Abstract
Purpose
Neurogenic erectile dysfunction (ED) is a common side effect of radical prostatectomy (RP) because of cavernous nerve damage. In these patients, the production of nitric oxide (NO), which is important for erection, is decreased in the corpus cavernosum. Therefore, NO donors are useful for post-RP ED. However, short half-life and systemic side effects are problems of NO application in ED therapy. To avert these problems, we developed a red-light controllable NO releaser, NORD-1. This study aimed to investigate the effect of NORD-1 and red-light irradiation on neurogenic ED using a rat model of bilateral cavernous nerve injury (BCNI).
Materials and Methods
BCNI and sham operations were conducted on 8-week-old rats. After 4 weeks, erectile function was evaluated using changes in intracavernous pressure (ICP) during electrostimulation of the cavernous nerve. ICP was measured under three conditions; without NORD-1 and red-light irradiation, with NORD-1 and without red-light irradiation, and with NORD-1 and red-light irradiation. SiR650 which absorbs red-light but does not release NO was used for the negative control. After the experiment, localization of NORD-1 was observed using a microscope.
Results
Erectile function in a BCNI rat model was significantly decreased compared to sham-operated rats (p<0.05). After injecting NORD-1 into the penis, erectile function did not change without red-light irradiation. However, the combination of NORD-1 and red-light irradiation significantly improved erectile function (p<0.05) without affecting systemic arterial pressure. In contrast, when SiR650 was used, erectile function did not change in all three conditions. NORD-1 was detected only in the corpus cavernosum and not in the urethra and dorsal vein.
Conclusions
NORD-1 combined with red-light irradiation is effective for ED induced by cavernous nerve injury. This treatment may have low risks of hypotension and urinary incontinence, and it can replace the current treatment for post-RP ED.
Keywords: Erectile dysfunction, Light, Nerve, Nitric oxide, Prostatectomy, Smooth muscle
INTRODUCTION
Neurogenic erectile dysfunction (ED) frequently occurs after radical prostatectomy (RP) due to cavernous nerve damage [1]. Despite the advances in surgical techniques, including nerve-sparing surgery, the prevalence of neurogenic ED following RP has not improved [2,3]. Phosphodiesterase (PDE)-5 inhibitors are used as first-line therapy for patients with post-RP ED. However, PDE-5 inhibitors have not completely restored erectile function in these patients; therefore, more effective therapy is needed [4].
Nitric oxide (NO) is a colorless gas involved in important chemical signaling in humans and other animals [5]. NO activates soluble guanylyl cyclase (sGC), which catalyzes the conversion of guanosine triphosphate to cyclic guanosine monophosphate (cGMP). The NO-sGC-cGMP signaling pathway regulates vasodilation and smooth muscle relaxation; thus, NO plays an important role in maintaining an erection [6]. However, it has been reported that NO production is reduced in patients with post-RP ED; thus, NO donors are considered beneficial therapy for these patients [7]. However, there are some critical problems with NO application for ED therapy. First, it is difficult to control NO release in vivo because of its short half-life [8]. Second, NO has systemic side effects such as headache and hypotension. To solve these problems, we focused on light-reactive NO releasers that could temporally and spatially regulate NO release, thereby controlling the beginning and the end of drug effects and preventing systemic side effects. We have previously reported the use of blue light-reactive NO releaser and yellowish-green light-reactive NO releasers [9,10,11]. However, it may be difficult to regulate erectile response because these lights have low tissue permeability. Therefore, we improved these NO releasers and developed NORD-1, a red-light-reactive NO releaser. Red light has a longer wavelength and higher tissue permeability than blue and yellowish-green light [12]. We have previously reported that the combination of NORD-1 and red-light irradiation increased intracavernous pressure (ICP), indicating an increase in erectile function in intact rats [13]. Thus, we hypothesized that NORD-1 would be effective in treating neurogenic ED. In this study, we aimed to investigate the effect of NORD-1 combined with red-light irradiation in a neurogenic ED model rat of bilateral cavernous nerve injury (BCNI).
MATERIALS AND METHODS
1. Ethics statement
These experiments complied with the National Institutes of Health guidelines. The procedures used and the care of animals were approved by the Institutional Animal Care and Use Committee (IACUC) in Nagoya City University (Approval No. H25-P-09).
2. Reagents
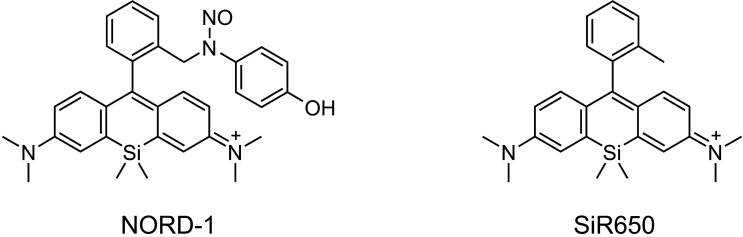
NORD-1 was prepared as reported previously [13]. In this study, SiR650, the antenna moiety for NORD-1, was used as the negative reference compound because it absorbs red-light but does not release NO during red-light irradiation. The chemical structures of NORD-1 and SiR650 are presented in Fig. 1.
Fig. 1. The chemical structures of NORD-1 and SiR650. NORD-1 and SiR650 were prepared as previously reported [13]. SiR650 was used as negative reference compound because it does not release nitric oxide (NO) by red-light irradiation.
