Abstract
The introduction of novel therapeutic agents for advanced prostate cancer has led to a wide range of treatment options for patients with metastatic castration-resistant prostate cancer (mCRPC). In the past decade, new treatment options for mCRPC, including abiraterone, enzalutamide, docetaxel, cabazitaxel, sipuleucel-T, radium-223, 177Lu-PSMA-617, and Olaparib, have demonstrated a survival benefit in phase 3 trials. Bone-modifying agents have become part of the overall treatment strategy for mCRPC, in which denosumab and zoledronic acid reduce skeletal-related events. Recently, androgen receptor-signaling inhibitors (ARSIs) and docetaxel have been used upfront against metastatic castration-sensitive prostate cancer. Further, triplet therapy with ARSI, docetaxel, and androgen deprivation therapy is emerging. However, cross-resistance may occur between these treatments, and the optimal treatment sequence must be considered. The sequential administration of ARSIs, such as abiraterone and enzalutamide, is associated with limited efficacy; however, cabazitaxel is effective for patients with mCRPC who were previously treated with docetaxel and had disease progression during treatment with ARSI. Radioligand therapy with 177Lu-PSMA-617 is a new effective class of therapy for patients with advanced PSMA-positive mCRPC. Tumors with gene alterations that affect homologous recombination repair, such as BRCA1 and BRCA2 alterations, are sensitive to poly (adenosine diphosphate–ribose) polymerase (PARP) inhibitors in mCRPC. This review sought to highlight recent advances in systemic therapy for mCRPC and strategies to support patient selection and treatment sequencing.
Keywords: Androgen receptor antagonists, Androgen synthesis inhibitors, Chemotherapy, Docetaxel, PARP inhibitor, Prostate cancer
INTRODUCTION
Prostate cancer (PCa) is the second most frequent cancer among males and was the cause of an estimated 375,000 deaths worldwide in 2020 [1]. In the 1940s, Charles Huggins found that patients with metastatic PCa respond to androgen deprivation therapy (ADT), which ultimately initiated therapies for PCa [2]. Nearly all patients respond to ADT; however, the duration of response varies from months to years. Thereafter, the disease progresses to a lethal stage called castration-resistant PCa (CRPC). In 2004, docetaxel, a taxane chemotherapy, was first demonstrated to prolong the survival of men with metastatic CRPC (mCRPC) and has since become the standard therapy for mCRPC [3,4]. In the past decade, new treatment options for mCRPC have been approved, including abiraterone [5,6], enzalutamide [7,8], cabazitaxel [9,10,11,12], sipuleucel-T [13], radium-223 [14], 177Lu-PSMA-617 [15], and olaparib [16,17]. These therapeutic agents have led to improved survival of mCRPC patients in phase 3 clinical trials [3,4,5,6,7,8,9,12,13,14,15,16,17,18] (Table 1). Recently, androgen receptor-signaling inhibitors (ARSIs) and docetaxel have been used upfront against metastatic castration-sensitive PCa (mCSPC) [19,20,21,22,23,24,25], and nonmetastatic CRPC [26,27,28]. However, cross-resistance may occur between these treatments, and the optimal treatment sequence must be considered [29]. mCRPC remains an incurable and fatal disease despite the existence of multiple treatments that prolong survival. This review provides an updated summary on current therapies for the management of mCRPC.
Table 1. Active therapies for mCRPC.
| Agent | Clinical trial | Patient status | Intervention | Comparison | Primary endpoint | Outcome | |
|---|---|---|---|---|---|---|---|
| ARSI | |||||||
| Abiraterone | COU-AA-301 [5] | Postdocetaxel mCRPC | Abiraterone+Prednisone | Placebo+Prednisone | OS | HR, 0.65; 95% CI, 0.54–0.77; p<0.001 | |
| COU-AA-302 [6] | Chemotherapy-naiive mCRPC | Abiraterone+Prednisone | Placebo+Prednisone | rPFS and OS | rPFS: HR, 0.53; 95% CI, 0.45–0.62; p<0.001 | ||
| OS: HR, 0.75; 95% CI, 0.61–0.93; p=0.01 | |||||||
| Enzalutamide | AFFIRM [7] | Postdocetaxel mCRPC | Enzalutamide | Placebo | OS | HR, 0.63; 95% CI, 0.53–0.75; p<0.001 | |
| PREVAIL [8] | Chemotherapy-naiive mCRPC | Enzalutamide | Placebo | rPFS and OS | rPFS: HR, 0.19; 95% CI, 0.15–0.23; p<0.001 | ||
| OS: HR, 0.71; 95% CI, 0.60–0.84; p<0.001 | |||||||
| Chemotherapy | |||||||
| Docetaxel | TAX 327 [3] | mCRPC | Docetaxel+Prednisone | Mitoxantrone+Prednisone | OS | Docetaxel every 3 wk: HR, 0.76; 95% CI, 0.62–0.94; p=0.009 | |
| SWOG 99-16 [4] | mCRPC | Docetaxel+Estramustine | Mitoxantrone+Prednisone | OS | HR, 0.80; 95% CI, 0.67–0.97; p=0.02 | ||
| Cabazitaxel | TROPIC [9] | Postdocetaxel mCRPC | Cabazitaxel+Prednisone | Mitoxantrone+Prednisone | OS | HR, 0.70; 95% CI, 0.59–0.83; p<0.0001 | |
| CARD [12] | Postdocetaxel and post-ARSI mCRPC | Cabazitaxel+Prednisone | Other ARSI (either Abiraterone+Prednisone or Enzalutamide) | rPFS | HR, 0.54; 95% CI, 0.40–0.73; p<0.001 | ||
| Immunotherapy | |||||||
| Sipuleucel-T | IMPACT [13] | mCRPC without visceral metastases | Sipuleucel-T | Placebo | OS | HR, 0.78; 95% CI, 0.61–0.98; p=0.03 | |
| Bone-targeted therapy | |||||||
| Radium-223 | ALSYMPCA [14] | mCRPC with bone metastases | Radium-223 | Placebo | OS | HR, 0.70; 95% CI, 0.58?0.83; p<0.001 | |
| Radioligand therapy | |||||||
| 177Lu-PSMA-617 | VISION [15] | Postdocetaxel and post-ARSI mCRPC | 177Lu-PSMA-617+Standard care | Standard care | rPFS, OS | rPFS: HR, 0.40; 99.2% CI, 0.29–0.57; p<0.001 | |
| OS: HR, 0.62; 95% CI, 0.52–0.74; p<0.001 | |||||||
| PARP inhibitor | |||||||
| Olaparib2 | PROfound [16] | Post-ARSI mCRPC with HRRm | Olaparib | Enzalutamide or Abiraterone | rPFS | HR, 0.34; 95% CI, 0.25–0.47; p<0.001 (cohort A) | |
| PROpel [17] | Previously untreated mCRPC, regardless of HRRm status | Olaparib+Abiraterone | Placebo+Abiraterone | rPFS | HR, 0.66; 95% CI, 0.54–0.81; p<0.001 | ||
| AKT inhibitor | |||||||
| Ipatasertib | IPATential150 [18] | Previously untreated mCRPC | Ipatasertib+Abiraterone | Placebo+Abiraterone | rPFS | HR, 0.77; 95% CI, 0.61–0.98; p=0.034; significant at α=0.04 (no significant difference in the ITT population) | |
ARSI: androgen receptor-signaling inhibitor, CI: confidence interval, HR: hazard ratio, HRRm: homologous recombination repair gene mutation, ITT: intention-to-treat, mCRPC: metastatic castration-resistant prostate cancer, OS: overall survival, rPFS: radiographic progression-free survival.
