Abstract
In the so called COVID19 era, headache, fever and gastrointestinal symptoms are highly suggestive for SARS-CoV-2 infection, but in all the cases presenting to the emergency room, clinicians should always keep in mind alternative diagnoses, particularly if the patient is pregnant. Life-threatening diseases, such as eclampsia and posterior reversible encephalopathy syndrome (PRES), should be promptly recognized and treated. Eclampsia is defined as a seizure occurring in association with pre-eclampsia, and it represents one of the major and serious obstetric disorders associated with significant maternal and perinatal morbidity and mortality. PRES is a distinctive clinical and imaging syndrome characterized by acute headaches, visual impairment, seizures, and altered sensorium, that can be associated with severe eclampsia. Emergency clinicians should always consider eclampsia in the differential diagnosis of headache in pregnant women. The prompt and accurate diagnosis of eclampsia/PRES is crucial to prevent adverse maternal and perinatal outcomes. Here we describe the case of a young pregnant woman admitted to our emergency department for fever, dyspnea, headache, nausea and vomiting, who developed generalized tonic clonic seizures and a subsequent status epilepticus due to eclampsia and PRES. (www.actabiomedica.it)
Keywords: eclampsia, pregnancy complication, brain edema, posterior reversible encephalopathy syndrome, cerebral vasospasm, COVID19, SARS-CoV-2
Introduction
In the current “COVID19 (coronavirus disease 19) era” fever, headache, dyspnea and gastrointestinal symptoms are suggestive for SARS-CoV-2 infection, that should be always considered in the differential diagnosis for the best management of the patients and to avoid the spread of the virus in the emergency department and the so called “no COVID wards”. Particular attention should be given to pregnant patients because life-threatening diseases, such eclampsia and posterior reversible encephalopathy syndrome (PRES), can mimic COVID19. The prompt recognition and treatment of these two conditions is pivotal to avoid fatal consequences both for the patient and the fetus. Eclampsia is a multisystem disorder defined as the occurrence of one or more seizures before, during or after delivery in women with signs or symptoms of pre-eclampsia (1). Pre-eclampsia is a serious hypertensive complication of pregnancy, that occurred in 5% of pregnancies (2). Pre-eclampsia is defined by the presence of new-onset hypertension (>140/90 mmHg) and occurrence of proteinuria (≥0.3 g in 24-hour urine or ≥+1 on dipstick in a random urine sample) after the 20th week of gestation (3). The incidence of progression to eclampsia is about 0.5% in patients with mild pre-eclampsia and 2 - 3% in those with severe pre-eclampsia, as defined by a systolic blood pressure of 160 mmHg or higher, nephrotic-range proteinuria (>3.5 g/24-hour urine), renal function impairment, thrombocytopenia, and/or evidence of microangiopathic hemolytic anemia, hepatocellular injury, pulmonary edema, and neurologic disturbances (4). The pathophysiology of pre-eclampsia/eclampsia is not properly understood. Maternal, fetal and placental factors are involved in the pathogenesis of the disease (5). Based on these observations, pre-eclampsia must be considered as a multisystem disorder that often involves the central nervous system. Common prodromal symptoms and signs of pre-eclampsia include headache and visual changes (6). Most women have a classic presentation of pre-eclampsia (hypertension and proteinuria) beyond 20 weeks of gestation and/or less than 48 hours after delivery, although “atypical cases” presenting with hypertension or proteinuria outside these time intervals, have been recently described (7). The estimated incidence of eclampsia is 1.4% (8). About 15% of maternal deaths worldwide are due to pre-eclampsia/eclampsia, despite in developed countries maternal mortality associated with eclampsia is extremely low (9). Neurologic signs and symptoms of eclampsia include hyperreflexia, headache, visual disorders, seizures, confusion, or disturbances of consciousness and cerebral hemorrhage. The pathogenesis of eclamptic convulsions remains unknown. Cerebral imaging suggests that cerebral abnormalities in eclampsia (mostly vasogenic edema) are similar to those found in hypertensive encephalopathy (10). As reported and validated in large, randomized placebo-controlled trials, magnesium sulphate is the drug of choice to prevent and treat eclampsia, recurrent seizures, and intra- and immediately post-partum complications (11).
