Key Points
Question
Is there a temporal association between SARS-CoV-2 infection and the development of type 1 diabetes–associated autoimmunity in children?
Findings
In this longitudinal cohort study of 885 infants with an elevated genetic risk of type 1 diabetes, the incidence rate of islet autoantibodies developing concurrently with or soon after SARS-CoV-2 antibodies were detected was 7.8 per 100 person-years and was 3.5 per 100 person-years in children without SARS-CoV-2 antibodies, a significant difference.
Meaning
In young children with high genetic risk of type 1 diabetes, SARS-CoV-2 infection was temporally associated with the development of islet autoantibodies.
Abstract
Importance
The incidence of diabetes in childhood has increased during the COVID-19 pandemic. Elucidating whether SARS-CoV-2 infection is associated with islet autoimmunity, which precedes type 1 diabetes onset, is relevant to disease etiology and future childhood diabetes trends.
Objective
To determine whether there is a temporal relationship between SARS-CoV-2 infection and the development of islet autoimmunity in early childhood.
Design, Setting, and Participants
Between February 2018 and March 2021, the Primary Oral Insulin Trial, a European multicenter study, enrolled 1050 infants (517 girls) aged 4 to 7 months with a more than 10% genetically defined risk of type 1 diabetes. Children were followed up through September 2022.
Exposure
SARS-CoV-2 infection identified by SARS-CoV-2 antibody development in follow-up visits conducted at 2- to 6-month intervals until age 2 years from April 2018 through June 2022.
Main Outcomes and Measures
The development of multiple (≥2) islet autoantibodies in follow-up in consecutive samples or single islet antibodies and type 1 diabetes. Antibody incidence rates and risk of developing islet autoantibodies were analyzed.
Results
Consent was obtained for 885 (441 girls) children who were included in follow-up antibody measurements from age 6 months. SARS-CoV-2 antibodies developed in 170 children at a median age of 18 months (range, 6-25 months). Islet autoantibodies developed in 60 children. Six of these children tested positive for islet autoantibodies at the same time as they tested positive for SARS-CoV-2 antibodies and 6 at the visit after having tested positive for SARS-CoV-2 antibodies. The sex-, age-, and country-adjusted hazard ratio for developing islet autoantibodies when the children tested positive for SARS-CoV-2 antibodies was 3.5 (95% CI, 1.6-7.7; P = .002). The incidence rate of islet autoantibodies was 3.5 (95% CI, 2.2-5.1) per 100 person-years in children without SARS-CoV-2 antibodies and 7.8 (95% CI, 5.3-19.0) per 100 person-years in children with SARS-CoV-2 antibodies (P = .02). Islet autoantibody risk in children with SARS-CoV-2 antibodies was associated with younger age (<18 months) of SARS-CoV-2 antibody development (HR, 5.3; 95% CI, 1.5-18.3; P = .009).
Conclusion and relevance
In young children with high genetic risk of type 1 diabetes, SARS-CoV-2 infection was temporally associated with the development of islet autoantibodies.
This longitudinal study of young children in 5 European countries investigates whether there is a temporal association between SARS-CoV-2 infection and islet autoimmunity when children are most susceptible.
Introduction
Type 1 diabetes in childhood is characteristically preceded by the development of autoantibodies against multiple pancreatic islet β-cell proteins.1 There is a peak period of susceptibility for developing these autoantibodies at approximately 1 year of age.2 Children who develop multiple islet autoantibodies usually progress to clinical type 1 diabetes within 10 years.3 Susceptibility for early islet autoimmunity is conferred by genes involved in immunity, islet β-cell function, and responses to viral infections.4 Although the cause of islet autoimmunity is unknown, suspected contributors include early viral infections5,6 including respiratory viral infections.4,7
The COVID-19 pandemic likely modified infectious exposures during childhood. Increased incidence rates of type 1 diabetes have been observed during the pandemic in several regions as well as an association between type 1 diabetes incidence and a COVID-19 diagnosis in some studies.8,9,10,11 Like other viruses associated with islet autoimmunity and type 1 diabetes, the SARS-CoV-2 virus can enter and infect islet β cells.12,13,14 Therefore, it is plausible that SARS-CoV-2 infection may also increase the susceptibility for islet autoimmunity, which has potentially important implications for future type 1 diabetes incidence. However, no study to our knowledge has been able to link the timing of SARS-CoV-2 infection to the appearance of islet autoantibodies.
From 2018 to 2021, the Primary Oral Insulin Trial (POINT) recruited infants with a genetically defined risk of at least 10% for developing multiple islet autoantibodies.15 The genetic selection represents approximately 1% of all infants and captures up to 25% of those who will develop type 1 diabetes during childhood. Infants were enrolled between the ages of 4 and 7 months with blood samples collected longitudinally for the detection of islet autoantibodies. Therefore, this study provides a rare setting to investigate whether there is a temporal association between SARS-CoV-2 infection and islet autoimmunity when children are most susceptible to testing positive for islet autoantibodies. Herein, antibodies to SARS-CoV-2 were measured, and their relationship to the concurrent and subsequent temporal development of islet autoantibodies was examined.
Methods
Participants
Between February 2018 and March 2021, 1050 infants (517 girls) aged 4.0-7.0 months with elevated genetic risk of developing type 1 diabetes were enrolled (ClinicalTrials.gov identifier: NCT03364868).15 Infants were eligible if their estimated risk of developing multiple islet autoantibodies by 6 years of age was more than 10%.16,17 Risk was derived from genetic typing, which was performed at 47 single-nucleotide variants. Eligible infants were randomly assigned to receive either oral insulin powder or oral placebo powder daily until age 3 years. Blood samples were collected at baseline (age 4-7 months), visit 2 (age 6-10 months), visit 3 (age 8-12 months), visit 4 (age 12-16 months), visit 5 (age 17-20 months), and visit 6 (age 21-25 months) and then every 6 months until they reached age 6.5 years. The ongoing trial is being conducted in Germany, Poland, Sweden, Belgium, and the UK and is organized through the Global Platform for the Prevention of Autoimmune Diabetes (GPPAD). The study was approved by local ethics committees and regulatory authorities of the Technische Universität München (326/17 Af), the Medical University of Warsaw (199/2017), the UK Health Research Authority (18/SC/0019), Onderzoek UZ/KU Leuven (S60711), and Regionala etikprövningsnämnden i Lund (2017/918). Written informed consent to participate in the study was obtained for all study participants from their parents or their legal guardians. As part of the study protocol, additional consent was obtained for biobank storage of blood samples to perform ancillary studies related to the mechanism of study drug action and to understanding the pathogenesis of type 1 diabetes. The viral infection ancillary study was proposed and approved in October 2021.
