcinoma: a systematic review and meta-analysis. World J Gastroenterol. 2017;23(44):7917–7929. doi: 10.3748/wjg.v23.i44.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Klein E.A., Bianco F.J., Serio A.M., et al. Surgeon experience is strongly associated with biochemical recurrence after radical prostatectomy for all preoperative risk categories. J Urol. 2008;179(6):2212–2217. doi: 10.1016/j.juro.2008.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hiller J.G., Perry N.J., Poulogiannis G., Riedel B., Sloan E.K. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15(4):205–218. doi: 10.1038/nrclinonc.2017.194. [DOI] [PubMed] [Google Scholar]
- 104.Sonoshita M., Aoki M., Fuwa H., et al. Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer Cell. 2011;19(1):125–137. doi: 10.1016/j.ccr.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 105.Bai L., Mao G.P., Cao C.P. Effects of inflammatory cytokines on the recurrence of liver cancer after an apparently curative operation. J Dig Dis. 2007;8(3):154–159. doi: 10.1111/j.1443-9573.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- 106.Ning L., Wentworth L., Chen H., Weber S.M. Down-regulation of Notch1 signaling inhibits tumor growth in human hepatocellular carcinoma. Am J Transl Res. 2009;1(4):358–366. [PMC free article] [PubMed] [Google Scholar]
- 107.Huang S., He J., Zhang X., et al. Activation of the hedgehog pathway in human hepatocellular carcinomas. Carcinogenesis. 2006;27(7):1334–1340. doi: 10.1093/carcin/bgi378. [DOI] [PubMed] [Google Scholar]
- 108.Nusse R., Clevers H. Wnt/β-Catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 109.Schwab R.H.M., Amin N., Flanagan D.J., Johanson T.M., Phesse T.J., Vincan E. Wnt is necessary for mesenchymal to epithelial transition in colorectal cancer cells. Dev Dyn. 2018;247(3):521–530. doi: 10.1002/dvdy.24527. [DOI] [PubMed] [Google Scholar]
- 110.Konsavage W.M., Jr., Yochum G.S. Intersection of Hippo/YAP and wnt/β-catenin signaling pathways. Acta Biochim Biophys Sin. 2013;45(2):71–79. doi: 10.1093/abbs/gms084. [DOI] [PubMed] [Google Scholar]
- 111.Perra A., Kowalik M.A., Ghiso E., et al. YAP activation is an early event and a potential therapeutic target in liver cancer development. J Hepatol. 2014;61(5):1088–1096. doi: 10.1016/j.jhep.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 112.Konsavage W.M., Kyler S.L., Rennoll S.A., Jin G., Yochum G.S. Wnt/β-Catenin signaling regulates yes-associated protein (YAP) gene expression in colorectal carcinoma cells∗. J Biol Chem. 2012;287(15):11730–11739. doi: 10.1074/jbc.M111.327767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li H., Wolfe A., Septer S., et al. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int Off J Int Assoc Study Liver. 2012;32(1):38–47. doi: 10.1111/j.1478-3231.2011.02646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Inman G.J. Switching TGFβ from a tumor suppressor to a tumor promoter. Curr Opin Genet Dev. 2011;21(1):93–99. doi: 10.1016/j.gde.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 115.Principe D.R., Doll J.A., Bauer J., et al. TGF-β: duality of function between tumor prevention and carcinogenesis. JNCI J Natl Cancer Inst. 2014;106(2):djt369. doi: 10.1093/jnci/djt369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Calon A., Espinet E., Palomo-Ponce S., et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22(5):571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsushima H., Ito N., Tamura S., et al. Circulating transforming growth factor beta 1 as a predictor of liver metastasis after resection in colorectal cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2001;7(5):1258–1262. [PubMed] [Google Scholar]
- 118.Picon A., Gold L.I., Wang J., Cohen A., Friedman E. A subset of metastatic human colon cancers expresses elevated levels of transforming growth factor beta1. Cancer Epidemiol Biomarkers Prev. 1998;7(6):497–504. [PubMed] [Google Scholar]