Case presentation
A 31-year-old primigravida woman at 35 weeks gestation was admitted to our emergency department complaining of occipital headache, fever, nausea, vomiting, and focal neurological signs over three days. According to the current triage-protocol process adopted in our emergency department, the patient was assigned to the “COVID19 area” (12). Her past medical history was unremarkable. At admission she was slowed and confused. She had high blood pressure (190/90 mmHg), high cardiac rate (130 beats/minute) and peripheral oxygen saturation at the lower limit of the normal range (SpO2 96% at room ambient), with normal respiratory rate and body temperature. Sinus tachycardia was detected on ECG. Urine dipstick resulted positive for protein (3+). Point-of-care lung ultrasound (LUS) showed diffuse B lines with slight bilateral pleural effusion as observed in pulmonary edema. Echocardiography documented normal ventricular function in absence of indirect signs of pulmonary embolism. Inferior vena cava was dilated and fixed, as sign of hypervolemia. Such evidence confirmed the diagnosis of pulmonary edema (13), excluding pulmonary embolism and acute respiratory distress syndrome, that can common complications in COVID19 patients (14-15).
Immediately after clinical evaluation, she developed general severe convulsive seizures and a subsequent status epilepticus. A diagnosis of eclamptic seizures was promptly done. Magnesium sulphate (loading dose: 4 g intravenous in 5-10 minutes; maintenance dose by pump infusion: 1 g/h for 24 hours after last seizure and 24 hours after birth), midazolam (5 mg intravenous bolus), labetalol (10 mg in repeated intravenous boluses until the target blood pressure 140-90 mmHg) and high-flow oxygen (Venti-mask, FiO2 50%) were promptly administered to control seizures and blood pressure. The patient was intubated, and a caesarean section was immediately performed. The baby was born healthy with no signs of severe respiratory distress. The biopsy of the placenta confirmed the diagnosis of eclampsia, documenting diffuse multiple infarctions and syncytiotrophoblastic hyperplasia, that are the most obvious morphologic alterations of the placenta in eclamptic pregnancy.
Laboratory findings showed significant proteinuria (1000 mg/dL), a slight increase in transaminases (AST 35 U/L, ALT 32 U/L, normal value 10-31) and LDH (307 U/L, normal value 0-247). Blood counts, C-reactive protein, bilirubin, renal function, serum electrolytes, glucose, lipase and CPK were all in the normal range. Repeated urine and blood cultures resulted negative. Mycoplasma, Legionella and Streptococcus pneumoniae infection were excluded. Two nasopharyngeal swabs ruled out SARS-CoV-2 infection.
After delivery, the patient underwent magnetic resonance imaging (MRI) of the brain, that revealed diffuse vasogenic edema in the occipital, parietal and frontal lobes white matter (figure. 1 A-C). MR angiography showed vasospasm of the right occipital artery (figure. 2B), brain perfusion the absence of impaired cerebral blood flow (figure. 2C). The radiological picture was compatible with PRES and reversible cerebral vasoconstriction syndrome (RCVS). Pulmonary angiography and high-resolution chest CT scan confirmed the diagnosis of pulmonary edema and excluded pulmonary embolism and interstitial pneumoniae. The patient was admitted to the intensive care unit and treated with nimodipine (a calcium channel blocker used to prevent brain damage caused by reduced blood flow), labetalol, levetiracetam, dexamethasone, magnesium sulphate, low molecular weight heparin, ceftriaxone and enteral nutrition. She was extubated after 2 days with a progressive improvement in her neurological conditions, as documented by EEGs repeated over time. Seven days after initial imaging, follow-up MRI revealed near-complete resolution of the typical PRES hallmarks, with only a residual vasogenic edema in the parietal and occipital lobes (figure 2 E-F), and the complete resolution of cerebral vasospasm (figure 2B).
