Key Points
Question
Is premorbid sociodemographic status associated with multiple sclerosis severity, independent of treatment?
Findings
In this nationwide cohort study of 4557 patients in a universal health care context, higher premorbid educational attainment and income were associated with up to 47% milder subsequent disability and patient-reported symptoms in both relapse-onset and progressive-onset disease, after adjusting for individual treatment. Marital separation was associated with up to 35% higher disease severity in relapse-onset disease.
Meaning
These findings suggest that predisease education, income, and marital status are associated with severity of future disability and symptom severity, and they should be considered in risk stratification.
This cohort study examines whether premorbid education, income, and marital status are associated with future multiple sclerosis (MS) disability and symptom severity, independent of treatment, in a universal health care context.
Abstract
Importance
Multiple sclerosis (MS) severity may be informed by premorbid sociodemographic factors.
Objective
To determine whether premorbid education, income, and marital status are associated with future MS disability and symptom severity, independent of treatment, in a universal health care context.
Design, Setting, and Participants
This nationwide observational cohort study examined data from the Swedish MS Registry linked to national population registries from 2000 to 2020. Participants included people with MS onset from 2005 to 2015 and of working age (aged 23 to 59 years) 1 year and 5 years preceding disease onset.
Exposures
Income quartile, educational attainment, and marital status measured at 1 and 5 years preceding disease onset.
Main Outcome and Measures
Repeated measures of Expanded Disability Status Scale (EDSS) scores and patient-reported Multiple Sclerosis Impact Scale (MSIS-29) scores. Models were adjusted for age, sex, relapses, disease duration, and treatment exposure. Secondary analyses further adjusted for comorbidity. All analyses were stratified by disease course (relapse onset and progressive onset).
Results
There were 4557 patients (mean [SD] age, 37.5 [9.3] years; 3136 [68.8%] female, 4195 [92.1%] relapse-onset MS) with sociodemographic data from 1-year preonset of MS. In relapse-onset MS, higher premorbid income and education correlated with lower disability (EDSS, −0.16 [95% CI, −0.12 to −0.20] points) per income quartile; EDSS, −0.47 [95% CI, −0.59 to −0.35] points if tertiary educated), physical symptoms (MSIS-29 physical subscore, −14% [95% CI, −11% to −18%] per income quartile; MSIS-29 physical subscore, −43% [95% CI, −35% to −50%] if tertiary educated), and psychological symptoms (MSIS-29 psychological subscore, −12% [95% CI, −9% to −16%] per income quartile; MSIS-29 psychological subscore, −25% [95% CI, −17% to −33%] if tertiary educated). Marital separation was associated with adverse outcomes (EDSS, 0.34 [95% CI, 0.18 to 0.51]; MSIS-29 physical subscore, 35% [95% CI, 12% to 62%]; MSIS-29 psychological subscore, 25% [95% CI, 8% to 46%]). In progressive-onset MS, higher income correlated with lower EDSS (−0.30 [95% CI, −0.48 to −0.11] points per income quartile) whereas education correlated with lower physical (−34% [95% CI, −53% to −7%]) and psychological symptoms (−33% [95% CI, −54% to −1%]). Estimates for 5-years preonset were comparable with 1-year preonset, as were the comorbidity-adjusted findings.
Conclusions and relevance
In this cohort study of working-age adults with MS, premorbid income, education, and marital status correlated with disability and symptom severity in relapse-onset and progressive-onset MS, independent of treatment. These findings suggest that socioeconomic status may reflect both structural and individual determinants of health in MS.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system. It is the most common nontraumatic cause of neurological disability in young people, and the severity is highly variable. Commonly studied disease and treatment factors only partially explain this variation in outcomes, and recent years have seen growing interest in the effect of sociodemographic factors on MS severity.1,2,3,4,5 The importance of social determinants of health have long been acknowledged in the field of public health,6 where a commonly used framework implicates education, income, and employment in facilitating better health literacy, access to quality health care, and health-optimizing environments and behaviors.7,8,9
Aside from socioeconomic factors, intermediary ecological factors such as interpersonal relationships have also been implicated in health outcomes.10 Previous studies show partnered individuals have lower morbidity and mortality, which is partially attributed to facilitation of access to health care.11,12 Recent studies in the MS context demonstrate associations between socioeconomic status (SES), measured at or after onset or diagnosis, and clinical measures of MS severity.3,4,5 Given the timing of the exposure, one cannot infer the direction of the relationship from these studies, as the disease may have influenced SES. Furthermore, previous studies have not addressed the question of whether marital status influences MS severity.