Antibody Detection
IgG SARS-CoV-2 antibodies to the receptor binding domain (amino acids 309-544 of the original variant sequence), the S2 portion of the spike protein (amino acids 697-1224), and the nucleocapsid protein (amino acids 1-422) were measured at the Dresden University of Technology using luciferase immunoprecipitation system assays, as described previously18 (eFigure 1 in Supplement 1). Samples at visit 6 (n = 765) or, if children had not reached this time point, visit 5 (n = 116) or visit 4 (n = 4) were screened for antibodies against the receptor binding domain protein. Samples that tested positive (≥1 arbitrary unit, equivalent to 5 binding antibody units) were subsequently tested against the S2 and the nucleocapsid protein. Samples were defined as positive for SARS-CoV-2 antibodies if they were positive against the receptor binding domain and the nucleocapsid protein antigens. Using these thresholds in samples from 3000 children before the pandemic and samples from 75 patients with polymerase chain reaction (PCR)–confirmed COVID-19, the assay specificity and sensitivity were 100% and 97.3%, respectively. Antibodies to the influenza A(H1N1) virus (A Germany 7405 2018) HA1 antigen (amino acids 19-344 of HA) were measured using a luciferase immunoprecipitation system assay using the same format (eFigure 1 in Supplement 1). If children tested positive for SARS-CoV-2 or influenza A(H1N1) antibodies, samples collected at earlier visits were tested to determine the earliest positive test result (eFigure 2 in Supplement). IgA antibodies against the virus antigens were measured in samples that had tested positive at the baseline visit to determine positivity owing to maternally transferred antibodies, which were defined as IgG antibody-positive status from the earliest tested sample, a more than 2-fold reduction in the IgG antibody titer per month, and the absence of IgA antibody titers. Viral antibody measurements were performed blinded to the islet autoantibody test result in the samples.
Islet Autoantibodies
Islet autoantibodies were centrally measured at 2 independent laboratories located at the Institute of Diabetes Research, Helmholtz Munich, Germany, and at the University of Bristol, Medical School, Diabetes and Metabolism, Learning and Research, Southmead Hospital, Bristol, UK (for confirmation of results), as described previously.19 All samples were measured in Munich for autoantibodies against insulin, glutamate acid decarboxylase 65 (GAD65), insulinoma-associated antigen 2 (IA-2), and zinc transporter 8 (ZnT8) using radiobinding assays.20,21,22 The respective sensitivities and specificities were 54% and 99% for autoantibodies against insulin, 66% and 99% for autoantibodies against GAD65, 76% and 100% for autoantibodies against IA-2, and 56% and 99% for autoantibodies against ZnT8, according to the Islet Autoantibody Standardization Program 2016 Workshop. Serum samples that tested positive for autoantibodies against GAD65 were tested using an enzyme-linked immunosorbent assay (RSR Ltd) and a radiobinding assay in Bristol22 and were considered positive if autoantibodies against GAD65 were detected in all 3 assays. Serum samples that tested positive for autoantibodies against insulin, IA-2, and ZnT8 were each tested by radiobinding assays in Bristol22,23,24 and considered positive if they were detected in both laboratories.
The primary outcome was either the development of persistent confirmed multiple β-cell autoantibodies, defined as autoantibodies against insulin; GAD65; insulinoma–associated antigen 2; or ZnT8 in 2 consecutive samples and a confirmed second antibody in 1 sample or 1 or more of the antibodies and diabetes. Maternally transferred islet autoantibodies were excluded and identified if the infant tested positive at the first sample, had declining antibody titers on follow-up, and subsequently became negative for islet autoantibodies. For infants classified as having tested positive for islet autoantibodies, the seroconversion time point was defined as the first sample that had tested positive.
Statistical Analyses
The incidence rates of viral antibodies and islet autoantibodies were calculated as the number of events per follow-up period and are reported per 100 person-years with Poisson 95% CIs. The incidence rates in relation to SARS-CoV-2 antibody status were examined from February 1, 2018, through September 30, 2022, and also from July 1, 2020, through September 30, 2022. The period until the last SARS-CoV-2 antibody-negative test result was defined as SARS-CoV-2 negative. For children who developed SARS-CoV-2 antibodies, the time after the last SARS-CoV-2 antibody–negative test result was defined as SARS-CoV-2 positive. The incidence rates of antibodies and autoantibodies were compared among groups using the χ2 statistic. Associations between time-varying SARS-CoV-2 antibodies and islet autoantibodies were also analyzed using Cox models, adjusting for sex, age at January 1, 2020, and country as fixed effects to assess hazard ratios (HRs) with corresponding 95% CIs. Kaplan-Meier curves were used to determine the cumulative frequency of islet autoantibodies. Follow-up was defined as the time until the last measured sample or when the first positive islet autoantibody test result occurred. SARS-CoV-2 antibody development was included as a time-dependent variable so that children who developed SARS-CoV-2 antibodies were censored and thus contributed to the number at risk of both groups for a certain time. CIs were calculated using the log-log transformed method (exponential Greenwood formula), and groups were compared using the log-rank (Mantel-Cox) test. The Cox proportional hazards model was used to analyze covariates associated with islet autoantibody development in children who tested positive for SARS-CoV-2 antibodies. For all comparisons, a 2-tailed P value of .05 was considered significant. Data analyses were conducted using Graphpad PRISM version 9.4.1 (GraphPad Software, http://www.graphpad.com), MedCalc version 20.2 (http://www.medcalc.org), and the survival Python package, version 0.27.4 (Python Software Foundation).
Results
Of 1050 enrolled, 885 (441 girls) infants had biobank consent and tested negative for islet autoantibodies at baseline and were included in the ancillary study (eTable 1 in Supplement 1; Figure 1A). Of these children, 407 were from Germany, 216 from Poland, 160 from Sweden, 71 from Belgium, and 31 from the UK (eTable 2 in Supplement 1).