METHODS
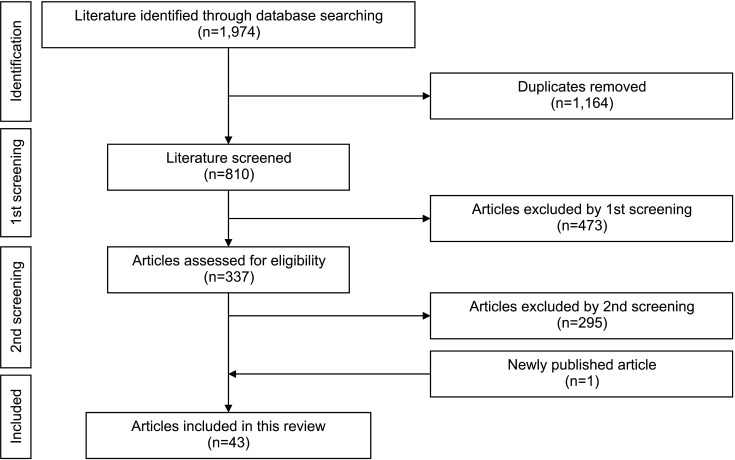
A literature search was carried out using PubMed/Medline database in January 2022 to identify relevant publications (Fig. 1). The following keywords were used: prostate cancer, metastatic castration-resistant prostate cancer, abiraterone, enzalutamide, docetaxel, cabazitaxel, olaparib, radium-223, and clinical randomized controlled trial. Clinical trials of systemic therapy for mCRPC were eligible for inclusion in this review. Newly published articles up to August 2022 were included. The authors' knowledge of the current literature was utilized to ensure proper final literature selection.
Fig. 1. Literature screening to identify relevant publications. A literature search was performed using PubMed/Medline database in January 2022. Thereafter, the duplicates were removed. At the first screening, the clinical trials for systemic therapy for mCRPC were selected. At the second screening, the clinical randomized controlled trials regarding active agents for mCRPC were selected. One article published between January 2022 and August 2022 was included in the analysis. mCRPC: metastatic castration-resistant prostate cancer.
ARSIs
Although the response rates to ADT are initially high, most PCa patients inevitably develop CRPC overtime. According to the current European Association of Urology guideline, CRPC is defined as castrate serum testosterone <50 ng/dL or 1.7 nmol/L plus one of the following types of progression: biochemical progression (three consecutive increases in prostate specific antigen [PSA] 1 wk apart, resulting in two 50% increases over the nadir, and PSA >2 ng/mL) or radiological progression [30]. A similar definition for castrate serum testosterone levels has been provided in the Prostate Cancer Clinical Trials Working Group (PCWG) 2 criteria and the PCWG 3 criteria updated in 2016 [31,32]. PCWG 3 defined that PSA 1.0 ng/mL is the minimal starting value if a confirmed rise in PSA is the only indication of progression, unless pure small-cell carcinoma [32].
The androgen receptor (AR) pathway is often found to be activated in CRPC [33]. AR plays pivotal roles in CRPC; PCa changes the AR status and adapts to survive under castration levels of androgen. The underlying mechanisms include AR mutations, AR overexpression, changes in androgen biosynthesis, constitutively active AR splice variants, and changes in androgen cofactors [34,35]. ARSIs, abiraterone and enzalutamide, are currently extensively used in the clinic against mCRPC.
1. Abiraterone
Abiraterone blocks CYP17, a critical enzyme in testosterone synthesis, thereby blocking androgen synthesis by the adrenal glands, testes, and within the prostate tumor. The concurrent administration of low-dose prednisone with abiraterone is required to prevent mineralocorticoid-related adverse events, including fluid retention, hypertension, and hypokalemia.
In the COU-AA-301 trial, patients with mCRPC who had previously been treated with docetaxel were randomly assigned to receive abiraterone or placebo. After a median follow-up of 12.8 months, the overall survival (OS) was longer in the abiraterone group than that in the placebo group (14.8 vs.10.9 months; hazard ratio [HR], 0.65; 95% confidence interval [CI], 0.54–0.77; p<0.001) [5]. Abiraterone also improved all secondary end points, including time to PSA progression (10.2 vs. 6.6 months, p<0.001), progression-free survival (PFS) (5.6 vs. 3.6 months, p<0.001), and PSA 50% response (29% vs. 6%, p<0.001) [5]. In the final analysis of the COUAA-301 trial, the median OS of the abiraterone group was longer than that of the placebo group (15.8 vs. 11.2 months; HR, 0.74; 95% CI, 0.64–0.86; p<0.0001) [36]. In the COU-AA-302 trial, abiraterone was evaluated in patients with chemotherapy-naive mCRPC without clinically significant cancer-related symptoms. The median radiographic PFS (rPFS) was 16.5 months with abiraterone and 8.3 months with placebo (HR, 0.53; 95% CI, 0.45–0.62; p<0.001) [6]. In the final analysis of the COU-AA-302 trial, median OS was significantly longer in the abiraterone group than that in the placebo group (34.7 vs. 30.3 months; HR, 0.81; 95% CI, 0.70–0.93; p=0.0033) [37]. Thus, abiraterone improved the OS of chemotherapy-treated and chemotherapy-naive patients with mCRPC.
Currently, abiraterone can be used upfront against mCSPC. The LATITUDE trial revealed that the addition of abiraterone to ADT significantly increased the OS and rPFS of patients with newly diagnosed high-risk mCSPC who had at least two of the three following high-risk factors associated with poor prognosis: a Gleason score of 8 or more, at least three bone lesions, and presence of visceral metastasis [20]. The STAMPEDE trial also revealed that ADT plus abiraterone was associated with significantly higher rates of OS and failure-free survival than ADT alone among men with locally advanced or metastatic PCa [38]. Recently, the PEACE-1 trial revealed that triplet therapy, which comprised ADT, docetaxel, and abiraterone, improved the OS and rPFS of patients with mCSPC [24]. Such evidence will change the treatment approach in clinical practice and influence the treatment sequence for mCRPC.
2. Enzalutamide
Enzalutamide is a second-generation, nonsteroidal AR inhibitor that competitively binds to the ligand-binding domain of the AR, inhibiting AR translocation to the cell nucleus, AR cofactor recruitment, and AR binding to DNA.
In the AFFIRM trial, patients with mCRPC after chemotherapy were randomly assigned to receive enzalutamide or placebo [7]. The median OS was 18.4 months in the enzalutamide group versus 13.6 months in the placebo group (HR, 0.63; 95% CI, 0.53–0.75; p<0.001). The superiority of enzalutamide over placebo was demonstrated for all secondary end points, including time to first skeletal-related event (SRE), pain control, and patient-reported health-related quality of life [7,39]. Fatigue was the most common clinically relevant adverse event associated with enzalutamide [7]. In the PREVAIL trial, enzalutamide was evaluated in patients with chemotherapy-naïve, and asymptomatic or mildly symptomatic mCRPC [8]. The rate of rPFS at 12 months was 65% among patients treated with enzalutamide and 14% among patients receiving placebo (HR, 0.19; 95% CI, 0.15–0.23; p<0.001). Enzalutamide significantly improved the OS compared with the placebo (HR, 0.71; 95% CI, 0.60–0.84; p<0.001) [8]. At the final analysis in the PREVAIL trial, median rPFS was 20.0 months in the enzalutamide arm and 5.4 months in the placebo arm, while median OS was 35.3 months in the enzalutamide arm and 31.3 months in the placebo arm [40]. Thus, enzalutamide improved OS in chemotherapy-treated and chemotherapy-naive patients with mCRPC.