Using linked data from Sweden’s health administrative and quality registries, our study aimed to assess whether premorbid income, educational attainment, or marital status are associated with MS severity, independently of treatment factors, and in a universal health care context. We hypothesized that higher premorbid education and income would be associated with more favorable clinical disability and patient-reported symptoms, and that having a partner may mitigate psychological symptoms, but not physical symptoms or disability, compared with nonpartnered states (single or separated). Furthermore, we postulated that the social determinants with effects mediated by health care may have a greater effect on the treatable, relapsing-onset subtype of MS, than on the more treatment-resistant, primary progressive subtype of MS.
Methods
Study Design, Participants, and Setting
This national observational cohort study accessed individual-level patient data from the (1) Swedish MS Registry, (2) Longitudinal Integrated Database for Health Insurance and Labor Market Studies, and (3) National Patient Register. The Swedish MS Registry records clinical data on approximately 84% of the prevalent MS cases in Sweden.13 Data were entered prospectively as part of routine clinical care nationwide since starting in 2001. Participation is voluntary and informed consent is obtained from all patients regarding the use of their data for research purposes. Data used for this study included birthdate, onset date, disease course, relapses, treatment (product, start and stop dates), Expanded Disability Status Scale (EDSS) scores, Multiple Sclerosis Impact Scale (MSIS-29) scores, and their corresponding dates.
The Longitudinal Integrated Database for Health Insurance and Labor Market Studies is a government-administered registry that records annual sociodemographic data for all inhabitants of Sweden over 15 years of age on December 31 each calendar year. Linked data for our study were extracted from calendar years 2000 to 2014 and included annual income, educational attainment, and marital status.
The National Patient Registry records primary and secondary diagnoses of all inpatient and specialist outpatient episodes in Sweden, along with the corresponding episode dates. Nationwide inpatient data were available from 1987 and outpatient data from 2001; data from inception until December 31, 2019, were extracted from this registry.
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.20 Participation in the Swedish MS Registry is voluntary, and written informed consent was obtained from all patients regarding the use of their data for research purposes. The remainder of the registries are mandatory, government-administered registries. Ethical approval for use of linked, anonymized data for the purposes of this study was granted by the Swedish Ethical Review Authority.
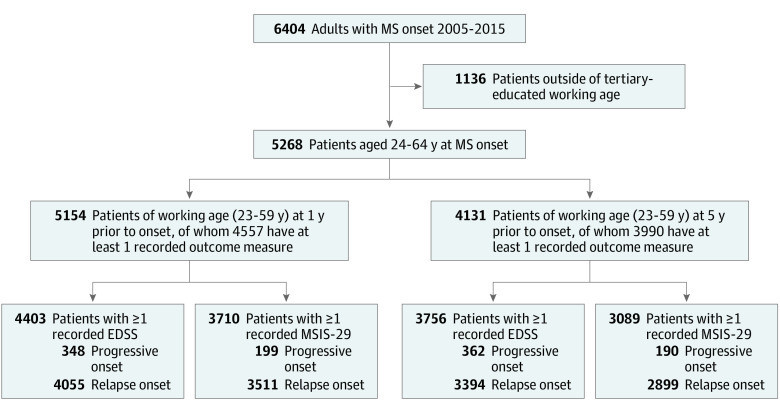
Study participants were persons with a diagnosis of MS made by a neurologist, with disease onset between January 1, 2005, and December 31, 2015, and of working age (23 to 59 years) at either of 2 index times: 1 calendar year or 5 calendar years prior to disease onset. This age threshold gave all participants the possibility of completing 3 years of tertiary education prior to the commencement of paid employment at the index times. Eligible participants required complete baseline clinical data (date of birth, sex, disease course, and MS onset date), as well as complete socioeconomic data from either of the 2 index times. Lastly, participants were required to have at least 1 recorded study outcome (EDSS or MSIS-29) (Figure 1).