3. Animals
Three experiments were performed in this study. In experiment I, 8-week-old male Wistar-ST rats (Japan SLC, Inc., Shizuoka, Japan) were used In experiment II and III, 12-week-old male Wistar-ST rats were used. All animals were housed in an environmentally (temperature and humidity) controlled room with a 12-h light or dark cycle and free access to laboratory chow and water.
4. Study design
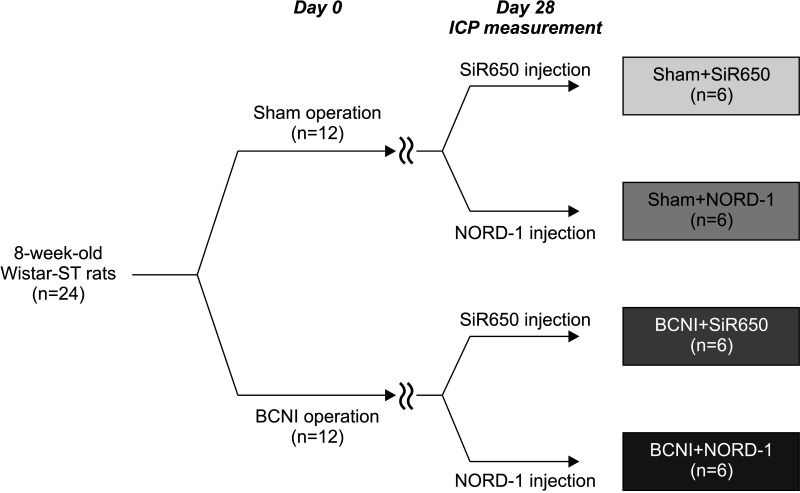
The study design of experiment I is depicted in Fig. 2. In total, 24 rats were used in experiment I. Rats randomly underwent sham or BCNI operations (n=12, respectively). After 4 weeks, erectile function was evaluated by measuring ICP. During the experiment, NORD-1 or SiR650 was injected into the rats. Finally, the rats were randomly assigned to one of four groups; Sham+SiR650, Sham+NORD-1, BCNI+SiR650, and BCNI+NORD-1 (n=6, respectively).
Fig. 2. Study design. BCNI: bilateral cavernous nerve injury, ICP: intracavernous pressure.
5. BCNI procedure
Rats were administered 3% and 1.5% isoflurane (Pfizer Inc., New York, NY, USA) to induce and maintain anesthesia, respectively. The prostate was exposed, and the cavernous nerve and major pelvic ganglion (MPG) were identified. Reverse action tweezers (Fontax®, Prilly, Switzerland; cat No.1x) held the cavernous nerve 5 mm distal to the MPG on both sides for 1 minute each, causing BCNI. In sham-operated rats, the prostate was exposed without cavernous nerve injury. After 4 weeks, erectile response was measured.
6. Evaluation of erectile function
Erectile function was assessed based on changes in ICP followed by electrostimulation of the cavernous nerves as previously reported [14,15]. Briefly, 3% and 1.5% isoflurane (Pfizer Inc.) were used to induce and maintain anesthesia, respectively, in the rats. The carotid artery was cannulated to monitor arterial pressure. The left crus of the penis was cannulated using a 23-gauge needle to monitor ICP. Subsequently, electrostimulation of the cavernous nerve was performed at a site closer to the MPG than the injury site with the following parameters: 1 minute at 5 V, 8 Hz, and a square wave duration of 5 ms. Systemic arterial pressure and ICP were recorded and analyzed with LabChart8 software (AD Instruments Pty. Ltd., New South Wales, Australia). Erectile function was evaluated using the maximum ICP to mean arterial pressure (MAP) ratio because ICP is influenced by systemic arterial pressure. In addition, the changes in arterial pressure during electrostimulation were evaluated by the MAP at the end of the electrostimulation (End-MAP) to the MAP at the start of the electrostimulation (Start-MAP) ratio.
