Abstract
Rituximab is a monoclonal antibody against the protein CD20. Various lymphomas as well as non-malignant immune disorders are treated with this antibody. Hypersensitivity reactions associated with the use of rituximab include urticaria, hypotension, chest tightness, vomiting, oxygen desaturation and bronchospasm. A very uncommon case of hypertensive crisis and pulmonary edema following rituximab-induced hypersensitivity reaction in an 80-year-old man receiving rituximab for non-Hodgkin lymphoma is reported. Anaphylaxis manifesting as coronary vasospasm following drug treatment, including rituximab, could be proved a serious condition in patients who need specific treatment. In these patients desensitization protocols seem to be mandatory. (www.actabiomedica.it)
Keywords: Anaphylaxis, hypertensive crisis, Kounis syndrome, pulmonary edema, rituximab
Introduction
Rituximab is a chimeric monoclonal antibody against the CD20 antigen, which is mainly found on the surface of immune system B lymphocytes (B cells). By binding to this protein, the drug induces cell death. Various lymphomas as well as nonmalignant immune disorders are treated with this antibody (1-4). Mild or severe allergic reactions are attributed to antibody’s portion of mouse protein, while the majority of the antibody is human protein (4,5). We report a unique case of hypertensive crisis and pulmonary edema associated with anaphylaxis following rituximab administration.
Case presentation
An 80-year-old male patient, suffering from non-Hodgkin lymphoma, with previous medical history of paroxysmal atrial fibrillation for which he was receiving apixaban 2,5 mg twice per day, developed uneasiness, chest discomfort, pallor, sweating, tachypnea, skin discoloration with flushing and netting, hypertension with systolic blood pressure of 210 mmHg culminating to acute pulmonary edema and new onset of rapid-ventricular-response atrial fibrillation following rituximab infusion (Figure 1).
Figure 1.
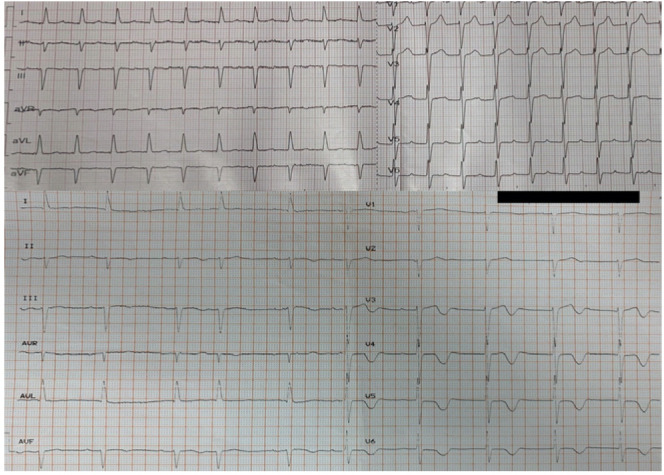
Atrial fibrillation with rapid ventricular response (above). Sinus rhythm with atrial extrasystole and negative T waves immediately after cardioversion (below).
A bolus of 500 mg of hydrocortisone had been administered before rituximab infusion. The patient was transferred to Intensive Care Unit where he was treated with high-dose oxygen, intravenous furosemide, high doses of nitrates and 300 mg amiodarone. An extra dose of 500 mg of hydrocortisone was also administered due to suspected allergic reaction. The arterial blood gases showed respiratory acidosis and increased levels of lactate. Due to hemodynamic instability and being on apixaban, emergent cardioversion was performed successfully. The electrocardiogram recorded after cardioversion showed 1st degree atrio-ventricular block with atrial extrasystole, left axis, biphasic T waves at lead V2 and negative T waves in V3-V6 precordial leads (Figure 1). The echocardiogram showed normal left ventricular dimensions and wall thickness, reduced left ventricular ejection fraction to 35%, paradoxical movement of interventricular septum, diastolic dysfunction grade II, moderate aortic valve stenosis, small regurgitation of both atrio-ventricular valves and increased pulmonary artery systolic pressure. Due to persistently low cardiac output signs, with marked vasoconstriction combined with pulmonary edema not responding to diuretics, he underwent hemodialysis. The troponin I levels rose up: from a baseline value before injection of rituximab of 14,7 pg/mL (upper reference level – URL - <34,2 pg/mL; Abbott Diagnostics), to 190,2 pg/mL during reaction, thus reducing in the next day to 70,9 pg/mL). C-reactive protein levels were also increased from 7.4 mg/L before injection of rituximab to 63,5 mg/L in the next day (normal values: 0-5mg/L). He subsequently underwent coronary angiography, which showed normal coronary arteries without hemodynamically significant stenosis (Figure 2).