Figure 1. Flow Diagram of Patient Selection.
EDSS indicates Expanded Disability Status Scale; MS, multiple sclerosis; MSIS-29, Multiple Sclerosis Impact Scale.
Exposure
The study exposures of interest were educational attainment, income quartile, and civil status at 1 and 5 years preceding disease onset. These index times were chosen to minimize the risk of reverse causation due to MS prodrome affecting academic, vocational, or social functioning.
Highest level of educational attainment (referred to henceforth as education), categorized as (1) presecondary (did not complete senior high school or A levels equivalent), (2) secondary (successfully completed senior high school or A levels equivalent), or (3) tertiary (completed at least 3 years of tertiary education, which is a standard duration of a bachelor degree in Sweden). Income quartile (referred to henceforth as income), which was the individual annual taxable income from any source, modeled in quartiles (ordinal variable, with 1 denoting the lowest income quartile and 4 denoting the highest) with reference to the study population. Marital status, categorized as single, partnered (married or civil partnership), or separated/divorced.
Outcomes
The study outcomes were repeated measures of clinical disability (EDSS), and patient-reported symptoms (MSIS-29). The EDSS14,15 is an ordinal scale from 0 (no disability) to 10 (death) points, with the smallest increment being 0.5, with the exception of an increment of 1 between scores 0 and 1. It is assessed approximately annually by a clinician at the time of clinic visits.
The MSIS-2916 is a disease-specific, patient-reported outcome measure, consisting of questions regarding the presence and severity of physical (20 items) and psychological (9 items) symptoms of MS experienced in the previous 2 weeks. The physical and psychological scores are converted to percentage scores out of 100, with higher values indicating more severe disability. Patients could complete MSIS-29 assessments at any time through the registry’s online patient portal and were encouraged to do so preceding their clinical visits.
Statistical Analysis
Analyses were completed separately for the index times 1 and 5 years prior to MS onset, and further stratified by disease course. Follow-up time for each patient was from their disease onset until the latest recorded outcome measure.
EDSS, although an ordinal variable, is typically modeled as a continuous variable, as the model outcomes are comparable with ordinal models.17 This study modeled EDSS using linear mixed models due to 2 levels of clustering.17 Model estimates for EDSS were reported as β coefficients. MSIS physical and psychological outcomes were modeled using generalized linear mixed models with a log link gamma function. Zeroes were handled by reassigning them the lowest possible nonzero value in their respective subscales. Model estimates for MSIS-29 were reported as relative difference compared with the reference estimate.
In all analyses, patient identifier was modeled as a random intercept to account for the dependency of repeated measures from the same patient. Calendar year of index was also modeled as a random intercept to account for shifts in baseline variable values over time. Additionally, the following variables were included in the main and secondary analyses: age at disease onset, sex, and disease duration at time of each outcome measurement. For patients with relapse-onset disease, number of relapses in the first 2 years of disease and treatment with high-efficacy or lower-efficacy therapies were also included. Treatment was modeled as the proportion of disease time (between onset date and the date of each outcome measure) under treatment with high-efficacy and lower-efficacy therapies. High-efficacy treatments included: rituximab, ocrelizumab, mitoxantrone, alemtuzumab, natalizumab, and hematopoietic stem cell transplant.18 Lower-efficacy therapy included interferon-β, glatiramer acetate, fingolimod, dimethyl fumarate, teriflunomide, cladribine, and iponimod.18 For all analyses, exposures of interest were all included in the same model after confirming variance inflation factor of less than 2. Unadjusted estimates for each variable modeled individually are provided for comparison.
To assess the association of comorbidity, a secondary analysis repeated the main analysis but with the inclusion of patients’ Charlson comorbidity index at disease onset, calculated using all primary and secondary diagnoses from inpatient and outpatient episodes in the 5-year period prior to the index date. The unadjusted index was stratified as 0 or greater than 1. Statistical significance was assessed by nonoverlap of 95% CIs with the null hypothesis. Statistical analyses were performed using R version 4.1.3 (R Project for Statistical Computing)19 from October 2021 to December 2022.