- 119.Lecharpentier A., Vielh P., Perez-Moreno P., Planchard D., Soria J.C., Farace F. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer. 2011;105(9):1338–1341. doi: 10.1038/bjc.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu Y., Hu B., Li Z., He X., Li Y., Lu L. An improved strategy to detect the epithelial-mesenchymal transition process in circulating tumor cells in hepatocellular carcinoma patients. Hepatol Int. 2016;10(4):640–646. doi: 10.1007/s12072-016-9732-7. [DOI] [PubMed] [Google Scholar]
- 121.Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 122.Van Cutsem E., Köhne C.-H., Hitre E., et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 123.Douillard J.-Y., Oliner K.S., Siena S., et al. Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 124.Sparrelid E., Johansson H., Gilg S., Nowak G., Ellis E., Isaksson B. Serial assessment of growth factors associated with liver regeneration in patients operated with associating liver partition and portal vein ligation for staged hepatectomy. Eur Surg Res Eur Chir Forschung Rech Chir Eur. 2018;59(1–2):72–82. doi: 10.1159/000488078. [DOI] [PubMed] [Google Scholar]
- 125.Sasturkar S.V., David P., Sharma S., Sarin S.K., Trehanpati N., Pamecha V. Serial changes of cytokines and growth factors in peripheral circulation after right lobe donor hepatectomy. Liver Transpl Off Publ Am Assoc Study Liver Dis Int Liver Transpl Soc. 2016;22(3):344–351. doi: 10.1002/lt.24373. [DOI] [PubMed] [Google Scholar]
- 126.Dłuzniewska J., Zolich D., Polański J., Zajac L., Sitkiewicz D., Łukomska B. Hepatocyte growth factor levels in liver and blood, and post-operative liver cell proliferation in patients with benign and malignant liver tumors after partial hepatectomy. Med Sci Monit Int Med J Exp Clin Res. 2002;8(10):CR690–C696. [PubMed] [Google Scholar]
- 127.Yoon S.S., Kim S.H., Gonen M., et al. Profile of plasma angiogenic factors before and after hepatectomy for colorectal cancer liver metastases. Ann Surg Oncol. 2006;13(3):353–362. doi: 10.1245/ASO.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 128.Ke A.-W., Shi G.-M., Zhou J., et al. Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma. Hepatology. 2009;49(2):491–503. doi: 10.1002/hep.22639. [DOI] [PubMed] [Google Scholar]
- 129.Kaposi-Novak P., Lee J.-S., Gòmez-Quiroz L., Coulouarn C., Factor V.M., Thorgeirsson S.S. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest. 2006;116(6):1582–1595. doi: 10.1172/JCI27236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang D., Zheng X., Fu B., et al. Hepatectomy promotes recurrence of liver cancer by enhancing IL-11-STAT3 signaling. EBioMedicine. 2019;46:119–132. doi: 10.1016/j.ebiom.2019.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li H.-W., Shan J.-X. Effects of hepatocyte growth factor/scatter factor on the invasion of colorectal cancer cells in vitro. World J Gastroenterol. 2005;11(25):3877–3881. doi: 10.3748/wjg.v11.i25.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mohammed F.F., Pennington C.J., Kassiri Z., et al. Metalloproteinase inhibitor TIMP-1 affects hepatocyte cell cycle via HGF activation in murine liver regeneration. Hepatology. 2005;41(4):857–867. doi: 10.1002/hep.20618. [DOI] [PubMed] [Google Scholar]
- 133.Duarte S., Baber J., Fujii T., Coito A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015;44–46:147–156. doi: 10.1016/j.matbio.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang Q., Li J., Zheng J., Wei A. The carcinogenic role of the notch signaling pathway in the development of hepatocellular carcinoma. J Cancer. 2019;10(6):1570–1579. doi: 10.7150/jca.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hayashi Y., Osanai M., Lee G.-H. NOTCH2 signaling confers immature morphology and aggressiveness in human hepatocellular carcinoma cells. Oncol Rep. 2015;34(4):1650–1658. doi: 10.3892/or.2015.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morse M.A., Sun W., Kim R., et al. The role of angiogenesis in hepatocellular carcinoma. Clin Cancer Res. 2019;25(3):912–920. doi: 10.1158/1078-0432.CCR-18-1254. [DOI] [PubMed] [Google Scholar]
- 137.Xiang Q., Chen W., Ren M., et al. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin Cancer Res Off J Am Assoc Cancer Res. 2014;20(11):2959–2970. doi: 10.1158/1078-0432.CCR-13-2620. [DOI] [PubMed] [Google Scholar]