Figure 1.
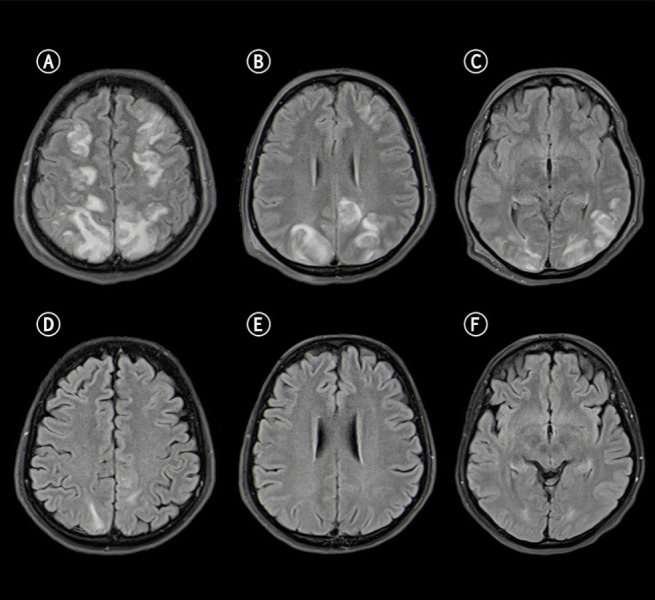
Fluid Attenuated Inversion Recovery (FLAIR) sequences show increased signal in the subcortical frontal (A), parietal (B), and occipital (C) lobes, cerebral cortex with swollen appearance and narrow sulci at the vertex. Apparent diffusion coefficient (ADC) was increased (not show) indicating vasogenic edema. MRI control performed days later documents near-normalization of the pathological findings (D-F).
Figure 2.
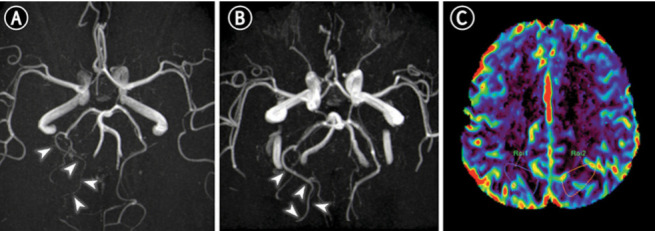
MR angiography and brain perfusion. On axial 3D reformatted Time Of Flight MR Angiography (TOF-MRA) there is diffuse narrowing of the right occipital artery (A, white arrowheads on distal branches,) with subsequent resolution of cerebral vasoconstriction (B) suggesting overlap syndrome (PRES/RCVS). Perfusion imaging was unremarkable with no abnormal Cerebral Blood Flow (CBF) values in both hemispheres (C).
The patient was discharged after 14 days of hospitalization in a good clinical condition with an anti-hypertensive and anti-epileptic therapy and scheduled neurological, cardiological and gynecological follow-up.