Results
Among the 4557 patients who fulfilled inclusion criteria for index time 1 year preceding MS onset, 3136 (68.8%) were female, 4195 (92.1%) had relapse-onset disease, and the mean (SD) age was 37.5 (9.3) years. The mean (SD) follow-up time was 8.5 (3.3) years. Baseline characteristics of those included in the EDSS and MSIS-29 analyses are in the Table.
Table. Patient Baseline Characteristics 1 Year Prior to Multiple Sclerosis Onset.
| Characteristic | Patients, No. (%) | |||
|---|---|---|---|---|
| EDSS analysis | MSIS-29 analysis | |||
| Progressive onset (n = 348) | Relapse onset (n = 4055) | Progressive onset (n = 199) | Relapse onset (n = 3511) | |
| Age at onset, mean (SD), y | 45.7 (8.5) | 36.9 (8.7) | 43.61 (8.7) | 36.3 (8.4) |
| Sex | ||||
| Male | 170 (48.9) | 1203 (29.7) | 97 (48.7) | 1048 (29.8) |
| Female | 178 (51.1) | 2852 (70.3) | 102 (51.3) | 2463 (70.2) |
| Income quartile | ||||
| 1 (Least affluent) | 76 (22.4) | 972 (24.9) | 41 (21.2) | 831 (24.5) |
| 2 | 67 (19.8) | 994 (25.5) | 38 (19.7) | 886 (26.1) |
| 3 | 96 (28.3) | 975 (25.0) | 57 (29.5) | 833 (24.5) |
| 4 (Most affluent) | 100 (29.5) | 957 (24.6) | 57 (29.5) | 844 (24.9) |
| Marital status | ||||
| Single | 148 (43.7) | 2179 (55.9) | 89 (46.1) | 1939 (57.1) |
| Partnered | 142 (41.9) | 1404 (36.0) | 81 (42.0) | 1200 (35.4) |
| Separated | 49 (14.5) | 315 (8.1) | 23 (11.9) | 255 (7.5) |
| Highest educational attainment | ||||
| Presecondary | 150 (44.5) | 1113 (28.8) | 73 (38.0) | 924 (27.4) |
| Secondary education | 122 (36.2) | 1636 (42.3) | 77 (40.1) | 1454 (43.1) |
| Tertiary | 65 (19.3) | 1121 (29.0) | 42 (21.9) | 994 (29.5) |
| Charlson comorbidity index | ||||
| 0 | 248 (92.2) | 3040 (94.4) | 153 (95.6) | 2662 (94.3) |
| >1 | 21 (7.8) | 180 (5.6) | 7 (4.4) | 160 (5.7) |
| No. of EDSS recorded at follow-up, median (IQR) | 5 (2-7) | 6 (4-10) | NA | NA |
| No. of MSIS-29 recorded at follow-up, median (IQR) | NA | NA | 2 (2-4) | 5 (3-7) |
| Follow-up time, mean (SD), y | 8.39 (3.30) | 8.22 (3.43) | 7.43 (3.21) | 8.10 (3.37) |
Abbreviations: EDSS, Expanded Disability Status Scale score; MSIS-29, Multiple Sclerosis Impact Scale score; NA, not applicable.
Relapse-Onset MS
After adjusting for disease and treatment parameters, individuals with higher educational attainment had less disability (secondary educated: EDSS, −0.30 [95% CI, −0.40 to −0.19] points; tertiary educated: EDSS, −0.47 [95% CI, −0.59 to −0.35] points) and self-reported physical symptoms (secondary educated: MSIS-29 physical subscore, −18% [95% CI, −27% to −8%]; tertiary educated: MSIS-29 physical subscore, −43% [95% CI, −50% to −35%]) and psychological symptoms (tertiary educated: MSIS-29 psychological subscore, 25% [95% CI, −33% to −17%]) compared with those who did not finish secondary school. A higher income quartile (compared with the quartile below) was similarly associated with better outcomes in all domains (EDSS, −0.16 [95% CI, −0.20 to −0.12] points; MSIS-29 physical subscore, −14% [95% CI, −18% to −11%]; MSIS-29 psychological subscore, −12% [95% CI, −16% to −9%]), whereas a marital status of divorced was associated with worse outcomes in all domains compared with participants who were single (EDSS, 0.34 [95% CI, 0.18 to 0.51] points; MSIS-29 physical subscore, 35% [95% CI, 12% to 62%; MSIS-29 psychological subscore, 25% [95% CI, 8% to 46%) (Figure 2A; eTable 1 in Supplement 1). There was no difference in outcomes between participants who were partnered vs single.