- 138.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 139.Hansen T.F., Qvortrup C., Pfeiffer P. Angiogenesis inhibitors for colorectal cancer. A review of the clinical data. Cancers (Basel) 2021;13(5) doi: 10.3390/cancers13051031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Krause P., Flikweert H., Monin M., et al. Increased growth of colorectal liver metastasis following partial hepatectomy. Clin Exp Metastasis. 2013;30(5):681–693. doi: 10.1007/s10585-013-9572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Harun N., Nikfarjam M., Muralidharan V., Christophi C. Liver regeneration stimulates tumor metastases. J Surg Res. 2007;138(2):284–290. doi: 10.1016/j.jss.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 142.Rupertus K., Kollmar O., Scheuer C., Junker B., Menger M.D., Schilling M.K. Major but not minor hepatectomy accelerates engraftment of extrahepatic tumor cells. Clin Exp Metastasis. 2007;24(1):39–48. doi: 10.1007/s10585-006-9054-6. [DOI] [PubMed] [Google Scholar]
- 143.Regimbeau J.M., Cosse C., Kaiser G., et al. Feasibility, safety and efficacy of two-stage hepatectomy for bilobar liver metastases of colorectal cancer: a LiverMetSurvey analysis. HPB. 2017;19(5):396–405. doi: 10.1016/j.hpb.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 144.Viganò L., Torzilli G., Cimino M., et al. Drop-out between the two liver resections of two-stage hepatectomy. Patient selection or loss of chance? Eur J Surg Oncol. 2016;42(9):1385–1393. doi: 10.1016/j.ejso.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 145.Ironside N., Bell R., Bartlett A., McCall J., Powell J., Pandanaboyana S. Systematic review of perioperative and survival outcomes of liver resections with and without preoperative portal vein embolization for colorectal metastases. HPB. 2017;19(7):559–566. doi: 10.1016/j.hpb.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 146.Schnitzbauer A.A. A comparison of pitfalls after ALPPS stage 1 or portal vein embolization in small-for-size setting hepatectomies. Visc Med. 2017;33(6):435–441. doi: 10.1159/000480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Truant S., Scatton O., Dokmak S., et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): impact of the inter-stages course on morbi-mortality and implications for management. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2015;41(5):674–682. doi: 10.1016/j.ejso.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 148.Schadde E., Raptis D.A., Schnitzbauer A.A., et al. Prediction of mortality after ALPPS stage-1: an analysis of 320 patients from the international ALPPS registry. Ann Surg. 2015;262(5):780–786. doi: 10.1097/SLA.0000000000001450. [DOI] [PubMed] [Google Scholar]
- 149.Lim C., Cauchy F., Azoulay D., Farges O., Ronot M., Pocard M. Tumour progression and liver regeneration—insights from animal models. Nat Rev Gastroenterol Hepatol. 2013;10(8):452–462. doi: 10.1038/nrgastro.2013.55. [DOI] [PubMed] [Google Scholar]
- 150.de Graaf W., van den Esschert J.W., van Lienden K.P., van Gulik T.M. Induction of tumor growth after preoperative portal vein embolization: is it a real problem? Ann Surg Oncol. 2009;16(2):423–430. doi: 10.1245/s10434-008-0222-6. [DOI] [PubMed] [Google Scholar]
- 151.Giglio M.C., Giakoustidis A., Draz A., et al. Oncological outcomes of major liver resection following portal vein embolization: a systematic review and meta-analysis. Ann Surg Oncol. 2016;23(11):3709–3717. doi: 10.1245/s10434-016-5264-6. [DOI] [PubMed] [Google Scholar]
- 152.Wong T.C.L., Ng K.K.C., Fung J.Y.Y., et al. Long-term survival outcome between living donor and deceased donor liver transplant for hepatocellular carcinoma: intention-to-treat and propensity score matching analyses. Ann Surg Oncol. 2019;26(5):1454–1462. doi: 10.1245/s10434-019-07206-0. [DOI] [PubMed] [Google Scholar]
- 153.Grant R.C., Sandhu L., Dixon P.R., Greig P.D., Grant D.R., McGilvray I.D. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transpl. 2013;27(1):140–147. doi: 10.1111/ctr.12031. [DOI] [PubMed] [Google Scholar]