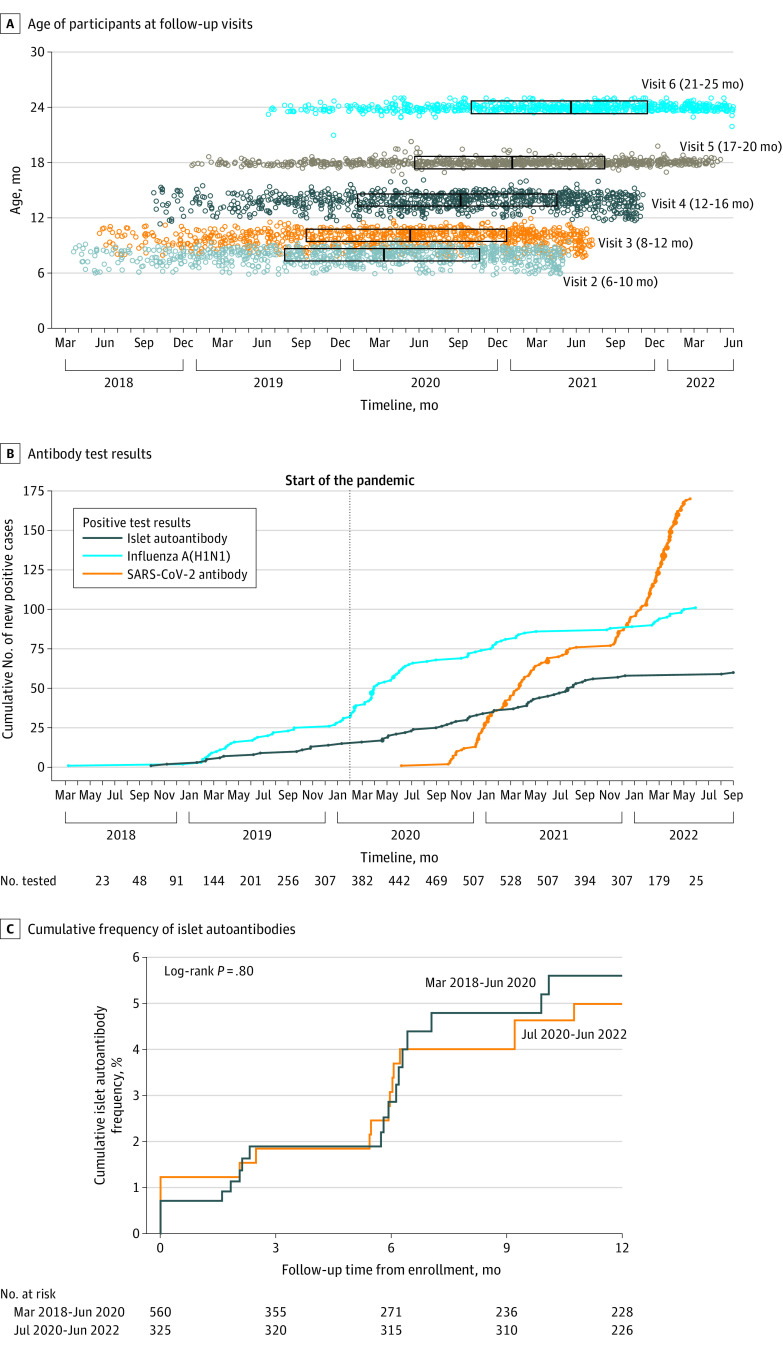
Figure 1. SARS-CoV-2 Antibodies, Influenza A(H1N1) Antibodies, and Islet Autoantibodies.
A, The age of the children and date of follow-up study visits of 885 children included in the study. The boxed areas represent the interquartile range (q1-q3) of dates for each study visit and the center line, the median.
B, The cumulative number of new cases for SARS-CoV-2 antibodies was 170 and for influenza A(H1N1) antibodies was 101 over time to age 2 years. The numbers of new cases of islet autoantibodies (multiple or single and diabetes) was 60 until 2.5 years of age. Each dot indicates a new case; the larger dots indicate multiple cases on the same day. The numbers underneath the x-axis indicate the number of children tested for all antibodies in each 3-month period and corresponding to the end of the indicated month.
C, Kaplan-Meier curves of the cumulative frequency of islet autoantibodies over 12 months from study entry of children recruited and followed up between February 2018 and June 2020 (n = 560) and children who had their first follow-up visit from July 2020 (n = 325).
SARS-CoV-2 Antibodies
SARS-CoV-2 antibodies developed in 170 children at a median age of 18 months (range, 6-25 months). The earliest date samples tested positive for SARS-CoV-2 antibodies was July 6, 2020 (Figure 1B; eFigure 3 in Supplement 1). All children who tested positive developed antibodies against both the receptor-binding domain and nucleocapsid regions, which was consistent with infection. The incidence rate of SARS-CoV-2 antibody positivity per 100 person-years was 0 from February 2018 through June 2020, 4.4 (95% CI, 2.3-7.7) from July 2020 to December 2020, 21.5 (95% CI, 16.3-27.8) from January 2021 through June 2021, 9.2 (95% CI, 5.4-14.7) from July 2021 through December 2021, and 81.7 (95% CI, 65.2-101.2) from January 2022 through June 2022 (eFigure 4A in Supplement 1). There were substantial differences in the incidence rates between countries (P < .001; eFigure 4B in Supplement 1; Table). Similar incidence rates of SARS-CoV-2 antibodies were observed in boys and girls, in children with or without a first-degree relative with type 1 diabetes, and in children with or without the human leukocyte antigen (HLA) DR3/DR4-DQ8 genotype.
Table. SARS-CoV-2 Antibodies, H1N1 Antibodies, and Islet Autoantibodies Until Age 2 Years.