7. Experimental protocol
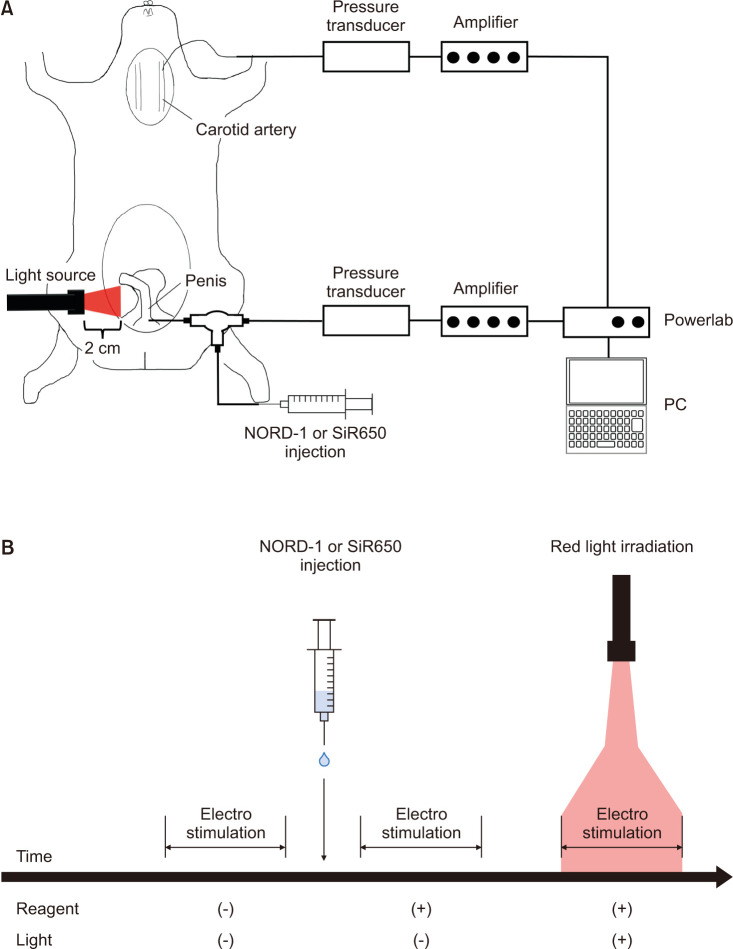
The schematic image of the experiment is shown in Fig. 3A. The experimental protocol for ICP measurement is shown in Fig. 3B. ICP was measured during electrostimulation for 1 minute at 8 Hz. Using a T-shape stopcock, 100 µL (10-4 M) NORD-1 or SiR650 was injected into the penis via the same route for ICP measurement. After few minutes, ICP was measured during electrostimulation for 1 minute at 8 Hz. Finally, ICP was measured during electrostimulation for 1 minute at 8 Hz with the penis irradiated 630–690 nm light (115 mW) at a distance of 2 cm using a MAX-302 xenon light source (Asahi Spectra Co., Ltd., Tokyo, Japan). Immediately after the experiment, penis samples were obtained and frozen in the optimal cutting temperature (O.C.T.) compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan).
Fig. 3. Schematic image and experimental protocol of intracavernous pressure (ICP) measurement Schematic image of ICP measurement experiment is shown in (A). Light source was set at a distance of 2 cm from the penis and red light was irradiated during electrostimulation. NORD-1 or SiR650 were injected into the penis using a T-shape stopcock. Experimental protocol is shown in (B). ICP was measured in three conditions; without injecting reagent and irradiating red-light, with reagent and without red-light, and with reagent and red-light, continuously.
8. Localization of NORD-1 and cavernosum smooth muscle
Ten-micron two-serial sections of penis samples were cut and mounted on microscope slides separately. One slide was observed using a BZ-X810 fluorescence microscope (KEYENCE Cop., Osaka, Japan) to detect NORD-1 or SiR650. NORD-1 and SiR650 emit fluorescence at approximately 690 nm when undergoing light excitation at approximately 650 nm. Therefore, a Cy5 filter (excitation; 620/60 nm, emission; 700/75 nm) was selected for the detection of NORD-1 or SiR650. Another slide was used for immunofluorescent staining of alpha-smooth muscle actin (α-SMA). The slide was fixed with 10% formaldehyde, blocked by treatment with 5% skim milk, and probed with anti-α-SMA (Abcam plc., Cambridge, UK; cat. No.; ab5694; 1:5000 dilution). Afterwards, slides were incubated with Alexa Fluor® 488 conjugate anti-rabbit secondary antibody (Cell Signaling Technology Inc., Danbers, MA, USA; cat. No.; 4412 1:5000 dilution) and visualized using a BZ-X800 fluorescence microscope. The images were analyzed using Photoshop CS4 (Adobe Systems Inc., San Jose, CA, USA). The pixel numbers of stained with α-SMA and whole cavernosum were analyzed. Smooth muscle content in the corpus cavernosum was evaluated by calculating the ratio of α-SMA area to cavernosum area.
9. Further evaluation of NORD-1 treatment
The detailed information was described in Supplement File.
10. Evaluation of the distribution of NORD-1 injecting to penis throughout the body
The detailed information was described in Supplement File.
11. Red-light permeability experiment
The detailed information was described in Supplement File.
12. Statistical analysis
All data conform to normal distribution and results are expressed as mean±standard deviation. For comparisons between the four groups, analysis of variance was carried out. If the results of the analysis of variance were significant, Bonferroni multiple t-tests were used and differences with p-values less than 0.05 were considered significant. All statistical analyses were performed using EZR (version 1.55; Saitama Medical Center, Jichi Medical University, Saitama, Japan) [16].
