Abstract
Purpose
Sperm DNA fragmentation (SDF) has been associated with male infertility and poor outcomes of assisted reproductive technology (ART). The purpose of this study was to investigate global practices related to the management of elevated SDF in infertile men, summarize the relevant professional society recommendations, and provide expert recommendations for managing this condition.
Materials and Methods
An online global survey on clinical practices related to SDF was disseminated to reproductive clinicians, according to the CHERRIES checklist criteria. Management protocols for various conditions associated with SDF were captured and compared to the relevant recommendations in professional society guidelines and the appropriate available evidence. Expert recommendations and consensus on the management of infertile men with elevated SDF were then formulated and adapted using the Delphi method.
Results
A total of 436 experts from 55 different countries submitted responses. As an initial approach, 79.1% of reproductive experts recommend lifestyle modifications for infertile men with elevated SDF, and 76.9% prescribe empiric antioxidants. Regarding antioxidant duration, 39.3% recommend 4–6 months and 38.1% recommend 3 months. For men with unexplained or idiopathic infertility, and couples experiencing recurrent miscarriages associated with elevated SDF, most respondents refer to ART 6 months after failure of conservative and empiric medical management. Infertile men with clinical varicocele, normal conventional semen parameters, and elevated SDF are offered varicocele repair immediately after diagnosis by 31.4%, and after failure of antioxidants and conservative measures by 40.9%. Sperm selection techniques and testicular sperm extraction are also management options for couples undergoing ART. For most questions, heterogenous practices were demonstrated.
Conclusions
This paper presents the results of a large global survey on the management of infertile men with elevated SDF and reveals a lack of consensus among clinicians. Furthermore, it demonstrates the scarcity of professional society guidelines in this regard and attempts to highlight the relevant evidence. Expert recommendations are proposed to help guide clinicians.
Keywords: Delphi method, Disease management, DNA fragmentation, Male infertility, Practice guideline, Survey
INTRODUCTION
An estimated 180 million couples or more are affected by infertility globally, with the male factor contributing to almost 50% of cases [1]. Male infertility has a complex nature and can be caused by a vast array of disorders. Besides some systematic illnesses or iatrogenic complications, any condition that can affect the male reproductive system including anatomical or functional anomalies, hormonal instabilities, and genetic or immunologic disorders can cause male infertility [2]. The assessment of male infertility relies primarily on conventional semen analysis. However, it is believed that semen analysis alone is insufficient to predict male fertility potential [3], as 15% of infertile men have normal semen parameters [4].
For this reason, new tests have been proposed to assess the functional competence of spermatozoa, including sperm DNA fragmentation (SDF) testing, which has been included and highlighted as a promising biomarker in the Sixth Edition of the World Health Organization (WHO) Laboratory Manual for the Examination and Processing of Human Semen [5]. Sperm DNA integrity is an important factor that can have a direct impact on male fertility potential [6] and sperm DNA strand breaks have been negatively correlated with fertilization rates in couples suffering from unexplained infertility [7]. Furthermore, impaired sperm DNA integrity can have adverse impacts on assisted reproductive technology (ART) success [8].
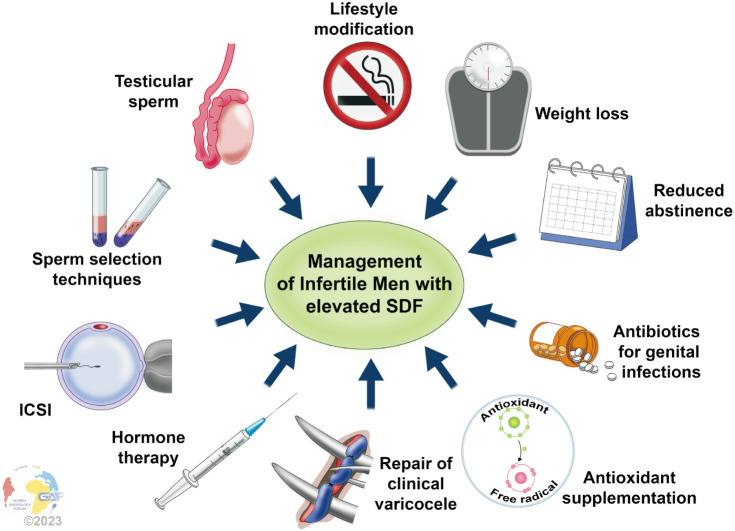
Different approaches have been suggested to lower SDF. Antioxidants play a key role in maintaining redox balance by scavenging reactive oxygen species (ROS) and have been shown to benefit infertile men with elevated SDF [9,10]. Short ejaculatory abstinence [11], weight loss [12,13], and using testicular sperm for intracytoplasmic sperm injection (ICSI) [14,15] are among other approaches that have been shown to reduce SDF. Recently, Agarwal et al [16] suggested a treatment algorithm using the available evidence in the literature that included the following strategies to lower SDF: recurrent ejaculation, antioxidants, lifestyle modification, control of infection/inflammation, varicocele repair (VR), and sperm processing and preparation.
Despite the many different approaches for lowering SDF that have been investigated, there is a lack of standardization on clinical grounds. This can mainly be attributed to the scarcity of professional society recommendations that specifically address the management of infertile men who are found to have elevated SDF. As such, it is important to determine the current worldwide practices related to the treatment of elevated SDF and how clinicians approach such cases, and whether they are in line with the current evidence and recommendations. To ensure adequate management of infertile men, a unified, evidence-based, and patient-centered approach to those found to have elevated SDF is crucial.
Therefore, the aims of this study are:
1) To investigate the global practices related to the management of infertile men with elevated SDF.
2) To summarize and present the professional society guidelines related to the management of infertile men with elevated SDF and compare them to our findings.
3) To provide expert recommendations on the management of infertile men with elevated SDF based on global practices, society guidelines, and evidence available in the literature.
MATERIALS AND METHODS
A global online survey was created, validated, and disseminated by the Global Andrology Forum (GAF) management team [17] (https://www.globalandrologyforum.com/). The survey included questions on clinical practices related to all aspects of SDF, including indications for SDF testing, technical aspects of SDF testing, management of elevated SDF, and barriers and limitations to incorporating SDF testing into clinical practice. The survey was targeted toward clinicians of various disciplines who manage infertility. Dissemination occurred via secure emails to GAF members, secure emails to clinicians recommended by GAF members, and andrology and urology professional societies. After the exclusion of invalid responses, 436 questionnaires were analyzed to capture global practices.
The results presented in this article are those related to the management of infertile men with elevated SDF (Survey questions 33–60; Supplement File 1), as well as advanced analyses conducted on some of these questions (Supplement File 2). In addition, the following professional society guidelines were screened for recommendations related to managing infertile men with elevated SDF:
1) Diagnosis and Treatment of Infertility in Men: American Urological Association/American Society for Reproductive Medicine (AUA/ASRM) Guideline [18,19].
2) European Association of Urology (EAU) Guidelines on sexual and reproductive health [20,21] and the EAU Guidelines Panel on Male Sexual and Reproductive Health: A Clinical Consultation Guide on the Indications for Performing Sperm DNA Fragmentation Testing in Men with Infertility and Testicular Sperm Extraction in Nonazoospermic Men [22].
3) European Society of Human Reproduction and Embryology (ESHRE) guideline: recurrent pregnancy loss [23].
4) European Academy of Andrology (EAA) guideline: Management of oligo-astheno-teratozoospermia [24].
5) Management of male factor infertility: position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS) [25].
6) Diagnosis and Treatment before Assisted Reproductive Treatments. Guideline of the German Society of Gynecology and Obstetrics (DGGG), the Austrian Society of Gynecology and Obstetrics (OEGGG), and the Swiss Society of Gynecology and Obstetrics (SGGG) [26].
Finally, expert recommendations regarding the management of infertile men with elevated SDF were proposed based on: (1) the survey results, (2) the professional society guideline recommendations, and (3) the evidence available in the literature [16,27]. Consensus was reached using the Delphi method [28]. The complete methodology is described in Supplement File 3, which also includes the Checklist for Reporting Results of Internet E-Surveys (CHERRIES), upon which the survey was based [29]. The complete survey and the invitation letter are provided in Supplement File 4. The methodology is also presented in Fig. 1.
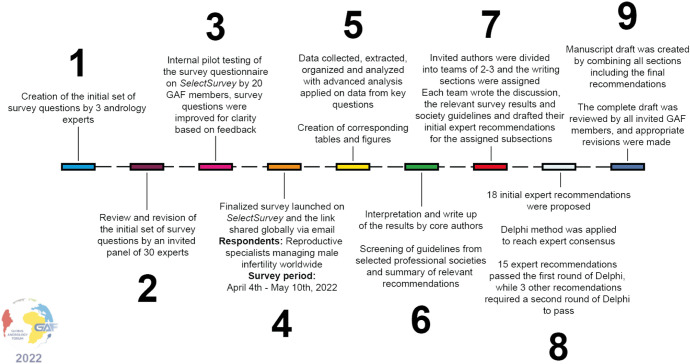
Fig. 1. Complete survey methodology. The complete survey consisted of 64 questions on SDF clinical practices divided into five sections: demographics, indications for SDF testing, technical aspects of SDF testing, management of elevated SDF, and barriers in incorporating SDF into clinical practice. A total of 18 recommendations were made as follows: seven for indications for SDF testing, ten for management of infertile men with elevated SDF, and one for technical aspects of SDF testing. Passing criteria for the Delphi method was set at >80% scoring the recommendation ≥7 in agreement. GAF: Global Andrology Forum, SDF: sperm DNA fragmentation.
RESULTS, GUIDELINES, DISCUSSION, AND EXPERT RECOMMENDATIONS
1. Participant demographics
Complete demographic information is provided in Supplement File 5. A total of 55 countries were represented in our survey. Respondents included urologists, andrologists, gynecologists, embryologists, and endocrinologists in a variety of practice settings and with different years of experience.
2. Professional society guidelines
The recommendations made by the latest AUA/ASRM, EAU, ESHRE, EAA, SIAMS, and DGGG, OEGGG, and SGGG, guidelines are summarized in Table 1 [18,19,20,21,22,23,24,25,26]. Pertinent aspects of the guidelines are expanded upon in the subsequent subsections.
Table 1. Summary of recommendations by professional society guidelines on management of infertile men with elevated SDF.
| Guidelines | AUA/ASRM [18,19] | EAU [20,21,22] | ESHRE [23] | EAA [24] | SIAMS [25] | DGGG, OEGGG,and SGGG [26] |
|---|---|---|---|---|---|---|
| General approach to elevated SDF | No specific recommendation | No specific recommendation, lifestyle changes discussed as first line | NA | No specific recommendation | No specific recommendation | No specific recommendation |
| Referring infertile men with elevated SDF to ART | No specific recommendation | No specific recommendation | NA | No specific recommendation | No specific recommendation | No specific recommendation |
| Managing RPL associated with elevated SDF | No specific recommendation | No specific recommendation | No specific recommendation regarding the management of elevated SDF, however lifestyle modification recommended for couples with RPL due to male factor | No specific recommendation | No specific recommendation | No specific recommendation |
| Managing infertile men with clinical varicocele, normal semen parameters, and elevated SDF | No specific recommendation | VR may be considered in men with elevated SDF with otherwise unexplained infertility or who have failed ART | NA | No specific recommendation | No specific recommendation, but evidence is provided on the benefit of VR in reducing SDF | No specific recommendation |
| Managing infertile men with subclinical varicocele and elevated SDF | Subclinical varicocele should not be repaired | Subclinical varicocele should not be repaired in general, no specific recommendation for elevated SDF with subclinical varicocele | NA | Subclinical varicocele should be monitored | Subclinical varicocele should be monitored | No specific recommendation |
| Use of antioxidants for elevated SDF | No specific recommendation regarding their use for elevated SDF | No specific recommendation regarding their use for elevated SDF | NA | No specific recommendation regarding their use for elevated SDF | No specific recommendation regarding their use for elevated SDF | No specific recommendation regarding their use for elevated SDF |
| Use of hormonal therapy for elevated SDF | No specific recommendation regarding their use for elevated SDF | No specific recommendation regarding their use for elevated SDF | NA | No specific recommendation regarding their use for elevated SDF | No specific recommendation regarding their use for elevated SDF, the benefit of FSH on SDF is discussed | No specific recommendation regarding their use for elevated SDF |
| Managing ART failure in a couple with elevated SDF in the man | No specific recommendation | No specific recommendation | NA | See testicular sperm (below) | No specific recommendation | No specific recommendation |
| Use of sperm selection techniques for infertile men with elevated SDF | No specific recommendation | No specific recommendation | Not recommended for RPL, no specific recommendation for elevated SDF | No specific recommendation | No specific recommendation | No specific recommendation |
| Use of testicular sperm for infertile men with elevated SDF | No specific recommendation, however, they do state that a clinician may use surgically-obtained sperm in cases of high SDF | Not recommended for non-azoospermic men outside clinical trials, but may be used for elevated SDF after the failure of other treatments | NA | Can be considered in cases of recurrent (two or more) ICSI failures with ejaculated sperm (low-quality evidence) | No specific recommendation | No specific recommendation |
ART: assisted reproductive technology, AUA/ASRM: American Urological Association/American Society for Reproductive Medicine, DGGG, OEGGG, and SGGG: Guideline of the German Society of Gynecology and Obstetrics, the Austrian Society of Gynecology and Obstetrics, and the Swiss Society of Gynecology and Obstetrics, EAA: European Academy of Andrology, EAU: European Association of Urology, ESHRE: European Society of Human Reproduction and Embryology, FSH: follicle-stimulating hormone, ICSI: intracytoplasmic sperm injection, NA: not applicable, RPL: recurrent pregnancy loss, SDF: sperm DNA fragmentation, SIAMS: Italian Society of Andrology and Sexual Medicine, VR: varicocele repair.
3. Results of the Delphi method
Sixty-three participants completed the questionnaire for the first round of voting. Of the ten recommendations pertaining to the management of elevated SDF in infertile men, eight recommendations passed. Those failing to meet the passing criteria were “managing recurrent pregnancy loss with elevated SDF in the male partner” and “management of infertile men with subclinical varicocele and elevated SDF”, with 75% and 73.4% of respondents giving them a score of ≥7 respectively. These recommendations were revised and submitted for the second round of voting, which was completed by 47 of the 63 experts. Both recommendations met the passing requirement and a consensus was reached without the need for discussion.
4. Treatment of elevated sperm DNA fragmentation
1) General approach
(1) Results
When asked how they would treat elevated SDF once diagnosed in infertile men, almost 80% of respondents recommend lifestyle modification and 76.9% would prescribe empiric antioxidants. Less frequently, 38.3% recommend reduced ejaculatory abstinence, 20.7% would refer directly to ART with advanced sperm selection techniques and 16.9% would repeat testing to confirm elevated SDF (Fig. 2). When results were stratified based on the cost of SDF, no significant difference was found between the cost of less $100 and more than $100 overall (p=0.6). Similarly, when specifically analyzing the option “repeat testing and confirm elevated SDF”, there was no significant difference in the number of participants who chose this practice based on the cost of SDF testing (p=0.2) (Fig. 3). When asked about the abstinence period as a management approach to lower SDF levels, approximately one-third of respondents recommend 24–48 hours, while almost 30% recommend 3–5 days (Fig. 4).
Fig. 2. General approach to managing elevated SDF in infertile men. Respondents were allowed to select more than one answer. The percentage for each answer was calculated by dividing the number of respondents who had selected it by the total number of respondents who had answered this question (n=402). ART: assisted reproductive technology, ICSI: intracytoplasmic sperm injection, SDF: sperm DNA fragmentation.
Fig. 3. Ordering a repeat SDF test as confirmation for elevated SDF, with results stratified according to the cost of SDF testing. The majority do not order a confirmation test regardless of the cost of testing, as there is no significant difference when responses were compared between costs less than $100 and more than $100 (p=0.2). SDF: sperm DNA fragmentation.
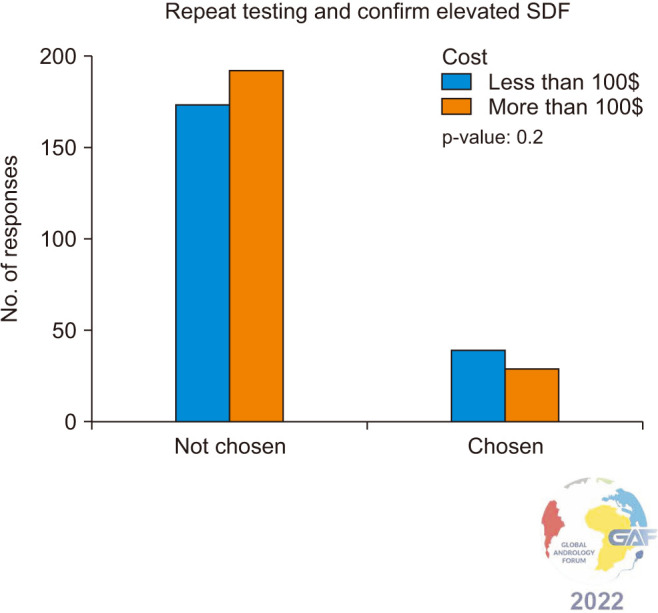
Fig. 4. Duration of abstinence recommended by respondents as a means to lower SDF. ART: assisted reproductive technology, SDF: sperm DNA fragmentation.

(2) Society guidelines
There are no specific recommendations regarding the general approach to managing infertile men with elevated SDF in the guidelines [18,19,20,21,22,23,24,25,26].
The AUA/ASRM guidelines state risk factors for male infertility include advanced paternal age, obesity, lifestyle habits, medical conditions, and environmental chemical exposure [18,19]. They further highlight that clinicians should counsel patients that the data on these factors is limited. No specific recommendation for SDF is made.
The EAU guidelines recommend weight loss, increased physical activity, smoking cessation, and reduced alcohol intake for infertile men with idiopathic oligoasthenoteratozoospermia (OAT) to improve sperm quality and chances of conception [20,21]. In the clinical consultation guide, they state that most treatments for elevated SDF are lifestyle-based and list smoking cessation, weight loss, and alcohol limitation as examples [22]. This is followed by the acknowledgment of the lack of robust evidence to support these measures and hence, no direct recommendation is made.