The phase 2 trials, STRIVE and TERRAIN, revealed that enzalutamide significantly reduced the risk of PCa progression compared with the first-generation nonsteroidal AR inhibitor, bicalutamide, in patients with nonmetastatic or metastatic CRPC [41,42]. The OCUU-CRPC study also showed that enzalutamide provides superior clinical outcomes compared with the first-generation AR inhibitor, flutamide, in patients with CRPC [43].
Currently, enzalutamide is approved for upfront use for mCSPC and nonmetastatic CRPC. The ENZAMET and ARCHES trials demonstrated that enzalutamide is associated with significantly longer rPFS and OS than standard care in men with mCSPC receiving testosterone suppressiontreatment [22,23,44]. In the PROSPER trial, enzalutamide clinically significantly decreased (71%) risk of metastasis or death compared to placebo among patients with nonmetastatic CRPC with a PSA doubling time of 10 months or less [27]. The risk of death associated with enzalutamide treatment was 27%, lower than that with placebo [27].
The phase 2 CHEIRON trial assessed the efficacy of the combination of docetaxel and enzalutamide as a first-line treatment for mCRPC. The 6-month progression rate was 12.5% in the docetaxel and enzalutamide group and 27.8% in the docetaxel group (p=0.002). In the combination group, serious adverse events were more frequent, and no OS improvement was observed [45].
3. Apalutamide
Apalutamide is a novel nonsteroidal AR inhibitor that is fully antagonistic to AR overexpression, a common feature of CRPC, and inhibits AR transcriptional activity [46]. In the SPARTAN trial, apalutamide significantly improved metastasis-free survival and time to symptomatic progression among patients with nonmetastatic CRPC with a PSA doubling time of 10 months or less. Skin rash was the most common adverse event with apalutamide treatment [26,47]. In the TITAN trial comprising patients with mCSPC, the OS and rPFS were significantly longer with the addition of apalutamide to ADT than with placebo plus ADT [21,48].
In the phase 3 ACIS trial, Saad et al [49] investigated the combination treatment using apalutamide plus abiraterone, each of which suppresses the androgen signaling axis in a different manner. Patients with chemotherapy-naive mCRPC who had not been previously treated with ARSIs were randomly assigned to receive apalutamide plus abiraterone or placebo plus abiraterone. In the updated analysis, median rPFS was 24.0 months in the apalutamide plus abiraterone group versus 16.6 months in the abiraterone alone group (HR, 0.70; 95% CI, 0.60–0.83; p<0.0001). The median OS, a secondary endpoint in ACIS, surpassed 3 years with apalutamide plus abiraterone versus abiraterone alone (36.2 vs. 33.7 months), although there was no significant difference between the groups [49]. However, to date, the two active treatments evaluated in clinical trials for mCRPC have not displayed a significant OS advantage over their respective control arms [45,49]. Such result may be due to the uncertainty regarding the number of active life-prolonging treatments for mCRPC and optimal treatment sequence.
4. Darolutamide
Darolutamide is a novel, nonsteroidal, AR inhibitor that potently inhibits androgen binding to AR and androgen-induced nuclear translocation of AR in AR-overexpressing cells. Darolutamide blocks the activity of the mutant ARs arising in response to antiandrogen therapies, including the F876L mutation that confers resistance to enzalutamide and apalutamide. Darolutamide has low neurotoxicity owing to its low penetration of the blood–brain barrier [50]. In the ARAMIS study, metastasis-free survival and OS were significantly longer with darolutamide than with placebo among patients with nonmetastatic CRPC with a PSA doubling time of 10 months or less. [28,51] In the ARASENS trial comprising patients with mCSPC, OS was significantly longer with the triplet therapy, which consisted of darolutamide, ADT, and docetaxel, than with placebo plus ADT and docetaxel (HR, 0.68; 95% CI, 0.57–0.80; p<0.001). Further, the addition of darolutamide led to improvement in the key secondary end points, including time to CRPC and time to pain progression. The frequency of adverse events was similar between the two groups [25]. On August 5, 2022, the Food and Drug Administration (FDA) approved darolutamide combined with docetaxel for patients with mCSPC.
5. Sequencing of abiraterone and enzalutamide
Whether patients treated with one ARSI would benefit from treatment with the alternate agent at progression is unknown as the available data consistently revealed varying degrees of cross resistance. Among patients previously treated with abiraterone, the percentage of patients with a PSA 50% response to enzalutamide ranged from 12% to 40%, while the percentage of patients previously treated with enzalutamide responding to abiraterone was less than 10% [52]. Consistent with these results, only 1% of patients in the phase 3 PLATO trial had a PSA 50% response to abiraterone after progression with enzalutamide [53].
Khalaf et al [54] performed a randomized, phase 2, crossover trial, in which 202 patients with mCRPC were randomly administered either abiraterone until PSA progression and then enzalutamide, or the opposite sequence. Abiraterone and enzalutamide had similar first-line activity for mCRPC. Time to second PSA progression was longer in the abiraterone followed by enzalutamide group than in the opposite sequence group (median 19.3 vs. 15.2 months; HR, 0.66; 95% CI, 0.45–0.97; p=0.036). With regard to PSA 30% responses to second-line therapy, responses were observed in 36% patients for enzalutamide and in 4% for abiraterone (p<0.0001) [54]. Enzalutamide retains clinical activity as a second-line drug following abiraterone, whereas abiraterone has low activity as a second-line drug following enzalutamide.
6. Bipolar androgen therapy (BAT)
ARSIs have become a standard of care as they result in improved survival; however, resistance increases with each subsequent line of AR-directed therapy. PCa cells can acquire resistance to ADT by adaptively increasing AR expression upon castration. Preclinical studies revealed that temporary exposure to supraphysiologic testosterone can downregulate the AR levels, leading to potential resensitization to ADT [55]. Clinical studies have demonstrated the safety of BAT, defined as rapid cycling between high and low serum testosterone, in men with mCRPC [56].
Denmeade et al [57] performed the phase 2 TRANSFORMER study to compare monthly BAT with enzalutamide in asymptomatic patients with mCRPC progression during abiraterone treatment. The PFS was 5.7 months for both arms, and crossover was permitted at progression. At crossover, a PSA 50% response occurred in 77.8% of patients crossing to enzalutamide and 23.4% crossing to BAT. The PFS2 was 28.2 months for BAT to enzalutamide versus 19.6 months for enzalutamide to BAT (HR, 0.44; 95% CI, 0.22–0.88; p=0.02). Patient-reported quality of life consistently favored BAT. Thus, BAT is safe and can sensitize CRPC to subsequent antiandrogen therapy [57].
7. Current status and future directions for ARSIs
Currently, enzalutamide and apalutamide are approved and widely used for the treatment of mCSPC. In addition, high-risk mCSPC patients are treated with abiraterone. Recently, the combination of darolutamide and docetaxel has been approved for patients with mCSPC. Patients with nonmetastatic CRPC are treated with either enzalutamide, apalutamide, or darolutamide. Cross-resistance between these ARSIs is not fully elucidated, but the efficacy of abiraterone or enzalutamide for mCRPC will be affected by prior use of other ARSI. The main adverse event profiles of ARSI and taxane that can guide patient selection in treatment strategies are summarized in Table 2.