Discussion
The cerebral manifestations of eclampsia are the outcome of several mechanisms, including hypertensive encephalopathy and failure of cerebral blood flow autoregulation, that leads to cerebral edema and hemorrhage. Neuropathological findings show cerebral edema and, in complicated cases, ischemic or hemorrhagic lesions, that cause seizure foci and visual hallucination or cortical blindness (17). The incidence of PRES in eclampsia is not precisely known. In some studies, 92-98% of eclamptic patients had PRES (18, 19). PRES can also occur in puerperium and can be frequently misdiagnosed with syndromes sharing similar MRI findings such as hypoglycemic encephalopathy, extrapontine myelinolysis and deep venous thrombosis (20). The etiopathogenesis of PRES in eclampsia is not completely clear, but it seems to be related to two mechanisms: the altered cerebral flow autoregulation and the endothelial damage. Clinical and radiological signs of PRES are well explained by two widely accepted theories: the “vasogenic theory” and the “vasospasm theory” (21-23). Brain CT scan and MRI are radiological techniques of choice to evaluate complicated eclampsia. Typical findings are multiple areas of hypodensity in occipito-parietal regions and focal vasogenic cerebral edema. EEG in patients with eclampsia has revealed evidence of diffuse cerebral dysfunction (delta waves) and epileptiform activity (spikes or sharp waves) (24). PRES is a clinical-neuroradiological entity that can be associated with several well-known causes, the most common of which are severe eclampsia, hypertension, and immunosuppressive treatment (25). Radiologists may be the first to suggest the diagnosis of PRES. Brain CT and MR images in PRES can help in the early diagnosis and treatment: neuroimaging is characterized by cerebral edema predominantly affecting the parietal and occipital lobes (26). When promptly recognized and treated, PRES is usually reversible: symptoms generally resolve within a period of days or weeks while recovery of the MRI abnormalities takes longer (27). If unrecognized, PRES can progress to ischemia, massive infarction, and death. In pre-eclampsia/eclampsia patients, headache, seizures, altered sensorium, and vision loss are cardinal symptoms of PRES, although headache is significantly more frequent as initial PRES-related symptom (28). Management of eclampsia include anti-hypertensive, anticonvulsive and anti-edemigen drugs, and the delivery of the infant. Magnesium sulphate is the drug of choice for controlling convulsion and vasospasm (3). In case of ongoing seizures uncontrolled with magnesium sulphate, benzodiazepines should be used. Promptly treatment of hypertension is paramount (29). Severe hypertension (>160/110 mmHg) should be treated with intravenous anti-hypertensive drugs such as labetalol, hydralazine, or nifedipine. Aggressive blood pressure control is crucial in PRES to avoid permanent disability and even death (30). The obstetrical management depends on the time when eclampsia occurs and on the efficacy of the medical treatment. Once the patient is stabilized, the delivery of fetus should be prioritized. Headache, visual disorders, confusion, and right upper quadrant/epigastric pain forewarn recurrent and impending seizures, and they are an indication for a prompt delivery of the fetus. Major systemic complications of eclampsia include pulmonary edema, renal failure, disseminated intravascular coagulation, acute respiratory distress syndrome, stroke, or cardiac arrest. Attention must also be paid to correcting any associated dehydration and electrolyte imbalance, avoiding hyper-hydration that can result in pulmonary edema. Adequate oxygenation should always be ensured. As result, a multidisciplinary approach is mandatory for the correct management of eclamptic patients.
Conclusion