- 154.Lai Q., Sapisochin G., Gorgen A., et al. Evaluation of the intention-to-treat benefit of living donation in patients with hepatocellular carcinoma awaiting a liver transplant. JAMA Surg. 2021;156(9):e213112. doi: 10.1001/jamasurg.2021.3112. e213112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee E.C., Kim S.H., Shim J.R., Park S.-J. Small-for-size grafts increase recurrence of hepatocellular carcinoma in liver transplantation beyond milan criteria. Liver Transpl Off Publ Am Assoc Study Liver Dis Int Liver Transpl Soc. 2018;24(1):35–43. doi: 10.1002/lt.24868. [DOI] [PubMed] [Google Scholar]
- 156.Hu Z., Zhong X., Zhou J., et al. Smaller grafts do not imply early recurrence in recipients transplanted for hepatocellular carcinoma: a Chinese experience. Sci Rep. 2016;6 doi: 10.1038/srep26487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hwang S., Lee S.G., Ahn C.S., et al. Small-sized liver graft does not increase the risk of hepatocellular carcinoma recurrence after living donor liver transplantation. Transpl Proc. 2007;39(5):1526–1529. doi: 10.1016/j.transproceed.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 158.Zhai Y., Shen X.-D., Gao F., et al. CXCL10 regulates liver innate immune response against ischemia and reperfusion injury. Hepatology. 2008;47(1):207–214. doi: 10.1002/hep.21986. [DOI] [PubMed] [Google Scholar]
- 159.Li C.X., Ling C.C., Shao Y., et al. CXCL10/CXCR3 signaling mobilized-regulatory T cells promote liver tumor recurrence after transplantation. J Hepatol. 2016;65(5):944–952. doi: 10.1016/j.jhep.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 160.Yeung O.W.H., Lo C.-M., Ling C.-C., et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J Hepatol. 2015;62(3):607–616. doi: 10.1016/j.jhep.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 161.Shao Y., Lo C.M., Ling C.C., et al. Regulatory B cells accelerate hepatocellular carcinoma progression via CD40/CD154 signaling pathway. Cancer Lett. 2014;355(2):264–272. doi: 10.1016/j.canlet.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 162.Man K., Ng K.T., Lo C.M., et al. Ischemia-reperfusion of small liver remnant promotes liver tumor growth and metastases--activation of cell invasion and migration pathways. Liver Transpl Off Publ Am Assoc Study Liver Dis Int Liver Transpl Soc. 2007;13(12):1669–1677. doi: 10.1002/lt.21193. [DOI] [PubMed] [Google Scholar]
- 163.Lim C., Broqueres-You D., Brouland J.-P., et al. Hepatic ischemia-reperfusion increases circulating bone marrow-derived progenitor cells and tumor growth in a mouse model of colorectal liver metastases. J Surg Res. 2013;184(2):888–897. doi: 10.1016/j.jss.2013.04.069. [DOI] [PubMed] [Google Scholar]
- 164.Ling C.-C., Ng K.T.P., Shao Y., et al. Post-transplant endothelial progenitor cell mobilization via CXCL10/CXCR3 signaling promotes liver tumor growth. J Hepatol. 2014;60(1):103–109. doi: 10.1016/j.jhep.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 165.Hamada T., Fondevila C., Busuttil R.W., Coito A.J. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology. 2008;47(1):186–198. doi: 10.1002/hep.21922. [DOI] [PubMed] [Google Scholar]
- 166.Peiseler M., Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest. 2019;129(7):2629–2639. doi: 10.1172/JCI124616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Carmeliet P., Dor Y., Herbert J.M., et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394(6692):485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 168.Chu Q., Gu X., Zheng Q., Zhu H. Regulatory mechanism of HIF-1α and its role in liver diseases: a narrative review. Ann Transl Med. 2022;10(2):109. doi: 10.21037/atm-21-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wei M.C., Zong W.X., Cheng E.H., et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Frezza C., Zheng L., Tennant D.A., et al. Metabolic profiling of hypoxic cells revealed a catabolic signature required for cell survival. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0024411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Graeber T.G., Osmanian C., Jacks T., et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379(6560):88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 172.Zheng S.-S., Chen X.-H., Yin X., Zhang B.-H. Prognostic significance of HIF-1α expression in hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shimomura M., Hinoi T., Kuroda S., et al. Overexpression of hypoxia inducible factor-1 alpha is an independent risk factor for recurrence after curative resection of colorectal liver metastases. Ann Surg Oncol. 2013;20(3):527–536. doi: 10.1245/s10434-013-2945-2. [DOI] [PubMed] [Google Scholar]