| Antibodies | Islet autoantibodiesb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2a | Influenza A(H1N1) | |||||||||
| Cases, No./total (%) | Person-years | Incidence, per 100 person-years (95% CI) | Cases, No./total (%) | Person-years | Incidence, per 100 person-years (95% CI) | Cases, No./total (%) | Person-years | Incidence, per 100 person-years (95% CI) | ||
| Period interval | ||||||||||
| Feb 18-Jun 20 | 0/562 | 438 | 0 | 59/562 (10.5) | 430 | 13.7 (10.5-17.7) | 21/562 (3.7) | 430 | 4.9 (3.0-7.4) | |
| Jul 20-Dec 21 | 86/739 (11.6) | 722 | 11.9 (9.5-14.7) | 29/739 (3.9) | 718 | 4.0 (2.7-5.8) | 34/755 (4.5) | 829 | 4.1 (3.3-6.7) | |
| Jan 22-Jun 22 | 84/223 (37.7) | 103 | 81.7 (65.2-101.2) | 13/239 (5.4) | 110 | 11.8 (6.3-20.2) | ||||
| Sex | ||||||||||
| Girls | 84/364 (23.0) | 400 | 21.0 (16.8-26.0) | 56/437 (12.8) | 639 | 8.8 (6.6-11.4) | 21/441 (4.8) | 639 | 3.3 (2.1-5.1) | |
| Boys | 86/383 (22.5) | 425 | 20.2 (16.2-25.0) | 45/442 (10.2) | 619 | 7.3 (5.3-9.7) | 34/444 (7.7) | 620 | 5.5 (3.8-7.7) | |
| Country | ||||||||||
| Poland | 64/187 (34.2) | 196 | 32.7 (25.2-41.8) | 28/214 (17.8) | 194 | 14.4 (9.6-20.9) | 19/216 (8.8) | 308 | 6.2 (3.7-9.6) | |
| Belgium | 22/71 (31.0) | 98 | 22.4 (14.0-33.9) | 9/70 (12.9) | 71 | 12.7 (5.8-24.1) | 1/71 (1.4) | 105 | 1.0 (0.0-5.3) | |
| Sweden | 34/153 (22.2) | 172 | 19.7 (13.6-27.5) | 9/160 (5.6) | 140 | 6.4 (2.9-12.3) | 9/160 (5.6) | 233 | 3.9 (1.8-7.3) | |
| Germany | 48/306 (15.7) | 330 | 14.5 (10.7-19.2) | 53/404 (13.1) | 375 | 14.1 (10.6-18.5) | 24/407 (5.9) | 570 | 4.2 (2.7-6.3) | |
| UK | 2/30 (6.7) | 29 | 7.0 (0.8-25.3) | 2/31 (6.5) | 28 | 7.1 (0.9-20.8) | 2/31 (6.5) | 43 | 4.7 (5.6-16.8) | |
| First-degree relative with type 1 diabetes | ||||||||||
| Yes | 88/384 (22.9) | 412 | 21.5 (17.2-26.5) | 62/475 (13.0) | 674 | 9.2 (7.1-11.8) | 31/477 (6.5) | 675 | 4.6 (3.1-6.6) | |
| No | 82/363 (22.6) | 413 | 19.8 (15.8-24.6) | 39/404 (9.6) | 574 | 6.8 (4.8-9.3) | 24/408 (5.9) | 574 | 4.2 (2.7-6.3) | |
| Human leukocyte antigen DR-DQ genotype c | ||||||||||
| DR3/DR4-DQ8 | 97/416 (23.3) | 472 | 20.6 (16.7-25.1) | 45/473 (9.5) | 669 | 6.7 (4.9-9.0) | 36/478 (7.5) | 670 | 5.4 (3.8-7.5) | |
| Other | 73/331 (22.1) | 353 | 20.7 (16.2-26.0) | 56/406 (13.8) | 579 | 9.7 (7.3-12.6) | 19/407 (4.7) | 579 | 3.3 (2.0-5.1) | |
Abbreviation: HLA, human leukocyte antigen.
The frequency and incidence of SARS-CoV-2 antibodies were calculated from July 2020 to June 2022 for the variables other than period. The frequency and incidence for H1N1 antibodies and islet autoantibodies were calculated from February 2018 to June 2022.
Islet autoantibodies are given for cases until age 2 years.
HLA DR4-DQ/DR4-DQ8 refers to the HLA DR-DQ genotype conferring the highest risk of type 1 diabetes; other includes children who had the DR4-DQ8/DR4-DQ8 genotype or for children who had a first-degree relative containing DR4-DQ8 in the absence of a protective HLA DR or DQ allele.
Influenza A(H1N1) Antibodies
To assess infection by other viruses during the study period, antibodies to the HA1 antigen of influenza A(H1N1) were tested until age 24 months. After excluding maternally transferred antibodies, 101 children developed influenza A(H1N1) antibodies (Figure 1B). The incidence rate per 100 person-years of the first development of influenza A(H1N1) antibodies was 13.7 (95% CI, 10.5-17.7) from February 2018 to June 2020 and was 4.0 (95% CI, 2.7-5.8) from July 2020 to December 2021 (P < .001), and 11.8 (95% CI, 6.3-20.2) from January 2022 to June 2022 (P = .63; Table).
Islet Autoantibodies
Islet autoantibodies, as defined by the study primary outcome, developed in 55 children by age 24 months and in 5 additional children at age 30 months (Figure 1B) with a cumulative frequency of 6.8% (95% CI, 5.3%-7.6%) by age 30 months. All 60 children have continued to test positive through the last follow-up (December 31, 2022) and 20 (33.3%) have developed type 1 diabetes.
Twenty-one of the 60 children developed islet autoantibodies from October 2018, when the first child tested positive, through June 2020 and 39 from July 2020 to September 2022. The incidence rate per 100 person-years of developing islet autoantibodies was 4.9 (95% CI, 3.0-7.4) from February 2018 to June 2020 and 4.1 (95% CI, 2.9-5.8) from July 2020 to September 2022 (P = .46; Table). The cumulative risk of islet autoantibodies after 12 months of follow-up was 5.6% (95% CI, 3.5%-8.8%) for the 560 children with follow-up visits through June 2020 and 5.0% (95% CI, 3.1%-8.0%) in 325 children who had their first follow-up visit in July 2020 (P = .80; Figure 1C).
Islet Autoantibody and SARS-CoV-2 Antibodies
Six children of the 60 children developed islet autoantibodies at the same time as developing SARS-CoV-2 antibodies and 6 children developed islet autoantibodies at the next visit after testing positive for SARS-CoV-2 antibodies (Figure 2A). None of the children developed islet autoantibodies concurrently with or after having developed influenza A(H1N1) antibodies. The incidence rate per 100 person-years of islet autoantibodies from February 2018 through September 2022 was 4.0 (95% CI, 3.0-5.3) among children when they had tested negative for SARS-CoV-2 antibodies and 7.8 (95% CI, 4.9-14.0; P = .04) from the last SARS-CoV-2 antibody–negative time point among children who developed SARS-CoV-2 antibodies (Figure 2B). The incidence rate of islet autoantibodies from July 2020 was 3.5 (95% CI, 2.2-5.1) among children who tested negative for SARS-CoV-2 antibodies (P = .02). The incidence rate ratio for SARS-CoV-2 positive vs negative was 2.3 (95% CI, 1.02-4.80; P = .03). The attributable proportion of SARS-CoV-2 infection to the development of islet autoantibodies since July 2020 in the infected group was 57%, resulting in an estimated proportion of 18% of all islet autoantibody-positive cases since July 2020 attributable to SARS-CoV-2 infection. The incidence rate of islet autoantibodies in the children with SARS-CoV-2 antibodies was 7.3 (95% CI, 2.3-19.0) for the period concurrent with SARS-CoV-2 antibodies, 8.3 (95% CI, 3.1-18.1; P = .04 vs SARS-CoV-2 negative) for the period to the subsequent study visit, and 3.8 (95% CI, 0.8-11.2) for the period prior to SARS-CoV-2 antibodies (Figure 2B).
Figure 2. Islet Autoantibodies and Their Temporal Relationship to SARS-CoV-2 Antibodies.