Figure 2. One-Year Premorbid Sociodemographic Status and Multiple Sclerosis (MS) Severity.
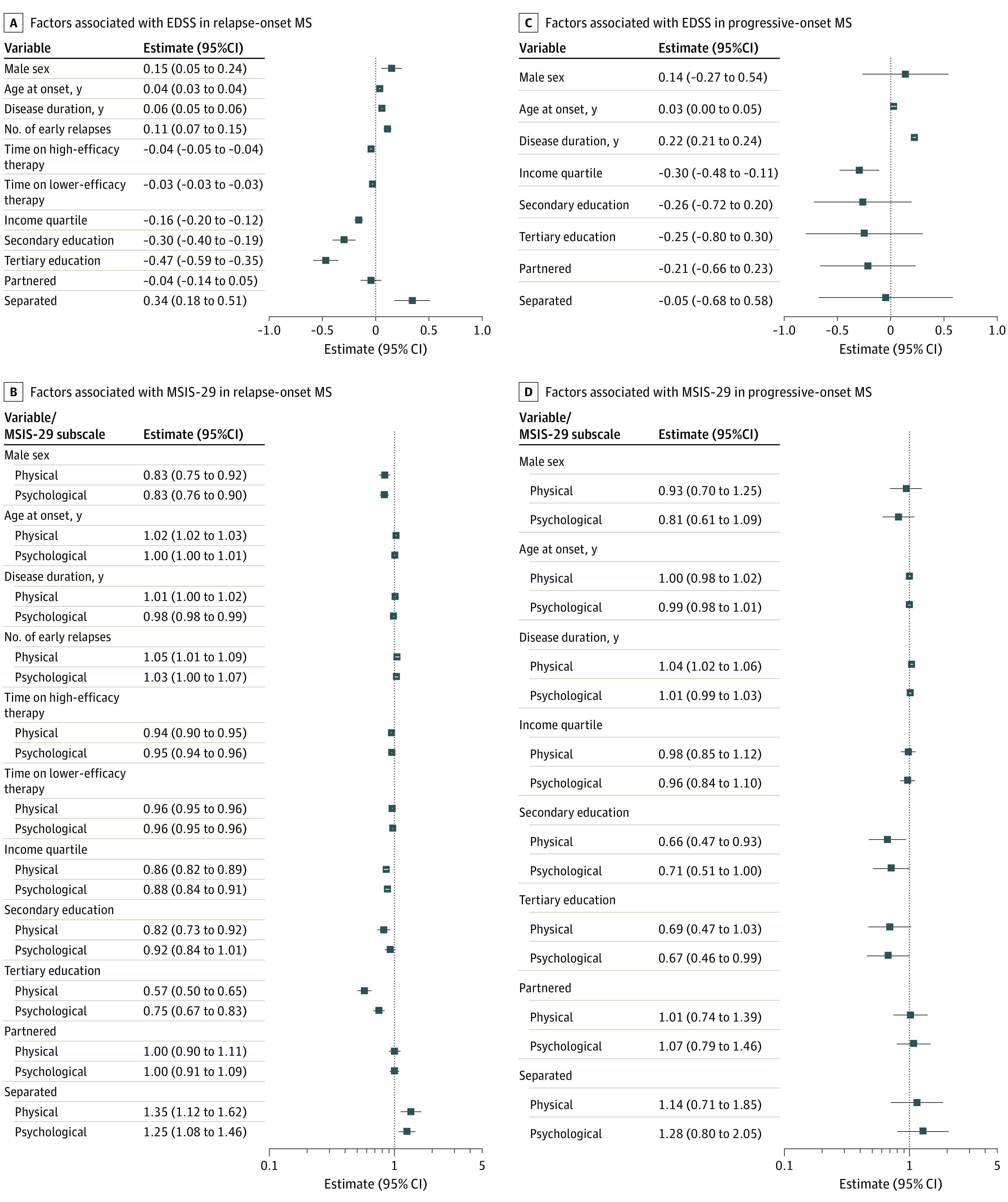
EDSS indicates Expanded Disability Status Scale; MSIS-29, Multiple Sclerosis Impact Scale.