Figure 2.
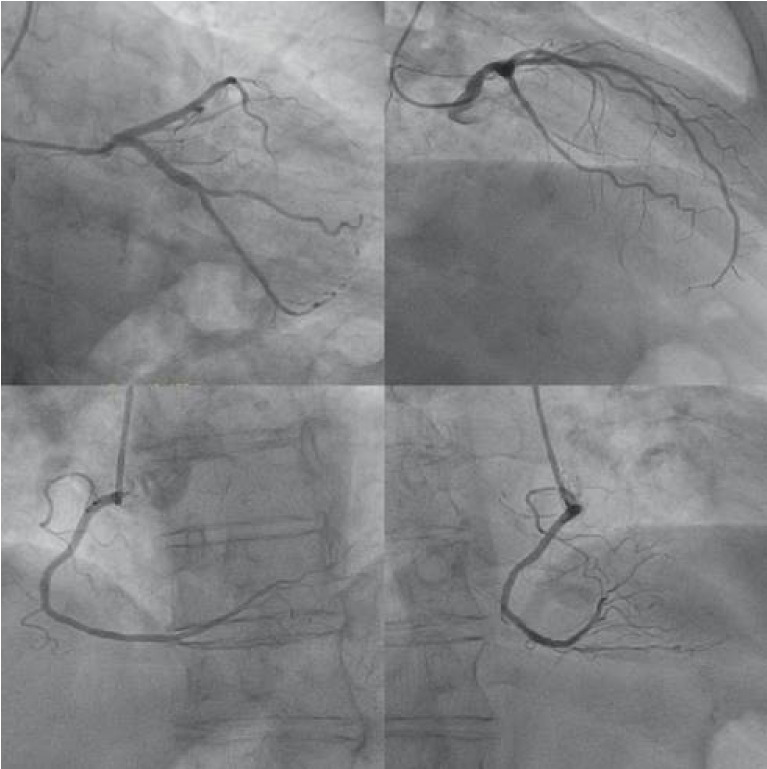
Coronary angiography: coronary arteries without significant stenosis.
The patient continued to receive high doses of diuretics and nitrates with progressive improvement and after 6 days he was discharged with the diagnosis of type 2 myocardial infarction due to demand ischemia from the hypertensive crisis, anaphylaxis and atrial fibrillation associated with Kounis syndrome of type I variant (3). One month later, echocardiography revealed ejection fraction of 50-55%, and improvement of diastolic function (type I).
Discussion
Rituximab infusion adverse effects are not rare and vary from severe anaphylactoid events with cardiogenic shock, death, acute respiratory distress syndrome, myocardial infarction and pulmonary infiltrates to less malignant ones such as angioedema, bronchospasm and urticaria (4). The incidence of hypersensitivity reactions induced by infusion of this drug in non-Hodgkin lymphoma is referred to be up to 77% (4,7,8). Reactions to rituximab can be either due to type I immunoglobulin E (IgE) mediated reaction or due to massive cytokine release-mediated reaction (5). First-time rituximab administration can provoke hypersensitivity reactions; however, this can happen even after administration of the subsequent therapeutic sessions. The majority of early drug reactions occur within minutes to a few hours after the administration of a drug previously well-tolerated (5,9).
Kounis syndrome is a disease manifesting as coronary artery spasm, myocardial infarction or coronary thrombosis (including stent thrombosis) and its pathophysiologic basis is either due to type I IgE-mediated reaction or due to massive release of cytokine (cytokine-mediated) or due to mix of both (6). The cardiovascular and respiratory symptoms are the most prominent and most dangerous characteristics of anaphylaxis (10). Coronary artery spasm progressing to acute myocardial infarction, thrombotic myocardial infarction or even sudden death have been reported on several occasions with the use of rituximab (11). In a case of rituximab-induced Kounis syndrome coronary angiography showed atypical takotsubo pattern of left mid-ventricular ballooning and the patient was treated with stenting (12).