RESULTS
1. ICP measurement
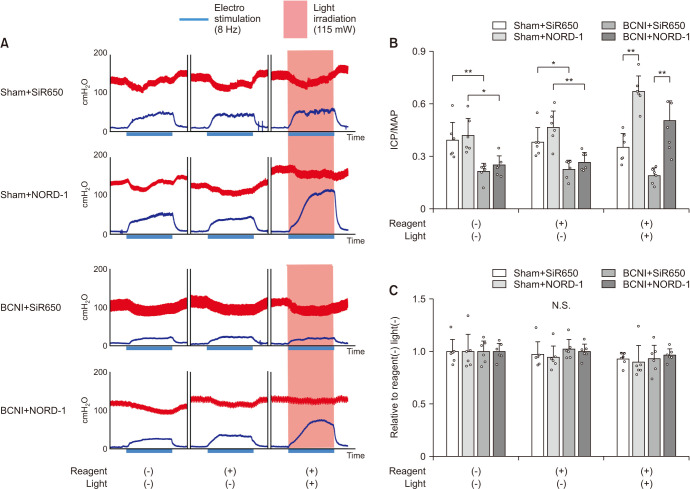
Representative arterial pressure and ICP charts in the four groups are shown in Fig. 4A. The ICP/MAP ratio in the four groups is shown in Fig. 4B. Before injecting SiR650, ICP/MAP during 8 Hz electrostimulation in the BCNI+SiR650 group was lower than in the Sham+SiR650 group (p<0.01). Also before injecting NORD-1, ICP/MAP in the BCNI+NORD-1 group was lower than in the Sham+NORD-1 group (p<0.05). After injecting SiR650 or NORD-1, ICP/MAP did not change compared to those before injection in the four groups in the condition without irradiating 630–690 nm light. On the other hand, during 8 Hz electrostimulation with the penis irradiated with 630–690 nm light, ICP/MAP in the Sham+NORD-1 group was higher than in the Sham+SiR650 group (p<0.01). ICP/MAP in the BCNI+NORD-1 group was also higher than in the BCNI+SiR650 group (p<0.01). Subsequently, we analyzed the changes in Start-MAP to End-MAP in three different conditions (without reagent and light, with reagent and without light, and with reagent and light). We calculated the ratios of End-MAP to Start-MAP (End-MAP/Start-MAP) in three different conditions, and the results were expressed relative to without reagent and light (Fig. 4C). The End-MAP/Start-MAP were not different among the three conditions in all four groups.
Fig. 4. Representative intracavernous pressure (ICP) and mean arterial pressure (MAP) results. Charts show ICP (blue line) and arterial pressure (red line), in four groups, during electrostimulation without reagent and light, with reagent and without light, and with reagent and light (A). The electrostimulation period is shown by the blue thicker bar along time axis. Red-light irradiation (630–690 nm, 115 mW) period is indicated by the red background. ICP to MAP ratios in each group were measured (B). The ratio of MAP at the end of electrostimulation (End-MAP) to MAP at the start of electrostimulation (Start-MAP) was calculated. End-MAP/Start-MAP ratios in three different conditions were expressed relative to without reagent and light (C). Data are reported as mean±standard deviation (n=6 per group). NS: not significant. *p<0.05, **p<0.01 using Bonferroni’s multiple t-tests.
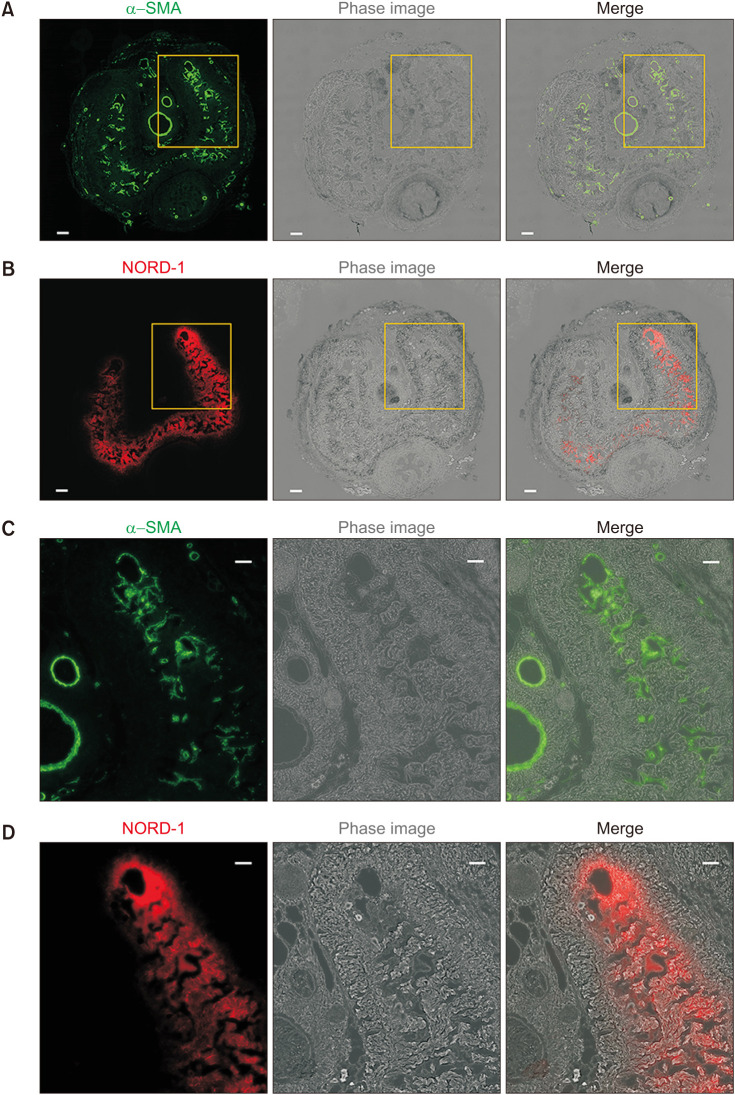
2. Localization of NORD-1
Representative images of corpus cavernosum from a rat after NORD-1 injection into the crura of the penis are provided in Fig. 5. In Fig. 5A, 5C, the smooth muscle was detected by immunofluorescence staining with antibody to α-SMA. In Fig. 5B, 5D, NORD-1 was detected by approximately 620 nm excitation light. Fluorescence of NORD-1 was detected only in the corpus cavernosum, including cavernosum smooth muscle. In addition, NORD-1 was not detected outside of tunica albuginea, including the urethra and vein.