Table 2. Major AE profiles of ARSI and taxane.
| Agent | Abiraterone | Enzalutamide | Apalutamide | Darolutamide | Docetaxel | Cabazitaxel |
|---|---|---|---|---|---|---|
| Major AEs | Fluid retention | Fall/Fracture | Fall/Fracture | Fatigue | Alopecia | Diarrhea |
| Hepatic disorders | Fatigue | Fatigue | Change in taste | Fatigue | ||
| Hypertension | Hot flashes | Hypothyroidism | Diarrhea | Infection | ||
| Hypokalemia | Hypertension | Rash | Fatigue | Neutropenia | ||
| Nail changes | ||||||
| Neutropenia | ||||||
| Peripheral edema | ||||||
| Sensory neuropathy | ||||||
| Stomatitis | ||||||
| Clinical trial | COU-AA-301 [5] | AFFIRM [7] | TITAN [21] | ARAMIS [28] | TAX 327 [3] | TROPIC [9] |
| COU-AA-302 [6] | PREVAIL [8] | SPARTAN [26] | CHAARTED [19] | CARD [12] | ||
| LATITUDE [20] | ENZAMET [22] | |||||
| STAMPEDE [38] | ARCHES [23] | |||||
| PROSPER [27] |
AE: adverse event, ARSI: androgen receptor-signaling inhibitor.
CHEMOTHERAPY
1. Docetaxel
Docetaxel, a taxane-based anticancer drug, has been widely used as a standard treatment for mCRPC since its first demonstration to prolong life in 2004. In the TAX 327 study, patients with mCRPC were randomly assigned to receive mitoxantrone, docetaxel every 3 weeks (75 mg/m2), or weekly docetaxel (30 mg/m2). Docetaxel every 3 weeks had superior OS compared to mitoxantrone plus prednisone (HR, 0.76; 95% CI, 0.62–0.94; p=0.009) [3]. In the SWOG 99-16 trial, the median OS was longer in patients with mCRPC treated with docetaxel (60 mg/m2) and estramustine than that in those treated with mitoxantrone and prednisone (17.5 vs. 15.6 months; HR, 0.80; 95% CI, 0.67–0.97; p=0.02) [4].
To date, several clinical trials have focused on the use of docetaxel in patients with mCSPC. In the GETUG-AFU15 trial, the OS of patients with mCSPC was not significantly improved by the upfront docetaxel therapy. This result may be partly due to the fact that most patients in the ADT arm (79%) received salvage docetaxel within a median of 18.5 months [58]. In the CHAARTED trial, the median OS for mCSPC was 13.6 months longer with ADT plus docetaxel than that with ADT alone (57.6 vs. 44.0 months; HR, 0.61; 95% CI, 0.47–0.80; p<0.001). The survival benefit was more apparent in the subgroup with high-volume disease, which was defined as the presence of visceral metastases or ≥4 bone lesions with ≥1 beyond the vertebral bodies and pelvis [19]. In the STAMPEDE trial, the OS was significantly improved by the combination of docetaxel with ADT in patients with mCSPC [59]. To date, upfront docetaxel combined with ADT is the standard of care for patients with mCSPC, especially those with a high tumor burden. The retrospective data from the GETUG-AFU 15 trial revealed that docetaxel rechallenge following progression to mCRPC after upfront ADT plus docetaxel for mCSPC was active in a limited number of patients [60]. A PSA 50% response following treatment with first- or second-line docetaxel for mCRPC was observed in 45% of patients in the ADT arm and 14% in the ADT plus docetaxel arm [60].
2. Cabazitaxel
Cabazitaxel is a next-generation taxane approved for the treatment of mCRPC in patients previously administered docetaxel. The safety profile of cabazitaxel differs from that of docetaxel, and is associated with a higher incidence of febrile neutropenia and lower incidence of peripheral neuropathy, peripheral edema, and nail disorders.
The phase 3 TROPIC trial sought to compare cabazitaxel (25 mg/m2) with mitoxantrone in patients with mCRPC with progressive disease after docetaxel. The median OS was 15.1 months in the cabazitaxel group and 12.7 months in the mitoxantrone group (HR, 0.70; 95% CI, 0.59–0.83; p<0.0001) [9]. In the phase 3 PROSELICA trial, the noninferiority of cabazitaxel 20 mg/m2 compared to cabazitaxel 25 mg/m2 was confirmed in patients with post-docetaxel mCRPC [10]. In the phase 3 FIRSTANA trial, cabazitaxel 20 mg/m2 or cabazitaxel 25 mg/m2 did not demonstrate superiority for OS compared to docetaxel 75 mg/m2 in patients with chemotherapy naïve mCRPC [11] (Table 3).
Table 3. Comparison between cabazitaxel 20 mg/m2, cabazitaxel 25 mg/m2, docetaxel 75 mg/m2 .
| Clinical trial | Patient status | Intervention | Comparison | Primary endpoint | Outcome |
|---|---|---|---|---|---|
| PROSELICA [10] | Postdocetaxel mCRPC | Cabazitaxel (20 mg/m2) | Cabazitaxel (25 mg/m2) | OS (noninferiority) | The upper boundary of the HR CI was 1.184 (less than the 1.214 noninferiority margin). |
| FIRSTANA [11] | Chemotherapy-naiive mCRPC | Cabazitaxel (20 mg/m2) or (25 mg/m2) | Docetaxel (75 mg/m2) | OS | HR for C20 vs. D75 was 1.01 (95% CI, 0.85–1.20; p=0.997) and HR for C25 vs. D75 was 0.97 (95% CI, 0.82–1.16; p=0.757). |
CI: confidence interval, C20: cabazitaxel (20 mg/m2), C25: cabazitaxel (25 mg/m2), D75: docetaxel (75 mg/m2), HR: hazard ratio, mCRPC: metastatic castration-resistant prostate cancer, OS: overall survival.
The CARD trial was designed to determine the efficacy of cabazitaxel (25 mg/m2) compared with an ARSI (abiraterone or enzalutamide) for patients with mCRPC who were previously treated with docetaxel and had progression within 12 months while receiving an alternative ARSI (abiraterone or enzalutamide). The median rPFS was 8.0 months with cabazitaxel and 3.7 months with the ARSI (HR, 0.54; 95% CI, 0.40–0.73; p<0.001) [12].
Annala et al [61] performed a phase 2 trial to determine the optimal treatment for patients with ARSI naïve mCRPC with poor prognosis features (e.g., presence of liver metastases or progression to mCRPC after <12 months of ADT). Patients were randomly assigned to receive cabazitaxel or the physician's choice of enzalutamide or abiraterone. The first-line clinical benefit rate (defined as PSA 50% response, radiographic response, or stable disease ≥12 weeks) was greater in the cabazitaxel group than that in the ARSI group (80% vs. 62%, p=0.039) [61].
3. Platinum-based chemotherapy
Platinum-based chemotherapy has antitumor effects against mCRPC, but has not improved survival in large clinical trials. Emerging evidence suggests that the status of DNA damage-repair pathways may influence sensitivity to platinum-based chemotherapy, such as cisplatin and carboplatin [62,63]. mCRPC harboring BRCA2 mutations has a high response to platinum-based chemotherapy [64,65,66].
The aggressive variant of PCa tumors shares molecular features with platinum-sensitive small-cell prostate carcinomas and is characterized by defects in at least two of the following genes: TP53, RB1, and PTEN [67]. Corn et al [68] performed a phase 1-2 trial to determine whether adding carboplatin to cabazitaxel would improve the outcomes of men with mCRPC. The combination improved the median PFS from 4.5 months to 7.3 months (HR, 0.69; 95% CI, 0.50–0.95; p=0.018). Subgroup analyses suggested that treatment with the combination seemed to benefit patients with aggressive variant PCa features, but not those without such features [68].