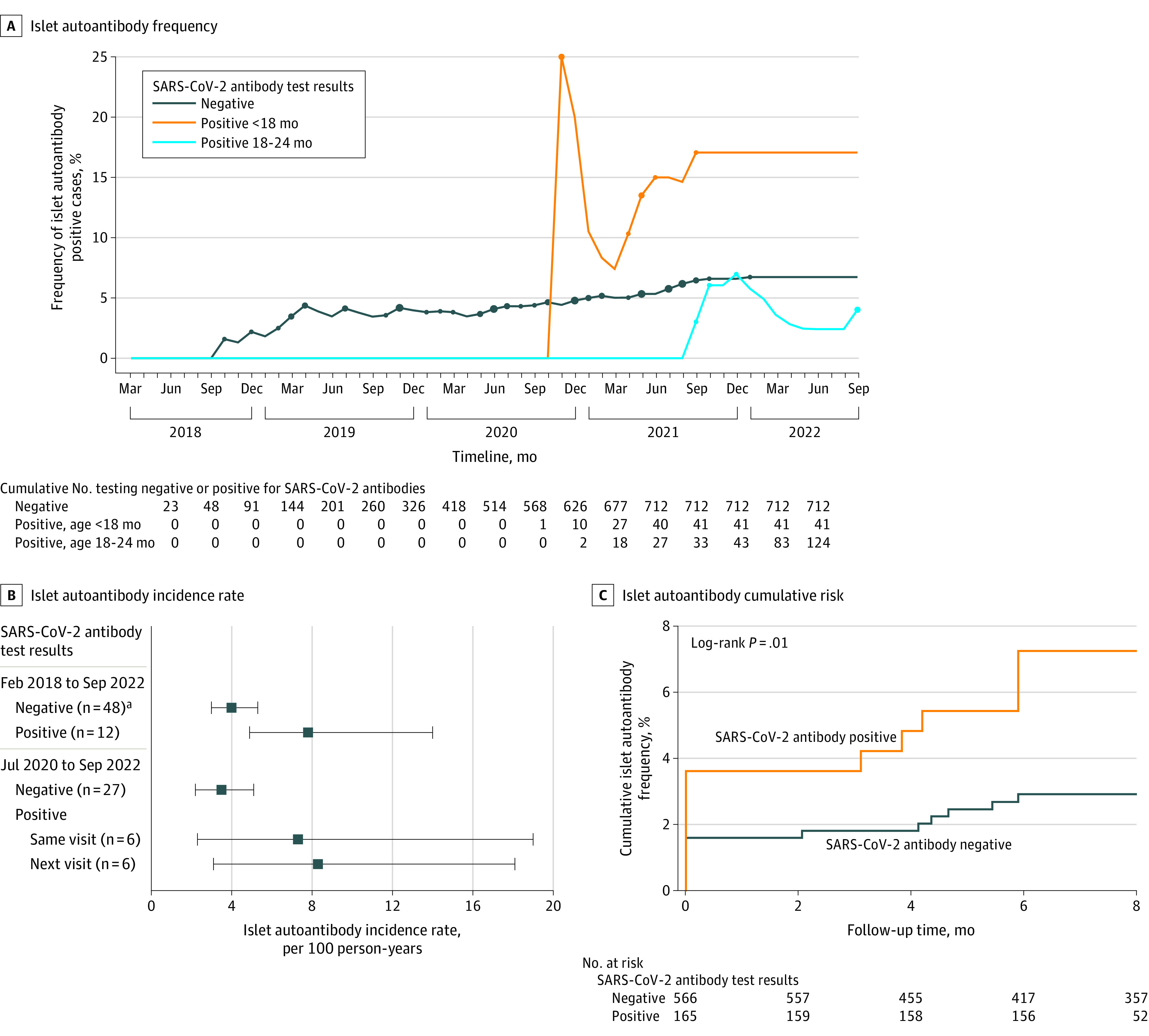
A, Frequency of children who tested positive for islet autoantibodies at the end of each month of the study is shown for 712 children who tested negative for SARS-CoV-2 antibodies and 165 children who tested positive for SARS-CoV-2 antibodies before age 18 months (visits 2, 3, or 4) or at age 18 or 24 months (visits 5 or 6). Five children who tested positive for SARS-CoV-2 antibodies developed islet autoantibodies prior to testing positive for SARS-CoV-2 antibodies and are included in the SARS-CoV-2 antibody–negative group. Dots indicate months when there were new cases of children who tested positive for islet autoantibodies. The size of the dot indicates the number of new cases (1, 2, or 3). The cumulative number of children in each group are presented at the end of each 3-month period and corresponding to the end of the indicated month.
B, From February 2018 through September 2022, the incidence rate of islet autoantibodies in 877 children is stratified by when they tested negative for SARS-CoV-2 (48 islet autoantibody–positive cases) and when they tested positive for SARS-CoV-2 antibodies (12 islet autoantibody-\–positive cases). For the children who tested positive for SARS-CoV-2, the incidence rate is calculated for islet autoantibodies that developed either in the same visit or after the first visit after testing positive for SARS-CoV-2. The incidence rate of islet autoantibodies is also shown from July 2020 to September 2022 for 735 children through the last time they tested negative for SARS-CoV-2 (27 islet autoantibody–positive cases) and for the 170 children who tested positive for SARS-CoV-2 from the last visit they tested negative for SARS-CoV-2 antibodies to the first visit they tested positive for SARS-CoV-2 antibodies (6 islet autoantibody–positive cases) and from the first visit they tested positive for SARS-CoV-2 antibodies and the next visit (6 islet autoantibody cases). The data markers represent the incidence rate and the whiskers, 95% CIs.
aThe number of children who developed islet autoantibodies.
C, Kaplan-Meier curves of the cumulative frequency of islet autoantibody seroconversion among 731 children who were islet autoantibody negative in June 2020 from the time of first SARS-CoV-2 antibodies (n = 165) and for children who did not develop SARS-CoV-2 antibodies from the first sample after June 2020 (n = 566). Five children who developed islet autoantibodies prior to the development of SARS-CoV-2 antibodies are not included in the SARS-CoV-2 antibody–positive group.
The relationship between islet autoantibodies and SARS-CoV-2 antibodies from July 2020 in the remaining 735 children who were negative for islet autoantibodies was also examined using time-varying analyses. The sex-, age-, and country-adjusted HR for developing islet autoantibodies when children tested positive for SARS-CoV-2 antibodies was 3.5 (95% CI, 1.6-7.7; P = .002) in a time-dependent Cox model. The cumulative risk of developing islet autoantibodies was 7.3% (95% CI, 4.2%-12.7%) within 6 months of testing positive for SARS-CoV-2 antibodies and 2.9% (95% CI, 1.8%-4.8%) among children who did not develop SARS-CoV-2 antibodies (P = .01; Figure 2C).