- 174.Li H., Rokavec M., Jiang L., Horst D., Hermeking H. Antagonistic effects of p53 and HIF1A on microRNA-34a regulation of PPP1R11 and STAT3 and hypoxia-induced epithelial to mesenchymal transition in colorectal cancer cells. Gastroenterology. 2017;153(2):505–520. doi: 10.1053/j.gastro.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 175.Alvarez-Tejado M., Naranjo-Suarez S., Jiménez C., Carrera A.C., Landázuri M.O., del Peso L. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: protective role in apoptosis. J Biol Chem. 2001;276(25):22368–22374. doi: 10.1074/jbc.M011688200. [DOI] [PubMed] [Google Scholar]
- 176.Rius J., Guma M., Schachtrup C., et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453(7196):807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Demaria M., Giorgi C., Lebiedzinska M., et al. A STAT3-mediated metabolic switch is involved in tumour transformation and STAT3 addiction. Aging (Albany NY) 2010;2(11):823–842. doi: 10.18632/aging.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yu H.-C., Qin H.-Y., He F., et al. Canonical notch pathway protects hepatocytes from ischemia/reperfusion injury in mice by repressing reactive oxygen species production through JAK2/STAT3 signaling. Hepatology. 2011;54(3):979–988. doi: 10.1002/hep.24469. [DOI] [PubMed] [Google Scholar]
- 179.Orci L.A., Lacotte S., Delaune V., et al. Effects of the gut–liver axis on ischaemia-mediated hepatocellular carcinoma recurrence in the mouse liver. J Hepatol. 2018;68(5):978–985. doi: 10.1016/j.jhep.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 180.Oldani G., Crowe L.A., Orci L.A., et al. Pre-retrieval reperfusion decreases cancer recurrence after rat ischemic liver graft transplantation. J Hepatol. 2014;61(2):278–285. doi: 10.1016/j.jhep.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 181.Orci L.A., Lacotte S., Oldani G., et al. Effect of ischaemic preconditioning on recurrence of hepatocellular carcinoma in an experimental model of liver steatosis. Br J Surg. 2016;103(4):417–426. doi: 10.1002/bjs.10080. [DOI] [PubMed] [Google Scholar]
- 182.Yang F., Zhang Y., Ren H., et al. Ischemia reperfusion injury promotes recurrence of hepatocellular carcinoma in fatty liver via ALOX12-12HETE-GPR31 signaling axis. J Exp Clin Cancer Res. 2019;38(1):489. doi: 10.1186/s13046-019-1480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.van der Bilt J.D.W., Kranenburg O., Nijkamp M.W., et al. Ischemia/reperfusion accelerates the outgrowth of hepatic micrometastases in a highly standardized murine model. Hepatology. 2005;42(1):165–175. doi: 10.1002/hep.20739. [DOI] [PubMed] [Google Scholar]
- 184.van der Bilt J.D.W., Kranenburg O., Borren A., van Hillegersberg R., Borel Rinkes I.H.M. Ageing and hepatic steatosis exacerbate ischemia/reperfusion-accelerated outgrowth of colorectal micrometastases. Ann Surg Oncol. 2008;15(5):1392–1398. doi: 10.1245/s10434-007-9758-0. [DOI] [PubMed] [Google Scholar]
- 185.Nicoud I.B., Jones C.M., Pierce J.M., et al. Warm hepatic ischemia-reperfusion promotes growth of colorectal carcinoma micrometastases in mouse liver via matrix metalloproteinase-9 induction. Cancer Res. 2007;67(6):2720–2728. doi: 10.1158/0008-5472.CAN-06-3923. [DOI] [PubMed] [Google Scholar]
- 186.Jiao S.-F., Sun K., Chen X.-J., et al. Inhibition of tumor necrosis factor alpha reduces the outgrowth of hepatic micrometastasis of colorectal tumors in a mouse model of liver ischemia-reperfusion injury. J Biomed Sci. 2014;21(1):1. doi: 10.1186/1423-0127-21-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Germanova D., Keirsse J., Köhler A., et al. Myeloid tumor necrosis factor and heme oxygenase-1 regulate the progression of colorectal liver metastases during hepatic ischemia-reperfusion. Int J Cancer. 2021;148(5):1276–1288. doi: 10.1002/ijc.33334. [DOI] [PubMed] [Google Scholar]