Progressive-Onset MS
In progressive-onset MS, individuals with higher educational attainment had milder self-reported physical (secondary educated: lower MSIS-29 physical subscore, −34% [95% CI, −53% to −7%]) and psychological symptoms (secondary educated: lower MSIS-29 psychological subscore, −29% [95% CI, −49% to 0%]; tertiary educated: lower MSIS-29 psychological subscore, −33% [95% CI, −54% to −1%]). There were no statistically significant associations between education and marital status and clinical disability among patients with progressive-onset MS were in the same direction as for patients with relapsing-remitting MS. Those with higher income had lower clinical disability (EDSS lower by 0.30 [95% CI, 0.11 to 0.48] points for every quartile above the lowest income group) (Figure 2; eTable 1 in Supplement 1).
When modeling socioeconomic indices from 5 years prior to MS onset (Figure 3; eTable 2 in Supplement 1; baseline characteristics of this cohort provided in eTable 3 in Supplement 1), the same pattern of results was observed for the relapse-onset cohort. In the progressive onset cohort, 5-year premorbid socioeconomic indicators did not correlate to clinical disability nor MSIS-29 scores (Table).
Figure 3. Five-Year Premorbid Sociodemographic Status and Multiple Sclerosis (MS) Severity.
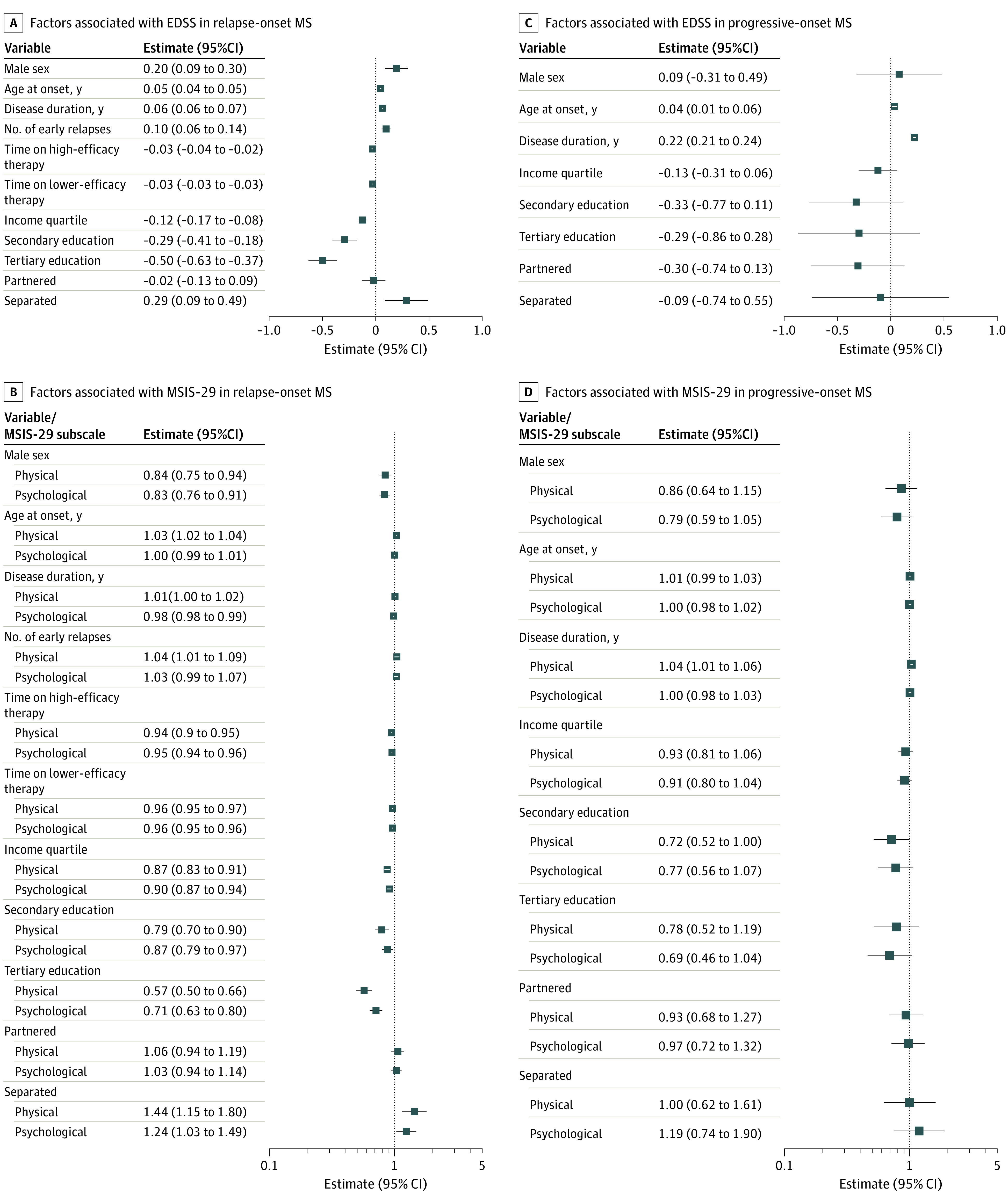
EDSS indicates Expanded Disability Status Scale; MSIS-29, Multiple Sclerosis Impact Scale.
The secondary analyses including the Charlson comorbidity index replicated the main results in full (see eTable 4 in Supplement 1). Unadjusted estimates of the degree of association between each sociodemographic variable and each outcome measure are provided in eTable 5 in Supplement 1; the estimates support the main findings.
Discussion
In Sweden’s universal health care context, premorbid sociodemographic indicators were significantly associated with subsequent MS severity in relapse-onset disease, independent of treatment exposure and comorbidity. In the progressive-onset group, income correlated with subsequent disability, while educational attainment was associated with self-reported symptom burden. Taken together, our study’s results suggest that premorbid sociodemographic factors play an important role in the prognosis of all types of MS.