The first electrocardiogram after the Rituximab infusion showed rapid atrial fibrillation supporting the view of type 2 myocardial infarction due to demand ischemia from the hypertensive crisis, atrial fibrillation and anaphylaxis. The T wave inversion post cardioversion could be due to cardiac memory phenomenon. Cardiac memory appears with T wave inversion after a change in the activation sequence of the heart. It may mimic cardiac ischemia and may mask any condition that appears with T wave abnormality on the electrocardiogram (13). The hypertensive crisis and atrial fibrillation with rapid ventricular response would explain the patient symptoms and elevated troponin level. Anaphylaxis can trigger atrial fibrillation (14) and Kounis syndrome associated with atrial fibrillation has been reported (15). Electrocardiographic changes ranging from ST segment elevation or depression to any degree of heart block and cardiac arrhythmias resembling digitalis intoxication are commonly associated with the cardiac symptoms and signs of Kounis syndrome (16).
The point that was most striking in our patient, was the severe output reduction presented as netting with extreme sweating along with high blood pressure (210 mmHg). This may be explained by the fact that during anaphylactic reaction compensatory vasopressor response to the secretion of mediators from basophils and mast cells is mandatory in order to fight their deleterious results on respiratory and cardiovascular system. This response is mediated by substances such as serotonin -which plays a significant role in the development of hypertension -, as well as by catecholamines, endothelin-1 and activation of the angiotensin system and may induce extreme hypertensive anaphylactic reactions (17). Emergency conditions require prompt and meticulous evaluation and treatment. Our patient was treated with conventional anti-hypertensive drugs and hemodialysis. Although we suspected an anaphylactic reaction, we did not administer epinephrine due to severely unstable hemodynamic condition such as extreme hypertension with pulmonary edema, respiratory acidosis and increased lactate levels. Rarely anaphylactic reactions could be accompanied by transient hypertension (18). It is worth to mention that our patient had undergone full body screening within his follow-up (4 months before episode) and thus pheochromocytoma was excluded as a potential cause of the patient’s hypertension (19). Although most of the hypersensitivity reactions occur more often during the first rituximab infusions, some can occur in patients who have already tolerated previous infusions (5). Intradermal skin testing helps to confirm the diagnosis and to risk stratification of desensitization. This is recommended at least 3 weeks after the initial reaction to avoid false-negative results (5). Most of the patients who develop reactions to rituximab have a positive intradermal skin tests (20).
Skin testing was not performed in our patient on ethical grounds and tryptase levels were not measured due to more than 2 hours elapsing time from the acute event (21). Rapid desensitization therapy is recommended in instances where specific treatment is mandatory. In a recent report, rapid drug desensitization was proved safe and valid because 98.5% of such protocols were completed successfully (22).
This case shows that reactions to rituximab are common even not being expressed at the first infusion. Although hypotension is one of the main symptoms that characterizes anaphylaxis, severe hypertension can also happen and poses difficulties in the therapeutic management because the use of epinephrine injection could be dangerous. For all the aforementioned reasons, it is well understood that rapid drug desensitization protocols are of paramount importance, in order to overcome hypersensitivity reactions to rituximab since its therapeutic impact on many diseases is indisputable.