IMMUNOTHERAPY
1. Sipuleucel-T
Sipuleucel-T, an autologous cellular immunotherapy, is the first immunotherapeutic agent to exhibit a survival benefit in patients with mCRPC. In the phase 3 IMPACT study, patients with asymptomatic or minimally symptomatic mCRPC without visceral metastases were randomly assigned to receive either sipuleucel-T or placebo group [13]. The sipuleucel-T group had a relative reduction of 22% in the risk of death compared with the placebo group (HR, 0.78; 95% CI, 0.61–0.98; p=0.03).
2. Immune checkpoint inhibitors
The presence of inflammatory cells and T-cell infiltration in PCa tissue suggests that host immune effectors may mediate antitumor response. Ipilimumab is a monoclonal antibody that increases antitumor T cell responses by binding to cytotoxic T lymphocyte antigen 4. In the phase 3 CA184-095 trial, chemotherapy-naive patients with asymptomatic or minimally symptomatic mCRPC without visceral metastases were randomly assigned to receive ipilimumab or placebo. Ipilimumab did not improve OS, the primary end point, in patients with mCRPC, despite increasing the PFS [69].
Radiotherapy is a rational modality to stimulate immune priming that can be amplified by ipilimumab. In the phase 3 CA184-043 trial, patients with mCRPC with at least one bone metastasis that progressed after docetaxel were randomly assigned to receive bone-directed radiotherapy followed by either ipilimumab or placebo [70]. At the final analysis, the OS assessment revealed crossing of the curves at 7–8 months, followed by persistent separation of the curves beyond that point, favoring the ipilimumab arm [71].
Pembrolizumab is a humanized antibody that targets programmed cell death protein 1 (PD-1). Pembrolizumab is approved by the FDA for the treatment of patients with unresectable or metastatic solid tumors with high microsatellite instability (MSI-H), deficient in mismatch repair (dMMR), or high mutational burden, that have progressed following prior treatment and lack satisfactory alternative treatment options [72,73]. Abida et al [74] reported that among 1,033 patients with PCa who had adequate tumor quality, 32 (3.1%) had MSI-H/dMMR. Eleven patients with MSI-H/dMMR CRPC received an anti–PD-1 or anti-programmed death-ligand 1 therapy. Six of these patients (54.5%) had a PSA 50% response, four of whom had radiographic responses [74].
BONE-TARGETED THERAPY
PCa has a high preference for metastasis to bone, and 90% of men who die owing to PCa have bone metastasis [75]. Management of bone metastases is important for the prevention of SREs, such as pathological fractures, spinal cord compression, and the painful metastatic lesions. The osteoclast-targeted agents, zoledronic acid and denosumab, reduce SREs in mCRPC, while radium-223 improves OS and reduces SREs.
1. Radium-223
Radium-223 is an alpha-emitting radiopharmaceutical agent that selectively binds to sites of increased bone turnover in bone metastases. In the phase 3 ALSYMPCA trial, patients with mCRPC with symptomatic bone metastases and no known visceral metastases were randomly assigned to receive radium-223 or placebo. Radium-223 significantly improved OS compared with placebo (median 14.9 vs. 11.3 months; HR, 0.70; 95% CI, 0.58–0.83; p<0.001). Radium-223 significantly prolonged the time to the first SRE compared with placebo (median 15.6 vs. 9.8 months; HR, 0.66; 95% CI, 0.52–0.83; p<0.001) [14].
An early access trial showed that radium-223 may safely prolong survival in patients treated with concomitant abiraterone, enzalutamide, or denosumab [76]. ERA 223 is the first randomized controlled phase 3 trial to demonstrate the efficacy and safety of the combination of radium-223 and abiraterone in patients with mCRPC. Patients with asymptomatic or mildly symptomatic, chemotherapy-naive, mCRPC with bone metastases were scheduled to receive abiraterone and then randomly assigned to receive radium-223 or placebo. The addition of radium-223 to abiraterone therapy did not improve the SRE-free survival of patients with mCRPC with bone metastases, and was associated with an increased frequency of bone fractures compared with placebo (any grade, 29% vs. 11%). In the post-hoc analyses, the use of bone-modifying agents at baseline was substantially less common in patients who had a fracture than those without fracture in both groups [77].
2. Bone-modifying agent
Bone metastases of PCa are often characterized as osteoblastic, but usually have an underlying osteoclastic component. Bisphosphonate is a pyrophosphate analog that blocks bone destruction. Saad F et al. conducted a randomized, phase 3 clinical trial to evaluate zoledronic acid and determine its effectiveness and safety in reducing SREs associated with mCRPC with bone disease [78]. Fewer patients in the zoledronic acid group than in the placebo group had at least one SRE (38 vs. 49%; difference –11%; 95% CI –20.2% to –1.3%; p=0.028) [79].
Denosumab is a monoclonal antibody against RANKL, which inhibits osteoclast-mediate bone destruction. Fizazi et al [80] carried out a randomized phase 3 study to compare denosumab with zoledronic acid for the prevention of SREs in patients with mCRPC with bone metastases. The median time to first SRE was 20.7 months with denosumab, while it was 17.1 months with zoledronic acid (HR, 0.82; 95% CI, 0.71–0.95; p=0.0002). More hypocalcemia events occurred in the denosumab group (13%) than in the zoledronic acid group (6%, p<0.0001) [80]. In the TRAPEZE trial, the clinical effectiveness was compared between docetaxel alone or with strontium-89, zoledronic acid, or both in patients with bony mCRPC [81]. Strontium-89 combined with docetaxel modestly improved clinical PFS but did not improve SREs, while zoledronic acid with docetaxel did not improve clinical PFS but significantly improved SREs. A post hoc analysis of COUAA-302 trial displayed a trend of prolongation of life for mCRPC patients treated with abiraterone in combination with bone-targeted therapy [82]. Bone-targeted therapy has now become part of the overall treatment strategy for mCRPC.
RADIOLIGAND THERAPY
Prostate-specific membrane antigen (PSMA) is a transmembrane glutamate carboxypeptidase that is highly expressed in mCRPC [83]. 177Lu-PSMA-617 is a radioligand therapy that delivers beta-particle radiation to PSMA-expressing PCa cells and the surrounding microenvironment. In the phase 3 VISION trial, patients who had mCRPC, were previously treated with ARSI and taxane, and were 68Ga-PSMA-positive were randomly assigned to receive either 177Lu-PSMA-617 plus standard care or standard care alone groups. Compared with the standard care, 177Lu-PSMA-617 plus standard care significantly prolonged the rPFS (median, 8.7 vs 3.4 months; HR, 0.40; 99.2% CI, 0.29–0.57; p<0.001) and OS (median, 15.3 vs. 11.3 months; HR, 0.62; 95% CI, 0.52–0.74; p<0.001) [15].
Hofman et al [84] performed a phase 2 TheraP trial to compare 177Lu-PSMA-617 with cabazitaxel in patients with mCRPC that were previously treated with docetaxel. The eligibility criteria for the trial were PSMA-positive disease and no sites of metastatic disease with discordant 18F-fluorodeoxyglucose-positive and 68Ga-PSMA-negative findings. PSA 50% responses were more frequent among men in the 177Lu-PSMA-617 group than in the cabazitaxel group (66% vs. 37%; difference 29%; 95% CI, 16–42; p<0.0001) [84]. Radioligand therapy with 177Lu-PSMA-617 is a new effective class of therapy for patients with advanced PSMA-positive mCRPC.
