This cohort study assesses the risk of fractures associated with sodium-glucose cotransporter 2 inhibitors vs incretin-based drugs of dipeptidyl-peptidase 4 inhibitors and glucagon-like peptide 1 receptor agonists, separately, in postmenopausal individuals with type 2 diabetes.
Key Points
Question
Do sodium-glucose cotransporter 2 inhibitors (SGLT2i) increase the risk of fractures among postmenopausal individuals with type 2 diabetes?
Findings
In this Korean nationwide cohort study among the population susceptible for bone fragility, SGLT2i was not associated with the increased risk of fractures compared with the incretin-based drugs of dipeptidyl-peptidase 4 inhibitors and glucagon-like peptide 1 receptor agonists, separately.
Meaning
In this study, SGLT2i use was associated with either similar or lower risks of fractures than incretin-based drugs even in a population with high risk of fractures.
Abstract
Importance
Postmenopausal individuals with type 2 diabetes are susceptible to fractures due to the interaction of elevated blood glucose levels and a deficiency of the hormone estrogen. Despite continued concerns of fracture risks associated with sodium-glucose cotransporter 2 inhibitors (SGLT2i), existing evidence in this high-risk population is lacking.
Objective
To assess the risk of fractures associated with SGLT2i vs incretin-based drugs of dipeptidyl-peptidase 4 inhibitors (DPP4i) and glucagon-like peptide 1 receptor agonists (GLP1RA), separately, in postmenopausal individuals with type 2 diabetes.
Design, Setting, and Participants
This active-comparator, new-user cohort study used nationwide claims data of Korea and took place from January 1, 2013, to December 31, 2020. Postmenopausal individuals (aged ≥45 years) with type 2 diabetes were included.
Exposures
New users of SGLT2i or comparator drugs.
Main Outcomes and Measures
The primary outcome was overall fractures, comprising vertebral, hip, humerus, and distal radius fractures. Patients were followed up from the day after drug initiation until the earliest of outcome occurrence, drug discontinuation (90-day grace period) or switch, death, or end of the study period. After propensity score fine stratification, hazard ratios (HRs) with 95% CIs were estimated using weighted Cox models.
Results
Among 37 530 (mean [SD] age, 60.6 [9.7] years) and 332 004 (mean [SD] age, 60.6 [9.9] years) new users of SGLT2i and DPP4i, respectively, a lower rate of incident overall fractures was presented with SGLT2i vs DPP4i (weighted HR, 0.78; 95% CI, 0.72-0.84). Among 111 835 (mean [SD] age, 61.4 [9.8] years) and 8177 (mean [SD] age, 61.1 [10.3] years) new users of SGLT2i and GLP1RA, respectively, no association with an increased risk of overall fractures was presented with SGLT2i vs GLP1RA (weighted HR, 0.92; 95% CI, 0.68-1.24). Results from several subgroup and sensitivity analyses presented consistent results from main analysis.
Conclusions and relevance
This population-based cohort study suggests that SGLT2i was not associated with an increased rate of incident fractures compared with DPP4i and GLP1RA, separately, among postmenopausal individuals with type 2 diabetes.
Introduction
Postmenopausal individuals are at high risk of fractures due to declining estrogen levels that disrupt the homeostasis of bone metabolism.1 If these individuals also have type 2 diabetes, risk of fractures could be further heightened given that type 2 diabetes itself is an independent risk factor for fractures2,3,4; it might be attributed to chronic hyperglycemia and accumulation of advanced glycation end products, subsequently leading to altered bone metabolism and skeletal fragility.5 Therefore, potential fracture risks should be carefully weighed when choosing an optimal pharmacologic regimen for glycemic control in this population.
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) have demonstrated substantial cardio-kidney benefits across several pivotal randomized clinical trials,6,7,8,9 and accordingly their usage in clinical practice is increasing.10 However, due to its unique glucose-controlling mechanism through kidney proximal tubules, SGLT2i may also affect calcium and phosphate homeostasis to possibly pose harm to bone mineral density.11 In support, one particular landmark trial of SGLT2i7 reported significantly increased rate of incident fractures compared with placebo (hazard ratio [HR], 1.26; 95% CI, 1.04-1.52). However, recent meta-analyses of trials have found no significant associations between SGLT2i and fractures.12,13,14 While several observational studies on this clinical topic are present,15,16,17,18,19 none to our knowledge have specifically assessed the association between SGLT2i use and fracture risk in postmenopausal individuals.
Given the current knowledge gap, we aimed to evaluate whether the use of SGLT2i is associated with an increased risk of fractures in a population that carries a greater underlying risk of bone fragility. Accordingly, we conducted a nationwide, population-based cohort study by comparing SGLT2i with incretin-based drugs in 2 independent cohorts of (1) SGLT2i vs dipeptidyl peptidase 4 inhibitors (DPP4i) and (2) SGLT2i vs glucagon-like peptide 1 receptor agonists (GLP1RA).
Methods
Data Source
We used health administrative claims data (January 1, 2013, to December 31, 2020) obtained from the National Health Insurance Service, a single provider for health insurance in South Korea. The national health insurance database contains health insurance claims data for roughly 97% of the entire Korean population (>50 million). Sociodemographic variables such as age, sex, residence, income level, eligibility status, and health insurance types are included. Health care utilization information such as diagnosis, prescription, medical procedures, and health examinations records are also available. Diagnosis records are coded according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, and drugs are coded based on a domestic drug chemical code that is mapped to the Anatomical Therapeutic Chemical classification of the World Health Organization. This study was approved by the institutional review board of Sungkyunkwan University, where requirement of informed consent was waived as this study used anonymized administrative data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.20
Study Population
We selected 2 active comparators for comparison with the SGLT2i and constructed 2 separate cohorts accordingly. Each cohort was constructed independently with patients with type 2 diabetes (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes E11-E14), and all female individuals 45 years or older were included. We defined age of 45 years as a criterion for menopause based on the postmenopausal individuals definition of the Canagliflozin Cardiovascular Assessment Study (CANVAS) trial. Initially, we identified all individuals who initiated a SGLT2i or a comparator incretin-based drug (DPP4i or GLP1RA for each cohort) during the study period. The index date was defined as the date of the first prescription of either SGLT2i or a comparator incretin-based drug, whichever came first, from September 1, 2014 (ie, first date of SGLT2i reimbursement in Korea), to December 31, 2020; the forementioned drug classes were reimbursed exclusively for type 2 diabetes during this study period of interest. Patients whose date of the first prescription of either SGLT2i or a comparator drug was before September 1, 2014, were excluded. In this way, SGLT2i initiators who had previously used the comparator drug were excluded, while patients initiating comparator drug were restricted to those without a prior history of SGLT2i use.
Of these eligible individuals, we excluded those with severe kidney impairment (eg, end-stage kidney disease or received dialysis) within the year prior to the index date to consider for contraindication to SGLT2i. Since recurrences of fractures may not be independent, we further excluded patients who had records of fractures within the year prior to the index date. We also excluded patients with history of intensive care unit admission or diagnosis of cancer within the year prior to the index date. Last, we excluded patients who initiated both SGLT2i and a comparator incretin-based drug on the same date to avoid exposure misclassification (eFigures 1, 2, and 5 in Supplement 1).
Exposure and Follow-Up
The drug of interest was SGLT2i (dapagliflozin, empagliflozin, ipragliflozin, or ertugliflozin). In each cohort, we chose DPP4i (alogliptin, evogliptin, gemigliptin, linagliptin, saxagliptin, sitagliptin, teneligliptin, or vildagliptin) and GLP1RA (albiglutide, dulaglutide, exenatide, or lixisenatide) as the active comparator with SGLT2i as they share the same line of therapy with SGLT2i in type 2 diabetes (ie, second- or third-line antihyperglycemic drug). Sulfonylureas and thiazolidinediones were not selected as appropriate active comparators with SGLT2i given their possible nonneutral effects on fractures.