The EAA guideline recommends that subfertile men with OAT should quit cigarette smoking, reduce weight, and reduce alcohol consumption (if excessive) to improve the chance for the couple to achieve the desired pregnancy [24]. There is no direct mention of high SDF. The EAA guideline recommends against asking men with OAT to do the following: quit any physical activity, apply scrotal cooling and changes in clothing, or seek working conditions leading to decrease scrotal heating, as means to improve the chance of the couple achieving the desired pregnancy. There is no direct mention of high SDF.
The SIAMS guideline recommends lifestyle changes (including decreased alcohol intake, weight loss, increased physical activity, and smoking cessation) in men with infertility to improve general health [25]. There is no mention of these approaches for infertile men with elevated SDF specifically, however, the evidence section discusses the relationship between obesity and smoking with increased SDF. SIAMS does recommend the use of antibiotics for the treatment of male genital tract infections, however, no specific recommendations regarding the effects of antibiotic therapy on SDF were made, which was attributed to the lack of properly sized and designed trials evaluating this aspect.
Recommendations regarding: ART management, antioxidant use, hormonal therapy, varicocele management, and use of testicular sperm will be presented in subsequent sections.
(3) Discussion
When asked about their initial approach, only 16.9% of the participants would order confirmatory testing for elevated SDF. The most commonly chosen strategies were lifestyle changes, empiric antioxidants, and reduced abstinence. The cost of SDF testing did not impact the treatment strategy or the clinicians’ decision to order confirmatory testing.
Evolving evidence supports different treatment strategies in relieving elevated SDF levels [30]. Lifestyle changes might benefit men with high SDF values. Exposure to environmental and lifestyle factors including smoking, airborne pollutants, ionizing radiation, and pesticides have far-reaching implications on male fertility [31,32,33,34,35,36,37,38]. Although no robust evidence of lifestyle modification impact on SDF exists [12], weight loss and dietary changes have been shown to alleviate SDF in patients [39,40]. Our survey showed that almost all respondents recommend lifestyle modification to their patients with high SDF.
Moreover, current evidence suggests that male accessory gland infection (MAGI) promotes inflammation with elevated oxidative stress (OS), leukocytospermia, and ROS production, which can ultimately negatively affect sperm chromatin integrity [41]. Identification of bacteria and targeted antibiotic therapy has been reported to reduce seminal leukocytes and ROS levels, as well as significantly reduce DNA damage [42]. Empirical antibiotic therapy for leukocytospermia may also ameliorate spontaneous pregnancy rates [43], however, a recent meta-analysis failed to find a difference in the SDF rate between patients with leukocytospermia without symptoms of urinary tract infections and controls without leukocytospermia, suggesting an unclear role in the benefit of treating this condition [44]. In agreement, only a very small proportion of survey participants prescribe empirical antibiotic therapy to treat SDF.
Furthermore, reduced abstinence may be a simple non-invasive measure to improve SDF, especially if applied within the context of assisted reproduction [45,46,47]. Shorter abstinence has been reported to reduce SDF and improve pregnancy outcomes [48]. Agarwal et al [11] compared SDF levels in men according to abstinence periods and reported significantly lower SDF in the group with less than 2 days abstinence (9.9%) compared to both 2–7 days abstinence (12.8%) and >7 days abstinence (17.8%) (p<0.05 for both comparisons), and also reported a significant increase in SDF percentage as abstinence duration increases (p<0.001). Pons et al [49] reported similarly promising results, as more than 80% of infertile men with >30% DNA fragmentation index (DFI) were able to reduce their SDF levels to less than 30% on the first ejaculate after a 24-hour abstinence protocol. Other studies also highlight that an extremely short period of abstinence (1–3 h) might exert the best benefits on SDF [50,51,52], suggesting this procedure as a possible treatment to improve the outcome of ART [50]. Our survey showed that more than one-third of participants suggest reducing the period of abstinence to improve SDF.
Since there is no direct mention of SDF treatment in any of the guidelines, a direct comparison of our results with what the guidelines suggest is not possible. However, as also confirmed by the SIAMS and EAA guidelines, lifestyle changes are the first step of infertile male management. Few participants in the survey attribute a role to empiric antibiotic treatment in the management of SDF, and this is also in line with the SIAMS guidelines’ statement on the lack of quality studies that have evaluated the effects of empirical antibiotic treatment on SDF.
(4) Expert recommendations
Ordering a second confirmation test for elevated SDF is not necessary for diagnosis.
For infertile men with elevated SDF, lifestyle modification strategies should be recommended including maintaining a healthy lifestyle to overcome obesity, cessation of smoking and alcohol use, as well as treating genital infections, and eliminating toxic exposure.
Reduced ejaculatory abstinence of 12–24 hours before attempting conception (natural or by ART) is recommended as a means to lower SDF and improve pregnancy outcomes.
2) ART referral for infertile men with elevated SDF
(1) Results
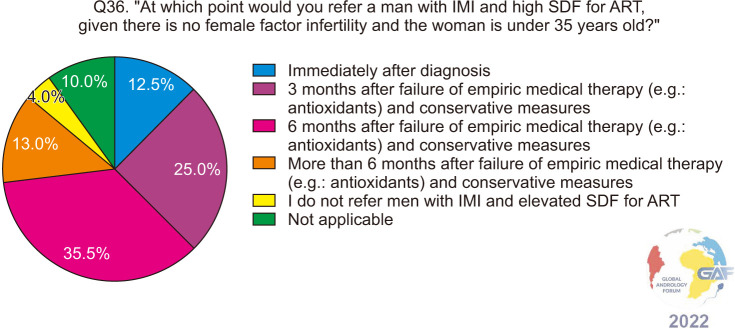
When asked when they would refer men diagnosed with unexplained male infertility (UMI) or idiopathic male infertility (IMI) and were found to have elevated SDF to ART, given there is no female factor and the female partner is younger than 35 years, responses for both groups of men are fairly similar (Fig. 5, 6), with the majority of participants referring to ART six months after the failure of conservative measures and medical management such as antioxidants.
Fig. 5. ART referral for men with unexplained infertility and elevated SDF. ART: assisted reproductive technology, SDF: sperm DNA fragmentation, UMI: unexplained male infertility.
Fig. 6. ART referral for men with idiopathic infertility and elevated SDF. ART: assisted reproductive technology, IMI: idiopathic male infertility, SDF: sperm DNA fragmentation.
(2) Society guidelines
AUA/ASRM, EAU, and SIAMS guidelines do not provide a specific statement regarding ART referral for infertile men with elevated SDF [18,19,20,21,22,25].
The EAA guideline recommends performing SDF analysis when a couple is referred for ART. The EAA guideline recommends couples with male partners with OAT consider ART to improve their chance of achieving pregnancy in cases when other treatment options are not available or not efficient [24]. There is no specific comment concerning ART referral for infertile men with elevated SDF.
The DGGG, OEGGG, and SGGG guidelines state that SDF testing is a potentially useful clinical biomarker, but the conclusive predictive value of this test for IVF and/or ICSI treatment is still unclear [26]. So, there is no specific recommendation regarding ART referral for infertile men with elevated SDF in the guidelines of the DGGG, OEGGG, and SGGG.
(3) Discussion
Elevated SDF can have a deleterious impact on the ability of a couple to achieve natural pregnancy [53,54]. In a cohort study that followed 2,713 couples who had failed to conceive naturally after one year and subsequently underwent ART, elevated DFI as measured by sperm chromatin structure assay (SCSA) was associated with poor outcomes after IVF including fertilization and live birth rates (LBRs), however, no such adverse effect was found for couples who underwent ICSI [55]. Many meta-analyses have been published over the past decade that investigated ART outcomes between high and low SDF. In general, SDF has been associated with poor outcomes after IUI [56] and IVF [57,58,59]. As for ICSI, there are conflicting reports as to whether SDF can affect clinical pregnancy rates after ICSI [57,58,59], however, a higher miscarriage rate has been demonstrated after ICSI with high SDF [58].
SDF can still exert its adverse impact on ART and therefore it is not reasonable to use ART as a management strategy for infertile men who are found to have elevated SDF. This conclusion is in line with the responses to our survey, such that the majority would attempt conservative management for UMI and IMI patients with high SDF and would only refer to ART following 6 months of failure with conservative strategies.
(4) Expert recommendations
Different ART methods are not recommended as first-line treatment strategies for infertile men found to have elevated SDF. Instead, known underlying causes should be addressed first as well as conservative management to lower SDF.
3) Managing couples experiencing RPL after spontaneous conception with elevated SDF in the man
(1) Results
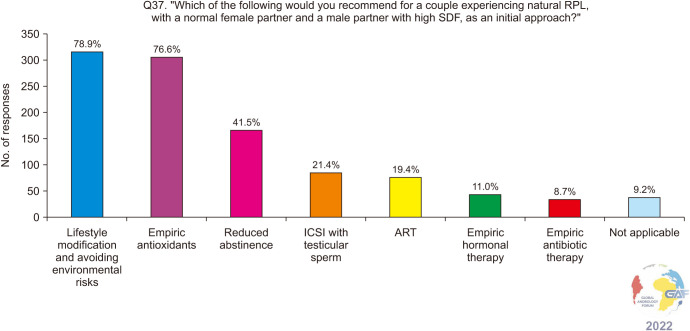
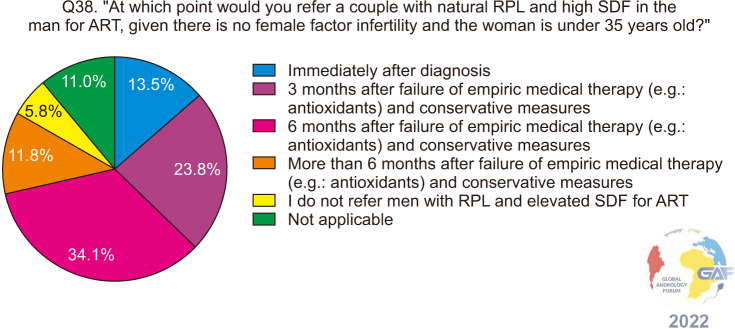
For couples experiencing RPL after spontaneous conception with a normal female partner and elevated SDF in the male partner, lifestyle modification and empiric antioxidants still remain the highest chosen treatment strategies at 78.9% and 76.6% respectively (Fig. 7). Only 19.4% chose ART, while 21.4% chose ICSI with testicular sperm. When asked when they would refer such a couple to ART, given the female partner is younger than 35 years of age, more than one third (136/399, 34.1%) chose six months after the failure of empiric medical therapy and conservative measures (Fig. 8). The percentages for this question were also very similar to the ones pertaining to ART referral for men with UMI and IMI, which are described in the previous section. Regarding ART referral for couples with RPL and elevated SDF in the man, results were compared between urologists/andrologists and other specialties, yielding significant differences (p<0.001). Most urologists and andrologists refer to ART six months after the failure of medical and conservative treatment compared to other specialties (43% vs. 19.3%), while other specialties including gynecology, endocrinology, and IVF specialists refer to ART immediately after diagnosis or three months after the failure of medical and conservative treatment compared to urologists/andrologists (48.7% vs. 30.5%).
Fig. 7. Management approach for those who have elevated SDF and are experiencing recurrent pregnancy loss after spontaneous conception. Respondents were allowed to select more than one answer. The percentage for each answer was calculated by dividing the number of respondents who had selected it by the total number of respondents who had answered this question (n=402). ART: assisted reproductive technology, ICSI: intracytoplasmic sperm injection, RPL: recurrent pregnancy loss, SDF: sperm DNA fragmentation.
Fig. 8. ART referral for recurrent pregnancy loss and men with elevated SDF. ART: assisted reproductive technology, RPL: recurrent pregnancy loss, SDF: sperm DNA fragmentation.
(2) Society guidelines
AUA/ASRM guideline recommends that for couples with RPL, men should be evaluated with karyotype (Expert Opinion) and SDF (Moderate recommendation; Evidence Level: Grade C) [18,19]. There is no further specific recommendation for the management of SDF in couples with RPL.
EAU guideline recommends SDF testing to be performed in the assessment of couples with RPL from natural conception and ART or men with unexplained infertility (Strong recommendation) [20,21,22]. There is no specific recommendation regarding the management of couples with RPL and elevated SDF in the EAU guidelines.
ESHRE guideline recommends assessing SDF in couples with RPL for explanatory purposes, based on indirect evidence [23]. There is no specific recommendation regarding the management of elevated SDF, however for couples with RPL due to male factor, the guideline recommends smoking cessation, a normal body weight, limited alcohol consumption, and a normal exercise pattern.
(3) Discussion
Elevated SDF is often associated with a significantly increased risk of RPL [60,61]. The management of couples with RPL and elevated SDF values aims at providing pertinent treatment strategies directed at lowering SDF levels.
Our results highlight that the highest percentage of treatment strategies chosen is represented by lifestyle modification and empiric antioxidants treatment. Some studies in the literature recommend analyzing SDF in couples with RPL. In addition to counseling on the same lifestyle interventions as mentioned above, studies propose that antioxidant supplementation may play a role in the treatment of RPL [62].
As such, there is no consensus or guideline on how to manage these cases in clinical practice. This is further highlighted by the reported differences in practices between urologists and other specialties, with regards to referring such couples to ART. In addition, this demonstrates the importance of a multidisciplinary approach in the management of couples’ infertility.
Given that controversies still exist currently regarding the optimal strategies to improve SDF, managing the expectations of the couple, educating about the role of nutraceutical and lifestyle modifications, and counseling on ART by expert clinicians are necessary to assist couples in their journey to start a family.
(4) Expert recommendations
In couples with RPL following spontaneous pregnancy, associated with elevated SDF in the male partner and no female factor infertility, an appropriate initial approach should include addressing known risk factors of elevated SDF and other causes associated with male infertility. These men may also be supplemented with oral antioxidant therapy, particularly if there is no associated underlying cause for their infertility. The decision to refer such a couple to ART should be determined on a case-by-case scenario and after adequate management of elevated SDF.
4) Managing infertile men with clinical varicocele, normal semen parameters, and elevated SDF
(1) Results
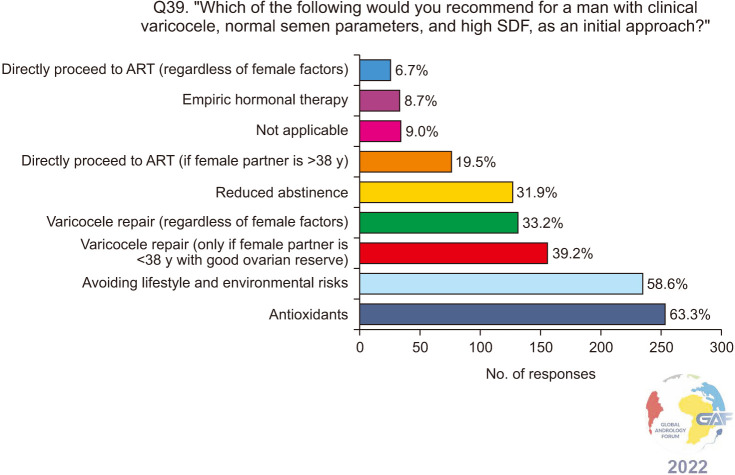
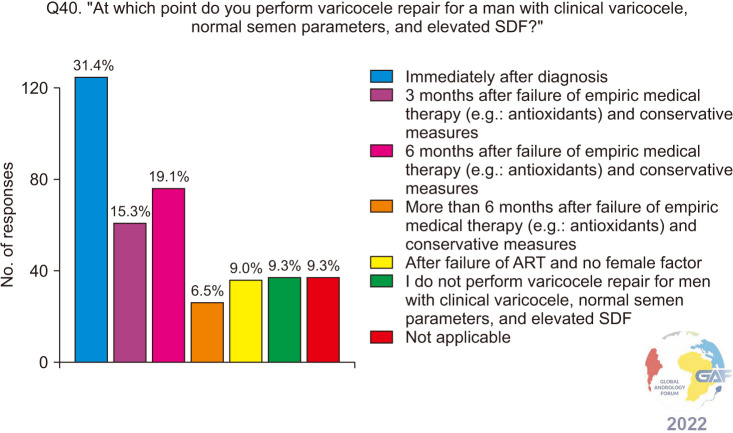
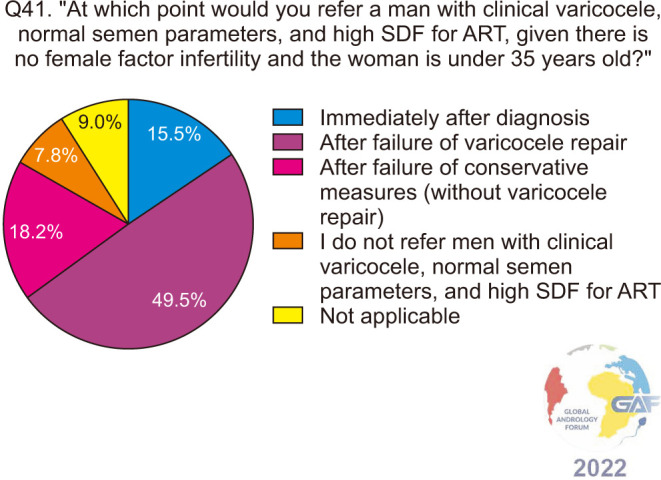
When asked how they would manage a man with clinical varicocele and elevated SDF with normal conventional semen parameters, antioxidants and conservative methods were chosen by a majority of the respondents, at 63.3% and 58.6% respectively. 39.2% would perform VR only if the female partner is <38 years with good ovarian reserve, while 33.2% would perform VR regardless of female factors. Lower percentages of respondents would refer such a patient to ART. The results of this question are presented in Fig. 9. When asked specifically about the timing of VR in such a patient, 31.4% chose immediately after diagnosis, while 15.3%, 19.1%, and 6.5% would perform VR after the failure of antioxidants and conservative measures for a duration of 3 months, 6 months, and more than 6 months respectively (Fig. 10), and when responses were stratified based on level of specialization, no significant difference in responses was found between general urologists and fellowship-trained reproductive urologists (p=0.5). Regarding ART failure in such men, almost half (49.5%) would refer to ART only after failure of VR, while 18.2% do so after failure of conservative measures without VR, and 15.5% immediately after diagnosis (Fig. 11).
Fig. 9. Management approach for infertile men who have a clinical varicocele, normal conventional semen parameters, and elevated SDF. Respondents were allowed to select more than one answer. The percentage for each answer was calculated by dividing the number of respondents who had selected it by the total number of respondents who had answered this question (n=401). ART: assisted reproductive technology, SDF: sperm DNA fragmentation.