- 188.Doi K., Horiuchi T., Uchinami M., et al. Hepatic ischemia–reperfusion promotes liver metastasis of colon cancer. J Surg Res. 2002;105(2):243–247. doi: 10.1006/jsre.2002.6356. [DOI] [PubMed] [Google Scholar]
- 189.Famularo S., Giani A., Di Sandro S., et al. Does the Pringle maneuver affect survival and recurrence following surgical resection for hepatocellular carcinoma? A western series of 441 patients. J Surg Oncol. 2018;117(2):198–206. doi: 10.1002/jso.24819. [DOI] [PubMed] [Google Scholar]
- 190.Huang J., Tang W., Hernandez-Alejandro R., et al. Intermittent hepatic inflow occlusion during partial hepatectomy for hepatocellular carcinoma does not shorten overall survival or increase the likelihood of tumor recurrence. Medicine (Baltimore) 2014;93(28) doi: 10.1097/MD.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Al-Saeedi M., Ghamarnejad O., Khajeh E., et al. Pringle maneuver in extended liver resection: a propensity score analysis. Sci Rep. 2020;10(1):8847. doi: 10.1038/s41598-020-64596-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ferrero A., Russolillo N., Viganò L., Lo Tesoriere R., Muratore A., Capussotti L. Does Pringle maneuver affect survival in patients with colorectal liver metastases? World J Surg. 2010;34(10):2418–2425. doi: 10.1007/s00268-010-0682-2. [DOI] [PubMed] [Google Scholar]
- 193.Lee K.F., Chong C.C.N., Cheung S.Y.S., et al. Impact of intermittent Pringle maneuver on long-term survival after hepatectomy for hepatocellular carcinoma: result from two combined randomized controlled trials. World J Surg. 2019;43(12):3101–3109. doi: 10.1007/s00268-019-05130-8. [DOI] [PubMed] [Google Scholar]
- 194.Liu S., Li X., Li H., et al. Longer duration of the Pringle maneuver is associated with hepatocellular carcinoma recurrence following curative resection. J Surg Oncol. 2016;114(1):112–118. doi: 10.1002/jso.24271. [DOI] [PubMed] [Google Scholar]
- 195.Nijkamp M.W., van der Bilt J.D.W., Snoeren N., et al. Prolonged portal triad clamping during liver surgery for colorectal liver metastases is associated with decreased time to hepatic tumour recurrence. Eur J Surg Oncol. 2010;36(2):182–188. doi: 10.1016/j.ejso.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 196.Yamashita S., Venkatesan A.M., Mizuno T., et al. Remnant liver ischemia as a prognostic factor for cancer-specific survival after resection of colorectal liver metastases. JAMA Surg. 2017;152(10):e172986. doi: 10.1001/jamasurg.2017.2986. e172986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cho J.Y., Han H.-S., Choi Y., et al. Association of remnant liver ischemia with early recurrence and poor survival after liver resection in patients with hepatocellular carcinoma. JAMA Surg. 2017;152(4):386–392. doi: 10.1001/jamasurg.2016.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Teng D.H., Zhu Z.J., Zheng H., et al. Effect of steatosis donor liver transplantation on hepatocellular carcinoma recurrence: experience at a single institution. Hepatogastroenterology. 2012;59(115):858–862. doi: 10.5754/hge12007. [DOI] [PubMed] [Google Scholar]
- 199.Cusumano C., De Carlis L., Centonze L., et al. Advanced donor age does not increase risk of hepatocellular carcinoma recurrence after liver transplantation: a retrospective two-centre analysis using competing risk analysis. Transpl Int. 2021;34(10):1948–1958. doi: 10.1111/tri.13950. [DOI] [PubMed] [Google Scholar]