Applying an as-treated approach, patients were followed up from the index date until the earliest of outcome occurrence, treatment change (discontinuation, switching to, or adding the comparator drug), death, or end of the study period (December 31, 2020). We introduced a 90-day grace period to determine treatment discontinuation; thus, patients were considered as exposed until 90 days after the end of days’ supply.
Outcome Definition
The primary outcome was overall fractures, which comprised vertebral, hip, humerus, and distal radius fractures. Secondary outcomes were the individual components of the primary composite outcome. All outcomes were identified through diagnosis codes in the primary and secondary position, which have been previously reported to have a sensitivity of 63% to 93% and a positive predictive value of 60% to 97%.21 To further increase the validity of the outcomes, domestic procedure codes related to conservative therapy or procedure of each fracture sites were incorporated in sensitivity analyses (eg, stringent operational definition) (eTable 14 in Supplement 1).22,23,24,25,26,27
Covariates
We assessed calendar year and age (45-65, >65 years) on the index date and the number of antidiabetic medications (other than SGLT2i, incretin-based drugs) prescribed in the year prior to index date. We also defined levels of diabetes treatment into 3 levels depending on the number of antidiabetic medication (excluding the study drugs of interest), prescribed in the year preceding the index date: level 1, as taking none or only 1 class of antidiabetic medication other than insulin, level 2, as taking 2 or more different classes of antidiabetic medication without insulin, and level 3, as taking insulin with or without other classes of antidiabetic medication. Moreover, as proxies for health-seeking behavior, the number of outpatient visits and the number of hospitalizations were assessed within the year prior to the index date. Clinical characteristics including the Charlson Comorbidity Index, comorbidities, and comedications, were also assessed within the year prior to the index date. Comorbidities of interest, identified using relevant diagnostic codes (eTable 15 in Supplement 1), were diabetes-related conditions (nephropathy, neuropathy, retinopathy, and hypoglycemia), asthma, cancer, chronic kidney disease, congestive heart failure, chronic obstructive pulmonary disease, dementia, epilepsy, gout, hyperlipidemia, hypertension, ischemic heart disease, liver cirrhosis, osteoarthritis, osteoporosis, rheumatoid arthritis, stroke, and thyroid disease. Use of fall-related medications (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, anticholinergics, benzodiazepines, β-blockers, calcium channel blockers, diuretics, nitrates, opioids, sedative hypnotics, tricyclic antidepressant, and typical antipsychotics), osteoporosis medications (bisphosphonates, calcium/vitamin D, parathyroid hormone/calcitonin, receptor activator of nuclear factors kappa-B ligand inhibitors, and selective estrogen receptor modulators), and other comedications (anticoagulants, anticonvulsants, antidepressants, aromatase inhibitors, oral corticosteroids, hormone replacement therapy, immunosuppressants, nonsteroidal anti-inflammatory drugs, platelet inhibitors, proton-pump inhibitors, and statins) were also evaluated (eTable 16 in Supplement 1). Laboratory test results (missing rates, 27.9%-57.6%) from biennial health examination data were assessed within 3 years prior to the index date and included in the propensity score model for sensitivity analysis (eTable 10 in Supplement 1). Results of bone mineral density test using dual-energy x-ray absorptiometry were also assessed within 3 years prior to the index date among a subset of population.
Statistical Analysis
Descriptive statistics were used to compare patients’ baseline characteristics in each cohort. Continuous variables were presented as means and SDs, and categorial variables were summarized as frequency and proportions. We used the propensity score fine stratification weighting method28 to control for potential confounding within each cohort (SGLT2i vs DPP4i; SGLT2i vs GLP1RA). Multivariable logistic regression model was used to estimate the predicted probability of initiating SGLT2i vs incretin-based drugs (DPP4i or GLP1RA) given all baseline covariates mentioned above. Patients from nonoverlapping regions of propensity score distributions were trimmed to focus the estimation of treatment effects in a population with clinical equipoise. We created 50 strata based on the distribution of propensity score in the SGLT2i group. Within each stratum, patients exposed to SGLT2i were assigned a weight of 1, while patients exposed to comparator drugs were weighted in proportion to the number of SGLT2i-exposed patients in the stratum into which they fell; this would then measure the mean treatment effect among treated patients. Absolute standardized differences larger than 0.1 were considered as significant covariate imbalance between treatment groups. We also estimated postweighting C statistics that serves as a measure of balance (0.5 denotes balance) in aggregate over all included covariates. Weighted incidence rates based on the Poisson distribution were estimated for study outcomes by dividing the number of weighted events by the total number of weighted 100 patients-years at risk. Weighted Kaplan-Meier plots were used to visualize cumulative incidence of the primary outcome (eFigure 3 in Supplement 1). Weighted Cox proportional hazard models were used to estimate weighted HRs and 95% CIs for the risk of fractures associated with SGLT2i vs incretin-based drugs.
Subgroup analyses were conducted to test for potential effect modification. First, we stratified on age (45-60, 61-75, >75 years) to identify whether higher age cutoffs for menopause modify effect estimates. Second, we also stratified on history of osteoporosis or use of osteoporosis medications, history of osteoarthritis, history of neurological dysfunction, and prior use of drugs that increase fracture risks (thiazolidinediones, proton pump inhibitor, systemic corticosteroid, selective serotonin reuptake inhibitor). Third, we examined the effect of individual SGLT2i associated with fractures. In all subgroup analyses, propensity score were reestimated. P values for interaction less than .05 were used to denote a significant heterogeneity among subgroups.
Range of sensitivity analyses were conducted for the primary outcome to assess the robustness of main analysis. First, we repeated the main analysis among patients with a history of hysterectomy or ovarian resection (eg, conditions that lead to surgical menopause) any time before the index date or patients with a history of hormone replacement therapy any time before the index date to increase the validity of age-based definition. Second, we repeated the main analysis including laboratory test results as covariates for the propensity score model. Third, we extended the assessment period for baseline comorbidities to include any time before the index date. Fourth, we varied the length of the grace period to 30- and 60-day periods to consider potential exposure misclassifications. Fifth, we adopted a more stringent outcome definition by using domestic procedural codes related to conservative therapy or procedures of each fracture sites to minimize outcome misclassification. Sixth, to indirectly assess the presence of potential unmeasured confounding, we used 2 control outcomes: (1) herpes zoster virus infection as a negative control outcome, where a null association was expected and (2) hospitalization for heart failure as a positive control outcome, where a lower risk with SGLT2i was anticipated. These analyses were based on the same cohort as the one used in the main analysis but involved excluding patients diagnosed with herpes zoster virus infection or heart failure a year prior to the index date. Finally, we repeated the main analysis among a subset of participants with bone mineral density test results, including the results as covariates for the propensity score model. All statistical analyses were conducted with SAS software, version 9.4 (SAS Institute Inc).
Results
A total of 369 570 patients were selected for the first cohort: 37 532 patients initiating SGLT2i (mean [SD] age, 60.6 [9.7] years) and 332 038 patients initiating DPP4i (mean [SD] age, 66.0 [11] years) (eFigure 1 in Supplement 1). Regarding the second cohort, a total of 121 803 patients were selected: 113 622 patients initiating SGLT2i (mean [SD] age, 61.4 [9.8] years) and 8181 patients initiating GLP1RA (mean [SD] age, 62.5 [10.2] years) (eFigure 2 in Supplement 1). Patients in the second cohort presented a higher prevalence of diabetes-related conditions, insulin usage, and a greater number of diabetic medications taken, indicating a generally more severe diabetic profile compared with the first cohort (eTable 1 in Supplement 1).