Fig. 5. Localization of NORD-1. Representative image of two-serial sections of the penis after NORD-1 injection into the crura of the penis. In one slide, smooth muscle was detected by immunofluorescence staining with antibody to alpha-smooth muscle actin (α-SMA) (A, C). In another slide, NORD-1 was detected by approximately 620 nm excitation light (B, D). (A) and (B) reveal whole images of the corpus cavernosum (scale bar: 200 µm). (C) and (D) show enlargement images surrounded by yellow flame in (A) and (B) (scale bar: 100 µm).
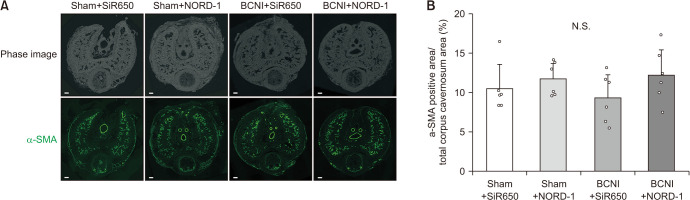
3. Evaluation of smooth muscle content in corpus cavernosum
Representative images of the corpus cavernosum stained with α-SMA in the four groups are shown in Fig. 6A. The ratio of α-SMA area to whole cavernosum area was not different among the four groups (Fig. 6B).
Fig. 6. Evaluation of smooth muscle content in the corpus cavernosum. Representative images of the corpus cavernosum stained with alpha-smooth muscle actin (α-SMA) in each group (A; scale bar: 200 µm). The ratio of α-SMA area to the whole cavernosum area was evaluated (B). Data are reported as mean±standard deviation (n=6 per group). N.S. means not significant using Bonferroni’s multiple t-tests.
4. Further evaluation of NORD-1 treatment
Representative ICP and arterial pressure charts in experiment II are provided in Supplement Fig. 1, 2. As shown in Supplement Fig. 1, ICP was increased during electrostimulation of cavernous nerve. Then, ICP was further increased by irradiating red-light to penis. When the red-light irradiation was stopped, ICP was back to baseline. In addition, as represented in Supplement Fig. 2, ICP did not change from baseline during red-light irradiation without electrostimulation of cavernous nerve.
5. Distribution of NORD-1 injected to penis throughout the body
Representative images of penis and kidney samples one hour after injecting NORD-1 into penis are provided in Supplement Fig. 3. NORD-1 fluorescent was detected in both corpus cavernosum and in kidney.
6. Red-light permeability in muscle tissue
A table in Supplement Fig. 4 indicates red-light illuminance when the light source was turned off (baseline), when 630–690 nm light was irradiated without meat (0 cm), and when 630–690 nm light was irradiated toward the meats whose thickness varied from 0.5 cm to 1.0 cm. Although the illuminance was weakened as the thickness of the meat increased, red-light reached optical power meter partially even when the thickness of the meat was 1.0 cm.
DISCUSSION
We previous reported NORD-1 combined with red-light irradiation enhanced erectile function in intact rats [13]. Thus, in this study, we investigated the effect of NORD-1 treatment on erectile function in a rat model of cavernous nerve injury. Erectile function was significantly decreased in the BCNI rat model compared to the sham-operated rats. Therefore, the BCNI operation induced neurogenic ED. Erectile function did not improve with NORD-1 injection, without red-light irradiation. However, the erectile function of the BCNI rat model significantly improved to that of sham-operated rats with NORD-1 and irradiating red light. This indicates that the combination of NORD-1 and red light is effective for treating ED induced by BCNI. We evaluated the systemic effect of this treatment by analyzing the change in arterial pressure during electrostimulation because NO induces vascular smooth muscle relaxation, which sometimes results in hypotension. In this study, NORD-1 and red-light irradiation localized to the penis did not affect systemic arterial pressure, implying that it may have no systemic effect. However, this is limited under anesthesia. Therefore, further studies should be performed to investigate whether the combination of NORD-1 and red-light irradiation has systemic effect under arousal.
In this study, the localization of NORD-1 after the experiment was investigated. The result reveals that NORD-1 was localized only in the corpus cavernosum and reached the cavernosum smooth muscle. Importantly, NORD-1 was not detected in the urethra and dorsal vein. These results indicate two things. First, NORD-1, reaching the cavernosum smooth muscle, released NO by irradiating red light. Released NO is considered to activate sGC which increased cGMP levels in cavernosum smooth muscle. Increased cGMP level induced smooth muscle relaxation and thus, resulted in the enhancement of erectile function. Second, it may have a low risk of side effects induced by smooth muscle relaxation in the urethra and dorsal vein, such as urinary incontinence. However, as previously reported, NORD-1 and red-light irradiation localized in the urethra can cause smooth muscle relaxation [17]. This study did not investigate the effect of NORD-1 localized to the corpus cavernosum on urethral smooth muscle. Therefore, further study should be conducted to evaluate the possibility of urinary incontinence during the treatment.