Age of Infection
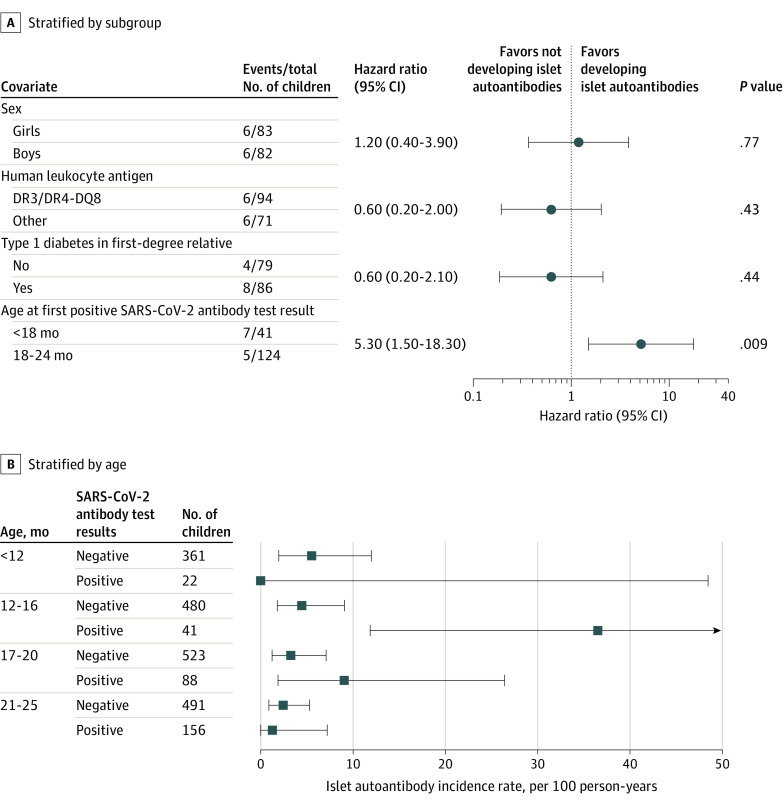
Factors associated with the development of islet autoantibodies were examined in children who tested positive for SARS-CoV-2 antibodies using Cox proportional hazards (Figure 3A). SARS-CoV-2 antibodies at visits prior to age 18 months was associated with an increased risk of islet autoantibodies (HR, 5.3; 95% CI, 1.5-18.3; P = .009). The association with age was pronounced at age 12 to 16 months (Figure 3B), which corresponded to visit 4 and to the previously described peak incidence of islet autoantibodies among children with a genetic susceptibility for type 1 diabetes.2,25,26,27 The incidence rate was 36.5 (95% CI, 11.9-85.2) among children who first tested positive for SARS-CoV-2 antibodies at age 12 to 16 months compared with 4.4 (95% CI, 1.8-9.2) among children who tested negative for SARS-CoV-2 antibodies until this age (P < .001), with an incidence rate ratio of 8.2 (95% CI, 2.1-30.1; P = .002). Islet autoantibodies developed at age 12 to 16 months in 5 of 41 children (12.2%) with SARS-CoV-2 antibodies compared with 7 of 480 (1.5%) who were negative at this age from July 2020 (P < .001) and 8 of 341 (2.3%) prior to July 2020 (P < .001). Similarly, among the children who developed SARS-CoV-2 antibodies between the ages of 12 and 16 months (visit 4), islet autoantibodies were first detected at the same visit in 4 of 19 (21.1%) and in the next visit at age 18 months (visit 5) in 2 of the remaining 15 (13.3%) compared with children who tested negative for the SARS-CoV-2 antibody until visit 4, in whom 15 of 821 (1.8%) tested positive for islet autoantibodies at the same visit (P < .001) and 9 of 755 (1.2%) at the next visit (P = .02; eTable 3 in Supplement).
Figure 3. Stratified Analyses of the Association Between SARS-CoV-2 Infection and Islet Autoantibody Development.
A, Univariable Cox proportional hazards model performed on 165 SARS-CoV-2 antibody–positive children who were negative for islet autoantibodies at the time of SARS-CoV-2 antibody seroconversion. Each covariate was binary with the risk category and the number of islet autoantibody–positive events and total number in each category indicated.
B, Incidence rate of islet autoantibodies from July 2020 in children younger than age 12 months (study visits 2 and 3), age 12 to 16 months (study visit 4), age 17 to 20 months (study visit 5), and age 21 to 25 months (study visit 6) stratified for their SARS-CoV-2 antibody status as positive or negative at each age category. The SARS-CoV-2 antibody–positive category at each age group includes cases that were positive up to and including the indicated age group. The incidence rate at each age category is calculated for the number of new islet autoantibody events observed in the interval from the preceding age category. For the children positive for SARS-CoV-2, new islet autoantibody events observed together with or after a previous SARS-CoV-2 antibody seroconversion are included.
Discussion
Antibodies to the SARS-CoV-2 virus and the influenza A(H1N1) virus were longitudinally measured across the COVID-19 pandemic period in young children with a high genetic risk of type 1 diabetes. An association was found between the development of islet autoantibodies concurrent with or soon after the development of SARS-CoV-2 antibodies but not with H1N1 antibodies. This association was strongest in children infected before 18 months of age. The findings indicate that SARS-CoV-2 infections are associated with an increased risk of islet autoimmunity in young children with a high genetic risk of type 1 diabetes.
This study is one of a few studies to actively recruit and longitudinally follow-up young children for islet autoimmunity during the COVID-19 pandemic. A unique feature of this study was the frequent follow-up of genetically at-risk children at an age with greatest susceptibility to islet autoimmunity both before and during the pandemic. This allowed a comparison of the incidences and risks in these periods and, unlike previous studies, the ability to define the likely timing and age of infection and islet autoantibody development. In contrast, a previous cross-sectional analysis by Rewers et al28 of children in Germany and the US, which found no association between islet autoantibodies and SARS-CoV-2 antibodies in children aged 1 and three-quarters to 18 years, could not determine whether SARS-CoV-2 antibodies appeared prior to the development of islet autoantibodies. In hindsight, the children in those cross-sectional studies were not selected for their genetic susceptibility and were past the age of greatest susceptibility. Therefore, it is possible that most of the children may have developed islet autoantibodies prior to SARS-CoV-2 infection. Indeed, a major strength of the measurements in this study is that the children were followed up during their peak risk period for developing islet autoantibodies, a feature that proved particularly relevant for the outcome of interest.
Another strength of the study was that other viral infections, which are potential confounders when examining associations between a specific infection and the development of autoimmunity, were likely to be reduced during the pandemic period. Evidence for this reduction was the 3-fold decrease in the incidence rate of influenza A(H1N1) antibodies in study participants observed in 2020 and 2021. Also relevant is that, despite the increased risk in children who developed SARS-CoV-2 antibodies, there was no overall increase in the incidence of islet autoantibodies during the pandemic period. This would be consistent with a corresponding reduction in other infectious exposures associated with islet autoantibody risk. Indeed, the pandemic and the lifestyle changes related to social distancing during the pandemic created an unprecedented opportunity to examine the association between a newly introduced virus and islet autoimmunity. The absence of an overall increase in islet autoantibody incidence rate may also seem contradictory to increased incidence rate of disease during the pandemic.8,9,10,11 However, the proportion of islet autoantibody–positive cases attributable to SARS-CoV-2 infection in the current study was 18% and the majority of these are only expected to develop clinical diabetes in subsequent years. Therefore, the association observed with islet autoantibody development is unlikely to underly the rapid increase in type 1 diabetes incidence rate early in the pandemic period but is likely to be relevant to future type 1 diabetes incidence rates.