After propensity score weighting and trimming, the first cohort had 37 530 patients initiating SGLT2i and 332 004 patients initiating DPP4i, whereas the second cohort had 111 835 patients initiating SGLT2i and 8177 patients initiating GLP1RA. In both cohorts, the exposure and comparator groups were well balanced, with absolute standardized differences for all baseline covariates less than 0.10 after propensity score weighting (Table 1). The overall balance was further confirmed using the postweighting C statistics (0.504 for the first cohort, 0.567 for the second cohort) (eFigure 5 in Supplement 1).
Table 1. Baseline Characteristics of Patients Who Received SGLT2i or Comparator Drugs After Weighting by Propensity Score Fine Stratification.
| Baseline characteristic | Patients, No. (%) | |||||
|---|---|---|---|---|---|---|
| SGLT2i vs DPP4i | SGLT2i vs GLP1RA | |||||
| SGLT2i (n = 37 530) | DPP4i (n = 332 004) | ASD | SGLT2i (n = 111 835) | GLP1 RA (n = 8177) | ASD | |
| Cohort entry year | ||||||
| 2014 | 1232 (3.3) | 10 957 (3.3) | 0.001 | 2499 (2.2) | 166 (2) | 0.014 |
| 2015 | 3921 (10.4) | 34 628 (10.4) | 0.001 | 9419 (8.4) | 714 (8.7) | 0.011 |
| 2016 | 5220 (13.9) | 46 175 (13.9) | <0.001 | 16 845 (15.1) | 1225 (15) | 0.002 |
| 2017 | 6310 (16.8) | 55 960 (16.9) | 0.001 | 19 557 (17.5) | 1545 (18.9) | 0.036 |
| 2018 | 5815 (15.5) | 51 432 (15.5) | <0.001 | 18 336 (16.4) | 1286 (15.7) | 0.018 |
| 2019 | 7414 (19.8) | 65 512 (19.7) | 0.001 | 23 212 (20.8) | 1663 (20.3) | 0.01 |
| 2020 | 7618 (20.3) | 67 340 (20.3) | <0.001 | 21 967 (19.6) | 1579 (19.3) | 0.008 |
| Age, mean (SD), y | 60.6 (9.7) | 60.6 (9.9) | 0.001 | 61.4 (9.8) | 61.1 (10.3) | 0.039 |
| Age group | ||||||
| 45-65 | 26 760 (71.3) | 237 418 (71.5) | 0.001 | 75 939 (67.9) | 5613 (68.6) | 0.016 |
| >65 | 10 770 (28.7) | 94 626 (28.5) | 35 896 (32.1) | 2564 (31.4) | ||
| Diabetic medications | ||||||
| Alpha-glucosidase inhibitors | 1141 (3) | 10 117 (3) | <0.001 | 3141 (2.8) | 259 (3.2) | 0.021 |
| GLP1RA | 126 (0.3) | 868 (0.3) | 0.014 | NA | NA | NA |
| DPP4i | NA | NA | NA | 68 426 (61.2) | 4864 (59.5) | 0.035 |
| Insulin | 3258 (8.7) | 28 607 (8.6) | 0.002 | 15 717 (14.1) | 1265 (15.5) | 0.04 |
| Meglitinides | 191 (0.5) | 1681 (0.5) | <0.001 | 580 (0.5) | 49 (0.6) | 0.011 |
| Metformin | 21 188 (56.5) | 187 378 (56.4) | <0.001 | 90 052 (80.5) | 6372 (77.9) | 0.064 |
| Sulfonylureas | 9388 (25) | 83 027 (25) | <0.001 | 53 286 (47.6) | 4034 (49.3) | 0.034 |
| Thiazolidinediones | 2708 (7.2) | 23 821 (7.2) | 0.002 | 15 904 (14.2) | 1264 (15.5) | 0.035 |
| No. of diabetic medications being taken | ||||||
| 0-1 | 26 469 (70.5) | 234 486 (70.6) | 0.002 | 29 365 (26.3) | 2197 (26.9) | 0.014 |
| 2-3 | 10 752 (28.6) | 94 809 (28.6) | 0.002 | 69 311 (62) | 4851 (59.3) | 0.054 |
| ≥4 | 309 (0.8) | 2709 (0.8) | 0.001 | 13 159 (11.8) | 1129 (13.8) | 0.061 |
| Level of diabetes treatmenta | ||||||
| 1 | 25 926 (69.1) | 229 636 (69.2) | 0.002 | 28 629 (25.6) | 2144 (26.2) | 0.014 |
| 2 | 8346 (22.2) | 73 761 (22.2) | 0.001 | 67 489 (60.3) | 4767 (58.3) | 0.042 |
| 3 | 3258 (8.7) | 28 607 (8.6) | 0.002 | 15 717 (14.1) | 1265 (15.5) | 0.04 |
| Diabetes related conditions | ||||||
| Diabetic nephropathy | 1148 (3.1) | 10 107 (3) | 0.001 | 5811 (5.2) | 401 (4.9) | 0.014 |
| Diabetic neuropathy | 4595 (12.2) | 40 551 (12.2) | 0.001 | 20 639 (18.5) | 1543 (18.9) | 0.011 |
| Diabetic retinopathy | 5730 (15.3) | 50 450 (15.2) | 0.002 | 25 723 (23) | 1818 (22.2) | 0.018 |
| Hypoglycemia | 130 (0.3) | 1154 (0.3) | <0.001 | 543 (0.5) | 34 (0.4) | 0.01 |
| Charlson Comorbidity Index | ||||||
| 0 | 19 584 (52.2) | 173 208 (52.2) | <0.001 | 49 318 (44.1) | 3450 (42.2) | 0.039 |
| 1 | 10 586 (28.2) | 93 614 (28.2) | <0.001 | 40 709 (36.4) | 3116 (38.1) | 0.035 |
| 2 | 4385 (11.7) | 38 826 (11.7) | <0.001 | 10 796 (9.7) | 782 (9.6) | 0.003 |
| ≥3 | 2975 (7.9) | 26 356 (7.9) | <0.001 | 11 012 (9.8) | 830 (10.2) | 0.01 |
| No. of outpatients visits | ||||||
| 0-2 | 1038 (2.8) | 9264 (2.8) | 0.002 | 1451 (1.3) | 177 (2.2) | 0.067 |
| 3-5 | 1738 (4.6) | 15 415 (4.6) | 0.001 | 3034 (2.7) | 231 (2.8) | 0.007 |
| ≥6 | 34 754 (92.6) | 307 325 (92.6) | 0.001 | 107 350 (96) | 7769 (95) | 0.047 |
| No. of hospitalizations | ||||||
| 0 | 30 118 (80.3) | 266 471 (80.3) | <0.001 | 85 439 (76.4) | 5882 (71.9) | 0.102 |
| 1-2 | 6815 (18.2) | 60 230 (18.1) | <0.001 | 23 680 (21.2) | 2060 (25.2) | 0.095 |
| ≥3 | 597 (1.6) | 5303 (1.6) | 0.001 | 2716 (2.4) | 235 (2.9) | 0.028 |
| Comorbidities | ||||||
| Asthma | 3249 (8.7) | 28 665 (8.6) | 0.001 | 9514 (8.5) | 745 (9.1) | 0.021 |
| Chronic kidney disease | 201 (0.5) | 1830 (0.6) | 0.002 | 1160 (1) | 97 (1.2) | 0.014 |
| Congestive heart failure | 1481 (3.9) | 13 012 (3.9) | 0.001 | 4769 (4.3) | 310 (3.8) | 0.024 |
| COPD | 2036 (5.4) | 18 027 (5.4) | <0.001 | 6164 (5.5) | 528 (6.5) | 0.04 |
| Dementia | 1001 (2.7) | 8994 (2.7) | 0.003 | 3701 (3.3) | 292 (3.6) | 0.014 |
| Epilepsy | 227 (0.6) | 2011 (0.6) | <0.001 | 685 (0.6) | 54 (0.7) | 0.006 |
| Gout | 260 (0.7) | 2314 (0.7) | 0.001 | 823 (0.7) | 54 (0.7) | 0.009 |
| Hyperlipidemia | 16 975 (45.2) | 149 714 (45.1) | 0.003 | 52 526 (47) | 3772 (46.1) | 0.017 |