Eclampsia is rare, but a serious and potential life-threatening condition. Prompt recognition and appropriate management is crucial to reduce maternal and perinatal morbidity and mortality. Emergency physicians should consider the diagnosis of eclampsia in in a woman who is pregnant or in the puerperium (up to 4 weeks post-partum), presenting with pulsating bilateral (temporal, frontal, occipital or diffuse) headache, vision changes, elevated blood pressure, or seizures (31). Women with a history of eclampsia are at increased risk of eclampsia (1-2%) and pre-eclampsia (22-35%) in subsequent pregnancies (10). PRES is a serious complication of eclampsia. Early recognition of PRES is mandatory for preventing permanent neurological sequelae such as permanent vision loss. Early correction of increased blood pressure and treatment of seizures are the cornerstones of eclamptic encephalopathy treatment. Finally, in the current era of high incidence of COVID19, emergency physicians should always keep in mind that not all the patients admitted to the emergency room complaining suggestive symptoms for SARS-CoV-2 infection, such as the case reported, are COVID19 patients. Hence the importance of a correct approach to the patient based on the patient’s medical history, the development of signs and symptoms, and the clinical course. Clinicians should always consider alternative diagnoses, particularly for life-threatening diseases, which can have a complete restitutio ad integrum if promptly recognized and treated, such as eclampsia and PRES.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- The Eclampsia Trial Collaborative Group. Which anticonvulsant for women with eclampsia? Evidence from the collaborative eclampsia trial. Lancet. 1995;345:1455–63. [PubMed] [Google Scholar]
- Pearce CF, Hansen WF. Headache and neurological disease in pregnancy. Clin Obstet Gynecol. 2012;55(3):810–828. doi: 10.1097/GRF.0b013e31825d7b68. doi: 10.1097/GRF.0b013e31825d7b68. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- Lindheimer MD, Taler SJ, Cunningham FG. ASH position paper: hypertension in pregnancy. J Clin Hypertens (Greenwich) 2009;11(4):214–225. doi: 10.1111/j.1751-7176.2009.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro PT. Management of eclampsia in the accident and emergency department. J Accid Emerg Med. 2000;17(1):7–11. doi: 10.1136/emj.17.1.7. doi: 10.1136/emj.17.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karumanchi SA, Lindheimer MD. Advances in the understanding of eclampsia. Curr Hypertens Rep. 2008;10(4):305–12. doi: 10.1007/s11906-008-0057-3. doi: 10.1007/s11906-008-0057-3. [DOI] [PubMed] [Google Scholar]
- Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. 2009;200(5):481.e1–7. doi: 10.1016/j.ajog.2008.07.048. doi: 10.1016/j.ajog.2008.07.048. Epub 2008 Nov 18. [DOI] [PubMed] [Google Scholar]
- Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1–7. doi: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–33. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- Sibai Baha M. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402–10. doi: 10.1097/01.AOG.0000152351.13671.99. doi: 10.1097/01.AOG.0000152351.13671.99. [DOI] [PubMed] [Google Scholar]
- Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877–90.16. doi: 10.1016/s0140-6736(02)08778-0. [DOI] [PubMed] [Google Scholar]
- Poggiali E, Vercelli A, Cillis MG, Ioannilli E, Iannicelli T, Magnacavallo A. Triage decision-making at the time of COVID-19 infection: the Piacenza strategy. Intern Emerg Med. 2020;15(5):879–882. doi: 10.1007/s11739-020-02350-y. doi: 10.1007/s11739-020-02350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copetti R, Soldati G, Copetti R. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovascular Ultrasound. 2008;6:16. doi: 10.1186/1476-7120-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggiali E, Bastoni D, Ioannilli E, Vercelli A, Magnacavallo A. Deep Vein Thrombosis and Pulmonary Embolism: Two Complications of COVID-19 Pneumonia? Eur J Case Rep Intern Med. 2020;7(5):001646. doi: 10.12890/2020_001646. doi: 10.12890/2020_001646. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni Luciano, Chiumello Davide, Rossi Sandra. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24:154. doi: 10.1186/s13054-020-02880-z. Published online 2020 Apr 16. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J O. Eclamptic hypertensive encephalopathy. Semin Neurol. 1988;8(3):230–3. doi: 10.1055/s-2008-1041383. doi: 10.1055/s-2008-1041383. [DOI] [PubMed] [Google Scholar]