The age of SARS-CoV-2 infection appeared to be critical in the association with islet autoimmunity. It has been postulated that the peak incidence of islet autoantibodies at around 12 months of age may reflect either a relative abundance of initial exposures to diabetogenic insults or an increased vulnerability for islet autoimmunity at this age. This study provided the possibility of assessing these hypotheses. The study was particularly suitable because SARS-CoV-2 was newly introduced into the community with little or no maternally transferred protection and no vaccine-induced protection because vaccination had not been approved for children younger than 5 years in Europe until after this study concluded. The finding that the association between SARS-CoV-2 antibodies and islet autoantibodies was strongest around the peak age of islet autoantibody risk is consistent with an increased vulnerability of islet autoimmunity at around 12 months of age. The mechanisms for the association with age are speculative and may include the possibility that SARS-CoV-2 may infect islet cells at a vulnerable age of β-cell activity, as has been postulated for coxsackievirus B,29 leading to changes that unmask islet β cells to autoreactive T cells in susceptible children.30
Limitations
The current study has several limitations. First, the findings are limited to those with high genetic susceptibility to type 1 diabetes. The study obtained samples from more than 700 children within the pandemic period. These children represent the 1% of newborns with the greatest genetic risk of developing type 1 diabetes. However, the selection process excludes at least 75% of all cases who will develop the disease during childhood16 and may not be generalizable to individuals of all ethnicities.31 Second, the study used SARS-CoV-2 antibodies to define infection and did not have access to verification via PCR. Nevertheless, the antibody measurements and strategy were previously shown to be highly specific and sensitive.18,32 Third, although the sampling was relatively frequent, it was not possible to determine whether SARS-CoV-2 infection occurred prior to the development of islet autoantibodies in the children who tested positive for SARS-CoV-2 antibodies and islet autoantibodies in the same sample. This introduces bias that favors an association between the 2 antibodies. However, the incidence rate and cumulative risk of islet autoantibodies remained increased when only events that occurred after the first SARS-CoV-2 antibody–positive visit were considered. Fourth, the total number of children who developed islet autoantibodies during the pandemic was small and there are unlikely to be other studies recruiting a sufficient number of young children during 2020 and 2021 that could be used to validate the findings. Fifth, reverse causality such as a potential increased susceptibility to infection in children who will develop islet autoantibodies or confounders that increase both the likelihood of SARS-CoV-2 antibodies and islet autoantibodies could not be excluded. Sixth, children were randomly assigned to receive daily oral insulin or placebo and, although highly unlikely, the possibility that the association between SARS-CoV-2 infection and islet autoimmunity was influenced by the administration of the study drug cannot be excluded.
This longitudinal study has identified SARS-CoV-2 infection as a risk factor for islet autoimmunity in young children at increased genetic risk of type 1 diabetes. The magnitude of risk is similar or greater than the risks previously reported for other viruses, including enteroviruses.4,5 Further monitoring of children is needed to determine whether the increase in islet autoimmunity will be followed by continued increases in the incidence rate of childhood type 1 diabetes.
Conclusions
In young children with high genetic risk of type 1 diabetes, SARS-CoV-2 infection was temporally associated with the development of islet autoantibodies.
eTable 1. Age and Period of Follow-up Visits in 885 Children of Study
eTable 2. Characteristics of Children Included in Follow-up SARS-CoV-2 Antibody Measurements
eTable3. Temporal Development of Islet Autoantibodies in Relation to SARS-CoV-2 Antibodies at Age 12 to 16 months
eFigure 1. Linear Schematic of Constructs Encoding Antigens Fused to Nanoluc Used in the Luciferase Immunoprecipation System (LIPS) Assay
eFigure 2. Examples of SARS-CoV-2 (A) and H1N1 Antibody Titres (B,C) Over Time in Children
eFigure 3. Children in POInT Study Tested in the Ancillary Study
eFigure 4. Incidence of SARS-CoV-2 Antibodies during the Pandemic Period (A) at Different Study Sites (B)
Nonauthor Collaborators. The GPPAD collaborators
Data Sharing Statement
References
- 1.Ziegler AG, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity. 2010;32(4):468-478. doi: 10.1016/j.immuni.2010.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krischer JP, Lynch KF, Schatz DA, et al. ; TEDDY Study Group . The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58(5):980-987. doi: 10.1007/s00125-015-3514-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473-2479. doi: 10.1001/jama.2013.6285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krischer JP, Liu X, Lernmark Å, et al. ; TEDDY Study Group . Predictors of the initiation of islet autoimmunity and progression to multiple autoantibodies and clinical diabetes: the TEDDY study. Diabetes Care. 2022;45(10):2271-2281. doi: 10.2337/dc21-2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laitinen OH, Honkanen H, Pakkanen O, et al. Coxsackievirus B1 is associated with induction of β-cell autoimmunity that portends type 1 diabetes. Diabetes. 2014;63(2):446-455. doi: 10.2337/db13-0619 [DOI] [PubMed] [Google Scholar]
- 6.Vehik K, Lynch KF, Wong MC, et al. ; TEDDY Study Group . Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat Med. 2019;25(12):1865-1872. doi: 10.1038/s41591-019-0667-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyerlein A, Wehweck F, Ziegler AG, Pflueger M. Respiratory infections in early life and the development of islet autoimmunity in children at increased type 1 diabetes risk: evidence from the BABYDIET study. JAMA Pediatr. 2013;167(9):800-807. doi: 10.1001/jamapediatrics.2013.158 [DOI] [PubMed] [Google Scholar]
- 8.Kamrath C, Rosenbauer J, Eckert AJ, et al. Incidence of type 1 diabetes in children and adolescents during the COVID-19 pandemic in Germany: results from the DPV registry. Diabetes Care. 2022;45(8):1762-1771. doi: 10.2337/dc21-0969 [DOI] [PubMed] [Google Scholar]