| Hypertension | 20 253 (54) | 178 733 (53.8) | 0.003 | 60 294 (53.9) | 4389 (53.7) | 0.005 |
| Ischemic heart disease | 3273 (8.7) | 28 763 (8.7) | 0.002 | 11 078 (9.9) | 758 (9.3) | 0.022 |
| Liver cirrhosis | 144 (0.4) | 1289 (0.4) | 0.001 | 635 (0.6) | 48 (0.6) | 0.003 |
| Osteoarthritis | 12 546 (33.4) | 110 917 (33.4) | <0.001 | 38 494 (34.4) | 2830 (34.6) | 0.004 |
| Osteoporosis | 3268 (8.7) | 28 820 (8.7) | 0.001 | 9972 (8.9) | 682 (8.3) | 0.02 |
| Parkinson disease | 129 (0.3) | 1165 (0.4) | 0.001 | 497 (0.4) | 45 (0.6) | 0.015 |
| Rheumatoid arthritis | 778 (2.1) | 6901 (2.1) | <0.001 | 2363 (2.1) | 178 (2.2) | 0.004 |
| Stroke | 1068 (2.8) | 9506 (2.9) | 0.001 | 3902 (3.5) | 289 (3.5) | 0.003 |
| Thyroid disease | 2951 (7.9) | 25 947 (7.8) | 0.002 | 8143 (7.3) | 634 (7.8) | 0.018 |
| Fall-related medications | ||||||
| ACE inhibitors or ARBs | 19 035 (50.7) | 167 677 (50.5) | 0.004 | 60 846 (54.4) | 4471 (54.7) | 0.005 |
| Anticholinergics | 30 173 (80.4) | 266 797 (80.4) | 0.001 | 91 291 (81.6) | 6711 (82.1) | 0.011 |
| Benzodiazepines | 13 565 (36.1) | 119 957 (36.1) | <0.001 | 41 469 (37.1) | 3061 (37.4) | 0.007 |
| β-Blockers | 1848 (4.9) | 16 290 (4.9) | 0.001 | 5512 (4.9) | 398 (4.9) | 0.003 |
| Calcium channel blockers | 14 617 (38.9) | 128 939 (38.8) | 0.002 | 43 791 (39.2) | 3177 (38.9) | 0.006 |
| Diuretics | 9571 (25.5) | 84 374 (25.4) | 0.002 | 28 869 (25.8) | 2205 (27) | 0.026 |
| Nitrates | 470 (1.3) | 4120 (1.2) | 0.001 | 1643 (1.5) | 86 (1.1) | 0.037 |
| Opioids | 2875 (7.7) | 25 364 (7.6) | 0.001 | 9612 (8.6) | 914 (11.2) | 0.087 |
| Sedative hypnotics | 3976 (10.6) | 35 143 (10.6) | <0.001 | 12 507 (11.2) | 937 (11.5) | 0.009 |
| Tricyclic antidepressant | 2633 (7) | 23 285 (7) | <0.001 | 9056 (8.1) | 716 (8.8) | 0.024 |
| Typical antipsychotics | 1227 (3.3) | 10 837 (3.3) | <0.001 | 3875 (3.5) | 355 (4.3) | 0.045 |
| Osteoporosis medications | ||||||
| Bisphosphonates | 1737 (4.6) | 15 346 (4.6) | <0.001 | 5529 (4.9) | 320 (3.9) | 0.05 |
| Calcium/vitamin D | 2903 (7.7) | 25 547 (7.7) | 0.002 | 9346 (8.4) | 671 (8.2) | 0.005 |
| Parathyroid hormone/calcitonin | 108 (0.3) | 964 (0.3) | <0.001 | 384 (0.3) | 19 (0.2) | 0.021 |
| RANKL inhibitors | 134 (0.4) | 1181 (0.4) | <0.001 | 470 (0.4) | 32 (0.4) | 0.004 |
| SERMs | 440 (1.2) | 3882 (1.2) | <0.001 | 1348 (1.2) | 78 (1) | 0.024 |
| Comedications | ||||||
| Anticoagulants | 1287 (3.4) | 11 337 (3.4) | 0.001 | 4617 (4.1) | 398 (4.9) | 0.036 |
| Anticonvulsants | 4301 (11.5) | 37 984 (11.4) | 0.001 | 17 293 (15.5) | 1358 (16.6) | 0.031 |
| Antidepressants | 2806 (7.5) | 24 661 (7.4) | 0.002 | 9172 (8.2) | 815 (10) | 0.061 |
| Aromatase inhibitors | 2 (0) | 16 (0) | 0.001 | 0 (0) | 0 (0) | NA |
| Oral corticosteroids | 14 825 (39.5) | 131 199 (39.5) | <0.001 | 41 631 (37.2) | 3136 (38.4) | 0.023 |
| Hormone replacement therapy | 2180 (5.8) | 19 339 (5.8) | 0.001 | 6300 (5.6) | 494 (6) | 0.018 |
| Immunosuppressants | 113 (0.3) | 1016 (0.3) | 0.001 | 382 (0.3) | 49 (0.6) | 0.038 |
| NSAIDs | 25 971 (69.2) | 229 672 (69.2) | 0.001 | 79 021 (70.7) | 5773 (70.6) | 0.001 |
| Platelet inhibitors | 23 792 (63.4) | 210 321 (63.3) | 0.001 | 75 427 (67.4) | 5511 (67.4) | 0.001 |
| Proton-pump inhibitors | 14 685 (39.1) | 129 908 (39.1) | <0.001 | 46 368 (41.5) | 3480 (42.6) | 0.022 |
| Statins | 22 388 (59.7) | 197 423 (59.5) | 0.004 | 79 510 (71.1) | 5537 (67.7) | 0.073 |
Abbreviations: ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers; ASD, absolute standardized difference; COPD, chronic obstructive pulmonary disorder; DPP4i, dipeptidyl peptidase 4 inhibitors; GLP1RA, glucagon-like peptide 1 receptor agonists; NA, not applicable; NSAIDs, nonsteroidal anti-inflammatory drugs; RANKL, receptor activator of nuclear factors kappa B ligand; SERMs, selective estrogen receptor modulator; SGLT2i, sodium glucose cotransporter 2 inhibitors.
Defined depending on the number of antidiabetic medication (excluding the study drugs of interest), prescribed in the year preceding the index date: level 1, taking none or 1 class of antidiabetic medication other than insulin; level 2, taking 2 or more different classes of antidiabetic medication without insulin; and level 3, taking insulin with or without other classes of antidiabetic medication.
During a mean (SD) follow-up of 1.45 (1.42) years and 2.09 (1.79) years, users of SGLT2i and DPP4i yielded a weighted incidence of 1.41 and 1.81 events per 100 person-years for the overall fractures, respectively, corresponding to a 22% lower rate of incident overall fractures with SGLT2i vs DPP4i (weighted HR, 0.78; 95% CI, 0.72-0.84). Meanwhile, over a mean (SD) follow-up of 1.43 (1.3) years and 0.82 (0.85) years, the weighted incidence of overall fractures was 1.67 per 100 person-years for SGLT2i and 1.92 per 100 person-years for GLP1RA. Use of SGLT2i, compared with GLP1RA, was not associated with overall fractures (weighted HR, 0.92; 95% CI, 0.68-1.24) (Table 2; eTables 8 and 9 and eFigure 4 in Supplement 1). Similar trends were found for the secondary outcomes across both cohorts, where SGLT2i use did not increase the risk of fractures, regardless of the comparator incretin-based drug (eTables 2 and 5 in Supplement 1).