A NEW ERA OF TARGETED THERAPY
Cancer genomic profiling provides a useful guide regarding treatment selection for mCRPC [85]. The CRPC tumors have a highly complex genomic landscape compared to primary prostate tumors; a number of mCRPC harbor clinically actionable molecular alterations, including mutations in homologous recombination repair (HRR) (e.g., BRCA1, BRCA2, and ATM) and PTEN/phosphoinositide 3-kinase signaling.
1. PARP inhibitors
DNA repair alterations are observed in up to 30% of PCa, and the most commonly mutated genes include BRCA1, BRCA2, and ATM [86]. These gene alterations can occur at either a somatic or germline level. The germline mutations in BRCA1, BRCA2, and ATM are associated with PCa risk and an aggressive PCa phenotype [85]. Tumors with gene alterations that affect HRR are sensitive to poly (adenosine diphosphate– ribose) polymerase (PARP) inhibitors in prostate and other cancers [87,88]. In the TOPARP-B trial, Mateo et al [89] demonstrated the antitumor activity of olaparib in mCRPC with specific DDR gene aberrations. The high response rates observed in mCRPC patients with germline or somatic BRCA1/2 alterations and the durability of many of these responses support the use of olaparib in this subpopulation [89]. In the phase 2 TRITON2 study, Abida et al [90] found that the PARP inhibitor, rucaparib, had antitumor activity in mCRPC patients with deleterious BRCA alterations.
The PROfound trial is a phase 3 trial comprising patients with mCRPC who had disease progression while receiving an ARSI (e.g., enzalutamide or abiraterone). Patients with qualifying alterations in genes involved in HRR were randomly assigned to receive the PARP inhibitor, olaparib, or either enzalutamide or abiraterone (control group). The primary outcome was efficacy, which was assessed based on rPFS in patients with alterations in BRCA1, BRCA2, or ATM (cohort A). In cohort A, rPFS was significantly longer in the olaparib group than that in the control group (median, 7.4 vs. 3.6 months; HR, 0.34; 95% CI, 0.25–0.47; p<0.001). Significant differences were also observed with respect to objective response rate and time to pain progression. Anemia and nausea are the main toxicities in patients treated with olaparib [16]. The median OS in cohort A was 19.1 months in the olaparib group and 14.7 months in the control group (HR, 0.69; 95% CI, 0.50–0.97; p=0.02). Despite substantial crossover from control to olaparib, patients initially assigned to receive olaparib had significantly longer OS than those assigned to receive enzalutamide or abiraterone as control therapy [91].
Preclinical studies suggest a synergy between PARP inhibitors and ARSIs. This synergy may be due to the involvement of PARP in the positive co-regulation of AR signaling, which leads to enhanced AR target gene suppression when PARP/AR signaling is corepressed [92]. ARSIs have been reported to inhibit the transcription of some HRR genes, leading to HRR deficiency and increased sensitivity to PARP inhibitors via nongenetic mechanisms [93]. These preclinical findings were confirmed in a phase 2 trial, which showed that olaparib combined with abiraterone significantly prolonged rPFS compared to abiraterone and placebo in patients with mCRPC who had previously received docetaxel and were not selected based on their HRR mutation status (HR, 0.65; 95% CI, 0.44–0.97; p=0.034) [94]. The PROpel trial is a phase 3 trial comparing abiraterone and olaparib to abiraterone and placebo as the first-line treatment for patients with mCRPC. Patients were enrolled regardless of their HRR gene mutation status. Based on the primary analysis at the first data cutoff, the median rPFS was significantly longer in the abiraterone and olaparib group than that in the abiraterone and placebo group (24.8 vs. 16.6 months; HR, 0.66; 95% CI, 0.54–0.81; p<0.001). However, at this data cutoff, the OS data were immature [17].
2. AKT inhibitor
The PI3K/AKT and AR pathways are dysregulated in approximately 40%–60% of patients with mCRPC, and AKT signaling is hyperactivated in tumors with functional PTEN-loss [33]. The PI3K/AKT and AR pathways exhibit crosstalk regulation in mCRPC [95]. Ipatasertib is a selective ATP-competitive small-molecule inhibitor of the three isoforms of AKT. In a phase 2 study, de Bono JS et al. found that the rPFS was prolonged in patients with mCRPC administered ipatasertib plus abiraterone compared with that in those administered placebo plus abiraterone, with a greater effect observed in patients with PTEN-loss tumors [96]. In the phase 3 IPATential150 trial, patients with previously untreated asymptomatic or mildly symptomatic mCRPC were randomized to receive ipatasertib plus abiraterone or placebo plus abiraterone. For patients who had tumors with PTEN loss, the median rPFS was 16.5 months in the placebo plus abiraterone group and 18.5 months in the ipatasertib plus abiraterone group (HR, 0.77; 95% CI, 0.61–0.98; p=0.034); however, there was no significant difference between the groups in the intention-to-treat population [18].
CONCLUSIONS
Currently, abiraterone, enzalutamide, apalutamide, and docetaxel are approved and widely used to treat mCSPC in combination with ADT. Furthermore, the triplet therapy comprising docetaxel, ARSI, and ADT has emerged for the treatment for mCSPC. This intensification of treatment upfront in the disease trajectory has undoubtedly been an important step forward. However, cross-resistance between drugs may reduce the effectiveness of downstream therapies for mCRPC, giving rise to a more aggressive, treatment-resistant disease phenotype. The sequential administration of ARSIs, such as abiraterone and enzalutamide, is associated with limited efficacy. For patients with mCRPC who were previously treated with docetaxel and had disease progression while receiving an ARSI, cabazitaxel is recommended and should be applied early if the patient is still eligible for chemotherapy. In addition, several novel agents have been clinically applied for the treatment of mCRPC. In particular, Lu-PSMA and PARP inhibitors are emerging as effective therapeutic options. PSMA-PET is used to determine the eligibility for Lu-PSMA therapy. Companion diagnostics are used to identify patients with mCRPC with HRR mutations, making them eligible for treatment with the PARP inhibitors.
Footnotes
Conflict of Interest: KH has received grants/research supports from Astellas Global. NN has nothing to disclose.
Funding: This research was supported by the Japan Society for the Promotion of Science KAKENHI (grant number 22K09447).
- Conceptualization: KH, NN.
- Data curation: KH.
- Formal analysis: KH.
- Funding acquisition: KH, NN.
- Investigation: KH.
- Methodology: KH.
- Project administration: KH, NN.
- Resources: KH.
- Software: KH.
- Supervision: NN.
- Validation: KH, NN.
- Visualization: KH.
- Writing – original draft: KH.