- 200.Zhou J., Huang Z., Chen Z., Xu F., Tong R., Zheng S. Impact of donor age on liver transplant outcomes in patients with hepatocellular carcinoma: analysis of the SRTR database. BMC Gastroenterol. 2021;21(1):195. doi: 10.1186/s12876-021-01786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Siegel A.B., Lim E.A., Wang S., et al. Diabetes, body mass index, and outcomes in hepatocellular carcinoma patients undergoing liver transplantation. Transplantation. 2012;94(5):539–543. doi: 10.1097/TP.0b013e31825c58ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Adeniji N., Arjunan V., Prabhakar V., et al. Posttransplant outcomes in older patients with hepatocellular carcinoma are driven by non-hepatocellular carcinoma factors. Liver Transpl Off Publ Am Assoc Study Liver Dis Int Liver Transpl Soc. 2021;27(5):684–698. doi: 10.1002/lt.25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kornberg A., Witt U., Kornberg J., Friess H., Thrum K. Extended ischemia times promote risk of HCC recurrence in liver transplant patients. Dig Dis Sci. 2015;60(9):2832–2839. doi: 10.1007/s10620-015-3541-z. [DOI] [PubMed] [Google Scholar]
- 204.Scalea J.R., Redfield R.R., Foley D.P. Liver transplant outcomes using ideal donation after circulatory death livers are superior to using older donation after brain death donor livers. Liver Transpl. 2016;22(9):1197–1204. doi: 10.1002/lt.24494. [DOI] [PubMed] [Google Scholar]
- 205.Silverstein J., Roll G., Dodge J.L., Grab J.D., Yao F.Y., Mehta N. Donation after circulatory death is associated with similar posttransplant survival in all but the highest-risk hepatocellular carcinoma patients. Liver Transpl. 2020;26(9):1100–1111. doi: 10.1002/lt.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Khorsandi S.E., Yip V.S., Cortes M., et al. Does donation after cardiac death utilization adversely affect hepatocellular cancer survival? Transplantation. 2016;100(9) doi: 10.1097/TP.0000000000001150. https://journals.lww.com/transplantjournal/Fulltext/2016/09000/Does_Donation_After_Cardiac_Death_Utilization.23.aspx [DOI] [PubMed] [Google Scholar]
- 207.Croome K.P., Lee D.D., Burns J.M., et al. The use of donation after cardiac death allografts does not increase recurrence of hepatocellular carcinoma. Am J Transpl. 2015;15(10):2704–2711. doi: 10.1111/ajt.13306. [DOI] [PubMed] [Google Scholar]
- 208.Martinez-Insfran L.A., Ramirez P., Cascales P., et al. Early outcomes of liver transplantation using donors after circulatory death in patients with hepatocellular carcinoma: a comparative study. Transpl Proc. 2019;51(2):359–364. doi: 10.1016/j.transproceed.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 209.Mao X.-L., Cai Y., Chen Y.-H., et al. Novel targets and therapeutic strategies to protect against hepatic ischemia reperfusion injury. Front Med. 2021;8 doi: 10.3389/fmed.2021.757336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Su Z.-R., Cui Z.-L., Ma J.-L., et al. Beneficial effects of S-adenosyl-L-methionine on post-hepatectomy residual liver function: a prospective, randomized, controlled clinical trial. Hepatogastroenterology. 2013;60(125):1136–1141. doi: 10.5754/hge11072. [DOI] [PubMed] [Google Scholar]
- 211.Neumann U.P., Kaisers U., Langrehr J.M., et al. Administration of prostacyclin after liver transplantation: a placebo controlled randomized trial. Clin Transpl. 2000;14(1):70–74. doi: 10.1034/j.1399-0012.2000.140113.x. [DOI] [PubMed] [Google Scholar]
- 212.Bruno S., Chiabotto G., Camussi G. Extracellular vesicles: a therapeutic option for liver fibrosis. Int J Mol Sci. 2020;21(12) doi: 10.3390/ijms21124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Anger F., Camara M., Ellinger E., et al. Human mesenchymal stromal cell-derived extracellular vesicles improve liver regeneration after ischemia reperfusion injury in mice. Stem Cell Dev. 2019;28(21):1451–1462. doi: 10.1089/scd.2019.0085. [DOI] [PubMed] [Google Scholar]
- 214.Jin G., Qiu G., Wu D., et al. Allogeneic bone marrow-derived mesenchymal stem cells attenuate hepatic ischemia-reperfusion injury by suppressing oxidative stress and inhibiting apoptosis in rats. Int J Mol Med. 2013;31(6):1395–1401. doi: 10.3892/ijmm.2013.1340. [DOI] [PubMed] [Google Scholar]
- 215.Teratani T., Kasahara N., Fujimoto Y., et al. Mesenchymal stem cells secretions enhanced ATP generation on isolated islets during transplantation. Islets. 2022;14(1):69–81. doi: 10.1080/19382014.2021.2022423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Liu P., Mao Y., Xie Y., Wei J., Yao J. Stem cells for treatment of liver fibrosis/cirrhosis: clinical progress and therapeutic potential. Stem Cel Res Ther. 2022;13(1):356. doi: 10.1186/s13287-022-03041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang H., Zhang T., Zhong F., Xia X. Effects of remote ischemic preconditioning on liver injury following hepatectomy: a systematic review and meta-analysis of randomized control trials. Surg Today. 2021;51(8):1251–1260. doi: 10.1007/s00595-020-02205-1. [DOI] [PubMed] [Google Scholar]