Table 2. Association Between the Use of SGLT2i and the Risk of Overall Fractures Among Weighted Populations.
| Exposure | No. of patientsa | No. of Events | Person-years | Weighted IR (95% CI)b | Weighted HR (95% CI) |
|---|---|---|---|---|---|
| SGLT2i vs DPP4i | |||||
| SGLT2i | 37 530 | 768 | 54 619 | 1.41 (1.31-1.51) | 0.78 (0.72-0.84) |
| DPP4i | 332 004 | 18 800 | 693 818 | 1.81 (1.78-1.85) | 1.00 [Reference] |
| SGLT2i vs GLP1RA | |||||
| SGLT2i | 111 835 | 2723 | 164 967 | 1.67 (1.60-1.73) | 0.92 (0.68-1.24) |
| GLP1RA | 8177 | 156 | 6737 | 1.92 (1.59-2.30) | 1.00 [Reference] |
Abbreviations: DPP4i, dipeptidyl peptidase 4 inhibitors; GLP1RA, glucagon-like peptide 1 receptor agonists; HR, hazard ratio; IR, incidence rate; SGLT2i, sodium-glucose cotransporter 2 inhibitors.
Patients remained after trimming patients in the nonoverlapping regions of the propensity score distributions.
Per 100 person-years.
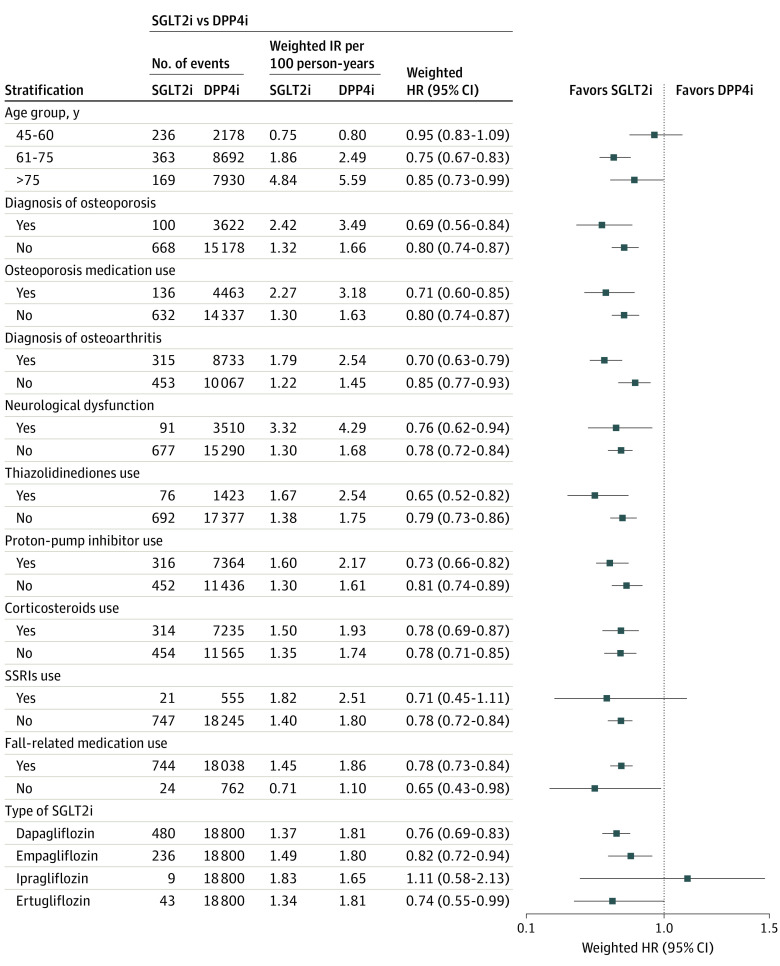
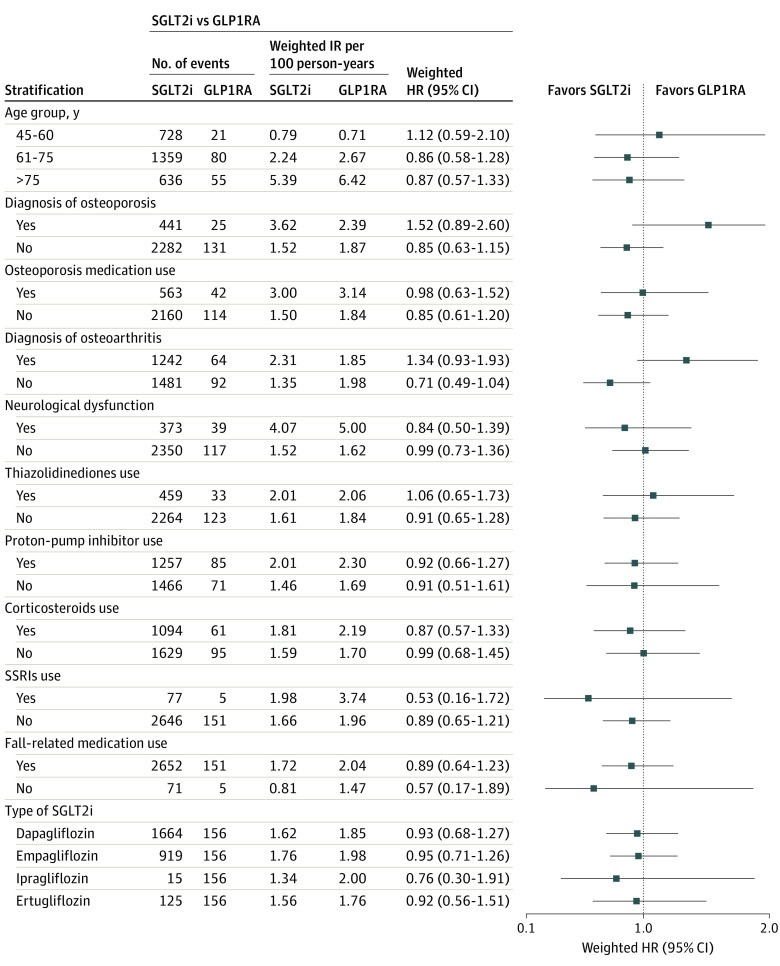
Results of subgroup analyses found no significant effect modification by age and prespecified covariates known to increase fracture risks, except for history of osteoarthritis; patients with a history of osteoarthritis (HR, 0.70; 95% CI, 0.63-0.79) had a lower rate of incident fractures with SGLT2i vs DPP4i than their counterpart (HR, 0.85; 95% CI, 0.77-0.93; P for interaction = .02) (Figure 1 and Figure 2; eTable 3 in Supplement 1). On the contrary, patients with a history of osteoarthritis (HR, 1.34; 95% CI, 0.93-1.93) had a significantly higher rate of incident fractures with SGLT2i vs GLP1RA, while their counterpart had a significantly lower rate of outcome (HR, 0.71; 95% CI, 0.49-1.04; P for interaction = .03) (eTable 6 in Supplement 1). All ingredients of SGLT2i-generated HR estimates below the null, although ipragliflozin had very wide CIs likely due to the relatively low numbers of events.
Figure 1. Results of Subgroup Analyses With Events for the Association Between the Use of Sodium-Glucose Cotransporter 2 Inhibitors (SGLT2i) vs Dipeptidyl Peptidase 4 Inhibitors (DPP4i) and the Risk of Overall Fractures.
HR indicates hazard ratio; IR, incidence rate; SSRI, selective serotonin reuptake inhibitor.
Figure 2. Results of Subgroup Analyses With Events for the Association Between the Use of Sodium-Glucose Cotransporter 2 Inhibitors (SGLT2i) vs Glucagon-Like Peptide 1 Receptor Agonists (GLP1RA) and the Risk of Overall Fractures.
HR indicates hazard ratio; IR, incidence rate; SSRI, selective serotonin reuptake inhibitor.