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Williams C, Cable C, Choi J. Treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma presenting simultaneously with acquired hemophilia and warm autoimmune hemolytic anemia. Proc (Bayl Univ Med Cent) 2017;30:343–345. doi: 10.1080/08998280.2017.11929642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J, Duddempudi AT, Sana MM, et al. Screening for hepatitis B in patients with lymphoma. Proc (Bayl Univ Med Cent) 2015;28:438–442. doi: 10.1080/08998280.2015.11929300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas V, Dobson R, Mennel R. Primary cutaneous large B-cell lymphoma, leg type. Proc (Bayl Univ Med Cent) 2011;24:350–353. doi: 10.1080/08998280.2011.11928757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth JD. Safety of rituximab in the treatment of B cell malignancies: implications for rheumatoid arthritis. Arthritis Res Ther. 2003;5(Supp 4):S12–16. doi: 10.1186/ar1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PJ, Rodriguez Bouza T, Hsu FI, Sloane DE, Castells MC. Hypersensitivity reactions to mAbs: 105 desensitizations in 23 patients, from evaluation to treatment. J Allergy Clin Immunol. 2009;124:1259–1266. doi: 10.1016/j.jaci.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Kounis NG, Mazarakis A, Tsigkas G, Giannopoulos S, Goudevenos J. Kounis syndrome: a new twist on an old disease. Future Cardiology. 2011;7:805–824. doi: 10.2217/fca.11.63. [DOI] [PubMed] [Google Scholar]
- Ataca P, Atilla E, Kendir R, Bavbek S, Ozcan M. Successful desensitization of a patient with rituximab hypersensitivity. Case Reports Immunol. 2015:524507. doi: 10.1155/2015/524507. doi:10.1155/2015/524507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rituxan (package insert), South San Francisco, Calif, USA: Biogen Idec and Genentech. 2013 [Google Scholar]
- Öztürk E, Özyiğit LP, Öztürk AB, Akay MO, Çetiner M, Ferhanoğlu B. Rituximab desensitization in three patients with severe rituximab allergy. Curr Probl Cancer. 2017;41:349–354. doi: 10.1016/j.currproblcancer.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Simons FER. World Allergy Organization survey on global availability of essentials for the assessment and management of anaphylaxis by allergy–immunology specialists in health care settings. Ann Allergy Asthma Immunol. 2010;104:405–412. doi: 10.1016/j.anai.2010.01.023. [DOI] [PubMed] [Google Scholar]
- Sharif K, Watad A, Bragazzi NL, et al. Anterior ST-elevation myocardial infarction induced by rituximab infusion: A case report and review of the literature. J Clin Pharm Ther. 2017;42:356–362. doi: 10.1111/jcpt.12522. [DOI] [PubMed] [Google Scholar]
- Gori T, Münzel T. A case of coronary hypersensitivity (Kounis) syndrome associated with mid-ventricular ballooning pattern, intracoronary thrombosis and troponin elevation. Int J Cardiol. 2011;149:377–378. doi: 10.1016/j.ijcard.2011.02.066. [DOI] [PubMed] [Google Scholar]
- Sovari AA, Farokhi F. When the heart remembers. Am J Emerg Med. 2007;25:831–833. doi: 10.1016/j.ajem.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Kounis NG, Koniari I, Tzanis G, Soufras GD, Velissaris D, Hahalis G. Anaphylaxis-induced atrial fibrillation and anesthesia: Pathophysiologic and therapeutic considerations. Ann Card Anaesth. 2020;23:1–6. doi: 10.4103/aca.ACA_100_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akçay M. Ibuprofen-induced Kounis syndrome with diffuse ST segment depression and atrial fibrillation. Anatol J Cardiol. 2017 Nov;18:380–381. doi: 10.14744/AnatolJCardiol.2017.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounis NG. Kounis syndrome: an update on epidemiology, pathogenesis, diagnosis and therapeutic management. Clin Chem Lab Med. 2016;54:1545–1559. doi: 10.1515/cclm-2016-0010. [DOI] [PubMed] [Google Scholar]
- Solmazgul E, Kutlu A, Dogru S, et al. Anaphylactic reactions presenting with hypertension. Springer plus. 2016;5:1223. doi: 10.1186/s40064-016-2913-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldbott GL. Hypertension Associated with Allergy. JAMA. 1930;94:1390–1392. [Google Scholar]
- Fent GJ, Kamaruddin H, Garg P, Iqbal A, Kelland NF, Hall IR. Hypertensive emergency and type 2 myocardial infarction resulting from pheochromocytoma and concurrent Capnocytophaga canimorsus infection. Open Cardiovasc Med J. 2014;8:43–47. doi: 10.2174/1874192401408010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Carmen Sancho M, Breslow R, Sloane D, Castells M. Desensitization for hypersensitivity reactions to medications. Chem Immunol Allergy. 2012;97:217–233. doi: 10.1159/000335637. [DOI] [PubMed] [Google Scholar]
- Kounis NG. Serum tryptase levels and Kounis syndrome. Int J Cardiol. 2007;114:407–408. doi: 10.1016/j.ijcard.2005.11.088. [DOI] [PubMed] [Google Scholar]
- Görgülü B, Seval GC, Kendirlinan R, Toprak SK, Özcan M, Bavbek S. Rapid Drug Desensitization with Rituximab in 24 Cases: A Single-Center Experience. J Investig Allergol Clin Immunol. 2019;29:468–470. doi: 10.18176/jiaci.0445. [DOI] [PubMed] [Google Scholar]