- 9.Gottesman BL, Yu J, Tanaka C, Longhurst CA, Kim JJ. Incidence of new-onset type 1 diabetes among US children during the COVID-19 global pandemic. JAMA Pediatr. 2022;176(4):414-415. doi: 10.1001/jamapediatrics.2021.5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKeigue PM, McGurnaghan S, Blackbourn L, et al. Relation of incident type 1 diabetes to recent COVID-19 infection: cohort study using e-health record linkage in Scotland. Diabetes Care. 2023;46(5):921-928. doi: 10.2337/dc22-0385 [DOI] [PubMed] [Google Scholar]
- 11.Weiss A, Donnachie E, Beyerlein A, Ziegler AG, Bonifacio E. Type 1 diabetes incidence and risk in children with a diagnosis of COVID-19. JAMA. 2023;329(23):2089-2091. doi: 10.1001/jama.2023.8674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fignani D, Licata G, Brusco N, et al. SARS-CoV-2 receptor angiotensin i-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front Endocrinol (Lausanne). 2020;11:596898. doi: 10.3389/fendo.2020.596898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller JA, Groß R, Conzelmann C, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3(2):149-165. doi: 10.1038/s42255-021-00347-1 [DOI] [PubMed] [Google Scholar]
- 14.Wu CT, Lidsky PV, Xiao Y, et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021;33(8):1565-1576.e5. doi: 10.1016/j.cmet.2021.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler AG, Achenbach P, Berner R, et al. ; GPPAD Study group . Oral insulin therapy for primary prevention of type 1 diabetes in infants with high genetic risk: the GPPAD-POINT (Global Platform for the Prevention of Autoimmune Diabetes Primary Oral Insulin Trial) study protocol. BMJ Open. 2019;9(6):e028578. doi: 10.1136/bmjopen-2018-028578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkler C, Haupt F, Heigermoser M, et al. ; GPPAD Study Group . Identification of infants with increased type 1 diabetes genetic risk for enrollment into Primary Prevention Trials-GPPAD-02 study design and first results. Pediatr Diabetes. 2019;20(6):720-727. doi: 10.1111/pedi.12870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonifacio E, Beyerlein A, Hippich M, et al. ; TEDDY Study Group . Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: a prospective study in children. PLoS Med. 2018;15(4):e1002548. doi: 10.1371/journal.pmed.1002548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hippich M, Holthaus L, Assfalg R, et al. A public health antibody screening indicates a 6-fold higher SARS-CoV-2 exposure rate than reported cases in children. Med. 2021;2(2):149-163.e4. doi: 10.1016/j.medj.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warncke K, Weiss A, Achenbach P, et al. ; GPPAD and POInT Study Groups . Elevations in blood glucose before and after the appearance of islet autoantibodies in children. J Clin Invest. 2022;132(20):e162123. doi: 10.1172/JCI162123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naserke HE, Dozio N, Ziegler AG, Bonifacio E. Comparison of a novel micro-assay for insulin autoantibodies with the conventional radiobinding assay. Diabetologia. 1998;41(6):681-683. doi: 10.1007/s001250050968 [DOI] [PubMed] [Google Scholar]
- 21.Achenbach P, Lampasona V, Landherr U, et al. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia. 2009;52(9):1881-1888. doi: 10.1007/s00125-009-1438-0 [DOI] [PubMed] [Google Scholar]
- 22.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab. 2010;95(7):3360-3367. doi: 10.1210/jc.2010-0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams AJ, Norcross AJ, Chandler KA, Bingley PJ. Non-specific binding to protein A Sepharose and protein G Sepharose in insulin autoantibody assays may be reduced by pre-treatment with glycine or ethanolamine. J Immunol Methods. 2006;314(1-2):170-173. doi: 10.1016/j.jim.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 24.Long AE, Gooneratne AT, Rokni S, Williams AJ, Bingley PJ. The role of autoantibodies to zinc transporter 8 in prediction of type 1 diabetes in relatives: lessons from the European Nicotinamide Diabetes Intervention Trial (ENDIT) cohort. J Clin Endocrinol Metab. 2012;97(2):632-637. doi: 10.1210/jc.2011-1952 [DOI] [PubMed] [Google Scholar]
- 25.Bonifacio E, Weiß A, Winkler C, et al. ; TEDDY Study Group . An age-related exponential decline in the risk of multiple islet autoantibody seroconversion during childhood. Diabetes Care. 2021;44(10):2260-2268. doi: 10.2337/dc20-2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziegler AG, Bonifacio E; BABYDIAB-BABYDIET Study Group . Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia. 2012;55(7):1937-1943. doi: 10.1007/s00125-012-2472-x [DOI] [PubMed] [Google Scholar]
- 27.Parikka V, Näntö-Salonen K, Saarinen M, et al. Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia. 2012;55(7):1926-1936. doi: 10.1007/s00125-012-2523-3 [DOI] [PubMed] [Google Scholar]
- 28.Rewers M, Bonifacio E, Ewald D, et al. ; ASK Study Group and Fr1da Study Group . SARS-CoV-2 infections and presymptomatic type 1 diabetes autoimmunity in children and adolescents from Colorado, USA, and Bavaria, Germany. JAMA. 2022;328(12):1252-1255. doi: 10.1001/jama.2022.14092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ifie E, Russell MA, Dhayal S, et al. Unexpected subcellular distribution of a specific isoform of the Coxsackie and adenovirus receptor, CAR-SIV, in human pancreatic beta cells. Diabetologia. 2018;61(11):2344-2355. doi: 10.1007/s00125-018-4704-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Herrath M, Bonifacio E. How benign autoimmunity becomes detrimental in type 1 diabetes. Proc Natl Acad Sci U S A. 2021;118(44):e2116508118. doi: 10.1073/pnas.2116508118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redondo MJ, Gignoux CR, Dabelea D, et al. Type 1 diabetes in diverse ancestries and the use of genetic risk scores. Lancet Diabetes Endocrinol. 2022;10(8):597-608. doi: 10.1016/S2213-8587(22)00159-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Secchi M, Bazzigaluppi E, Brigatti C, et al. COVID-19 survival associates with the immunoglobulin response to the SARS-CoV-2 spike receptor binding domain. J Clin Invest. 2020;130(12):6366-6378. doi: 10.1172/JCI142804 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Age and Period of Follow-up Visits in 885 Children of Study
eTable 2. Characteristics of Children Included in Follow-up SARS-CoV-2 Antibody Measurements
eTable3. Temporal Development of Islet Autoantibodies in Relation to SARS-CoV-2 Antibodies at Age 12 to 16 months
eFigure 1. Linear Schematic of Constructs Encoding Antigens Fused to Nanoluc Used in the Luciferase Immunoprecipation System (LIPS) Assay
eFigure 2. Examples of SARS-CoV-2 (A) and H1N1 Antibody Titres (B,C) Over Time in Children
eFigure 3. Children in POInT Study Tested in the Ancillary Study
eFigure 4. Incidence of SARS-CoV-2 Antibodies during the Pandemic Period (A) at Different Study Sites (B)
Nonauthor Collaborators. The GPPAD collaborators
Data Sharing Statement