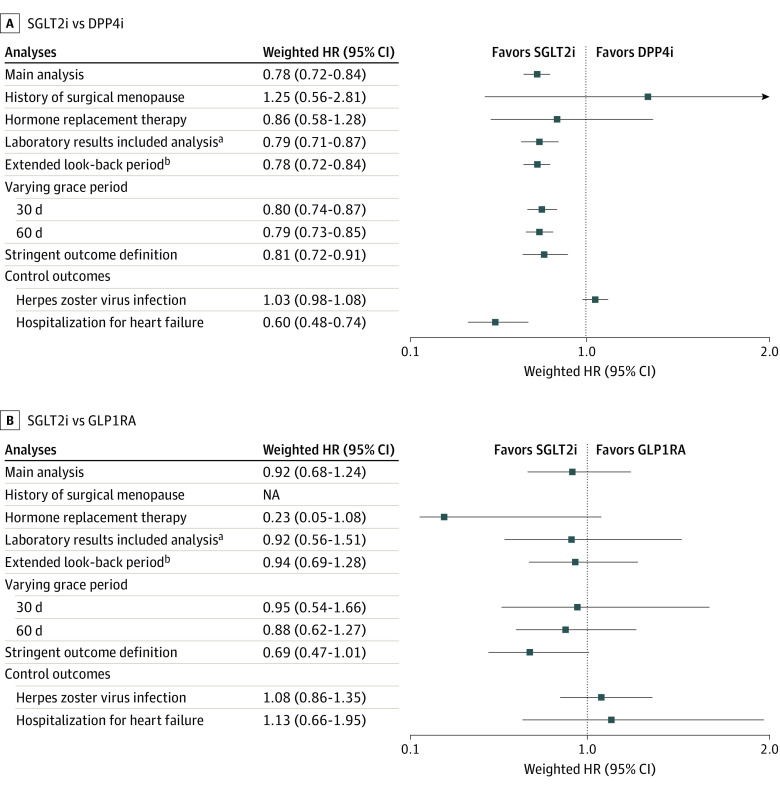
Across both cohorts, results of various sensitivity analyses were generally consistent, including the analysis that restricted to patients with a history of surgical menopause or hormone replacement therapy, the analysis that included laboratory results as covariates or extended comorbidity assessment period, and the analysis that used a stringent outcome definition. Moreover, null associations were consistently observed for the negative control outcomes, whereas a significantly lower risk was observed for the positive control outcome, which is in line with current knowledge (Figure 3; eTables 4, 7, 11-13 in Supplement 1).29
Figure 3. Results of Sensitivity Analyses for the Association Between the Use of Sodium-Glucose Cotransporter 2 Inhibitors (SGLT2i) vs Dipeptidyl Peptidase 4 Inhibitors (DPP4i) and the Use of SGLT2i vs Glucagon-Like Peptide 1 Receptor Agonists (GLP1RA) and the Risk of Overall Fractures.
HR indicates hazard ratio; NA, not applicable.
aLaboratory test results were assessed within 3 years prior to the index date and the most recent value was included. Included laboratory variables comprised waist circumference, body mass index, fasting blood glucose, systolic blood pressure, diastolic blood pressure, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, serum creatinine, estimated glomerular filtration rate, aspartate aminotransferase level, alanine aminotransferase level, gamma glutamyl transferase level, and smoking behavior, which presented varying missing rates of 27.9% to 60.5% for each variable.
bAssessment period for baseline comorbidities was extended to include any time before the index date.
Discussion
In this large-scale, nationwide cohort study that constructed 2 independent active comparator, new user cohorts, we found no increased risk of fractures among postmenopausal individuals with type 2 diabetes that initiated and continued treatment with SGLT2i vs DPP4i or GLP1RA. These findings were consistent across several subgroup and sensitivity analyses.
To our knowledge, no study, trial or observational, has specifically examined the risk of fractures with SGLT2i in postmenopausal individuals with type 2 diabetes, making it difficult to make any formal comparisons with existing data. Yet, our study was in agreement with a few studies that provide grounds for indirect comparisons. Several randomized clinical trials8,30 of SGLT2i other than canagliflozin and meta-analyses after the CANVAS trial have found little to no evidence of fracture risks.31,32 To date, 2 observational studies have assessed the risk of fractures associated with SGLT2i among patients with type 2 diabetes. The first cohort study, which used US Medicare claims data,18 found no association with an increased risk of fractures with SGLT2i among female individuals 66 years or older compared with DPP4i (HR, 0.88; 95% CI, 0.68-1.12) and GLP1RA (HR, 1.10; 95% CI, 0.83-1.45). Another cohort study using the UK Clinical Practice Research Datalink15 reported similar findings of no association with an increased risk of fractures among female individuals 40 years or older using SGLT2i vs DPP4i (HR, 0.88; 95% CI, 0.67-1.17). While these previous cohort studies did not investigate postmenopausal individuals specifically, their age cutoffs among female individuals support our findings of no increased risk of fractures with SGLT2i. Nevertheless, more studies in this population are needed to corroborate our findings.
Biologically, SGLT2i may potentially disrupt calcium and phosphate homeostasis in serum by increasing proximal tubular reabsorption of phosphate, which might have detrimental effects on bone and lead to skeletal fragility.11,33 This risk is expected to be further exacerbated in postmenopausal individuals given their decreased levels of estrogen hormones and consequent disruption of bone metabolism homeostasis. Moreover, SGLT2i have demonstrated an indirect elevation of bone turnover through weight loss. Increased levels of osteocalcin and C-terminal telopeptide of type I collagen, established indicators of bone turnover, have been detected subsequent to adipose tissue reduction, potentially exerting a detrimental influence on bone mineral density.34 Despite such biological plausibility, our results found a modest potential protective effect of SGLT2i against overall fractures, which may be explained by the role of SGLT2i in the trajectory of impaired bone metabolism caused by the accumulation of advanced glycation end products in bone collagen fibers in advanced diabetes.35,36 Persistent hyperglycemia accelerates the formation of advanced glycation end products such as pentosidine and N-carboxymethyl-lysine, which contributes to oxidative stress in the bone microenvironment to promote collagen cross-linking, making bone stiffer and more susceptible to fractures.37,38,39 Several experimental studies have reported that SGLT2i exhibit inhibitory effects on the advanced glycation end products–receptor for advanced glycation end products signaling pathway via ameliorating glucose toxic effects,41 and thereby having a protective effect against fractures. Nevertheless, there remains much to be understood regarding the exact biological mechanism SGLT2i, DPP4i, and GLP1RA have in bone metabolism homeostasis. Thus, further research is needed to better elucidate this observation.
Strengths and Limitations
Our study has several strengths. To our knowledge, this is the first study to assess the risk of fractures associated with commonly used antidiabetic regimens in routine practice among postmenopausal individuals with type 2 diabetes. Second, we constructed 2 separate cohorts to provide comprehensive comparative safety evidence on female diabetic patients using SGLT2i vs DPP4i and SGLTI2i vs GLP1RA, or medications that share treatment stage in type 2 diabetes management, which are therefore clinically meaningful. Third, by conducting several subgroup analyses that accounted for various risk factors for fractures, we validated the robustness of our main finding and established the safety of SGLT2i for the majority of patients, although those with osteoarthritis warrant further attention. Fourth, we minimized the potential for outcome misclassification by applying an algorithm of operational definition for fractures that included procedure codes stratified by skeletal sites, which have been previously validated. Fifth, we used propensity score fine stratification weights, allowing us to preserve most observations in the analysis and produce more precise effect estimates.28
This study has some limitations. First, residual confounding due to unmeasured covariates cannot be ruled out, although we adjusted for various measurable covariates; in particular, we assessed the severity of diabetes and multiple baseline risk factors of fracture. Second, the number of patients using GLP1RA was low, which may limit the generalizability of the SGLT2i vs GLP1RA comparison. Thus, further studies using more recent data are needed to better estimate this association. Third, since all study participants were Korean, our findings may not be generalized to other populations. Last, the lack of a standardized definition of postmenopausal status is a limitation in epidemiological studies using claims data. Nevertheless, several validation studies have shown that using age as a proxy was not significantly different from using other, more complex definitions.42 To further increase the validity, we used other measures (eg, surgery and hormone replacement therapy) that can complement the age-based definition as a sensitivity analysis, which revealed consistent findings. Also, in a subgroup analysis among patients 61 years or older, who were expected to be almost certain postmenopause, no trend of an increased risk of fracture with SGLT2i was observed.