- Writing – review & editing: KH, NN.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2:389–396. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. Erratum in: N Engl J Med 2013;368:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 8.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. PREVAIL Investigators. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberger M, Hardy-Bessard AC, Kim CS, Géczi L, Ford D, Mourey L, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35:3198–3206. doi: 10.1200/JCO.2016.72.1076. [DOI] [PubMed] [Google Scholar]
- 11.Oudard S, Fizazi K, Sengeløv L, Daugaard G, Saad F, Hansen S, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial-FIRSTANA. J Clin Oncol. 2017;35:3189–3197. doi: 10.1200/JCO.2016.72.1068. [DOI] [PubMed] [Google Scholar]
- 12.de Wit R, de Bono J, Sternberg CN, Fizazi K, Tombal B, Wülfing C, et al. CARD Investigators. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381:2506–2518. doi: 10.1056/NEJMoa1911206. [DOI] [PubMed] [Google Scholar]
- 13.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 14.Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al. ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 15.Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. VISION Investigators. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 17.Clarke NW, Armstrong AJ, Thiery-Vuillemin A, Oya M, Shore N, Loredo E, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid. 2022;1 doi: 10.1056/EVIDoa2200043. [DOI] [PubMed] [Google Scholar]
- 18.Sweeney C, Bracarda S, Sternberg CN, Chi KN, Olmos D, Sandhu S, et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2021;398:131–142. doi: 10.1016/S0140-6736(21)00580-8. [DOI] [PubMed] [Google Scholar]
- 19.Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. LATITUDE Investigators. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 21.Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. TITAN Investigators. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381:13–24. doi: 10.1056/NEJMoa1903307. [DOI] [PubMed] [Google Scholar]
- 22.Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. ENZAMET Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121–131. doi: 10.1056/NEJMoa1903835. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37:2974–2986. doi: 10.1200/JCO.19.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fizazi K, Foulon S, Carles J, Roubaud G, McDermott R, Fléchon A, et al. PEACE-1 Investigators. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2022;399:1695–1707. doi: 10.1016/S0140-6736(22)00367-1. [DOI] [PubMed] [Google Scholar]
- 25.Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, et al. ARASENS Trial Investigators. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386:1132–1142. doi: 10.1056/NEJMoa2119115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. SPARTAN Investigators. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408–1418. doi: 10.1056/NEJMoa1715546. [DOI] [PubMed] [Google Scholar]
- 27.Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378:2465–2474. doi: 10.1056/NEJMoa1800536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, et al. ARAMIS Investigators. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235–1246. doi: 10.1056/NEJMoa1815671. Erratum in: N Engl J Med 2022;387:860. [DOI] [PubMed] [Google Scholar]
- 29.Buck SAJ, Koolen SLW, Mathijssen RHJ, de Wit R, van Soest RJ. Cross-resistance and drug sequence in prostate cancer. Drug Resist Updat. 2021;56:100761. doi: 10.1016/j.drup.2021.100761. [DOI] [PubMed] [Google Scholar]
- 30.Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79:263–282. doi: 10.1016/j.eururo.2020.09.046. [DOI] [PubMed] [Google Scholar]
- 31.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Prostate Cancer Clinical Trials Working Group 3. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. Erratum in: Cell 2015;162:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701–711. doi: 10.1038/nrc4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita K, Nonomura N. Role of androgen receptor in prostate cancer: a review. World J Mens Health. 2019;37:288–295. doi: 10.5534/wjmh.180040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. COU-AA-301 Investigators. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 37.Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. COU-AA-302 Investigators. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 38.James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. STAMPEDE Investigators. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–351. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fizazi K, Scher HI, Miller K, Basch E, Sternberg CN, Cella D, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15:1147–1156. doi: 10.1016/S1470-2045(14)70303-1. Erratum in: Lancet Oncol 2014;15:e475. [DOI] [PubMed] [Google Scholar]
- 40.Beer TM, Armstrong AJ, Rathkopf D, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151–154. doi: 10.1016/j.eururo.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penson DF, Armstrong AJ, Concepcion R, Agarwal N, Olsson C, Karsh L, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34:2098–2106. doi: 10.1200/JCO.2015.64.9285. [DOI] [PubMed] [Google Scholar]
- 42.Shore ND, Chowdhury S, Villers A, Klotz L, Siemens DR, Phung D, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17:153–163. doi: 10.1016/S1470-2045(15)00518-5. [DOI] [PubMed] [Google Scholar]
- 43.Iguchi T, Tamada S, Kato M, Yasuda S, Machida Y, Ohmachi T, et al. Enzalutamide versus flutamide for castration-resistant prostate cancer after combined androgen blockade therapy with bicalutamide: the OCUU-CRPC study. Int J Clin Oncol. 2020;25:486–494. doi: 10.1007/s10147-019-01554-3. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong AJ, Azad AA, Iguchi T, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2022;40:1616–1622. doi: 10.1200/JCO.22.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caffo O, Ortega C, Nolè F, Gasparro D, Mucciarini C, Aieta M, et al. Docetaxel and prednisone with or without enzalutamide as first-line treatment in patients with metastatic castration-resistant prostate cancer: CHEIRON, a randomised phase II trial. Eur J Cancer. 2021;155:56–63. doi: 10.1016/j.ejca.2021.06.016. [DOI] [PubMed] [Google Scholar]
- 46.Clegg NJ, Wongvipat J, Joseph JD, Tran C, Ouk S, Dilhas A, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494–1503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79:150–158. doi: 10.1016/j.eururo.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Chi KN, Chowdhury S, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39:2294–2303. doi: 10.1200/JCO.20.03488. [DOI] [PubMed] [Google Scholar]
- 49.Saad F, Efstathiou E, Attard G, Flaig TW, Franke F, Goodman OB, Jr, et al. ACIS Investigators. Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): a randomised, placebo-controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2021;22:1541–1559. doi: 10.1016/S1470-2045(21)00402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moilanen AM, Riikonen R, Oksala R, Ravanti L, Aho E, Wohlfahrt G, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep. 2015;5:12007. doi: 10.1038/srep12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, et al. ARAMIS Investigators. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383:1040–1049. doi: 10.1056/NEJMoa2001342. [DOI] [PubMed] [Google Scholar]
- 52.Chi K, Hotte SJ, Joshua AM, North S, Wyatt AW, Collins LL, et al. Treatment of mCRPC in the AR-axis-targeted therapy-resistant state. Ann Oncol. 2015;26:2044–2056. doi: 10.1093/annonc/mdv267. [DOI] [PubMed] [Google Scholar]
- 53.Attard G, Borre M, Gurney H, Loriot Y, Andresen-Daniil C, Kalleda R, et al. PLATO collaborators. Abiraterone alone or in combination with enzalutamide in metastatic castration-resistant prostate cancer with rising prostate-specific antigen during enzalutamide treatment. J Clin Oncol. 2018;36:2639–2646. doi: 10.1200/JCO.2018.77.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khalaf DJ, Annala M, Taavitsainen S, Finch DL, Oja C, Vergidis J, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20:1730–1739. doi: 10.1016/S1470-2045(19)30688-6. [DOI] [PubMed] [Google Scholar]