Fig. 10. Varicocele repair for infertile men who have a clinical varicocele, normal conventional semen parameters and elevated SDF. ART: assisted reproductive technology, SDF: sperm DNA fragmentation.
Fig. 11. ART referral for infertile men who have a clinical varicocele, normal conventional semen parameters, and elevated SDF. ART: assisted reproductive technology, SDF: sperm DNA fragmentation.
(2) Society guidelines
AUA/ASRM guideline recommends surgical VR should be considered in men attempting to conceive, who have palpable varicoceles, infertility, and abnormal semen parameters except for azoospermic men (Moderate recommendation; Evidence Level: Grade B) [18,19]. There is no specific recommendation regarding the management of couples with clinical varicocele, normal semen parameters, and elevated SDF in the guidelines.
EAU guideline states that VR may be considered in men with elevated SDF with otherwise unexplained infertility or who have failed ART, including RPL, failure of embryogenesis, and implantation (Weak recommendation) [20,21,22]. EAU guideline also recommends treating infertile men with a clinical varicocele, abnormal semen parameters, and otherwise unexplained infertility in a couple where the female partner has a good ovarian reserve to improve fertility rates.
The SIAMS position statement suggests VR in infertile couples where the male partner has abnormal semen parameters, and the female partner has normal fertility or a potentially treatable cause of infertility and time to conception is not a concern [25]. No recommendation is made for men with clinical varicocele and normal semen parameters. Although elevated SDF is not listed as an indication for varicocele repair, the benefits of varicocele repair on improving SDF are discussed in the statement.
(3) Discussion
Varicocele is the most frequent surgically correctable cause of male infertility [20]. As a consequence of varicocele, excessive ROS are produced in the testes, which can lead to SDF [63]. Elevated SDF rates have been reported in all grades of clinical varicocele, but mainly in grades 2 and 3 [64]. Significantly higher values of sperm DFI were reported in normozoospermic men with clinical varicocele compared with healthy individuals, and levels remained abnormally high at 6 months of follow-up without VR [65].
A recent meta-analysis involving more than 1,000 patients demonstrated that varicocelectomy can decrease sperm DFI by 7.23% (95% CI, -8.86 to -5.59) in men with clinical varicocele, ranging from 2.3% to 16.3% decline in DFI among the included studies [66]. These findings are similar to another meta-analysis reporting a 6.14% (95% CI, -6.90 to -5.37) decrease in SDF after VR [67]. A study by Lara-Cerillo et al [68] revealed that VR was able to significantly decrease both single-strand SDF (68.5% vs. 56.5%; p=0.01) and double-strand SDF (53% vs. 47%; p=0.007). Furthermore, a recent pilot study showed that VR significantly lowered SDF rates and improved spontaneous pregnancy rates, in infertile men with clinically palpable varicoceles and normal conventional semen parameters [69].
Varicocele repair has been found to improve both spontaneous and ART pregnancy rates when performed for men with clinical varicocele, being associated with lower DFI levels [70]. One meta-analysis that included 4 studies with 870 ICSI cycles performed for non-azoospermic infertile men with clinical varicocele, examined the ICSI outcomes for those who underwent varicocelectomy before ART and compared them to the outcomes of ICSI without varicocelectomy [71]. They reported significantly improved clinical pregnancy rates (OR=1.59; p=0.002) and LBRs (OR=2.17; p<0.001) among men who underwent prior varicocelectomy compared to those who did not.
The practices of the respondents of this survey are in line with the aforementioned meta-analysis, with more than 70% choosing VR either regardless of female factors or if the female partner is young and with good ovarian reserve. Less than one-fifth directly refer to ART in case female age is over 38, and only 6.7% choosing direct ART regardless of female factors in cases of normozoospermic men with clinical varicocele and elevated SDF. Almost half would refer to ART only after VR fails to produce an outcome. Our results imply that clinicians are more inclined to offer VR to the male partner without considering female factors for ART referral. However, the timing of VR was very heterogenous, with only 31.4% offering it immediately after diagnosis, while almost 40% perform VR after different periods of antioxidants and conservative measures have failed.
Most professional society guidelines do not provide explicit recommendations on the management of infertile men with clinical varicoceles who have normal conventional semen parameters and elevated SDF. The EAU specifically, recommends VR in men with elevated SDF who have otherwise unexplained infertility or have failed ART.
The various practices of the clinicians who have completed this survey, highlight the need for implementing SDF levels into the decision-making process when managing this population of infertile men.
(4) Expert recommendations
In infertile men with clinical varicocele and normal semen parameters, VR should be offered if SDF is elevated. The persistence of abnormal postoperative SDF values is a poor predictor for both natural and assisted conception.
VR should be offered after diagnosis to lower SDF for both natural and assisted conception. ART could be performed after VR.
If there is a need or the couple wishes for ART to be performed on diagnosis, they should be counseled on the risk of failure that may be attributed to SDF with a known associated yet untreated cause (i.e., clinical varicocele), and other attempts to lower SDF should be considered including antioxidants, sperm selection techniques, and testicular sperm.
5) Managing infertile men with subclinical varicocele and elevated SDF
(1) Results
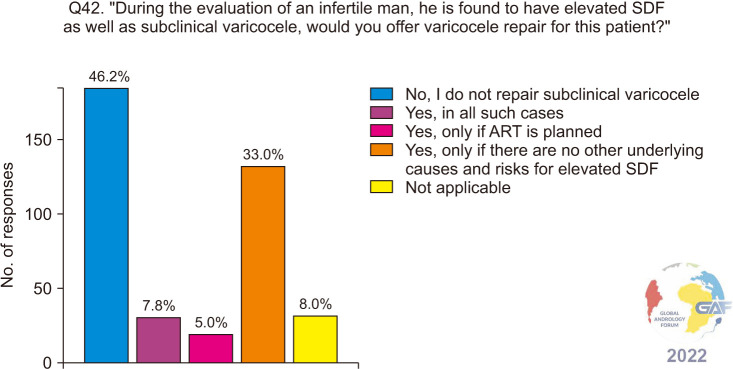
When asked if they would repair a subclinical varicocele in an infertile man if elevated SDF was found, 185/400 (46.2%) would not, and this was followed by 33.0% of respondents who would if there are no other underlying causes of risks for elevated SDF (Fig. 12).
Fig. 12. Varicocele repair for infertile men who have subclinical varicocele and elevated SDF. ART: assisted reproductive technology, SDF: sperm DNA fragmentation.
(2) Society guidelines
The AUA/ASRM and the EAU guidelines do not recommend the repair of subclinical non-palpable varicoceles [18,19,20,21,22]. There is no direct mention of SDF in this population of infertile men.
The EAA and SIAMS recommend monitoring subclinical varicoceles, as no significant improvement in fertility outcomes has been reported following the repair of subclinical varicoceles [24,25]. There is no direct mention of SDF.
(3) Discussion
Most evidence supports that a statistically significant reduction in SDF might occur only after VR of a clinically significant varicocele [72,73]. Even though SDF is used in some clinical settings as an indicator of the need for VR, SDF and pregnancy rates were not proven to improve in the group of men with subclinical varicocele [74]. Similarly, no significant difference in SDF levels was seen between men with subclinical varicocele who underwent treatment compared to those who did not [75].
Our survey identified that almost 46% of urologists do not repair a subclinical varicocele with elevated SDF associated with infertility, as also recommended by various professional society guidelines. Despite the lack of adequate evidence, a similar proportion of clinicians do repair subclinical varicocele with 5.0% choosing to perform VR before ART, 7.8% in all men with subclinical varicocele and elevated SDF, and 33.0% would repair a subclinical varicocele when no causative etiology of elevated SDF was identifiable.
Well-controlled studies that demonstrate whether there is a benefit of repairing subclinical varicocele on functional sperm parameters including SDF are warranted but may not be feasible given the ethical dilemma of subjecting patients to a potentially unnecessary surgical intervention.
(4) Expert recommendations
In men with elevated SDF and subclinical varicocele, varicocele repair is not recommended. Men with subclinical varicocele and elevated SDF need to be evaluated and treated similarly to men without a varicocele.
6) Use of antioxidants in managing infertile men with elevated SDF
(1) Results
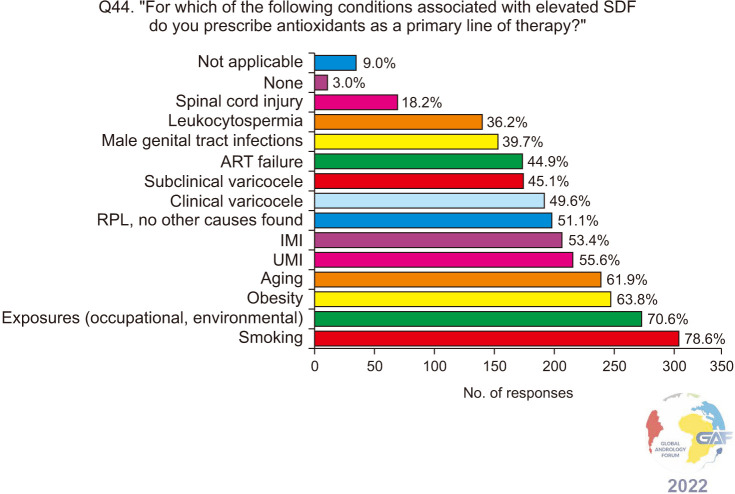
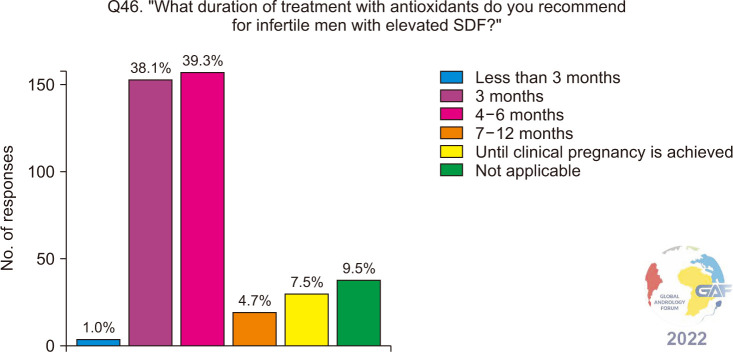
Regarding antioxidants, 49.8% of respondents always prescribe them empirically for infertile men with elevated SDF, while 38.5% consider other potential causes (Fig. 13). When asked about the associated conditions and risk factors associated with elevated SDF, for which antioxidants are prescribed, 78.6% selected smoking, followed by environmental and occupational exposures, obesity, aging, UMI, and IMI (Fig. 14). The most frequent antioxidants prescribed co-enzyme Q10, zinc, and L-carnitine. These are summarized in Table 2. Regarding duration of treatment, 39.3% recommend antioxidants for 4–6 months, while 38.1% recommend them for three months (Fig. 15). More than half (57.7%) of the respondents preferred follow-up SDF testing after antioxidant supplementation to confirm treatment response (Fig. 16).
Fig. 13. Prescribing antioxidants for infertile men with elevated SDF. SDF: sperm DNA fragmentation.
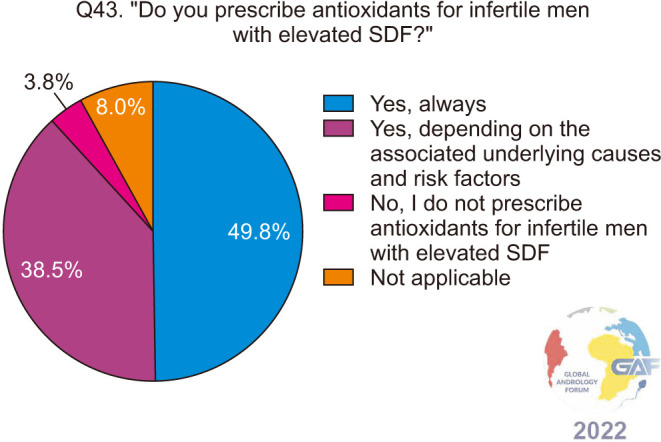
Fig. 14. Conditions associated with elevated SDF in infertile men, for which antioxidants are prescribed. Respondents were allowed to select more than one answer. The percentage for each answer was calculated by dividing the number of respondents who had selected it by the total number of respondents who had answered this question (n=401). ART: assisted reproductive technology, IMI: idiopathic male infertility, RPL: recurrent pregnancy loss, SDF: sperm DNA fragmentation, UMI: unexplained male infertility.
Table 2. Antioxidants prescribed for men with elevated SDF.
| Antioxidant | Number | Percentagea |
|---|---|---|
| Co-enzyme Q10 | 287 | 71.39 |
| Zinc | 286 | 71.14 |
| L-carnitine | 285 | 70.90 |
| Selenium | 248 | 61.69 |
| Vitamin E | 233 | 57.96 |
| Vitamin C | 204 | 50.75 |
| L-arginine | 189 | 47.01 |
| Acetyl carnitine | 182 | 45.27 |
| Folic acid (B9) | 170 | 42.29 |
| Glutathione | 155 | 38.56 |
| N-acetyl cysteine | 141 | 35.07 |
| Lycopene | 137 | 34.08 |
| Vitamin D | 107 | 26.62 |
| Vitamin A | 95 | 23.63 |
| Other B vitamins | 84 | 20.90 |
| Herbal products | 58 | 14.43 |
| Docosahexanoic acid (DHA) | 47 | 11.69 |
| Melatonin | 25 | 6.22 |
| Other | 24 | 5.97 |
| Not applicable | 47 | 11.69 |
SDF: sperm DNA fragmentation.
aMore than one option allowed, percentage calculated from the total number of responders to this question (n=402).
Fig. 15. Recommended duration of treatment with antioxidants for infertile men with elevated SDF. SDF: sperm DNA fragmentation.
Fig. 16. Follow-up on the success of antioxidant therapy in the management of infertile men with elevated SDF. SDF: sperm DNA fragmentation.

(2) Society guidelines
AUA/ASRM guideline states that clinicians should counsel patients that the benefits of supplements (e.g., antioxidants, vitamins) are of questionable clinical utility in treating male infertility [18,19]. Existing data are inadequate to provide recommendations for specific agents to use for this purpose (Conditional recommendation; Evidence Level: Grade B). There is no mention of SDF.
EAU guideline states that no clear recommendation can be made for the treatment of patients with idiopathic infertility using antioxidants, although antioxidant use may improve semen parameters (Weak recommendation) [20,21,22].
SIAMS guideline suggests considering the use of nutraceuticals/antioxidants in selected patients with idiopathic oligozoospermia and/or asthenozoospermia and/or clear signs of high OS since in some cases, they might improve sperm parameters (very low-quality evidence) [25]. There is no direct mention of SDF. However, in the evidence section, they mention that antioxidants may be considered for the treatment of IMI with proven sperm DNA damage.
(3) Discussion
The rationale behind the administration of antioxidants in cases with elevated SDF is to improve the total seminal antioxidant buffering capacity and reduce seminal ROS with the least possible adverse events [76]. Majzoub et al [77] reviewed the effectiveness of varied antioxidant therapy on cases with elevated SDF along 12 articles conveying a beneficial effect on SDF measures, semen parameters, and ICSI outcome. Lately, Humaidan et al [78] pointed out that a 3-month lifestyle intervention program combined with antioxidant therapy could reduce DFI in infertile men with elevated SDF and a history of failed IVF/ICSI. In many of these trials, combinations of antioxidants were used but the optimal dosages and durations were not defined.
Most of our respondents prescribe antioxidants in cases of infertile patients with high seminal SDF, either immediately or after consideration of other underlying contributing risk factors. OS due to high levels of ROS production and/or reduced antioxidants is an established cause of SDF [79]. Thus, antioxidant supplementation could potentially restore the seminal redox balance.
Among the underlying risk factors that could increase sperm DNA damage are aging, smoking, environmental toxins, and obesity [80]. Nearly 80% of our respondents were inclined to prescribe antioxidants to infertile patients who were smokers (Fig. 14). In their systematic review and meta-analysis, on infertile men (5257 smokers and 5566 non-smokers), Bundhun et al [81] observed the detrimental impact of smoking on sperm count and normal morphology. Additionally, smokers have higher chromatin decondensation compared to non-smokers [82]. While it would be ideal if infertile men would quit smoking, antioxidant supplementation could help alleviate some of smoking's negative impact on sperm quality.
The antioxidants that were commonly prescribed by our respondents were: co-enzyme Q10, zinc, and L-carnitine (Table 2). L-carnitine and coenzyme-Q10 are among the more efficacious antioxidants for improving sperm quality in IMI, as reported in a recent systematic review and meta-analysis of 23 randomized controlled trials involving 1917 patients [83]. L-carnitine was more effective in improving sperm motility and sperm morphology, while coenzyme Q10 therapy increased sperm motility and sperm concentration in patients with IMI [83]. The positive impact of coenzyme-Q10 on reducing SDF levels has also been documented [84]. Majzoub and Agarwal [85] addressed that these antioxidants along with N-acetyl cysteine, selenium, folic acid, vitamins C and E, and lycopene, alone or in combination, are among the most common antioxidant therapy in male infertility management.
Our respondents were almost equally likely to prescribe antioxidant therapy for either a 3-month or 4–6-month duration (Fig. 15). While both these durations are commonly used when prescribing antioxidants, few studies have determined the optimal duration to elicit improvement in sperm quality in these cases. However, a recent review observed that the average percentage change in sperm concentration, motility, progressive motility, and morphology at 3 months did not differ significantly from that at 6 months [86].
To determine if the antioxidant therapy was effective, the majority of the respondents (nearly 60%) choose to repeat SDF testing (Fig. 16). Indeed, studies have shown that antioxidant therapy yields a significant improvement in sperm OS and/or DNA damage levels [85,87]. Although most experts do not repeat SDF testing before initiating management, they agree to follow-up treatment success after prescribing antioxidants.
It is important to point out that not all evidence demonstrates desirable fertility outcomes with the use of antioxidants. A randomized clinical trial did not report any significant difference in SDF levels or clinical pregnancy rates after 3 months of antioxidants compared to placebo [88]. This conflicting evidence highlights the importance of careful patient selection and cautious prescription of antioxidants, as liberal use may shift the subtle balance between oxidants and antioxidants, towards reductive stress (RS) within the reproductive tract, which - similar to OS - can also lead to impaired sperm function and DNA abnormalities [89,90]. Thus, empiric antioxidant treatment is not devoid of potential adverse effects.