In this study, the smooth muscle content in the corpus cavernosum was not different between four groups. This result indicates that there might be no histological difference between Sham+SiR650 and Sham+NORD-1 group, or BCNI+SiR650 and BCNI+NORD-1 group. In addition, the smooth muscle content in the corpus cavernosum in the BCNI rat model was not different from sham-operated rats, although it has previously been reported that the expression levels of α-SMA were downregulated by cavernous nerve injury [18,19,20]. This difference may be due to the method used for crushing cavernous nerves. Chen et al achieved cavernous nerve injury by crushing cavernous nerves with a surgical clamp for 60 seconds [18]. Ouyang et al [19] and Wu et al [20] crushed cavernous nerves of rats using a hemostat clamp for 2 minute. On the other hand, in this study, cavernous nerve injury was achieved by crushing the cavernous nerve using a reverse action tweezer for 1 minute. Thus, in this study, the damage to the cavernous nerve is milder than previous reports. It is considered that preserved smooth muscle content in BCNI rat model may be one of the reasons why the combination of NORD-1 and red-light irradiation improved erectile function. However, it is unclear whether the treatment is effective in a more severe rat model of cavernous nerve injury.
In addition, we discuss about the efficacy of NORD-1 in a transected model rat. As indicated in Supplement Fig. 2, ICP did not change from baseline during red-light irradiation to penis without electrostimulation of cavernous nerve. It may imply relaxation of only corpus cavernosum is not enough to erect. In addition, relaxation of arteries involved to erection is necessary to induce erection. It is considered to be caused by cavernous nerve stimulation. Therefore, it might be difficult for NORD-1 injection and red-light irradiation to improve erectile function in the transected model.
In this study, the distribution of NORD-1 injected to penis throughout the body was evaluated using penis and kidney samples, given that kidney is one of the organs with increased blood flow. As a result, NORD-1 was detected in both corpus cavernosum and kidney one hour after the injection. This indicates NORD-1 injected to penis may distribute throughout the body. Therefore, further study should be conducted to investigate the effects of NORD-1 throughout the body.
It has been reported that longer wavelength light has higher tissue permeability [12]. However, the soft tissue permeability of red-light has not yet been investigated. Therefore, we investigated it using pork-shoulder meat. As a result, red-light penetrated the meat of thickness 1.0 cm, although the illuminance was weakened. This result may indicate red-light can penetrate muscle tissues even if they have a considerable level of thickness. In addition, smooth muscle in human penis is more abundant than that in rat penis [21]. Therefore, it is expected that the treatment of NORD-1 and red-light irradiation is effective not only in rats but also in humans.
NORD-1 may have a risk of tissue damage because peroxynitrite, produced by the reaction between released NO and another free radical, interacts with lipids, DNA, and protein. These reactions trigger oxidative injury, resulting in cell apoptosis [22]. Therefore, it is necessary to evaluate negative effect of the treatment in further study.
In this study, we could not measure NO concentrations in corpus cavernosum. It is difficult to measure NO because NO has short half-life [8]. It requires time to extract penis samples after ICP measurement experiment. Therefore, we should set another study to measure NO concentration in vivo.
For further investigation of the effect of NORD-1 treatment, ICP was measured when red-light was irradiated to penis in the middle of electrostimulation of cavernous nerve. As a result, ICP increased during red-light irradiation and returned to baseline when the red-light irradiation was stopped. This indicates the combination of NORD-1 injection and red-light irradiation may not be used for sexual intercourse situation, given that it is necessary to maintain erectile function even after red-light irradiation has been stopped in this situation. However, we propose that the NORD-1 injection and daily red-light irradiation will be expected to be applied for penile rehabilitation. Penile rehabilitation following RP involves using medicines and/or devices to recover erectile function to pre-surgery level [23]. The daily use of PDE-5 inhibitors has been widely used for penile rehabilitation [24]. However, it has been reported that no significant differences in erectile function were noticed between nightly and on-demand use of sildenafil in patients after RP [25]. Moreover, the daily use of PDE-5 inhibitors commenced after RP had no effect on drug-unassisted erectile function following the treatment [26,27]. In addition to these facts, it has been reported that NO production in the corpus cavernosum is decreased in patients with post-RP ED [7]. Therefore, we suggest that NORD-1 and red-light irradiation would be more effective than the current treatment because it increases NO levels in the corpus cavernosum. We conclude that the combination of NORD-1 and red-light irradiation could be a new standard treatment for penile rehabilitation. However, this study revealed only the effect of on-demand use of the treatment. Further studies should be conducted on whether the daily use of the treatment is also effective for ED-induced cavernous nerve injury.
CONCLUSIONS
We conclude that NORD-1 combined with red-light irradiation is effective for ED induced by BCNI. This treatment could replace the current treatment for patients with post-RP ED.
Acknowledgements
The authors are grateful for the assistance of the Research Equipment Sharing Center at the Nagoya City University. We also thank Prof. Tamihide Matsunaga at the Department of Clinical Pharmacy, Graduate School of Pharmaceutical Sciences, Nagoya City University for providing BZ-X810 microscope.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This work was supported by grant number 20K09583 (Y.H), 18K16742(Y.H), 19H03354 (H.N), 20K05752 (N.I.) and JST ACT-X grant number JPMJAX2011 (N.I.) and TOYOAKI SCHLORSHIP FOUNDATION 2018 (Y.H).
- Conceptualization: TM, YH, HN, KK.
- Data curation: TM, YH.
- Formal analysis: TM, TK.
- Investigation: TM, NI.
- Methodology: TM, YH.
- Project administration: TM, YH, NI.
- Resources: TM, NI, HN.