- 55.Isaacs JT, D'Antonio JM, Chen S, Antony L, Dalrymple SP, Ndikuyeze GH, et al. Adaptive auto-regulation of androgen receptor provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human prostate cancer. Prostate. 2012;72:1491–1505. doi: 10.1002/pros.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teply BA, Wang H, Luber B, Sullivan R, Rifkind I, Bruns A, et al. Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: an open-label, phase 2, multicohort study. Lancet Oncol. 2018;19:76–86. doi: 10.1016/S1470-2045(17)30906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denmeade SR, Wang H, Agarwal N, Smith DC, Schweizer MT, Stein MN, et al. TRANSFORMER: a randomized phase II study comparing bipolar androgen therapy versus enzalutamide in asymptomatic men with castration-resistant metastatic prostate cancer. J Clin Oncol. 2021;39:1371–1382. doi: 10.1200/JCO.20.02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gravis G, Boher JM, Joly F, Soulié M, Albiges L, Priou F, et al. GETUG. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol. 2016;70:256–262. doi: 10.1016/j.eururo.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 59.James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. STAMPEDE Investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lavaud P, Gravis G, Foulon S, Joly F, Oudard S, Priou F, et al. Anticancer activity and tolerance of treatments received beyond progression in men treated upfront with androgen deprivation therapy with or without docetaxel for metastatic castration-naïve prostate cancer in the GETUG-AFU 15 phase 3 trial. Eur Urol. 2018;73:696–703. doi: 10.1016/j.eururo.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 61.Annala M, Fu S, Bacon JVW, Sipola J, Iqbal N, Ferrario C, et al. Cabazitaxel versus abiraterone or enzalutamide in poor prognosis metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase II trial. Ann Oncol. 2021;32:896–905. doi: 10.1016/j.annonc.2021.03.205. [DOI] [PubMed] [Google Scholar]
- 62.Mota JM, Barnett E, Nauseef JT, Nguyen B, Stopsack KH, Wibmer A, et al. Platinum-based chemotherapy in metastatic prostate cancer with DNA repair gene alterations. JCO Precis Oncol. 2020;4:355–366. doi: 10.1200/PO.19.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmid S, Omlin A, Higano C, Sweeney C, Martinez Chanza N, Mehra N, et al. Activity of platinum-based chemotherapy in patients with advanced prostate cancer with and without DNA repair gene aberrations. JAMA Netw Open. 2020;3:e2021692. doi: 10.1001/jamanetworkopen.2020.21692. Erratum in: JAMA Netw Open 2020;3:e2029176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng HH, Pritchard CC, Boyd T, Nelson PS, Montgomery B. Biallelic inactivation of BRCA2 in platinum-sensitive metastatic castration-resistant prostate cancer. Eur Urol. 2016;69:992–995. doi: 10.1016/j.eururo.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pomerantz MM, Spisák S, Jia L, Cronin AM, Csabai I, Ledet E, et al. The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer. Cancer. 2017;123:3532–3539. doi: 10.1002/cncr.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Slootbeek PHJ, Duizer ML, van der Doelen MJ, Kloots ISH, Kuppen MCP, Westgeest HM, et al. Impact of DNA damage repair defects and aggressive variant features on response to carboplatin-based chemotherapy in metastatic castration-resistant prostate cancer. Int J Cancer. 2021;148:385–395. doi: 10.1002/ijc.33306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aparicio AM, Shen L, Tapia EL, Lu JF, Chen HC, Zhang J, et al. Combined tumor suppressor defects characterize clinically defined aggressive variant prostate cancers. Clin Cancer Res. 2016;22:1520–1530. doi: 10.1158/1078-0432.CCR-15-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corn PG, Heath EI, Zurita A, Ramesh N, Xiao L, Sei E, et al. Cabazitaxel plus carboplatin for the treatment of men with metastatic castration-resistant prostate cancers: a randomised, open-label, phase 1-2 trial. Lancet Oncol. 2019;20:1432–1443. doi: 10.1016/S1470-2045(19)30408-5. Erratum in: Lancet Oncol 2020;21:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35:40–47. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 70.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. CA184-043 Investigators. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fizazi K, Drake CG, Beer TM, Kwon ED, Scher HI, Gerritsen WR, et al. CA184-043 Investigators. Final analysis of the ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur Urol. 2020;78:822–830. doi: 10.1016/j.eururo.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25:3753–3758. doi: 10.1158/1078-0432.CCR-18-4070. [DOI] [PubMed] [Google Scholar]
- 73.Marcus L, Fashoyin-Aje LA, Donoghue M, Yuan M, Rodriguez L, Gallagher PS, et al. FDA approval summary: pembrolizumab for the treatment of tumor mutational burden-high solid tumors. Clin Cancer Res. 2021;27:4685–4689. doi: 10.1158/1078-0432.CCR-21-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abida W, Cheng ML, Armenia J, Middha S, Autio KA, Vargas HA, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5:471–478. doi: 10.1001/jamaoncol.2018.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gartrell BA, Coleman R, Efstathiou E, Fizazi K, Logothetis CJ, Smith MR, et al. Metastatic prostate cancer and the bone: significance and therapeutic options. Eur Urol. 2015;68:850–858. doi: 10.1016/j.eururo.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 76.Saad F, Carles J, Gillessen S, Heidenreich A, Heinrich D, Gratt J, et al. Radium-223 International Early Access Program Investigators. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016;17:1306–1316. doi: 10.1016/S1470-2045(16)30173-5. [DOI] [PubMed] [Google Scholar]
- 77.Smith M, Parker C, Saad F, Miller K, Tombal B, Ng QS, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:408–419. doi: 10.1016/S1470-2045(18)30860-X. Erratum in: Lancet Oncol 2019;20:e559. [DOI] [PubMed] [Google Scholar]
- 78.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 79.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Zoledronic Acid Prostate Cancer Study Group. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 80.Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.James ND, Pirrie SJ, Pope AM, Barton D, Andronis L, Goranitis I, et al. Clinical outcomes and survival following treatment of metastatic castrate-refractory prostate cancer with docetaxel alone or with strontium-89, zoledronic acid, or both: the TRAPEZE randomized clinical trial. JAMA Oncol. 2016;2:493–499. doi: 10.1001/jamaoncol.2015.5570. [DOI] [PubMed] [Google Scholar]
- 82.Saad F, Shore N, Van Poppel H, Rathkopf DE, Smith MR, de Bono JS, et al. Impact of bone-targeted therapies in chemotherapy-naïve metastatic castration-resistant prostate cancer patients treated with abiraterone acetate: post hoc analysis of study COU-AA-302. Eur Urol. 2015;68:570–577. doi: 10.1016/j.eururo.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paschalis A, Sheehan B, Riisnaes R, Rodrigues DN, Gurel B, Bertan C, et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur Urol. 2019;76:469–478. doi: 10.1016/j.eururo.2019.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. TheraP Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. doi: 10.1016/S0140-6736(21)00237-3. [DOI] [PubMed] [Google Scholar]
- 85.Hatano K, Nonomura N. Genomic profiling of prostate cancer: an updated review. World J Mens Health. 2022;40:368–379. doi: 10.5534/wjmh.210072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abida W, Armenia J, Gopalan A, Brennan R, Walsh M, Barron D, et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol. 2017;2017:PO.17.00029. doi: 10.1200/PO.17.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jonsson P, Bandlamudi C, Cheng ML, Srinivasan P, Chavan SS, Friedman ND, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019;571:576–579. doi: 10.1038/s41586-019-1382-1. Erratum in: Nature 2019;577:E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mateo J, Porta N, Bianchini D, McGovern U, Elliott T, Jones R, et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21:162–174. doi: 10.1016/S1470-2045(19)30684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abida W, Patnaik A, Campbell D, Shapiro J, Bryce AH, McDermott R, et al. TRITON2 Investigators. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol. 2020;38:3763–3772. doi: 10.1200/JCO.20.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hussain M, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. PROfound Trial Investigators. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. 2020;383:2345–2357. doi: 10.1056/NEJMoa2022485. [DOI] [PubMed] [Google Scholar]
- 92.Schiewer MJ, Goodwin JF, Han S, Brenner JC, Augello MA, Dean JL, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012;2:1134–1149. doi: 10.1158/2159-8290.CD-12-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li L, Karanika S, Yang G, Wang J, Park S, Broom BM, et al. Androgen receptor inhibitor-induced "BRCAness" and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal. 2017;10:eaam7479. doi: 10.1126/scisignal.aam7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19:975–986. doi: 10.1016/S1470-2045(18)30365-6. [DOI] [PubMed] [Google Scholar]
- 95.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Bono JS, De Giorgi U, Rodrigues DN, Massard C, Bracarda S, Font A, et al. Randomized phase II study evaluating akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin Cancer Res. 2019;25:928–936. doi: 10.1158/1078-0432.CCR-18-0981. [DOI] [PubMed] [Google Scholar]