All guidelines do not recommend prescribing antioxidants clearly and with confidence, and if so, they state that clinicians should counsel patients that their benefits are questionable. However, some guidelines advise antioxidants but do not mention them directly for high SDF [21,26]. In addition, there are no approved drugs or components recommended by recent guidelines to decrease SDF, so there is no specific dose or duration advised [21].
(4) Expert recommendations
Although there remains no unanimous consensus, empiric antioxidants may be prescribed for infertile men with elevated SDF, especially if they have risk factors and known conditions associated with elevated SDF, including idiopathic infertility, RPL, varicocele, leukocytospermia, smoking and other lifestyle and environmental risk factors.
There is no consensus on the type, dosage, and duration of antioxidant treatment that can be recommended, although a duration of 3–6 months has been proven successful.
The success of treatment should be guided by improved conventional semen parameters, decreased SDF levels, and improved reproductive outcomes (either natural or ART).
The current trend of prescribing antioxidants to all infertile men (even if SDF is not tested) is concerning, because improper prescription of these components may negatively impact semen parameters and fertility potentials of men.
7) Use of hormonal therapy in managing infertile men with elevated SDF
(1) Results
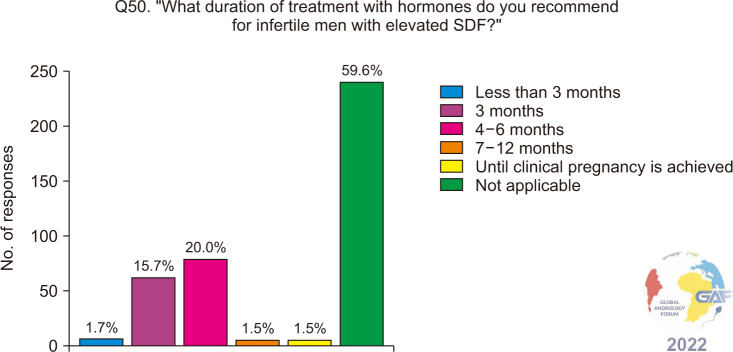
When asked if they prescribe empiric hormonal therapies for infertile men with elevated SDF, the majority of participants do not (197/399, 49.4%), while 34.6% prescribe them depending on associated conditions and risk factors (Fig. 17). Table 3 lists the hormones prescribed by the respondents of our survey, to infertile men with elevated SDF, with the most common being follicle-stimulating hormone (FSH). Participants were then asked about the duration of treatment with hormones and of those who do prescribe hormones, most chose 4–6 months, followed by 3 months (Fig. 18).
Fig. 17. Prescribing hormones for infertile men with elevated SDF. SDF: sperm DNA fragmentation.
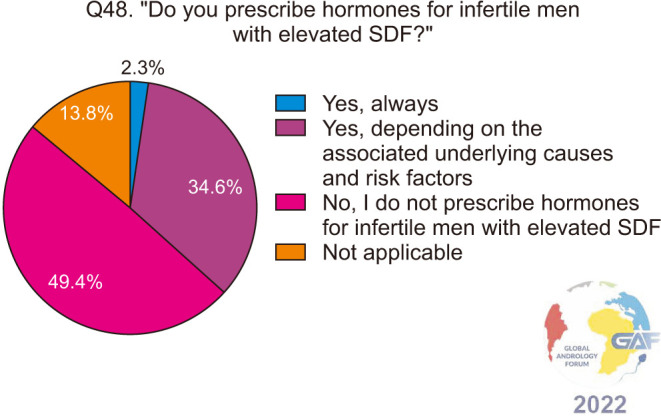
Table 3. Hormones prescribed for men with elevated SDF.
| Hormone | Number | Percentagea |
|---|---|---|
| FSH | 87 | 21.8 |
| SERMs, such as clomiphene citrate, tamoxifen | 81 | 20.3 |
| hCG | 63 | 15.8 |
| Aromatase inhibitors, such as letrozole, anastrozole, testolactone | 49 | 12.3 |
| GnRH | 19 | 4.8 |
| Other | 6 | 1.5 |
| Not applicable | 253 | 63.4 |
FSH: follicle stimulating hormone, GnRH: gonadotropin releasing hormone, hCG: human chorionic gonadotropin, SDF: sperm DNA fragmentation, SERM: selective estrogen receptor modulator.
aMore than one option allowed, percentage calculated from the total number of responders to this question (n=399).
Fig. 18. Recommended duration of treatment with hormones for infertile men with elevated SDF. SDF: sperm DNA fragmentation.
(2) Society guidelines
AUA/ASRM guideline recommends the use of aromatase inhibitors, hCG, and selective estrogen receptor modulators (SERMs) for infertile men with low testosterone (T); while recommending T monotherapy should not be prescribed for men interested in current or future fertility [18,19]. There is a limited benefit for the use of SERMs in the IMI patient relative to the results of ART. There is no specific mention of the use of hormonal therapies in relation to SDF.
There is no specific recommendation regarding the use of hormonal treatments in the management of infertile men with elevated SDF in the EUA guidelines [20,21,22]. However, they provide a weak recommendation on the benefit of FSH in men with idiopathic oligozoospermia and normal FSH levels, to improve spermatogenesis outcomes.
The EAA recommends against therapy with androgens [24]. This is not relevant to high SDF. Treatment with FSH can be suggested with low evidence in selected men from infertile couples (normogonadotropic men with idiopathic oligozoospermia or OAT) in order to improve quantitative and qualitative sperm parameters and pregnancy rate. There is no direct mention of high SDF. The EAA statement does not recommend either for or against SERMs (tamoxifen or clomiphene) or aromatase inhibitors in men with OAT. There is no direct mention of high SDF.
The SIAMS position statement describes the indications of FSH therapy, suggesting its use in selected men with oligozoospermia and/or asthenozoospermia, to increase the sperm quantity, quality, and pregnancy rate [25]. Even though there is no specific mention of SDF, in the evidence section there is mention of the efficacy of FSH on SDF. There is no mention of the effect of anti-estrogens or aromatase inhibitors on SDF.
(3) Discussion
Several intratesticular mechanisms can lead to SDF during spermatogenesis. These include (1) apoptosis; resulting from impairment of testicular function or derailment of chromatin condensation, and (2) DNA breaks; produced by sperm chromatin remodeling during spermiogenesis, which is normally repaired before mature spermatozoa are released, but can persist if testes are exposed to OS, resulting in the production of DNA fragmented sperm [91,92].
Spermatogenesis is under the synergistic effect of reproductive hormones, mainly FSH, T, and estradiol (E2) [93]. A high E2 level or impaired E2:T ratio will result in negative feedback on FSH production. FSH is essential for the initiation and maintenance of spermatogenesis and suppression of FSH may promote an increase in SDF [94]. Based on these facts, hormonal therapy may be offered as a method of improving SDF in infertile men through increasing FSH levels either directly or by decreasing negative feedback created by a high E2 or E2:T ratio.
The most commonly used hormonal treatment is recombinant FSH (rFSH). Ruvolo et al [95] reported significant improvement in SDF levels in men with oligozoospermia and hypogonadotropic hypogonadism given rFSH, especially with SDF >15%. Colacurci et al [96] also reported significant reductions in DFI (23.7% to 12.6%) when rFSH was given to infertile men with idiopathic OAT and normal hormone levels, compared to controls who received non-antioxidant supplements and did not show improvements in DFI levels. Simoni et al [97] reported improvement of SDF with rFSH in oligozoospermic men with FSH receptor homozygous genotype (p. N680S), highlighting a genetic role in modulating the response to FSH, and explaining why not all men with high SDF and oligozoospermia will respond to rFSH. Other hormonal therapies such as aromatase inhibitors (Letrozole) have also been shown to improve sperm chromatin integrity in men with idiopathic OAT and T:E2 ratio ≤10 [98].
The results of our survey showed that only 37% of the participants use hormonal therapy for high SDF. This is expected due to the lack of clear recommendations from different societies on the use of such treatment modality and the paucity of evidence in the literature with only a handful of studies with a small sample size discussing this treatment modality. A meta-analysis of articles assessing the effect of FSH on SDF included only 383 patients with marked heterogeneity between studies [99]. Furthermore, the small percentage of surveyors using hormones for elevated SDF reflects they should be carefully used for selected cases. Participants, prescribing hormonal therapy for SDF, the most commonly used rFSH, and for a duration of therapy of 4–6 months in accordance with the literature.
(4) Expert recommendations
No clear recommendation can be made for or against the use of hormonal therapy for high SDF. We recommend the use of hormone therapy only by well-trained fertility experts and in combination with other therapies (lifestyle modification, infections therapy, antioxidants).
Hormonal therapy could be effective in oligozoospermic, hypogonadotropic hypogonadal men, and those with FSH receptor homozygous genotype (p. N680S). Such men with high SDF can be counseled to use this treatment after being informed about the lack of clear recommendations and possible side effects.
Follow-up should also occur to determine whether higher pregnancy rates and most importantly whether LBRs are being achieved whether through natural pregnancy or ART.
8) Managing ART failure in a couple with elevated SDF in the male partner
(1) Results
In our survey, we asked participants how they would approach various scenarios of ART failure, with different types of failure of the different ART modalities. The responses to these questions were then stratified according to specialty and compared between urologists/andrologists and other specialties including gynecologists, endocrinologists, and ART specialists. These questions, along with the overall responses and stratified responses are presented in Table 4.
Table 4. Answers of questions related to management of ART failure and elevated SDF.
| Option | Overall responses | Urology/andrologya | Other specialtiesa | p-valuea | |
|---|---|---|---|---|---|
| In a couple with a normal female partner experiencing failure to achieve a clinical pregnancy after IUI, associated with elevated SDF in the male partner, what would your management strategy be? | |||||
| Repeat the procedure after applying conservative measures (shorter abstinence, antioxidants) | 133 (33.5) | 94 (37.5) | 39 (26.7) | 0.01 | |
| Refer for ICSI using techniques to select sperm with lower SDF | 68 (17.1) | 44 (17.5) | 24 (16.4) | ||
| Refer for ICSI with ejaculated sperm | 62 (15.6) | 29 (11.5) | 33 (22.6) | ||
| Repeat IUI using techniques to select sperm with lower SDF | 44 (11.1) | 29 (11.5) | 15 (10.3) | ||
| Refer for ICSI using testicular sperm | 34 (8.6) | 26 (10.4) | 8 (5.5) | ||
| Repeat the procedure with no additional intervention | 11 (2.8) | 5 (2.0) | 6 (4.1) | ||
| Not applicable | 45 (11.3) | 24 (9.6) | 21 (14.4) | ||
| Total | 397 (100) | 251 (100) | 146 (100) | ||
| In a couple with a normal female partner experiencing miscarriage after IUI, associated with elevated SDF in the male partner, what would your management strategy be? | |||||
| Repeat the procedure after applying conservative measures (shorter abstinence, antioxidants) | 115 (28.9) | 82 (32.8) | 33 (22.3) | 0.001 | |
| Refer for ICSI using techniques to select sperm with lower SDF | 86 (21.6) | 53 (21.2) | 33 (22.3) | ||
| Repeat IUI using techniques to select sperm with lower SDF | 56 (14.1) | 32 (12.8) | 24 (16.2) | ||
| Refer for ICSI with ejaculated sperm | 46 (11.6) | 22 (8.8) | 24 (16.2) | ||
| Refer for ICSI using testicular sperm | 38 (9.5) | 32 (12.8) | 6 (4.1) | ||
| Repeat the procedure with no additional intervention | 12 (3.0) | 5 (2.0) | 7 (4.7) | ||
| Not applicable | 45 (11.3) | 24 (9.6) | 21 (14.2) | ||
| Total | 398 (100) | 250 (100) | 148 (100) | ||
| In a couple with a normal female partner experiencing fertilization failure after IVF, associated with elevated SDF in the male partner, what would your management strategy be? | |||||
| Refer for ICSI using techniques to select sperm with lower SDF | 107 (26.7) | 62 (24.7) | 45 (30.0) | 0.05 | |
| Repeat the procedure after applying conservative measures (shorter abstinence, antioxidants) | 73 (18.2) | 50 (19.9) | 23 (15.4) | ||
| Refer for ICSI using testicular sperm | 63 (15.7) | 46 (18.3) | 17 (11.3) | ||
| Refer for ICSI with ejaculated sperm | 61 (15.2) | 32 (12.8) | 29 (19.3) | ||
| Repeat IVF using techniques to select sperm with lower SDF | 46 (11.5) | 34 (13.5) | 12 (8.0) | ||
| Repeat the procedure with no additional intervention | 5 (1.2) | 3 (1.2) | 2 (1.3) | ||
| Not applicable | 46 (11.5) | 24 (9.6) | 22 (14.7) | ||
| Total | 401 (100) | 251 (100) | 150 (100) | ||
| In a couple with a normal female partner experiencing failure to achieve a clinical pregnancy after IVF, associated with elevated SDF in the male partner, what would your management strategy be? | |||||
| Refer for ICSI using techniques to select sperm with lower SDF | 108 (26.9) | 69 (27.5) | 39 (26.0) | 0.3 | |
| Repeat the procedure after applying conservative measures (shorter abstinence, antioxidants) | 74 (18.5) | 50 (19.9) | 24 (16.0) | ||
| Refer for ICSI using testicular sperm | 73 (18.2) | 50 (19.9) | 23 (15.4) | ||
| Refer for ICSI with ejaculated sperm | 52 (13.0) | 26 (10.4) | 26 (17.3) | ||
| Repeat IVF using techniques to select sperm with lower SDF | 45 (11.2) | 30 (11.9) | 15 (10.0) | ||
| Repeat the procedure with no additional intervention | 5 (1.2) | 3 (1.2) | 2 (1.3) | ||
| Not applicable | 44 (11.0) | 23 (9.2) | 21 (14.0) | ||
| Total | 401 (100) | 251 (100) | 150 (100) | ||
| In a couple with a normal female partner experiencing miscarriage after IVF or ICSI, associated with elevated SDF in the male partner and no other abnormality, what would your management strategy be? | |||||
| Repeat IVF or ICSI using techniques to select sperm with lower SDF | 128 (32.0) | 74 (29.5) | 54 (36.2) | 0.05 | |
| Refer for ICSI using testicular sperm | 118 (29.5) | 86 (34.3) | 32 (21.5) | ||
| Repeat the procedure after applying conservative measures (shorter abstinence, antioxidants) | 92 (23.0) | 58 (23.1) | 34 (22.8) | ||
| Repeat the procedure with no additional intervention | 11 (2.7) | 5 (1.9) | 6 (4.0) | ||
| Transfer to another center | 5 (1.3) | 4 (1.6) | 1 (0.7) | ||
| Not applicable | 46 (11.5) | 24 (9.6) | 22 (14.8) | ||
| Total | 400 (100) | 251 (100) | 149 (100) | ||
Values are presented as number (%).
For each option, overall answers are presented in descending order in the first column. The answers are then stratified according to specialty into urology/andrology vs. other specialties.
ART: assisted reproductive technology, ICSI: intracytoplasmic sperm injection, IUI: intrauterine insemination, IVF: in vitro fertilization, SDF: sperm DNA fragmentation.
aThe p-value in the last column represents the overall comparison between the responses of urologists/andrologists when compared to other specialties (the two columns marked with a) using Fischer’s exact test; a value <0.05 is considered a significant difference.
In general, for both questions related to IUI failure (failure to achieve clinical pregnancy and miscarriage), the most common option chosen overall at 33.5% and 28.9% respectively, was to repeat IUI after instituting conservative measures including reduced abstinence and antioxidant supplementation. When the analysis was stratified according to specialties, significant differences were found in these two questions as urologists/andrologists were more inclined to repeat IUI after conservative measures while other specialists were more likely to refer to ICSI (p=0.01 for the question regarding failure to achieve a clinical pregnancy after IUI and p=0.001 for the question regarding miscarriage after IUI).
When asked how they would approach fertilization failure after IVF and elevated SDF, 26.7% of respondents would refer to ICSI using techniques to select sperm with lower SDF, followed by 18.2% who would repeat IVF after applying conservative measures, Only, 1.2% would repeat IVF with no additional intervention. When stratified according to specialty, no significant differences were found. Regarding their approach towards failure to achieve a clinical pregnancy after IVF, a very similar trend in the responses was seen with similar overall percentages, and when stratified no significant difference was seen between specialties.
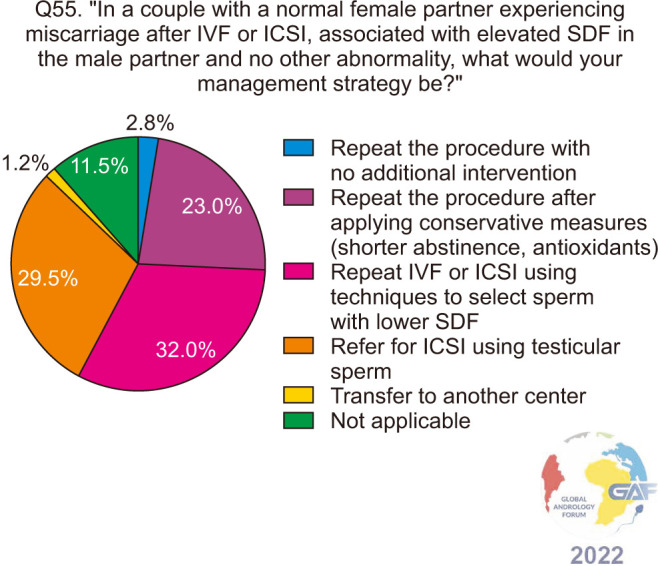
Regarding miscarriage after IVF or ICSI, 32.0% would repeat the procedure with techniques to select sperm with lower SDF, followed by referral to ICSI with testicular sperm at 29.5% (Fig. 19). When the analysis is stratified to compare specialties, differences did not reach statistical significance.
Fig. 19. Approach to miscarriage after IVF or ICSI and elevated SDF in the male partner. ICSI: intracytoplasmic sperm injection, IVF: in vitro fertilization, SDF: sperm DNA fragmentation.
(2) Society guidelines
AUA/ASRM, EAU, SIAMS, and DGGG, OEGGG and SGGG guidelines do not specifically address the management of ART failure with elevated SDF [18,19,20,21,22,25,26]. In the section on ART, however, the EAU recommends ICSI as the fertilization method when there is male factor infertility associated with elevated SDF [21].