Conclusions
The use of SGLT2i was not associated with an increased risk of overall fractures among postmenopausal patients with type 2 diabetes. This result remained consistent irrespective of the particular incretin-based drug as an active comparator. These findings indicate that SGLT2i has either similar or lower risks of fractures than incretin-based drugs even in a population at higher risk for fractures, providing reassurance to and helping health care professionals with their clinical decision-making.
eFigure 1. Flowchart of study participants selection
eFigure 2. Flowchart of study participants selection
eFigure 3. Cumulative incidence function plots for overall fractures among weighted populations
eFigure 4. Overall study design
eFigure 5. Propensity score distribution of before and after weighting in both cohorts
eTable 1. Baseline characteristics of patients received SGLT2 inhibitors or comparator drugs, before weighting by propensity score fine stratification
eTable 2. Association between the use of SGLT2 inhibitors and the risk of fractures compared with the use of DPP4 inhibitors
eTable 3. Results of subgroup analyses with events, person-years, weighted IRs (per 100 person-years), weighted HRs with 95% CIs, and p for interaction for the association between the use of SGLT2 inhibitors vs DPP4 inhibitors and the risk of overall fractures
eTable 4. Results of sensitivity analyses with events, person-years, weighted IRs (per 100 person-years), weighted HRs and 95% CIs for the association between the use of SGLT2 inhibitors vs DPP4 inhibitors and the risk of overall fractures
eTable 5. Association between the use of SGLT2 inhibitors and the risk of fractures compared with the use of GLP-1 receptor agonists
eTable 6. Results of subgroup analyses with events, person-years, weighted IRs (per 100 person-years), weighted HRs with 95% CIs, and p for interaction for the association between the use of SGLT2 inhibitors vs GLP-1 receptor agonists and the risk of overall fractures
eTable 7. Results of sensitivity analyses with events, person-years, weighted IRs (per 100 person-years), weighted HRs and 95% CIs for the association between the use of SGLT2 inhibitors vs GLP-1 receptor agonists and the risk of overall fractures
eTable 8. Reasons for the end of follow-up among weighted populations received SGLT2 inhibitors or DPP4 inhibitors
eTable 9. Reasons for the end of follow-up among weighted populations received SGLT2 inhibitors or GLP-1 receptor agonists
eTable 10. Laboratory test results and missing rates of patients receiving SGLT2 inhibitors or comparator drugs before propensity score based fine stratification
eTable 11. Baseline characteristics after weighting by propensity score stratification in patients receiving SGLT2 inhibitors or comparator drugs with bone mineral density test results present
eTable 12. Baseline characteristics before weighting by propensity score stratification in patients receiving SGLT2 inhibitors or comparator drugs with bone mineral density test results present
eTable 13. Association between the use of SGLT2 inhibitors and the risk of overall fractures among weighted populations with bone mineral density test results present
eTable 14. International Classification of Diseases-10 (ICD-10) and domestic procedure codes for identification of fractures
eTable 15. International Classification of Diseases-10 (ICD-10) codes for identification of covariates
eTable 16. Anatomical Therapeutic Chemical (ATC) classification codes for identification of covariates
Data Sharing Statement
References
- 1.Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155-170. doi: 10.1016/j.jsbmb.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thong EP, Milat F, Enticott JC, et al. The diabetes-fracture association in women with type 1 and type 2 diabetes is partially mediated by falls: a 15-year longitudinal study. Osteoporos Int. 2021;32(6):1175-1184. doi: 10.1007/s00198-020-05771-9 [DOI] [PubMed] [Google Scholar]
- 3.Behanova M, Haschka J, Zwerina J, et al. The doubled burden of diabetic bone disease: hip fracture and post-hip fracture mortality. Eur J Endocrinol. 2021;184(5):627-636. doi: 10.1530/EJE-20-1155 [DOI] [PubMed] [Google Scholar]
- 4.Khosla S, Samakkarnthai P, Monroe DG, Farr JN. Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17(11):685-697. doi: 10.1038/s41574-021-00555-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL; IOF Bone and Diabetes Working Group . Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13(4):208-219. doi: 10.1038/nrendo.2016.153 [DOI] [PubMed] [Google Scholar]
- 6.Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 7.Neal B, Perkovic V, Mahaffey KW, et al. ; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 8.Wiviott SD, Raz I, Bonaca MP, et al. ; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 9.Cannon CP, Pratley R, Dagogo-Jack S, et al. ; VERTIS CV Investigators . Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425-1435. doi: 10.1056/NEJMoa2004967 [DOI] [PubMed] [Google Scholar]
- 10.ElSayed NA, Aleppo G, Aroda VR, et al. ; on behalf of the American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi: 10.2337/dc23-S009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye Y, Zhao C, Liang J, Yang Y, Yu M, Qu X. Effect of sodium-glucose co-transporter 2 inhibitors on bone metabolism and fracture risk. Front Pharmacol. 2019;9:1517. doi: 10.3389/fphar.2018.01517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaze AD, Zhuo M, Kim SC, Patorno E, Paik JM. Association of SGLT2 inhibitors with cardiovascular, kidney, and safety outcomes among patients with diabetic kidney disease: a meta-analysis. Cardiovasc Diabetol. 2022;21(1):47. doi: 10.1186/s12933-022-01476-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng L, Li YY, Hu W, et al. Risk of bone fracture associated with sodium-glucose cotransporter-2 inhibitor treatment: a meta-analysis of randomized controlled trials. Diabetes Metab. 2019;45(5):436-445. doi: 10.1016/j.diabet.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 14.Tang HL, Li DD, Zhang JJ, et al. Lack of evidence for a harmful effect of sodium-glucose co-transporter 2 (SGLT2) inhibitors on fracture risk among type 2 diabetes patients: a network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2016;18(12):1199-1206. doi: 10.1111/dom.12742 [DOI] [PubMed] [Google Scholar]
- 15.Abrahami D, Douros A, Yin H, Yu OHY, Azoulay L. Sodium-glucose cotransporter 2 inhibitors and the risk of fractures among patients with type 2 diabetes. Diabetes Care. 2019;42(9):e150-e152. doi: 10.2337/dc19-0849 [DOI] [PubMed] [Google Scholar]
- 16.Ueda P, Svanström H, Melbye M, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ. 2018;363:k4365. doi: 10.1136/bmj.k4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fralick M, Kim SC, Schneeweiss S, Kim D, Redelmeier DA, Patorno E. Fracture risk after initiation of use of canagliflozin: a cohort study. Ann Intern Med. 2019;170(3):155-163. doi: 10.7326/M18-0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuo M, Hawley CE, Paik JM, et al. Association of Sodium-glucose cotransporter-2 inhibitors with fracture risk in older adults with type 2 diabetes. JAMA Netw Open. 2021;4(10):e2130762. doi: 10.1001/jamanetworkopen.2021.30762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Dalem J, Werkman NCC, van den Bergh JP, et al. Use of sodium-glucose co-transporter 2 inhibitors, changes in body mass index and risk of fracture: a population-based cohort study. Diabetes Res Clin Pract. 2022;190:109993. doi: 10.1016/j.diabres.2022.109993 [DOI] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 21.Lee YK, Park C, Won S, et al. Validation of an operational definition to identify distal radius fractures in a national health insurance database. J Hand Surg Am. 2021;46(11):1026.e1-1026.e7. doi: 10.1016/j.jhsa.2021.03.001 [DOI] [PubMed] [Google Scholar]