- 218.Jakubauskiene L., Jakubauskas M., Stiegler P., Leber B., Schemmer P., Strupas K. Ischemic preconditioning for liver transplantation: a systematic review and meta-analysis of randomized controlled trials. Visc Med. 2021;37(5):329–337. doi: 10.1159/000516608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ravaioli M., Germinario G., Dajti G., et al. Hypothermic oxygenated perfusion in extended criteria donor liver transplantation-A randomized clinical trial. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. 2022;22(10):2401–2408. doi: 10.1111/ajt.17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.van Rijn R., Schurink I.J., de Vries Y., et al. Hypothermic machine perfusion in liver transplantation - a randomized trial. N Engl J Med. 2021;384(15):1391–1401. doi: 10.1056/NEJMoa2031532. [DOI] [PubMed] [Google Scholar]
- 221.Schlegel A., Mueller M., Muller X., et al. A multicenter randomized-controlled trial of hypothermic oxygenated perfusion (HOPE) for human liver grafts before transplantation. J Hepatol. January 2023 doi: 10.1016/j.jhep.2022.12.030. [DOI] [PubMed] [Google Scholar]
- 222.He N., Jia J., Xie H., et al. 2018. Partial inhibition of HO-1 attenuates HMP-induced hepatic regeneration against liver injury in rats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jia J., Xie H., Li J., et al. 2019. Graft protection of the liver by hypothermic machine perfusion involves recovery of graft regeneration in rats. 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Reiling J., Butler N., Simpson A., et al. Assessment and transplantation of orphan donor livers: a back-to-base approach to normothermic machine perfusion. Liver Transpl Off Publ Am Assoc Study Liver Dis Int Liver Transpl Soc. 2020;26(12):1618–1628. doi: 10.1002/lt.25850. [DOI] [PubMed] [Google Scholar]
- 225.Markmann J.F., Abouljoud M.S., Ghobrial R.M., et al. Impact of portable normothermic blood-based machine perfusion on outcomes of liver transplant: the OCS liver PROTECT randomized clinical trial. JAMA Surg. 2022;157(3):189–198. doi: 10.1001/jamasurg.2021.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nasralla D., Coussios C.C., Mergental H., et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557(7703):50–56. doi: 10.1038/s41586-018-0047-9. [DOI] [PubMed] [Google Scholar]
- 227.Guo Z., He X., Tang Y., et al. Ischemia-free liver transplantation in pigs: ischemia reperfusion injury is avoidable? Am J Transpl. 2018;18:904. doi: 10.1111/ajt.14918LK. https://libkey.io/libraries/2953?sid=EMBASE&sid=EMBASE&issn=16006143&id=doi:10.1111%2Fajt.14918&atitle=Ischemia-free+liver+transplantation+in+pigs%3A+Ischemia+reperfusion+injury+is+avoidable%3F&stitle=Am.+J.+Transplant.&title=American+Journal+of+Transplantation&volume=18&issue=&spage=904&epage=&aulast=Guo&aufirst=Z.&auinit=Z.&aufull=Guo+Z.&coden=&isbn=&pages=904-&date=2018&auinit1=Z&auinitm= [DOI] [Google Scholar]
- 228.He X., Guo Z., Zhao Q., et al. The first case of ischemia-free organ transplantation in humans: a proof of concept. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. 2018;18(3):737–744. doi: 10.1111/ajt.14583. [DOI] [PubMed] [Google Scholar]
- 229.Tang Y., Wang T., Ju W., Li F., Zhang Q. Ischemic-free liver transplantation reduces the recurrence of hepatocellular carcinoma after liver transplantation. 2021;11(December):1–11. doi: 10.3389/fonc.2021.773535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mueller M., Kalisvaart M., O’Rourke J., et al. Hypothermic oxygenated liver perfusion (HOPE) prevents tumor recurrence in liver transplantation from donation after circulatory death. Ann Surg. 2020;272(5):759–765. doi: 10.1097/SLA.0000000000004258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.