The EAA recommends in cases of several (2 or more) ICSI failures after the use of ejaculated spermatozoa (with uncorrectable high DFI), the option of TESE and use of testicular spermatozoa for ICSI can be considered and discussed with the couple, with counseling that this approach is based on low-quality evidence [24].
(3) Discussion
Many studies have investigated the impact of elevated SDF on various outcomes of ART, which has allowed several meta-analyses to be published over the years highlighting various harmful impacts of SDF on different ART outcomes.
① SDF and IUI: A systematic review with meta-analysis including 10 studies, supported that a high SDF was associated with lower rates of clinical pregnancies and delivery rates following IUI [56]. However, this meta-analysis had some limitations such as the heterogeneity in terms of the SDF assay technique, and cut-off values used for DFI in the different studies included. Another meta-analysis also demonstrated lower pregnancy rates after IUI with high SDF but failed to demonstrate the role of SDF in predicting the outcomes of IUI [100]. Similarly, no significant association was reported between SDF and the rates of clinical pregnancy and pregnancy loss after IUI [101]. It does seem that elevated SDF can affect IUI success and should be considered and addressed in cases of IUI failure, however, no strong evidence supports the extent of this clinical impact.
② SDF and IVF: Low fertilization rates and total fertilization failure were found to be significantly correlated to DFI in men with asthenozoospermia [102]. A meta-analysis that included 4 studies with 770 IVF cycles, found fertilization rates to be lower with high sperm DNA damage (fertilization rate=55.4%) compared to low sperm DNA damage (fertilization rate=71.8%), however, pooled analysis did not yield statistical significance [103]. In a recent meta-analysis by Ribas-Maynou et al [59], that included 8 studies with 4,055 IVF cycles, lower implantation rates after IVF with high sperm DNA damage were reported (RR=0.68, p<0.01). As for clinical pregnancy rates after conventional IVF, the majority of published meta-analyses report a significantly lower clinical pregnancy rate after IVF for those with high sperm DNA damage [53,57,58,59,103,104]. On the other hand, a meta-analysis by Zhang et al [105] included 20 papers and did not confirm the predictive value of SDF on pregnancy outcomes after IVF and concluded that further studies are needed. Miscarriage rates were also reported to be significantly higher after IVF with higher SDF [58,106]. Lastly, two meta-analyses have also reported lower LBRs after IVF with higher SDF [59,107]. Although there are some contradicting reports, and evidence is based on heterogeneous studies that do not control for the several confounding variables including female factors and SDF testing methods and conditions, the harmful impact of SDF on all reproductive outcomes after conventional IVF must be acknowledged and taken into account when couples experience IVF failure or are planned for IVF.
③ SDF and ICSI: A previous study reported higher pregnancy rates in ICSI than in IVF when the SDF is high [108]. In 2015, a systematic review and meta-analysis including 6 studies showed no statistical difference in LBR between low and high SDF in ICSI while higher LBR was seen in men with low SDF after IVF [107]. Similarly, recent studies have failed to show the significant role of SDF in predicting reproductive outcomes in ICSI [109,110]. In contrast to conventional IVF, most meta-analyses do not report significant differences between high and low SDF in terms of fertilization rates, implantation rates, and clinical pregnancy rates after ICSI [53,58,59,104,105]. However, similar to IVF, miscarriage rates after ICSI with higher SDF were found to be significantly higher [58,105,106]. In general, the negative impacts of SDF on achieving fertilization and clinical pregnancy seem to be bypassed by ICSI, however, there is still a subsequent effect of high SDF that may lead to increased miscarriage rates, affecting final ICSI outcomes in these cases.
Almost a third of participants in the survey, manage IUI failure and elevated SDF by repeating the procedure after applying conservative measures such as a shorter abstinence period. This approach was supported by a prospective cohort study suggesting three hours of abstinence as an effective treatment for high SDF [51]. However, a clinical trial including 120 couples with unexplained infertility showed no effect of the ejaculatory abstinence period on SDF and outcomes of IUI cycles [111]. The practices of clinicians on how they approach IUI failure were heterogenous, with significant differences between disciplines. This is expected given the lack of standardized practices in addressing IUI failure as well as individual patient factors, financial factors, and a lack of consensus on how to manage SDF in general.
The results of our study show that after IVF failure with a high SDF, nearly 60% of participants propose a switch to ICSI with either ejaculated sperm, selected sperm, or testicular sperm. These practices are in line with the current evidence in that clinical pregnancy rates after ICSI are not significantly affected by SDF levels. Clinicians should still keep in mind the risk of miscarriage. Well-controlled randomized trials on the use of measures to reduce SDF prior to ICSI and their effect on miscarriage rates are needed and may in fact define appropriate management steps to undertake in these cases. On the other hand, less than 20% return to conventional IVF with or without conservative measures after IVF failure. Conservative measures such as shortening the abstinence period or taking antioxidant treatments can reduce SDF [51,77]. However, there is no evidence of the effect of these measures on conventional IVF outcomes.
In cases of recurrent miscarriage after IVF or ICSI, the options of ICSI with sperm selection or testicular sperm were the most chosen management options, and both have been associated with lower SDF and are discussed subsequently.
(4) Expert recommendations
When failure to achieve clinical pregnancy, or pregnancy loss occurs after IUI, associated with elevated SDF in the male partner, management of underlying causes of SDF as well as applying measures to lower SDF, including antioxidant supplementation and a shorter abstinence period before IUI, should be attempted when repeating IUI.
For couples failing to achieve fertilization or clinical pregnancy after conventional IVF, associated with elevated SDF in the male partner, management of underlying causes of SDF as well as applying measures to lower SDF, including antioxidant supplementation and a shorter abstinence period before IVF, may be considered, or alternatively, the couple may be referred for ICSI.
For couples experiencing ICSI failure or miscarriage after ICSI, associated with elevated SDF in the male partner, ICSI with sperm selection techniques or testicular sperm may be considered, in addition to conservative measures to lower SDF.
9) Use of Sperm Selection Techniques for infertile men with elevated SDF
(1) Results
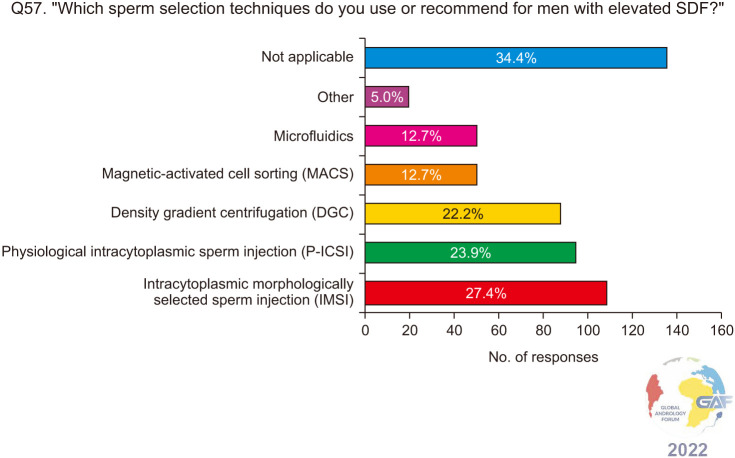
When asked about their practices regarding sperm selection techniques for infertile men with elevated SDF, 43.1% would recommend sperm selection for repeat ART after an initial failure if there is elevated SDF, while 32.3% would always recommend sperm selection if the couple is planned for ART and the man has elevated SDF. 24.6% do not recommend sperm selection techniques (Fig. 20). The most commonly chosen sperm selection methods are intracytoplasmic morphologically selected sperm injection (IMSI), physiological ICSI (P-ICSI), and density gradient centrifugation (DGC) at 27.4%, 23.9%, and 22.2% respectively (Fig. 21).
Fig. 20. Recommending sperm selection techniques for infertile men with elevated SDF. SDF: sperm DNA fragmentation.
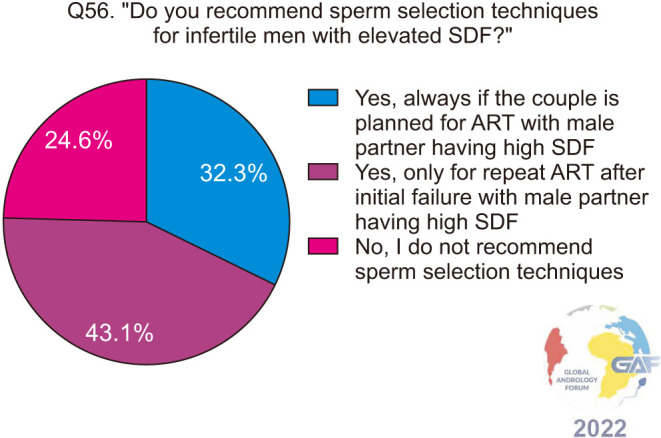
Fig. 21. Sperm selection techniques recommended by the respondents. Respondents were allowed to select more than one answer. The percentage for each answer was calculated by dividing the number of respondents who had selected it by the total number of respondents who had answered this question (n=401). SDF: sperm DNA fragmentation.
(2) Society guidelines
EAU guidelines do not make explicit recommendations for elevated treating elevated SDF by sperm selection [20,21,22]. They do state that traditional sperm preparation procedures for ICSI (swim-up and DGC) are unable to select sperm with optimal DNA integrity and go on to discuss IMSI, P-ICSI, and magnetic-activated sperm cell sorting (MACS), as advanced sperm selection techniques.
The recently updated ESHRE guidelines state that there is no evidence to support P-ICSI in couples with RPL [23]. There is no direct mention of SDF in this regard.
(3) Discussion
Based on our discussion in the previous section, it is clear that elevated SDF can still exert harmful impacts on the outcomes of various ART methods. Advanced sperm selection techniques may contribute towards selecting sperm with low levels of SDF. Conventional ICSI is performed by injecting single sperm directly into the oocyte. The sperm used for ICSI is chosen by the embryologist under the criteria of normal morphology and motility. IMSI is an ICSI technique that uses ultra-high magnification. The process is used to select motile spermatozoa with few vacuoles and normal nuclear morphology. The magnification used in IMSI is up to 6,000×, whereas in standard ICSI, the magnification is around 200–400×. Another improvement of the ICSI procedure, called P-ICSI, is with the use of hyaluronic acid (HA), which is the main component of the oocytes’ cumulus matrix. This technique is based on the fact that only the sperm cells that have successfully completed spermatogenesis and full maturation will express the HA receptors on their membrane, and this is correlated to a low SDF [112,113,114].
DGC is the most widespread sperm preparation technique which involves separating the spermatozoa as a function of density and motility, using a density gradient and then followed by centrifugation. Sperm preparation by DGC separates sperm cells based on their density, in which morphologically normal and abnormal spermatozoa have different densities [115]. MACS is a technique used to identify and eliminate apoptotic cells from the ejaculate by using annexin V. MACS is done by passing the ejaculate through a column where the apoptotic fraction remains retained and the viable fraction of a semen sample is collected [116]. Microfluidics is a technique that allows the motile fraction of sperm cells to swim through the flow and be collected in separate chambers, while the immotile sperm cells and debris reach the exit of the system. This method is based on the principle of natural sperm selection by passage through micro-barriers using disposable chips, mimicking in vivo natural environment of the female reproductive system [117].
The advantages and disadvantages of the various sperm selection techniques are presented in Table 5 [112,114,115,116,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136]. However, it should be underlined that the randomized clinical trials conducted to report on the effectiveness of these techniques did not focus strictly on men with elevated SDF, thus robust evidence is lacking on the effect of advanced sperm selection on this specific group of patients.
Table 5. Advantages and disadvantages of the various sperm selection techniques.
| Method | Advantages | Disadvantages |
|---|---|---|
| ICSI | There is a positive and reproducible correlation between teratozoospermia index and sperm DNA damage [118,119]. | There is no clear statement that every sperm which is morphologically normal will have a non-damaged DNA. The relationship between sperm morphology and sperm DNA damage still remains unclear [121]. |
| There is an inverse correlation between progressive motility and sperm DNA damage [119,120,122]. | ||
| Both reasons confirm ICSI as an appropriate technique to reduce sperm DNA damage. | ||
| IMSI | Provide better results in reducing SDF [123], since sperm with fragmented DNA tend to present vacuoles and ultra-morphological alterations. | Although the ultra-high magnification enables the operator to see the sperm clearer and better, there seems to be a lack of evidence to prove that IMSI is better than ICSI in selecting better sperm in terms of sperm DNA integrity [124]. |
| The use of expensive equipment and the long processing time is also a drawback of using this technique. | ||
| P-ICSI | Some studies demonstrated that this technique allows the selection of spermatozoa with a normal nucleus and without SDF [114]. | No specific drawbacks to P-ICSI are known at present [128], except in testicular sperm samples where no motile sperm is present. |
| Sperm that are mature and have intact DNA tend to bind to the hyaluronic acid, making them slow down, for easy choosing [112,125]. | ||
| Clinical evidence supporting its use is available [126]. Preferred for use in older females [127]. | ||
| DGC | DGC is easy to do, and already become the most widely used sperm preparation technique prior to ART [115]. | There is no consensus in the literature regarding the effect on DNA integrity, some studies demonstrated an improvement in SDF [115,129] while others report an increase in SDF [130]. |
| MACS | Several authors have shown the ability of this technique to select spermatozoa with reduced DNA fragmentation [116,131,132]. | Requires the use of expensive equipment [123]. |
| Preferred to be used in females younger than 30 years old [127]. | Several reports show no clinical benefits, probably because its use is not focused on the right population of patients [133,136]. | |
| Microfluidics | The ability to reduce SDF [134,135]. | Not widely available, and high cost [135]. |
| Easy to use. | Low volumes of ejaculate being processed, a considerable loss of sperm cells. |
ART: assisted reproductive technology, DGC: density gradient centrifugation, ICSI: intracytoplasmic sperm injection, IMSI: intracytoplasmic morphologically selected sperm injection, MACS: magnetic activated cell sorting, P-ICSI: physiologic intracytoplasmic sperm injection, SDF: sperm DNA fragmentation.
Our results show that 75.4% recommend the use of sperm selection techniques for infertile men with elevated SDF, which is in accordance with the literature that provides evidence on these techniques lowering SDF [114,116]. However, the indications remain unclear, and our results are mixed between 43.1% of the participants recommending sperm selection only for repeated ART after initial failure with a male partner having high SDF, and 32.3% always recommending sperm selection if the couple is planned for ART.
Regarding the choice of the sperm selection technique, according to our results, the most recommended techniques are IMSI (27.4%), P-ICSI (23.9%), and DGC (22.2%). Those results are in line with the current society guidelines as no clear recommendation is stated regarding the choice of a specific technique. The majority of our participants recommend the use of sperm selection for infertile men with elevated SDF, which is opposite to the current society guidelines that do not make recommendations in that regard.
Well-designed trials are needed to demonstrate the benefit of each sperm selection method and its effects on ART outcomes when used for elevated SDF.
(4) Expert recommendations
Sperm selection techniques may be used for infertile men with elevated SDF if the couple is planned for ART or in case of initial ART failure.
IMSI, MACS, P-ICSI, DGC, and microfluidic techniques have each shown advantages and drawbacks. No particular technique can be recommended over another, and none has shown a clear superiority.
Couples should be counseled that any choice and decision on how to select sperm to improve SDF and thus reproductive success after ART, is to date based on empirical evidence. Given this fact, these techniques should be limited to sporadic cases that have failed with classic methods, aiming at a different approach but being conscious of the lack of scientific robustness for its use.
10) Use of testicular sperm for infertile men with elevated SDF
(1) Results
When asked about the use of testicular sperm extraction for infertile men with elevated SDF, 58.2% would recommend or perform it in certain cases, while 18.9% would never and 9.2% routinely recommend it (Fig. 22). If they do recommend testicular sperm, most (38.5%) do so after failure of two or more ICSI cycles (Fig. 23). Those who do not recommend testicular sperm were asked to select reasons for their decision, and these are presented and summarized in Fig. 24.
Fig. 22. Recommending testicular for infertile men with elevated SDF undergoing ICSI. ICSI: intracytoplasmic sperm injection, SDF: sperm DNA fragmentation.
Fig. 23. Responses to when experts would recommend testicular sperm if they do for infertile men with elevated SDF. ICSI: intracytoplasmic sperm injection, SDF: sperm DNA fragmentation.

Fig. 24. Reasons for recommending against the use of testicular sperm for infertile men with elevated SDF. Respondents were allowed to select more than one answer. The percentage for each answer is calculated by dividing the number of respondents who have selected it by the total number of respondents who have answered this question (n=341). SDF: sperm DNA fragmentation.
(2) Society guidelines
The AUA/ASRM guidelines do not comment on the use of testicular sperm for men with elevated SDF [18,19]. In their discussion, however, they do state that a clinician may use surgically obtained sperm in cases of high SDF.
The EAU does not currently advocate the routine clinical use of testicular sperm in non-azoospermic men with high SDF (TESE-ICSI) outside of clinical trials [20,21,22]. They do state that urologists may offer this option after the failure of other treatments to address underlying etiologies but should counsel their patients that this practice is based on low-quality evidence.
EAA guideline states that due to a lack of solid evidence from randomized studies, they currently do not suggest performing routine TESE in OAT patients with high DNA fragmentation or patients with cryptozoospermia [24]. However, in cases of several (2 or more) ICSI failures after the use of ejaculated spermatozoa (with un-correctable high DFI), the option of TESE and use of testicular spermatozoa for ICSI can be considered and discussed with the couple, with counseling that this approach is based on low-quality evidence.
(3) Discussion
SDF increases in spermatozoa along the anatomic route as they pass through the male reproductive tract, DNA fragmentation increases from the testis to the vas deferens and the highest levels occur in the ejaculate [137]. Passage of sperm through the genital tract may lead to increased exposure to OS and thus resulting in elevated SDF [138]. As an underlying theory, it has been suggested that mature spermatozoa are exposed to OS during their transit along the seminiferous tubules and epididymis [139].
Some studies have shown that testicular sperm is superior to ejaculated sperm for ICSI in cases of elevated SDF, with higher pregnancy and delivery rates [140,141]. A systematic review and meta-analysis showed significantly lower miscarriage rates, higher LBRs, and clinical pregnancy rates in ICSI with testicular sperm compared to ICSI with ejaculated sperm among infertile men with high SDF [138]. However, the majority of evidence stems from observational studies with a rating of low and very low quality of evidence [142].