- 22.Park SM, Ahn SH, Kim HY, et al. Incidence and mortality of subsequent vertebral fractures: analysis of claims data of the Korea National Health Insurance Service from 2007 to 2016. Spine J. 2020;20(2):225-233. doi: 10.1016/j.spinee.2019.09.025 [DOI] [PubMed] [Google Scholar]
- 23.Jo YH, Lee BG, Kim HS, et al. Incidence and seasonal variation of distal radius fractures in Korea: a population-based study. J Korean Med Sci. 2018;33(7):e48. doi: 10.3346/jkms.2018.33.e48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jo YH, Lee KH, Lee BG. Surgical trends in elderly patients with proximal humeral fractures in South Korea: a population-based study. BMC Musculoskelet Disord. 2019;20(1):136. doi: 10.1186/s12891-019-2515-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung HS, Nho JH, Ha YC, et al. Incidence of osteoporotic refractures following proximal humerus fractures in adults aged 50 years and older in Korea. J Bone Metab. 2019;26(2):105-111. doi: 10.11005/jbm.2019.26.2.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YK, Ha YC, Park C, Yoo JJ, Shin CS, Koo KH. Bisphosphonate use and increased incidence of subtrochanteric fracture in South Korea: results from the National Claim Registry. Osteoporos Int. 2013;24(2):707-711. doi: 10.1007/s00198-012-2016-8 [DOI] [PubMed] [Google Scholar]
- 27.Yu MH, Hong N, Lee S, et al. Operational definition identifying osteoporotic vertebral fractures in the claims database. J Korean Med Sci. 2022;37(32):e249. doi: 10.3346/jkms.2022.37.e249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ. 2019;367:l5657. doi: 10.1136/bmj.l5657 [DOI] [PubMed] [Google Scholar]
- 29.Patorno E, Pawar A, Franklin JM, et al. Empagliflozin and the risk of heart failure hospitalization in routine clinical care. Circulation. 2019;139(25):2822-2830. doi: 10.1161/CIRCULATIONAHA.118.039177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zinman B, Lachin JM, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2016;374(11):1094. doi: 10.1056/NEJMc1600827 [DOI] [PubMed] [Google Scholar]
- 31.Chai S, Liu F, Yang Z, et al. Risk of fracture with dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis combining 177 randomized controlled trials with a median follow-up of 26 weeks. Front Pharmacol. 2022;13:825417. doi: 10.3389/fphar.2022.825417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hidayat K, Du X, Shi BM. Risk of fracture with dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors in real-world use: systematic review and meta-analysis of observational studies. Osteoporos Int. 2019;30(10):1923-1940. doi: 10.1007/s00198-019-04968-x [DOI] [PubMed] [Google Scholar]
- 33.Thrailkill KM, Clay Bunn R, Nyman JS, et al. SGLT2 inhibitor therapy improves blood glucose but does not prevent diabetic bone disease in diabetic DBA/2J male mice. Bone. 2016;82:101-107. doi: 10.1016/j.bone.2015.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bilezikian JP, Watts NB, Usiskin K, et al. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab. 2016;101(1):44-51. doi: 10.1210/jc.2015-1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asadipooya K, Uy EM. Advanced glycation end products (AGEs), receptor for AGEs, diabetes, and bone: review of the literature. J Endocr Soc. 2019;3(10):1799-1818. doi: 10.1210/js.2019-00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114(6):597-605. doi: 10.1161/CIRCULATIONAHA.106.621854 [DOI] [PubMed] [Google Scholar]
- 37.Shanbhogue VV, Mitchell DM, Rosen CJ, Bouxsein ML. Type 2 diabetes and the skeleton: new insights into sweet bones. Lancet Diabetes Endocrinol. 2016;4(2):159-173. doi: 10.1016/S2213-8587(15)00283-1 [DOI] [PubMed] [Google Scholar]
- 38.Hofbauer LC, Busse B, Eastell R, et al. Bone fragility in diabetes: novel concepts and clinical implications. Lancet Diabetes Endocrinol. 2022;10(3):207-220. doi: 10.1016/S2213-8587(21)00347-8 [DOI] [PubMed] [Google Scholar]
- 39.Nakano M, Nakamura Y, Suzuki T, et al. Pentosidine and carboxymethyl-lysine associate differently with prevalent osteoporotic vertebral fracture and various bone markers. Sci Rep. 2020;10(1):22090. doi: 10.1038/s41598-020-78993-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steven S, Oelze M, Hanf A, et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017;13:370-385. doi: 10.1016/j.redox.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ojima A, Matsui T, Nishino Y, Nakamura N, Yamagishi S. Empagliflozin, an inhibitor of sodium-glucose cotransporter 2 exerts anti-inflammatory and antifibrotic effects on experimental diabetic nephropathy partly by suppressing AGEs-receptor axis. Horm Metab Res. 2015;47(9):686-692. doi: 10.1055/s-0034-1395609 [DOI] [PubMed] [Google Scholar]
- 42.Phipps AI, Ichikawa L, Bowles EJ, et al. Defining menopausal status in epidemiologic studies: a comparison of multiple approaches and their effects on breast cancer rates. Maturitas. 2010;67(1):60-66. doi: 10.1016/j.maturitas.2010.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flowchart of study participants selection
eFigure 2. Flowchart of study participants selection
eFigure 3. Cumulative incidence function plots for overall fractures among weighted populations
eFigure 4. Overall study design
eFigure 5. Propensity score distribution of before and after weighting in both cohorts
eTable 1. Baseline characteristics of patients received SGLT2 inhibitors or comparator drugs, before weighting by propensity score fine stratification
eTable 2. Association between the use of SGLT2 inhibitors and the risk of fractures compared with the use of DPP4 inhibitors
eTable 3. Results of subgroup analyses with events, person-years, weighted IRs (per 100 person-years), weighted HRs with 95% CIs, and p for interaction for the association between the use of SGLT2 inhibitors vs DPP4 inhibitors and the risk of overall fractures
eTable 4. Results of sensitivity analyses with events, person-years, weighted IRs (per 100 person-years), weighted HRs and 95% CIs for the association between the use of SGLT2 inhibitors vs DPP4 inhibitors and the risk of overall fractures
eTable 5. Association between the use of SGLT2 inhibitors and the risk of fractures compared with the use of GLP-1 receptor agonists
eTable 6. Results of subgroup analyses with events, person-years, weighted IRs (per 100 person-years), weighted HRs with 95% CIs, and p for interaction for the association between the use of SGLT2 inhibitors vs GLP-1 receptor agonists and the risk of overall fractures
eTable 7. Results of sensitivity analyses with events, person-years, weighted IRs (per 100 person-years), weighted HRs and 95% CIs for the association between the use of SGLT2 inhibitors vs GLP-1 receptor agonists and the risk of overall fractures
eTable 8. Reasons for the end of follow-up among weighted populations received SGLT2 inhibitors or DPP4 inhibitors
eTable 9. Reasons for the end of follow-up among weighted populations received SGLT2 inhibitors or GLP-1 receptor agonists
eTable 10. Laboratory test results and missing rates of patients receiving SGLT2 inhibitors or comparator drugs before propensity score based fine stratification
eTable 11. Baseline characteristics after weighting by propensity score stratification in patients receiving SGLT2 inhibitors or comparator drugs with bone mineral density test results present
eTable 12. Baseline characteristics before weighting by propensity score stratification in patients receiving SGLT2 inhibitors or comparator drugs with bone mineral density test results present
eTable 13. Association between the use of SGLT2 inhibitors and the risk of overall fractures among weighted populations with bone mineral density test results present
eTable 14. International Classification of Diseases-10 (ICD-10) and domestic procedure codes for identification of fractures
eTable 15. International Classification of Diseases-10 (ICD-10) codes for identification of covariates
eTable 16. Anatomical Therapeutic Chemical (ATC) classification codes for identification of covariates
Data Sharing Statement