- Software: TK.
- Supervision: HN, KK.
- Validation: TM, YH, NI.
- Visualization: TM, YH.
- Writing–original draft: TM.
- Writing–review & editing: all authors.
Data Sharing Statement
The data required to reproduce these findings cannot be shared at this time due to technical and time limitations.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.220146.
Materials and Methods
Intracavernous pressure (ICP) and arterial pressure charts during electrostimulation and red-light irradiation in the middle of electrostimulation. ICP (blue line) and arterial pressure (red line) chart during electrostimulation of cavernous nerve in intact 12 week-old rats. Before the electrostimulation, 100 µL (10-4 M) NORD-1 was injected into penis. In the middle of electrostimulation, 630–690 nm light (115 mW) was irradiated to penis at a distance of 2 cm from penis. The electrostimulation period is represented by blue thicker bar along time axis. Light irradiation period is indicated by the red back ground.
Intracavernous pressure (ICP) and arterial pressure charts during red-light irradiation with penis. ICP (blue line) and arterial pressure (red line) chart during 630–690 nm light (115 mW) irradiation at a distance of 2 cm from penis in intact 12 weeks-old rats. Before the light irradiation, 100 µL (10-4 M) NORD-1 was injected into penis. Light irradiation period is indicated by the red back ground.
Localization of NORD-1 in the penis and kidney. Representative image of penis (upper) and kidney (lower) 1 hour after NORD-1 (100 µL, 10-4 M) injection into the crura of the penis. NORD-1 fluorescence was detected by approximately 620 nm excitation light. White bar in each image represents scale bar (penis: 200 µm, kidney: 1 mm).
Red-light permeability test using pork-shoulder meat. Schematic image of red-light permeability experiment using pork-shoulder meat. Light source and optical power meter were set and 630–690 nm light (115 mW) was irradiated toward optical power meter. Table represents red-light illuminance when red-light was turned off (baseline), when red-light was turned on without meat (0 cm), and when the meats, whose thickness were varied from 0.5 cm to 1.0 cm, were set between light source and optical power meter.
References
- 1.Resnick MJ, Koyama T, Fan KH, Albertsen PC, Goodman M, Hamilton AS, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436–445. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capogrosso P, Vertosick EA, Benfante NE, Eastham JA, Scardino PJ, Vickers AJ, et al. Are we improving erectile function recovery after radical prostatectomy? Analysis of patients treated over the last decade. Eur Urol. 2019;75:221–228. doi: 10.1016/j.eururo.2018.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian D, Wang XY, Zong HT, Zhang Y. Efficacy and safety of short- and long-term, regular and on-demand regimens of phosphodiesterase type 5 inhibitors in treating erectile dysfunction after nerve-sparing radical prostatectomy: a systematic review and meta-analysis. Clin Interv Aging. 2017;12:405–412. doi: 10.2147/CIA.S122273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albaugh J, Adamic B, Chang C, Kirwen N, Aizen J. Adherence and barriers to penile rehabilitation over 2 years following radical prostatectomy. BMC Urol. 2019;19:89. doi: 10.1186/s12894-019-0516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ignarro LJ, Bush PA, Buga GM, Wood KS, Fukuto JM, Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990;170:843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- 7.Karakus S, Musicki B, Burnett AL. Phosphodiesterase type 5 in men with vasculogenic and post-radical prostatectomy erectile dysfunction: is there a molecular difference? BJU Int. 2018;122:1066–1074. doi: 10.1111/bju.14433. [DOI] [PubMed] [Google Scholar]
- 8.Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotta Y, Ieda N, Fukamoto A, Kataoka T, Kawade Y, Maeda Y, et al. Light-controlled relaxation of the rat penile corpus cavernosum using NOBL-1, a novel nitric oxide releaser. Investig Clin Urol. 2016;57:215–220. doi: 10.4111/icu.2016.57.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ieda N, Hotta Y, Kawaguchi M, Kimura K, Nakagawa H. In cellullo and ex vivo availability of a yellowish-green-light-controllable NO releaser. Chem Pharm Bull (Tokyo) 2019;67:576–579. doi: 10.1248/cpb.c19-00112. [DOI] [PubMed] [Google Scholar]
- 11.Hotta Y, Kataoka T, Mori T, Kimura K. Review of a potential novel approach for erectile dysfunction: light-controllable nitric oxide donors and nanoformulations. Sex Med Rev. 2020;8:297–302. doi: 10.1016/j.sxmr.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Quek CH, Leong KW. Near-infrared fluorescent nanoprobes for in vivo optical imaging. Nanomaterials (Basel) 2012;2:92–112. doi: 10.3390/nano2020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ieda N, Hotta Y, Yamauchi A, Nishikawa A, Sasamori T, Saitoh D, et al. Development of a red-light-controllable nitric oxide releaser to control smooth muscle relaxation in vivo. ACS Chem Biol. 2020;15:2958–2965. doi: 10.1021/acschembio.0c00601. [DOI] [PubMed] [Google Scholar]
- 14.Hotta Y, Shiota A, Kataoka T, Motonari M, Maeda Y, Morita M, et al. Oral L-citrulline supplementation improves erectile function and penile structure in castrated rats. Int J Urol. 2014;21:608–612. doi: 10.1111/iju.12362. [DOI] [PubMed] [Google Scholar]