In addition, testicular sperm has been found to have 2–3-fold higher aneuploidy rates than ejaculated samples [143], although recent evidence is suggesting otherwise [144]. Besides, more problematic adverse effects occur with testicular sperm retrieval, with a reduction in T production, infection, hematoma formation, and testicular atrophy, all having been reported as potential complications [145].
More than half of the responders to our survey would recommend testicular sperm for elevated SDF, which is in line with the current literature trend.
The use of testicular sperm in the condition of elevated SDF is still a subject of debate. Most of the responders to our survey who recommend testicular sperm, use it after failed ART cycles as a salvage method, which is in line with EAA Guidelines. Conversely, although the AUA and EAU do discuss the use of testicular sperm for infertile men with impaired sperm DNA integrity, no recommendations are stated due to the lack of solid evidence.
(4) Expert recommendations
In infertile non-azoospermic men with high SDF that cannot be corrected with other treatment modalities and who have a history of unsuccessful ICSI, the option of ICSI with testicular sperm can be considered and discussed with the couple, who should be informed that this approach is based on low-quality evidence.
LIMITATIONS OF THE PRESENT STUDY
Our present survey and expert recommendations were limited in that they solely focused on the male partner, and female factors were not considered or were assumed normal in most questions. This may not be completely applicable in the clinical setting, given that infertility is a couple’s disease. Furthermore, factors other than SDF, that can affect fertility, embryo quality, and ART outcome were not considered specifically for questions addressing ART failure and management. For example, the effect of aneuploidy was not discussed and may also have a role in the approach of an infertile couple with elevated SDF, as one prospective cohort reported that SDF levels on the day of ICSI did not have a significant impact on fertilization, embryo development, implantation, and clinical pregnancy rates when only euploid blastocysts were transferred after preimplantation genetic testing [146]. Nevertheless, this manuscript provides a basis for all reproductive clinicians and includes practices and therapeutic approaches they can incorporate and utilize, should they encounter elevated SDF in the male partner when managing an infertile couple.
In addition, we were not able to accurately calculate the response rate of the survey, due to the various methods of dissemination including emails, direct communication between experts, and professional society websites. The complete survey was long, and many participants did not answer all the questions and submitted incomplete surveys which were included. Stratified analysis to compare various practices was also limited by the vast demographic variables and practice settings of the participating clinicians.
CONCLUDING REMARKS
With the growing evidence on the harmful impact of SDF on fertility, as well as the various groups of infertile men and couples, who might benefit from SDF testing [147], it is crucial to have a unified approach in managing a couple that is found to have elevated SDF in the male partner. The need for universal guidelines is further stressed by the many different disciplines that manage couple infertility, as well as the lack of solid recommendations by the professional reproductive societies regarding SDF management.
To the best of our knowledge, this is the largest survey that captures the various treatment approaches used by different reproductive clinicians in a variety of situations involving infertile men with elevated SDF. Although our results have displayed that the general approach is somewhat consistent, there are differences in practice between clinicians worldwide, which is expected given the scarcity of professional society recommendations.
By capturing global practices, expert recommendations were devised for specific situations associated with elevated SDF in the male partner, supported by the available professional society recommendations and evidence in the literature. These proposed recommendations were scrutinized and reviewed by renowned reproductive experts using the Delphi method and are presented in Table 6.
Table 6. Management of infertile men with elevated SDF based on expert recommendations.
| Scenario | Expert Recommendations |
|---|---|
| General approach to infertile men found to have elevated SDF | Ordering a second confirmation test for elevated SDF is not necessary for diagnosis. |
| For infertile men with elevated SDF, lifestyle modification strategies should be recommended including maintaining a healthy lifestyle to overcome obesity, cessation of smoking and alcohol use, as well as treating genital infections, and eliminating toxic exposure. | |
| Reduced ejaculatory abstinence of 12–24 hours before attempting conception (natural or by ART) is recommended as a means to lower SDF and improve pregnancy outcomes. | |
| ART referral for infertile men with elevated SDF | Different ART methods, are not recommended as first-line treatment strategies for infertile men found to have elevated SDF. Instead, known underlying causes should be addressed first as well as conservative management to lower SDF. |
| Managing couples experiencing RPL after spontaneous conception with elevated SDF in the man | In couples with RPL following spontaneous pregnancy, associated with elevated SDF in the male partner and no female factor infertility, an appropriate initial approach should include addressing known risk factors of elevated SDF and other causes associated with male infertility. These men may also be supplemented with oral antioxidant therapy, particularly if there is no associated underlying cause for their infertility. The decision to refer such a couple to ART should be determined on a case-by-case scenario and after adequate management of elevated SDF. |
| Managing infertile men with clinical varicocele, normal semen parameters, and elevated SDF | In infertile men with clinical varicocele and normal semen parameters, VR should be offered if SDF is elevated. The persistence of abnormal postoperative SDF values is a poor predictor for both natural and assisted conception. |
| VR should be offered after diagnosis to lower SDF for both natural and assisted conception. ART could be performed after VR. | |
| If there is a need or the couple wishes for ART to be performed on diagnosis, they should be counseled on the risk of failure that may be attributed to SDF with a known associated yet untreated cause (i.e., clinical varicocele), and other attempts to lower SDF should be considered including antioxidants, sperm selection techniques, and testicular sperm. | |
| Managing infertile men with subclinical varicocele and elevated SDF | In men with elevated SDF and subclinical varicocele, varicocele repair is not recommended. Men with subclinical varicocele and elevated SDF need to be evaluated and treated similarly to men without a varicocele. |
| Use of antioxidants in managing infertile men with elevated SDF | Although there remains no unanimous consensus, empiric antioxidants may be prescribed for infertile men with elevated SDF, especially if they have risk factors and known conditions associated with elevated SDF, including idiopathic infertility, RPL, varicocele, leukocytospermia, smoking and other lifestyle and environmental risk factors. |
| There is no consensus on the type, dosage, and duration of antioxidant treatment that can be recommended, although a duration of 3–6 months has been proven successful. | |
| The success of treatment should be guided by improved conventional semen parameters, decreased SDF levels, and improved reproductive outcomes (either natural or ART). | |
| The current trend of prescribing antioxidants to all infertile men (even if SDF is not tested) is concerning, because improper prescription of these components may negatively impact semen parameters and fertility potentials of men. | |
| Use of hormonal therapy in managing infertile men with elevated SDF | No clear recommendation can be made for or against the use of hormonal therapy for high SDF. We recommend the use of hormone therapy only by well-trained fertility experts and in combination with other therapies (lifestyle modification, infections therapy, antioxidants). |
| Hormonal therapy could be effective in oligozoospermic, hypogonadotropic hypogonadal men, and those with FSH receptor homozygous genotype (p. N680S). Such men with high SDF can be counseled to use this treatment after being informed about the lack of clear recommendations and possible side effects. | |
| Follow-up should also occur to determine whether higher pregnancy rates and most importantly whether live birth rates are being achieved whether through natural pregnancy or ART. | |
| Managing ART failure in a couple with elevated SDF in the male partner | When failure to achieve clinical pregnancy, or pregnancy loss occurs after IUI, associated with elevated SDF in the male partner, management of underlying causes of SDF as well as applying measures to lower SDF, including antioxidant supplementation and a shorter abstinence period before IUI, should be attempted when repeating IUI. |
| For couples failing to achieve fertilization or clinical pregnancy after conventional IVF, associated with elevated SDF in the male partner, management of underlying causes of SDF as well as applying measures to lower SDF, including antioxidant supplementation and a shorter abstinence period before IVF, may be considered, or alternatively, the couple may be referred for ICSI. | |
| For couples experiencing ICSI failure or miscarriage after ICSI, associated with elevated SDF in the male partner, ICSI with sperm selection techniques or testicular sperm may be considered, in addition to conservative measures to lower SDF. | |
| Use of sperm selection techniques for infertile men with elevated SDF | Sperm selection techniques may be used for infertile men with elevated SDF if the couple is planned for ART or in case of initial ART failure. |
| IMSI, MACS, P-ICSI, DGC, and microfluidic techniques have each shown advantages and drawbacks. No particular technique can be recommended over another, and none has shown a clear superiority. | |
| Couples should be counseled that any choice and decision on how to select sperm to improve SDF and thus reproductive success after ART, is to date based on empirical evidence. Given this fact, these techniques should be limited to sporadic cases that have failed with classic methods, aiming at a different approach but being conscious of the lack of scientific robustness for its use. | |
| Use of testicular sperm for infertile men with elevated SDF | In infertile non-azoospermic men with high SDF that cannot be corrected with other treatment modalities and who have a history of unsuccessful ICSI, the option of ICSI with testicular sperm can be considered and discussed with the couple, who should be informed that this approach is based on low-quality evidence. |
ART: assisted reproductive technology, DGC: density gradient centrifugation, FSH: follicle stimulating hormone, ICSI: intracytoplasmic sperm injection, IMI: idiopathic male infertility, IMSI: intracytoplasmic morphologically selected sperm injection, IUI: intrauterine insemination, IVF: in vitro fertilization, MACS: magnetic activated cell sorting, P-ICSI: physiologic intracytoplasmic sperm injection, RPL: recurrent pregnancy loss, SDF: sperm DNA fragmentation, UMI: unexplained male infertility, VR: varicocele repair.
Generally, once infertile men are found to have elevated SDF, there is no need for a repeat or confirmatory test. Underlying causes and risk factors should be addressed. These include VR for clinical varicocele, antibiotics for genital tract infections, weight loss for obesity and lifestyle modification, and exposure limitation for other risks. Reduced ejaculatory abstinence may also lower SDF before natural and assisted conception. Empiric antioxidants for 3-6 months can also be prescribed. Although hormonal therapies may be beneficial, their use must be limited to certain cases and by experienced clinicians. Finally, ART is not recommended as a first-line approach for the management of elevated SDF, however, if ART failure is associated with elevated SDF, clinicians should recommend the aforementioned strategies to lower SDF before repeating ART. SDF may further be reduced before ART by using advanced sperm selection methods such as IMSI or P-ICSI, or even testicular sperm in cases of recurrent ICSI failure, although the evidence on the benefit of these strategies is not robust. The different treatment strategies for elevated SDF are summarized in Fig. 25.
Fig. 25. Treatment strategies for infertile men with elevated SDF. ICSI: intracytoplasmic sperm injection, SDF: sperm DNA fragmentation.
In conclusion, there is a compelling need for universal recommendations by professional societies related to the management of infertile men with elevated SDF. In order to make such recommendations, high-quality solid evidence needs to exist, which can be accomplished by well-designed studies that demonstrate the benefit of various strategies in lowering SDF and improving reproductive outcomes after both natural and assisted methods of conception.
Acknowledgements
The authors are thankful to the following societies for promoting the online survey through the efforts of their members listed below:
1. AK Andrologie und Sexuelle Funktionsstörungen as part of the Österreichische Gesellschaft für Urologie und Andrologie (Germar-Michael Pinggera, MD, Austria).
2. Algerian Association of Urology (Nazim Gherabi, MD, Algeria).
3. Andrology Working Group, Society of Urologic Surgery in Turkey (Gökhan Çeker, MD, Turkey; Oğuzhan Kahraman, MD, Turkey; Erman Ceyhan, MD, Turkey).
4. Egyptian Society for Sexual Medicine & Surgery (Ahmed El-Sakka, MD, Egypt).
5. Egyptian Society of Andrology (Taymour Mostafa, MD, Egypt).
6. Indonesian Society of Andrological Urology (Gede Wirya Kusuma Duarsa, PhD, Indonesia).
7. Indonesian Urological Association (Ponco Birowo, MD, PhD Indonesia; Gede Wirya Kusuma Duarsa, PhD, Indonesia; Fahmi Bahar, MD, Indonesia).
8. Italian Society of Andrology and Sexual Medicine (Aldo E. Calogero, MD, Italy).
9. Italian Society of Human Reproduction (Carlo Trotta, MD, Italy; Giovanni M. Colpi, MD, Italy; Lucia Rocco, PhD, Italy).
10. Italian Society of Urology (Gian Maria Busetto, MD, PhD, Italy).
11. Lebanese Society of Urology (Mohamad Moussa, MD, Lebanon).
12. Malaysian Society of Andrology and the Study of the Aging Male (Christopher Ho, MD, Malaysia; Kay Seong, NGOO, MD, Malaysia).
13. Malaysian Urological Association (Teng Aik Ong, MD, Malaysia).
14. Mediterranean Society for Reproductive Medicine (Hassan Sallam, MD, PhD, Egypt).
15. Middle East Society for Sexual Medicine (Amr El Meliegy, MD, Egypt).
16. Romanian Association for Sexual Medicine (Catalina Zenoaga-Barbarosie, MSc, Romania).
17. Saudi Andrology Group (Naif Alhathal, MD, Saudi Arabia).
18. Society for Men's Health Singapore (King Chien; Joe Lee, MD, Singapore).
19. Society of Egyptian Fellows and Members of the Royal College of Obstetricians and Gynecologists (Hassan Sallam MD, PhD, Egypt).
20. Turkish Association of Urology (Arif Kalkanli, MD, Turkey; Ateş Kadıoğlu, MD, Turkey).
21. Vietnamese Society for Sexual Medicine (Quang Nguyen, MD, PhD; Ho Vinh Phuoc Nguyen, MD; Tan V. Le, MD; Quang Tien Long Tran, MD).
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: None.
- Conceptualization: Ashok Agarwal.
- Data curation: Shinnosuke Kuroda, Ahmed Harraz.
- Formal analysis: Ahmed Harraz.
- Methodology: Ashok Agarwal, Rupin Shah, Ala’a Farkouh, Ahmed Harraz.
- Project administration: Ashok Agarwal, Rupin Shah, Ala’a Farkouh.
- Supervision: Ashok Agarwal, Rupin Shah.
- Writing – original draft: Ala’a Farkouh, Damayanthi Durairajanayagam, Parviz Kavoussi, Mohamed Arafa, Murat Gul, Andrian Japari, Nicolas Garrido Puchalt, Nazim Gherabi, Andrea Crafa, Raneen Sawaid Kaiyal, Fotios Dimitriadis, Mara Simopoulou, Lucia Rocco, Eric Chung, Sara Darbandi, Giorgio Ivan Russo, Ioannis Sokolakis, Ramy Aou Ghayda, Vilvapathy Karthikeyan, Taymour Mostafa, Nasser Mogharabian, Konstantinos Makarounis, Shinnosuke Kuroda, Imad Ziouziou, Marion Bendayan, Kadir Bocu, Oguzhan Kahraman, Tan Le, Manaf Alhashmi.
- Writing – review & editing: All the authors.
Ethics statement: Submission of the survey was voluntary to all invited participants. Informed consent was described and obtained from all participants who agreed to submit the survey. No participant identifiers were shared with any third party. No patient information was elicited from any participant. No patients were involved in this study.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.230008.