- Brewer J, Owens MY, Wallace K, Reeves AA, Morris R, Khan M, Lamarca B, Martin JN., Jr Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am J Obstet Gynecol. 2013;208(6):468.e1–468.e6. doi: 10.1016/j.ajog.2013.02.015. doi: 10.1016/j.ajog.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Mayama M, Uno K, Tano S, Yoshihara M, Ukai M, Kishigami Y, et al. Incidence of posterior reversible encephalopathy syndrome in eclamptic and patients with preeclampsia with neurologic symptoms. Am J Obstet Gynecol. 2016;215:239.e1–5. doi: 10.1016/j.ajog.2016.02.039. [DOI] [PubMed] [Google Scholar]
- Morelli N, Gori S, Michelassi MC, Falorni M, Cafforio G, Bianchi MC, Cosottini M, Orlandi G, Murri L, Tartaglione A. Atypical posterior reversible encephalopathy syndrome in puerperium. Eur Neurol. 2008;59(3-4):195–7. doi: 10.1159/000114044. doi: 10.1159/000114044. Epub 2008 Jan 29. [DOI] [PubMed] [Google Scholar]
- Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036–1042. doi: 10.3174/ajnr.A0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata G, Greco A, Iannella G, Granata M, Manno A, Savastano E, et al. Posterior reversible encephalopathy syndrome—insight into pathogenesis, clinical variants and treatment approaches. Autoimmun Rev. 2015;14:830–6. doi: 10.1016/j.autrev.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: Clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914–259. doi: 10.1016/S1474-4422(15)00111-8. [DOI] [PubMed] [Google Scholar]
- Thomas SV. Neurological aspects of eclampsia. J Neurol Sci. 1998;155(1):37–43. doi: 10.1016/s0022-510x(97)00274-8. doi: 10.1016/s0022-510x(97)00274-8. [DOI] [PubMed] [Google Scholar]; Kaplan PW. Neurologic aspects of eclampsia. Neurol Clin. 2004;22(4):841–61. doi: 10.1016/j.ncl.2004.07.005. doi: 10.1016/j.ncl.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Camara-Lemarroy CR, Escobedo-Zúñiga N, Villarreal-Garza E, García-Valadez E, Góngora-Rivera F, Villarreal-Velázquez HJ. Posterior reversible leukoencephalopathy syndrome (PRES) associated with severe eclampsia: Clinical and biochemical features. Pregnancy Hypertens. 2017;7:44–49. doi: 10.1016/j.preghy.2017.01.003. doi: 10.1016/j.preghy.2017.01.003. Epub 2017 Jan 16. [DOI] [PubMed] [Google Scholar]
- Shankar J, Banfield J. Posterior Reversible Encephalopathy Syndrome: A Review. Can Assoc Radiol J. 2017;68(2):147–153. doi: 10.1016/j.carj.2016.08.005. doi: 10.1016/j.carj.2016.08.005. Epub 2017 Jan 26. [DOI] [PubMed] [Google Scholar]
- Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205–210. doi: 10.1001/archneurol.2007.46. [DOI] [PubMed] [Google Scholar]
- Liman TG, Bohner G, Heuschmann PU, Scheel M, Endres M, Siebert E. Clinical and radiological differences in posterior reversible encephalopathy syndrome between patients with preeclampsia-eclampsia and other predisposing diseases. Eur J Neurol. 2012;19(7):935–43. doi: 10.1111/j.1468-1331.2011.03629.x. doi: 10.1111/j.1468-1331.2011.03629.x. [DOI] [PubMed] [Google Scholar]
- Lew M, Klonis E. Emergency management of eclampsia and severe pre-eclampsia. Emerg Med (Fremantle) 2003;15(4):361–8. doi: 10.1046/j.1442-2026.2003.00475.x. doi: 10.1046/j.1442-2026.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- Wagner SJ, Acquah LA, Lindell EP, Craici IM, Wingo MT, Rose CH, White WM, August P, Garovic VD. Posterior reversible encephalopathy syndrome and eclampsia: pressing the case for more aggressive blood pressure control. Mayo Clin Proc. 2011;86(9):851–6. doi: 10.4065/mcp.2011.0090. doi: 10.4065/mcp.2011.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro A, Delaruelle Z, Ivanova TA, Khan S, Ornello R, Raffaelli B, Terrin A, Reuter U, Mitsikostas DD. European Headache Federation School of Advanced Studies (EHF-SAS). Headache and pregnancy: a systematic review. J Headache Pain. 2017;18(1):106. doi: 10.1186/s10194-017-0816-0. doi: 10.1186/s10194-017-0816-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