- 15.Mori T, Hotta Y, Nakamura D, Yahagi R, Kataoka T, Kimura K. Enhancement of the RhoA/Rho kinase pathway is associated with stress-related erectile dysfunction in a restraint water immersion stress model. Physiol Rep. 2021;9:e15064. doi: 10.14814/phy2.15064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda K, Hotta Y, Ieda N, Kataoka T, Nakagawa H, Kimura K. Control of rat bladder neck relaxation with NORD-1, a red light-reactive nitric oxide releaser: in vitro study. J Pharmacol Sci. 2021;146:226–232. doi: 10.1016/j.jphs.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Chen S, Huang X, Kong X, Sun Z, Zhao F, Huang W, et al. Hypoxia-induced phenotypic transformation of corpus cavernosum smooth muscle cells after cavernous nerve crush injury by down-regulating P38 mitogen-activated protein kinase expression. Sex Med. 2019;7:433–440. doi: 10.1016/j.esxm.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouyang X, Han X, Chen Z, Fang J, Huang X, Wei H. MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury. Stem Cell Res Ther. 2018;9:246. doi: 10.1186/s13287-018-1003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu YN, Chen KC, Liao CH, Liu CL, Chiang HS. Smooth muscle progenitor cells preserve the erectile function by reducing corporal smooth muscle cell apoptosis after bilateral cavernous nerve crush injury in rats. Biomed Res Int. 2019;2019:8520523. doi: 10.1155/2019/8520523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinheiro AC, Costa WS, Cardoso LE, Sampaio FJ. Organization and relative content of smooth muscle cells, collagen and elastic fibers in the corpus cavernosum of rat penis. J Urol. 2000;164:1802–1806. [PubMed] [Google Scholar]
- 22.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulhall JP, Bivalacqua TJ, Becher EF. Standard operating procedure for the preservation of erectile function outcomes after radical prostatectomy. J Sex Med. 2013;10:195–203. doi: 10.1111/j.1743-6109.2012.02885.x. [DOI] [PubMed] [Google Scholar]
- 24.Teloken P, Mesquita G, Montorsi F, Mulhall J. Post-radical prostatectomy pharmacological penile rehabilitation: practice patterns among the International Society for Sexual Medicine practitioners. J Sex Med. 2009;6:2032–2038. doi: 10.1111/j.1743-6109.2009.01269.x. [DOI] [PubMed] [Google Scholar]
- 25.Pavlovich CP, Levinson AW, Su LM, Mettee LZ, Feng Z, Bivalacqua TJ, et al. Nightly vs on-demand sildenafil for penile rehabilitation after minimally invasive nerve-sparing radical prostatectomy: results of a randomized double-blind trial with placebo. BJU Int. 2013;112:844–851. doi: 10.1111/bju.12253. [DOI] [PubMed] [Google Scholar]
- 26.Mulhall JP, Brock G, Oelke M, Fode M, Probst KA, Henneges C, et al. Effects of tadalafil once-daily or on-demand vs placebo on return to baseline erectile function after bilateral nerve-sparing radical prostatectomy--results from a randomized controlled trial (REACTT) J Sex Med. 2016;13:679–683. doi: 10.1016/j.jsxm.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Limoncin E, Gravina GL, Corona G, Maggi M, Ciocca G, Lenzi A, et al. Erectile function recovery in men treated with phosphodiesterase type 5 inhibitor administration after bilateral nerve-sparing radical prostatectomy: a systematic review of placebo-controlled randomized trials with trial sequential analysis. Andrology. 2017;5:863–872. doi: 10.1111/andr.12403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods
Intracavernous pressure (ICP) and arterial pressure charts during electrostimulation and red-light irradiation in the middle of electrostimulation. ICP (blue line) and arterial pressure (red line) chart during electrostimulation of cavernous nerve in intact 12 week-old rats. Before the electrostimulation, 100 µL (10-4 M) NORD-1 was injected into penis. In the middle of electrostimulation, 630–690 nm light (115 mW) was irradiated to penis at a distance of 2 cm from penis. The electrostimulation period is represented by blue thicker bar along time axis. Light irradiation period is indicated by the red back ground.
Intracavernous pressure (ICP) and arterial pressure charts during red-light irradiation with penis. ICP (blue line) and arterial pressure (red line) chart during 630–690 nm light (115 mW) irradiation at a distance of 2 cm from penis in intact 12 weeks-old rats. Before the light irradiation, 100 µL (10-4 M) NORD-1 was injected into penis. Light irradiation period is indicated by the red back ground.
Localization of NORD-1 in the penis and kidney. Representative image of penis (upper) and kidney (lower) 1 hour after NORD-1 (100 µL, 10-4 M) injection into the crura of the penis. NORD-1 fluorescence was detected by approximately 620 nm excitation light. White bar in each image represents scale bar (penis: 200 µm, kidney: 1 mm).
Red-light permeability test using pork-shoulder meat. Schematic image of red-light permeability experiment using pork-shoulder meat. Light source and optical power meter were set and 630–690 nm light (115 mW) was irradiated toward optical power meter. Table represents red-light illuminance when red-light was turned off (baseline), when red-light was turned on without meat (0 cm), and when the meats, whose thickness were varied from 0.5 cm to 1.0 cm, were set between light source and optical power meter.
Data Availability Statement
The data required to reproduce these findings cannot be shared at this time due to technical and time limitations.