Raw data on management of elevated SDF (Q33–60)
Advanced analysis of selected questions on the management of elevated SDF
Complete methodology with CHERRIES checklist
Complete survey questions and invitation
Complete demographic data
References
- 1.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Hum Reprod. 2017;32:1786–1801. doi: 10.1093/humrep/dex234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C, Swerdloff RS. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. 2014;102:1502–1507. doi: 10.1016/j.fertnstert.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal A, Allamaneni SS. Sperm DNA damage assessment: a test whose time has come. Fertil Steril. 2005;84:850–853. doi: 10.1016/j.fertnstert.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) WHO laboratory manual for the examination and processing of human semen. 6th ed. Geneva: WHO; 2021. [Google Scholar]
- 6.Winkle T, Rosenbusch B, Gagsteiger F, Paiss T, Zoller N. The correlation between male age, sperm quality and sperm DNA fragmentation in 320 men attending a fertility center. J Assist Reprod Genet. 2009;26:41–46. doi: 10.1007/s10815-008-9277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Høst E, Lindenberg S, Smidt-Jensen S. DNA strand breaks in human spermatozoa: correlation with fertilization in vitro in oligozoospermic men and in men with unexplained infertility. Acta Obstet Gynecol Scand. 2000;79:189–193. [PubMed] [Google Scholar]
- 8.Farkouh A, Salvio G, Kuroda S, Saleh R, Vogiatzi P, Agarwal A. Sperm DNA integrity and male infertility: a narrative review and guide for the reproductive physicians. Transl Androl Urol. 2022;11:1023–1044. doi: 10.21037/tau-22-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ménézo YJ, Hazout A, Panteix G, Robert F, Rollet J, Cohen-Bacrie P, et al. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online. 2007;14:418–421. doi: 10.1016/s1472-6483(10)60887-5. [DOI] [PubMed] [Google Scholar]
- 10.Tunc O, Thompson J, Tremellen K. Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod Biomed Online. 2009;18:761–768. doi: 10.1016/s1472-6483(10)60024-7. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal A, Gupta S, Du Plessis S, Sharma R, Esteves SC, Cirenza C, et al. Abstinence time and its impact on basic and advanced semen parameters. Urology. 2016;94:102–110. doi: 10.1016/j.urology.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 12.Samavat J, Cantini G, Lotti F, Di Franco A, Tamburrino L, Degl'Innocenti S, et al. Massive weight loss obtained by bariatric surgery affects semen quality in morbid male obesity: a preliminary prospective double-armed study. Obes Surg. 2018;28:69–76. doi: 10.1007/s11695-017-2802-7. [DOI] [PubMed] [Google Scholar]
- 13.Sepidarkish M, Maleki-Hajiagha A, Maroufizadeh S, Rezaeinejad M, Almasi-Hashiani A, Razavi M. The effect of body mass index on sperm DNA fragmentation: a systematic review and meta-analysis. Int J Obes (Lond) 2020;44:549–558. doi: 10.1038/s41366-020-0524-8. [DOI] [PubMed] [Google Scholar]
- 14.Bradley CK, McArthur SJ, Gee AJ, Weiss KA, Schmidt U, Toogood L. Intervention improves assisted conception intracytoplasmic sperm injection outcomes for patients with high levels of sperm DNA fragmentation: a retrospective analysis. Andrology. 2016;4:903–910. doi: 10.1111/andr.12215. [DOI] [PubMed] [Google Scholar]
- 15.Greco E, Scarselli F, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod. 2005;20:226–230. doi: 10.1093/humrep/deh590. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal A, Majzoub A, Baskaran S, Panner Selvam MK, Cho CL, Henkel R, et al. Sperm DNA fragmentation: a new guideline for clinicians. World J Mens Health. 2020;38:412–471. doi: 10.5534/wjmh.200128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal A, Saleh R, Boitrelle F, Cannarella R, Hamoda TAA, Durairajanayagam D, et al. The Global Andrology Forum (GAF): a world-wide, innovative, online initiative to bridge the gaps in research and clinical practice of male infertility and sexual health. World J Mens Health. 2022;40:537–542. doi: 10.5534/wjmh.220127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part I. Fertil Steril. 2021;115:54–61. doi: 10.1016/j.fertnstert.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part II. Fertil Steril. 2021;115:62–69. doi: 10.1016/j.fertnstert.2020.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Minhas S, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. EAU Working Group on Male Sexual and Reproductive Health. European Association of Urology guidelines on male sexual and reproductive health: 2021 update on male infertility. Eur Urol. 2021;80:603–620. doi: 10.1016/j.eururo.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. EAU Working Group on Male Sexual and Reproductive Health. European Association of Urology guidelines on sexual and reproductive health-2021 update: male sexual dysfunction. Eur Urol. 2021;80:333–357. doi: 10.1016/j.eururo.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Tharakan T, Bettocchi C, Carvalho J, Corona G, Jones TH, Kadioglu A, et al. EAU Working Panel on Male Sexual Reproductive Health. European Association of Urology guidelines panel on male sexual and reproductive health: a clinical consultation guide on the indications for performing sperm DNA fragmentation testing in men with infertility and testicular sperm extraction in nonazoospermic men. Eur Urol Focus. 2022;8:339–350. doi: 10.1016/j.euf.2020.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss: an update in 2022. Hum Reprod Open. 2023;2023:hoad002. doi: 10.1093/hropen/hoad002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colpi GM, Francavilla S, Haidl G, Link K, Behre HM, Goulis DG, et al. European Academy of Andrology guideline management of oligo-astheno-teratozoospermia. Andrology. 2018;6:513–524. doi: 10.1111/andr.12502. [DOI] [PubMed] [Google Scholar]
- 25.Ferlin A, Calogero AE, Krausz C, Lombardo F, Paoli D, Rago R, et al. Management of male factor infertility: position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS): endorsing organization: Italian Society of Embryology, Reproduction, and Research (SIERR) J Endocrinol Invest. 2022;45:1085–1113. doi: 10.1007/s40618-022-01741-6. [DOI] [PubMed] [Google Scholar]
- 26.Toth B, Baston-Büst DM, Behre HM, Bielfeld A, Bohlmann M, Bühling K, et al. Diagnosis and treatment before assisted reproductive treatments. Guideline of the DGGG, OEGGG and SGGG (S2k level, awmf register number 015-085, February 2019) - part 2, hemostaseology, andrology, genetics and history of malignant disease. Geburtshilfe Frauenheilkd. 2019;79:1293–1308. doi: 10.1055/a-1017-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esteves SC, Zini A, Coward RM, Evenson DP, Gosálvez J, Lewis SEM, et al. Sperm DNA fragmentation testing: summary evidence and clinical practice recommendations. Andrologia. 2021;53:e13874. doi: 10.1111/and.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Villiers MR, de Villiers PJ, Kent AP. The Delphi technique in health sciences education research. Med Teach. 2005;27:639–643. doi: 10.1080/13611260500069947. [DOI] [PubMed] [Google Scholar]
- 29.Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of Internet E-surveys (CHERRIES) J Med Internet Res. 2004;6:e34. doi: 10.2196/jmir.6.3.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal A, Cho CL, Majzoub A, Esteves SC. The Society for Translational Medicine: clinical practice guidelines for sperm DNA fragmentation testing in male infertility. Transl Androl Urol. 2017;6(Suppl 4):S720–S733. doi: 10.21037/tau.2017.08.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization laboratory methods for the examination of human semen. Eur Urol. 2016;70:635–645. doi: 10.1016/j.eururo.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Aboulmaouahib S, Madkour A, Kaarouch I, Sefrioui O, Saadani B, Copin H, et al. Impact of alcohol and cigarette smoking consumption in male fertility potential: looks at lipid peroxidation, enzymatic antioxidant activities and sperm DNA damage. Andrologia. 2018;50:e12926. doi: 10.1111/and.12926. [DOI] [PubMed] [Google Scholar]
- 33.Ranganathan P, Rao KA, Thalaivarasai Balasundaram S. Deterioration of semen quality and sperm-DNA integrity as influenced by cigarette smoking in fertile and infertile human male smokers-a prospective study. J Cell Biochem. 2019;120:11784–11793. doi: 10.1002/jcb.28458. [DOI] [PubMed] [Google Scholar]
- 34.Mostafa RM, Nasrallah YS, Hassan MM, Farrag AF, Majzoub A, Agarwal A. The effect of cigarette smoking on human seminal parameters, sperm chromatin structure and condensation. Andrologia. 2018;50:e12910. doi: 10.1111/and.12910. [DOI] [PubMed] [Google Scholar]
- 35.Rubes J, Selevan SG, Evenson DP, Zudova D, Vozdova M, Zudova Z, et al. Episodic air pollution is associated with increased DNA fragmentation in human sperm without other changes in semen quality. Hum Reprod. 2005;20:2776–2783. doi: 10.1093/humrep/dei122. [DOI] [PubMed] [Google Scholar]
- 36.Zhou DD, Hao JL, Guo KM, Lu CW, Liu XD. Sperm quality and DNA damage in men from Jilin province, China, who are occupationally exposed to ionizing radiation. Genet Mol Res. 2016;15:gmr8078. doi: 10.4238/gmr.15018078. [DOI] [PubMed] [Google Scholar]
- 37.Lafuente R, García-Blàquez N, Jacquemin B, Checa MA. Outdoor air pollution and sperm quality. Fertil Steril. 2016;106:880–896. doi: 10.1016/j.fertnstert.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Sánchez-Peña LC, Reyes BE, López-Carrillo L, Recio R, Morán-Martínez J, Cebrián ME, et al. Organophosphorous pesticide exposure alters sperm chromatin structure in Mexican agricultural workers. Toxicol Appl Pharmacol. 2004;196:108–113. doi: 10.1016/j.taap.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 39.Mir J, Franken D, Andrabi SW, Ashraf M, Rao K. Impact of weight loss on sperm DNA integrity in obese men. Andrologia. 2018 doi: 10.1111/and.12957. [Epub] [DOI] [PubMed] [Google Scholar]
- 40.Carette C, Levy R, Eustache F, Baron G, Coupaye M, Msika S, et al. Changes in total sperm count after gastric bypass and sleeve gastrectomy: the BARIASPERM prospective study. Surg Obes Relat Dis. 2019;15:1271–1279. doi: 10.1016/j.soard.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 41.Sharma R, Gupta S, Agarwal A, Henkel R, Finelli R, Parekh N, et al. Relevance of leukocytospermia and semen culture and its true place in diagnosing and treating male infertility. World J Mens Health. 2022;40:191–207. doi: 10.5534/wjmh.210063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallegos G, Ramos B, Santiso R, Goyanes V, Gosálvez J, Fernández JL. Sperm DNA fragmentation in infertile men with genitourinary infection by Chlamydia trachomatis and mycoplasma. Fertil Steril. 2008;90:328–334. doi: 10.1016/j.fertnstert.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 43.Hamada A, Agarwal A, Sharma R, French DB, Ragheb A, Sabanegh ES., Jr Empirical treatment of low-level leukocytospermia with doxycycline in male infertility patients. Urology. 2011;78:1320–1325. doi: 10.1016/j.urology.2011.08.062. [DOI] [PubMed] [Google Scholar]
- 44.Castellini C, D'Andrea S, Martorella A, Minaldi E, Necozione S, Francavilla F, et al. Relationship between leukocytospermia, reproductive potential after assisted reproductive technology, and sperm parameters: a systematic review and meta-analysis of case-control studies. Andrology. 2020;8:125–135. doi: 10.1111/andr.12662. [DOI] [PubMed] [Google Scholar]
- 45.Jurema MW, Vieira AD, Bankowski B, Petrella C, Zhao Y, Wallach E, et al. Effect of ejaculatory abstinence period on the pregnancy rate after intrauterine insemination. Fertil Steril. 2005;84:678–681. doi: 10.1016/j.fertnstert.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 46.Marshburn PB, Alanis M, Matthews ML, Usadi R, Papadakis MH, Kullstam S, et al. A short period of ejaculatory abstinence before intrauterine insemination is associated with higher pregnancy rates. Fertil Steril. 2010;93:286–288. doi: 10.1016/j.fertnstert.2009.07.972. [DOI] [PubMed] [Google Scholar]
- 47.Sánchez-Martín P, Sánchez-Martín F, González-Martínez M, Gosálvez J. Increased pregnancy after reduced male abstinence. Syst Biol Reprod Med. 2013;59:256–260. doi: 10.3109/19396368.2013.790919. [DOI] [PubMed] [Google Scholar]
- 48.Shen ZQ, Shi B, Wang TR, Jiao J, Shang XJ, Wu QJ, et al. Characterization of the sperm proteome and reproductive outcomes with in vitro, fertilization after a reduction in male ejaculatory abstinence period. Mol Cell Proteomics. 2019;18(Suppl 1):S109–S117. doi: 10.1074/mcp.RA117.000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pons I, Cercas R, Villas C, Braña C, Fernández-Shaw S. One abstinence day decreases sperm DNA fragmentation in 90 % of selected patients. J Assist Reprod Genet. 2013;30:1211–1218. doi: 10.1007/s10815-013-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manna C, Barbagallo F, Manzo R, Rahman A, Francomano D, Calogero AE. Sperm parameters before and after swim-up of a second ejaculate after a short period of abstinence. J Clin Med. 2020;9:1029. doi: 10.3390/jcm9041029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dahan MH, Mills G, Khoudja R, Gagnon A, Tan G, Tan SL. Three hour abstinence as a treatment for high sperm DNA fragmentation: a prospective cohort study. J Assist Reprod Genet. 2021;38:227–233. doi: 10.1007/s10815-020-01999-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kulkarni V, Kaingade P, Kulkarni N, Bhalerao T, Nikam A. Assessment of semen parameters in consecutive ejaculates with short abstinence period in oligospermic males. JBRA Assist Reprod. 2022;26:310–314. doi: 10.5935/1518-0557.20210073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med. 2011;57:78–85. doi: 10.3109/19396368.2010.515704. [DOI] [PubMed] [Google Scholar]
- 54.Malić Vončina S, Golob B, Ihan A, Kopitar AN, Kolbezen M, Zorn B. Sperm DNA fragmentation and mitochondrial membrane potential combined are better for predicting natural conception than standard sperm parameters. Fertil Steril. 2016;105:637–644.e1. doi: 10.1016/j.fertnstert.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 55.Malić Vončina S, Stenqvist A, Bungum M, Schyman T, Giwercman A. Sperm DNA fragmentation index and cumulative live birth rate in a cohort of 2,713 couples undergoing assisted reproduction treatment. Fertil Steril. 2021;116:1483–1490. doi: 10.1016/j.fertnstert.2021.06.049. [DOI] [PubMed] [Google Scholar]
- 56.Chen Q, Zhao JY, Xue X, Zhu GX. The association between sperm DNA fragmentation and reproductive outcomes following intrauterine insemination, a meta analysis. Reprod Toxicol. 2019;86:50–55. doi: 10.1016/j.reprotox.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Simon L, Zini A, Dyachenko A, Ciampi A, Carrell DT. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J Androl. 2017;19:80–90. doi: 10.4103/1008-682X.182822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng C, Li T, Xie Y, Guo Y, Yang QY, Liang X, et al. Sperm DNA fragmentation index influences assisted reproductive technology outcome: a systematic review and meta-analysis combined with a retrospective cohort study. Andrologia. 2019;51:e13263. doi: 10.1111/and.13263. [DOI] [PubMed] [Google Scholar]
- 59.Ribas-Maynou J, Yeste M, Becerra-Tomás N, Aston KI, James ER, Salas-Huetos A. Clinical implications of sperm DNA damage in IVF and ICSI: updated systematic review and meta-analysis. Biol Rev Camb Philos Soc. 2021;96:1284–1300. doi: 10.1111/brv.12700. [DOI] [PubMed] [Google Scholar]
- 60.McQueen DB, Zhang J, Robins JC. Sperm DNA fragmentation and recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2019;112:54–60.e3. doi: 10.1016/j.fertnstert.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Agarwal A, Farkouh A, Parekh N, Zini A, Arafa M, Kandil H, et al. Sperm DNA fragmentation: a critical assessment of clinical practice guidelines. World J Mens Health. 2022;40:30–37. doi: 10.5534/wjmh.210056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cho CL, Agarwal A. Role of sperm DNA fragmentation in male factor infertility: a systematic review. Arab J Urol. 2017;16:21–34. doi: 10.1016/j.aju.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeremias JT, Belardin LB, Okada FK, Antoniassi MP, Fraietta R, Bertolla RP, et al. Oxidative origin of sperm DNA fragmentation in the adult varicocele. Int Braz J Urol. 2021;47:275–283. doi: 10.1590/S1677-5538.IBJU.2019.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol. 2013;10:26–37. doi: 10.1038/nrurol.2012.198. [DOI] [PubMed] [Google Scholar]
- 65.Ni K, Steger K, Yang H, Wang H, Hu K, Zhang T, et al. A comprehensive investigation of sperm DNA damage and oxidative stress injury in infertile patients with subclinical, normozoospermic, and astheno/oligozoospermic clinical varicocoele. Andrology. 2016;4:816–824. doi: 10.1111/andr.12210. [DOI] [PubMed] [Google Scholar]
- 66.Lira Neto FT, Roque M, Esteves SC. Effect of varicocelectomy on sperm deoxyribonucleic acid fragmentation rates in infertile men with clinical varicocele: a systematic review and meta-analysis. Fertil Steril. 2021;116:696–712. doi: 10.1016/j.fertnstert.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 67.Qiu D, Shi Q, Pan L. Efficacy of varicocelectomy for sperm DNA integrity improvement: a meta-analysis. Andrologia. 2021;53:e13885. doi: 10.1111/and.13885. [DOI] [PubMed] [Google Scholar]
- 68.Lara-Cerrillo S, Gual-Frau J, Benet J, Abad C, Prats J, Amengual MJ, et al. Microsurgical varicocelectomy effect on sperm telomere length, DNA fragmentation and seminal parameters. Hum Fertil (Camb) 2022;25:135–141. doi: 10.1080/14647273.2019.1711204. [DOI] [PubMed] [Google Scholar]
- 69.Fathi A, Mohamed O, Mahmoud O, Alsagheer GA, Reyad AM, Abolyosr A, et al. The impact of varicocelectomy on sperm DNA fragmentation and pregnancy rate in subfertile men with normal semen parameters: a pilot study. Arab J Urol. 2021;19:186–190. doi: 10.1080/2090598X.2021.1889746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smit M, Romijn JC, Wildhagen MF, Veldhoven JL, Weber RF, Dohle GR. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol. 2010;183:270–274. doi: 10.1016/j.juro.2009.08.161. [DOI] [PubMed] [Google Scholar]
- 71.Esteves SC, Roque M, Agarwal A. Outcome of assisted reproductive technology in men with treated and untreated varicocele: systematic review and meta-analysis. Asian J Androl. 2016;18:254–258. doi: 10.4103/1008-682X.163269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sadek A, Almohamdy AS, Zaki A, Aref M, Ibrahim SM, Mostafa T. Sperm chromatin condensation in infertile men with varicocele before and after surgical repair. Fertil Steril. 2011;95:1705–1708. doi: 10.1016/j.fertnstert.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Tiseo BC, Esteves SC, Cocuzza MS. Summary evidence on the effects of varicocele treatment to improve natural fertility in subfertile men. Asian J Androl. 2016;18:239–245. doi: 10.4103/1008-682X.172639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baker K, McGill J, Sharma R, Agarwal A, Sabanegh E., Jr Pregnancy after varicocelectomy: impact of postoperative motility and DFI. Urology. 2013;81:760–766. doi: 10.1016/j.urology.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 75.García-Peiró A, Ribas-Maynou J, Oliver-Bonet M, Navarro J, Checa MA, Nikolaou A, et al. Multiple determinations of sperm DNA fragmentation show that varicocelectomy is not indicated for infertile patients with subclinical varicocele. Biomed Res Int. 2014;2014:181396. doi: 10.1155/2014/181396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Agarwal A, Majzoub A, Esteves SC, Ko E, Ramasamy R, Zini A. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol. 2016;5:935–950. doi: 10.21037/tau.2016.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Majzoub A, Agarwal A, Esteves SC. Antioxidants for elevated sperm DNA fragmentation: a mini review. Transl Androl Urol. 2017;6(Suppl 4):S649–S653. doi: 10.21037/tau.2017.07.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Humaidan P, Haahr T, Povlsen BB, Kofod L, Laursen RJ, Alsbjerg B, et al. The combined effect of lifestyle intervention and antioxidant therapy on sperm DNA fragmentation and seminal oxidative stress in IVF patients: a pilot study. Int Braz J Urol. 2022;48:131–156. doi: 10.1590/S1677-5538.IBJU.2021.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alahmar AT. Role of oxidative stress in male infertility: an updated review. J Hum Reprod Sci. 2019;12:4–18. doi: 10.4103/jhrs.JHRS_150_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Panner Selvam MK, Ambar RF, Agarwal A, Henkel R. Etiologies of sperm DNA damage and its impact on male infertility. Andrologia. 2021;53:e13706. doi: 10.1111/and.13706. [DOI] [PubMed] [Google Scholar]
- 81.Bundhun PK, Janoo G, Bhurtu A, Teeluck AR, Soogund MZS, Pursun M, et al. Tobacco smoking and semen quality in infertile males: a systematic review and meta-analysis. BMC Public Health. 2019;19:36. doi: 10.1186/s12889-018-6319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Omolaoye TS, El Shahawy O, Skosana BT, Boillat T, Loney T, du Plessis SS. The mutagenic effect of tobacco smoke on male fertility. Environ Sci Pollut Res Int. 2022;29:62055–62066. doi: 10.1007/s11356-021-16331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li KP, Yang XS, Wu T. The effect of antioxidants on sperm quality parameters and pregnancy rates for idiopathic male infertility: a network meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne) 2022;13:810242. doi: 10.3389/fendo.2022.810242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alahmar AT, Calogero AE, Singh R, Cannarella R, Sengupta P, Dutta S. Coenzyme Q10, oxidative stress, and male infertility: a review. Clin Exp Reprod Med. 2021;48:97–104. doi: 10.5653/cerm.2020.04175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Majzoub A, Agarwal A. Systematic review of antioxidant types and doses in male infertility: benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab J Urol. 2018;16:113–124. doi: 10.1016/j.aju.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuroda S, Agarwal A, Saleh R, Finelli R, Shah R. Antioxidant therapy for male infertility – 3 month or 6 months: which is better? Fertil Steril. 2021;116(3 Suppl):E352 [Google Scholar]
- 87.Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. 2011;26:1628–1640. doi: 10.1093/humrep/der132. [DOI] [PubMed] [Google Scholar]
- 88.Steiner AZ, Hansen KR, Barnhart KT, Cedars MI, Legro RS, Diamond MP, et al. Reproductive Medicine Network. The effect of antioxidants on male factor infertility: the males, antioxidants, and infertility (MOXI) randomized clinical trial. Fertil Steril. 2020;113:552–560.e3. doi: 10.1016/j.fertnstert.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Henkel R, Sandhu IS, Agarwal A. The excessive use of antioxidant therapy: a possible cause of male infertility? Andrologia. 2019;51:e13162. doi: 10.1111/and.13162. [DOI] [PubMed] [Google Scholar]
- 90.Torres-Arce E, Vizmanos B, Babio N, Márquez-Sandoval F, Salas-Huetos A. Dietary antioxidants in the treatment of male infertility: counteracting oxidative stress. Biology (Basel) 2021;10:241. doi: 10.3390/biology10030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93:1027–1036. doi: 10.1016/j.fertnstert.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 92.Leduc F, Nkoma GB, Boissonneault G. Spermiogenesis and DNA repair: a possible etiology of human infertility and genetic disorders. Syst Biol Reprod Med. 2008;54:3–10. doi: 10.1080/19396360701876823. [DOI] [PubMed] [Google Scholar]
- 93.Walczak-Jedrzejowska R, Slowikowska-Hilczer J, Marchlewsk K, Oszukowska E, Kula K. During seminiferous tubule maturation testosterone and synergistic action of FSH with estradiol support germ cell survival while estradiol alone has pro-apoptotic effect. Folia Histochem Cytobiol. 2007;45 Suppl 1:S59–S64. [PubMed] [Google Scholar]
- 94.Muratori M, Baldi E. Effects of FSH on sperm DNA fragmentation: review of clinical studies and possible mechanisms of action. Front Endocrinol (Lausanne) 2018;9:734. doi: 10.3389/fendo.2018.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruvolo G, Roccheri MC, Brucculeri AM, Longobardi S, Cittadini E, Bosco L. Lower sperm DNA fragmentation after r-FSH administration in functional hypogonadotropic hypogonadism. J Assist Reprod Genet. 2013;30:497–503. doi: 10.1007/s10815-013-9951-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Colacurci N, Monti MG, Fornaro F, Izzo G, Izzo P, Trotta C, et al. Recombinant human FSH reduces sperm DNA fragmentation in men with idiopathic oligoasthenoteratozoospermia. J Androl. 2012;33:588–593. doi: 10.2164/jandrol.111.013326. [DOI] [PubMed] [Google Scholar]
- 97.Simoni M, Santi D, Negri L, Hoffmann I, Muratori M, Baldi E, et al. Treatment with human, recombinant FSH improves sperm DNA fragmentation in idiopathic infertile men depending on the FSH receptor polymorphism p.N680S: a pharmacogenetic study. Hum Reprod. 2016;31:1960–1969. doi: 10.1093/humrep/dew167. [DOI] [PubMed] [Google Scholar]
- 98.Kooshesh L, Bahmanpour S, Zeighami S, Nasr-Esfahani MH. Effect of Letrozole on sperm parameters, chromatin status and ROS level in idiopathic oligo/astheno/teratozoospermia. Reprod Biol Endocrinol. 2020;18:47. doi: 10.1186/s12958-020-00591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Santi D, Spaggiari G, Simoni M. Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management - meta-analyses. Reprod Biomed Online. 2018;37:315–326. doi: 10.1016/j.rbmo.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 100.Sugihara A, Van Avermaete F, Roelant E, Punjabi U, De Neubourg D. The role of sperm DNA fragmentation testing in predicting intra-uterine insemination outcome: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;244:8–15. doi: 10.1016/j.ejogrb.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 101.Siddhartha N, Reddy NS, Pandurangi M, Muthusamy T, Vembu R, Kasinathan K. The effect of sperm DNA fragmentation index on the outcome of intrauterine insemination and intracytoplasmic sperm injection. J Hum Reprod Sci. 2019;12:189–198. doi: 10.4103/jhrs.JHRS_22_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang L, Rao M, Yang W, Yao Y, Luo Q, Lu L, et al. Predictive value of the sperm DNA fragmentation index for low or failed IVF fertilization in men with mild-to-moderate asthenozoospermia. J Gynecol Obstet Hum Reprod. 2021;50:101868. doi: 10.1016/j.jogoh.2020.101868. [DOI] [PubMed] [Google Scholar]
- 103.Li Z, Wang L, Cai J, Huang H. Correlation of sperm DNA damage with IVF and ICSI outcomes: a systematic review and meta-analysis. J Assist Reprod Genet. 2006;23:367–376. doi: 10.1007/s10815-006-9066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao J, Zhang Q, Wang Y, Li Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril. 2014;102:998–1005.e8. doi: 10.1016/j.fertnstert.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Z, Zhu L, Jiang H, Chen H, Chen Y, Dai Y. Sperm DNA fragmentation index and pregnancy outcome after IVF or ICSI: a meta-analysis. J Assist Reprod Genet. 2015;32:17–26. doi: 10.1007/s10815-014-0374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod. 2008;23:2663–2668. doi: 10.1093/humrep/den321. [DOI] [PubMed] [Google Scholar]
- 107.Osman A, Alsomait H, Seshadri S, El-Toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online. 2015;30:120–127. doi: 10.1016/j.rbmo.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 108.Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–179. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 109.Khalafalla K, Majzoub A, Elbardisi H, Bhathella A, Chaudhari A, Agarwal A, et al. The effect of sperm DNA fragmentation on intracytoplasmic sperm injection outcome. Andrologia. 2021;53:e14180. doi: 10.1111/and.14180. [DOI] [PubMed] [Google Scholar]
- 110.Le MT, Nguyen TV, Nguyen TTT, Nguyen HTT, Le DD, Nguyen VQH. Predictive significance of sperm DNA fragmentation testing in early pregnancy loss in infertile couples undergoing intracytoplasmic sperm injection. Res Rep Urol. 2021;13:313–323. doi: 10.2147/RRU.S315300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kabukçu C, Çil N, Çabuş U, Alataş E. Effect of ejaculatory abstinence period on sperm DNA fragmentation and pregnancy outcome of intrauterine insemination cycles: a prospective randomized study. Arch Gynecol Obstet. 2021;303:269–278. doi: 10.1007/s00404-020-05783-0. [DOI] [PubMed] [Google Scholar]
- 112.Choavaratana R, Thanaboonyawat I, Chongpensuklert Y, Prechapanich J, Phornwilardsiri S, Stavroula Maria M, et al. Additional hyaluronic acid binding selection decreased sperm DNA damage after conventional semen preparation in infertile patient with abnormal semen analysis. Siriraj Med J. 2018;70:213–218. [Google Scholar]
- 113.Huszar G, Ozkavukcu S, Jakab A, Celik-Ozenci C, Sati GL, Cayli S. Hyaluronic acid binding ability of human sperm reflects cellular maturity and fertilizing potential: selection of sperm for intracytoplasmic sperm injection. Curr Opin Obstet Gynecol. 2006;18:260–267. doi: 10.1097/01.gco.0000193018.98061.2f. [DOI] [PubMed] [Google Scholar]
- 114.Parmegiani L, Cognigni GE, Bernardi S, Troilo E, Ciampaglia W, Filicori M. "Physiologic ICSI": hyaluronic acid (HA) favors selection of spermatozoa without DNA fragmentation and with normal nucleus, resulting in improvement of embryo quality. Fertil Steril. 2010;93:598–604. doi: 10.1016/j.fertnstert.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 115.Ali AH, Ajina T, Ali MB, Mehdi M. Efficacy of density gradient centrifugation technique (DGC) in enhancing sperm cell DNA quality for assisted reproductive technique. Middle East Fertil Soc J. 2022;27:22 [Google Scholar]
- 116.Pacheco A, Blanco A, Bronet F, Cruz M, García-Fernández J, García-Velasco JA. Magnetic-activated cell sorting (MACS): a useful sperm-selection technique in cases of high levels of sperm DNA fragmentation. J Clin Med. 2020;9:3976. doi: 10.3390/jcm9123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nosrati R, Graham PJ, Zhang B, Riordon J, Lagunov A, Hannam TG, et al. Microfluidics for sperm analysis and selection. Nat Rev Urol. 2017;14:707–730. doi: 10.1038/nrurol.2017.175. [DOI] [PubMed] [Google Scholar]
- 118.Jakubik-Uljasz J, Gill K, Rosiak-Gill A, Piasecka M. Relationship between sperm morphology and sperm DNA dispersion. Transl Androl Urol. 2020;9:405–415. doi: 10.21037/tau.2020.01.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Campos LGA, Requejo LC, Miñano CAR, Orrego JD, Loyaga EC, Cornejo LG. Correlation between sperm DNA fragmentation index and semen parameters in 418 men seen at a fertility center. JBRA Assist Reprod. 2021;25:349–357. doi: 10.5935/1518-0557.20200079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Elbashir S, Magdi Y, Rashed A, Ibrahim MA, Edris Y, Abdelaziz AM. Relationship between sperm progressive motility and DNA integrity in fertile and infertile men. Middle East Fertil Soc J. 2018;23:195–198. [Google Scholar]
- 121.Nguyen HTT, Dang HNT, Nguyen TTT, Nguyen TV, Dang TC, Nguyen QHV, et al. Correlations between abnormalities of morphological details and DNA fragmentation in human sperm. Clin Exp Reprod Med. 2022;49:40–48. doi: 10.5653/cerm.2021.04777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang WL, Chang YK, Tung SY, Peng BH, Chang HC. Sperm motility is the best semen parameter to predict sperm DNA fragmentation. Urol Sci. 2021;32:157–163. [Google Scholar]
- 123.Esteves SC, Agarwal A, Majzoub A. Comparison of strategies to reduce sperm DNA fragmentation in couples undergoing ICSI. Transl Androl Urol. 2017;6(Suppl 4):S570–S573. doi: 10.21037/tau.2017.03.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Teixeira DM, Hadyme Miyague A, Barbosa MA, Navarro PA, Raine-Fenning N, Nastri CO, et al. Regular (ICSI) versus ultra-high magnification (IMSI) sperm selection for assisted reproduction. Cochrane Database Syst Rev. 2020;2:CD010167. doi: 10.1002/14651858.CD010167.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Avalos-Durán G, Cañedo-Del Ángel AME, Rivero-Murillo J, Zambrano-Guerrero JE, Carballo-Mondragón E, Checa-Vizcaíno MÁ. Physiological ICSI (PICSI) vs. conventional ICSI in couples with male factor: a systematic review. JBRA Assist Reprod. 2018;22:139–147. doi: 10.5935/1518-0557.20180027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.West R, Coomarasamy A, Frew L, Hutton R, Kirkman-Brown J, Lawlor M, et al. Sperm selection with hyaluronic acid improved live birth outcomes among older couples and was connected to sperm DNA quality, potentially affecting all treatment outcomes. Hum Reprod. 2022;37:1106–1125. doi: 10.1093/humrep/deac058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hasanen E, Elqusi K, ElTanbouly S, Hussin AE, AlKhadr H, Zaki H, et al. PICSI vs. MACS for abnormal sperm DNA fragmentation ICSI cases: a prospective randomized trial. J Assist Reprod Genet. 2020;37:2605–2613. doi: 10.1007/s10815-020-01913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Javed A, Mozafari F, Ashwini LS, Ganguly D. Commentary: physiological intracytoplasmic sperm injection (PICSI), an alternative to the standard ICSI procedure. MOJ Anat Physiol. 2015;1:43–45. [Google Scholar]
- 129.Wang M, Sun J, Wang L, Gao X, Lu X, Wu Z, et al. Assessment of density gradient centrifugation (DGC) and sperm chromatin dispersion (SCD) measurements in couples with male factor infertility undergoing ICSI. J Assist Reprod Genet. 2014;31:1655–1663. doi: 10.1007/s10815-014-0339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Muratori M, Tarozzi N, Carpentiero F, Danti S, Perrone FM, Cambi M, et al. Sperm selection with density gradient centrifugation and swim up: effect on DNA fragmentation in viable spermatozoa. Sci Rep. 2019;9:7492. doi: 10.1038/s41598-019-43981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee TH, Liu CH, Shih YT, Tsao HM, Huang CC, Chen HH, et al. Magnetic-activated cell sorting for sperm preparation reduces spermatozoa with apoptotic markers and improves the acrosome reaction in couples with unexplained infertility. Hum Reprod. 2010;25:839–846. doi: 10.1093/humrep/deq009. [DOI] [PubMed] [Google Scholar]
- 132.Degheidy T, Abdelfattah H, Seif A, Albuz FK, Gazi S, Abbas S. Magnetic activated-cell sorting: an effective method for reduction of sperm DNA fragmentation in varicocele men prior to assisted reproductive techniques. Andrologia. 2015;47:892–896. doi: 10.1111/and.12343. [DOI] [PubMed] [Google Scholar]
- 133.Gil Juliá M, Hervás I, Navarro-Gómez Lechón A, Quintana F, Amorós D, Pacheco A, et al. Sperm selection by magnetic-activated cell sorting before microinjection of autologous oocytes increases cumulative live birth rates with limited clinical impact: a retrospective study in unselected males. Biology (Basel) 2021;10:430. doi: 10.3390/biology10050430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Parrella A, Choi D, Keating D, Rosenwaks Z, Palermo GD. A microfluidic device for selecting the most progressively motile spermatozoa yields a higher rate of euploid embryos. Fertil Steril. 2018;110(4 Suppl):E342 [Google Scholar]
- 135.Samuel R, Feng H, Jafek A, Despain D, Jenkins T, Gale B. Microfluidic-based sperm sorting & analysis for treatment of male infertility. Transl Androl Urol. 2018;7(Suppl 3):S336–S347. doi: 10.21037/tau.2018.05.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Romany L, Garrido N, Motato Y, Aparicio B, Remohí J, Meseguer M. Removal of annexin V-positive sperm cells for intracytoplasmic sperm injection in ovum donation cycles does not improve reproductive outcome: a controlled and randomized trial in unselected males. Fertil Steril. 2014;102:1567–1575.e1. doi: 10.1016/j.fertnstert.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 137.Xie P, Keating D, Parrella A, Cheung S, Rosenwaks Z, Goldstein M, et al. Sperm genomic integrity by TUNEL varies throughout the male genital tract. J Urol. 2020;203:802–808. doi: 10.1097/JU.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 138.Esteves SC, Roque M, Bradley CK, Garrido N. Reproductive outcomes of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with high levels of DNA fragmentation in semen: systematic review and meta-analysis. Fertil Steril. 2017;108:456–467.e1. doi: 10.1016/j.fertnstert.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 139.Ollero M, Gil-Guzman E, Lopez MC, Sharma RK, Agarwal A, Larson K, et al. Characterization of subsets of human spermatozoa at different stages of maturation: implications in the diagnosis and treatment of male infertility. Hum Reprod. 2001;16:1912–1921. doi: 10.1093/humrep/16.9.1912. [DOI] [PubMed] [Google Scholar]
- 140.Zhang J, Xue H, Qiu F, Zhong J, Su J. Testicular spermatozoon is superior to ejaculated spermatozoon for intracytoplasmic sperm injection to achieve pregnancy in infertile males with high sperm DNA damage. Andrologia. 2019;51:e13175. doi: 10.1111/and.13175. [DOI] [PubMed] [Google Scholar]
- 141.Herrero MB, Lusignan MF, Son WY, Sabbah M, Buckett W, Chan P. ICSI outcomes using testicular spermatozoa in non-azoospermic couples with recurrent ICSI failure and no previous live births. Andrology. 2019;7:281–287. doi: 10.1111/andr.12591. [DOI] [PubMed] [Google Scholar]
- 142.Ambar RF, Agarwal A, Majzoub A, Vij S, Tadros NN, Cho CL, et al. The use of testicular sperm for intracytoplasmic sperm injection in patients with high sperm DNA damage: a systematic review. World J Mens Health. 2021;39:391–398. doi: 10.5534/wjmh.200084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moskovtsev SI, Alladin N, Lo KC, Jarvi K, Mullen JB, Librach CL. A comparison of ejaculated and testicular spermatozoa aneuploidy rates in patients with high sperm DNA damage. Syst Biol Reprod Med. 2012;58:142–148. doi: 10.3109/19396368.2012.667504. [DOI] [PubMed] [Google Scholar]
- 144.Cheung S, Schlegel PN, Rosenwaks Z, Palermo GD. Revisiting aneuploidy profile of surgically retrieved spermatozoa by whole exome sequencing molecular karyotype. PLoS One. 2019;14:e0210079. doi: 10.1371/journal.pone.0210079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Esteves SC, Miyaoka R, Agarwal A. Sperm retrieval techniques for assisted reproduction. Int Braz J Urol. 2011;37:570–583. doi: 10.1590/s1677-55382011000500002. [DOI] [PubMed] [Google Scholar]
- 146.Green KA, Patounakis G, Dougherty MP, Werner MD, Scott RT, Jr, Franasiak JM. Sperm DNA fragmentation on the day of fertilization is not associated with embryologic or clinical outcomes after IVF/ICSI. J Assist Reprod Genet. 2020;37:71–76. doi: 10.1007/s10815-019-01632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Agarwal A, Farkouh A, Saleh R, Abdel-Meguid TA, Harraz AM, Kavoussi P, et al. Controversy and consensus on indications for sperm DNA fragmentation testing in male infertility: A global survey, current guidelines, and expert recommendations. World J Mens Health. 2023 doi: 10.5534/wjmh.220282. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data on management of elevated SDF (Q33–60)
Advanced analysis of selected questions on the management of elevated SDF
Complete methodology with CHERRIES checklist
Complete survey questions and invitation
Complete demographic data