Most previous studies on SES in the context of MS have focused on susceptibility rather than severity, and results have been conflicting.21,22 A few studies looking at severity3,4,5,23,24 have demonstrated correlation to postcode-based socioeconomic indices,3,4,5,23,24 similar to our findings. Only 1 of these studies accounted for DMT exposure,5 and all measured SES at or after diagnosis. The present study accessed sociodemographic indices from several years prior to disease onset, which defines a temporal sequence of events and can help unravel the direction of this association.
Existing frameworks of social determinants of health have illustrated structural, societal level mechanisms by which sociodemographic factors such as education affect health outcomes. Such frameworks posit that the fundamental determinants of health, such as access to health care, health literacy, and health-optimizing resources and behaviors, differ by social group, while the extent of between-group health disparities is modifiable through governance and policy such as universal health care, public education, or universal basic income. However, despite Sweden’s universal health care system, differential health outcomes6,7,8,9,25,26,27 across socioeconomic strata have been identified in other illnesses.28 It is not clear whether sociodemographic factors directly affect disease severity on an individual biological level.
Higher educational attainment is correlated with several candidate factors that may explain the observed association, including lower incidence of obesity27 and smoking,28 which are known to be correlated with MS severity, putatively through chronic low-grade inflammation and oxidative stress. Some evidence for the effect of education on MS outcomes comes from a mendelian randomization study of an international cohort of persons with MS, wherein education was found to be causally related to MS disability.29 Finally, a Norwegian study found that maternal educational attainment was predictive of MS severity in the offspring.30 These studies indicate that both observed educational attainment as well as genetic determinants of educational attainment and intelligence are correlated with MS prognosis. In the mendelian randomization study, a causal relationship between obesity and MS severity was not observed, and while smoking was found to increase MS severity, the effect of education persisted independently of smoking.31
It is possible that education exerts a direct neuroprotective effect on brain structure and function by imparting greater neurological resilience to injury. This paradigm is referred to as the brain reserve hypothesis.32The presence of an effect of income and education on disability progression even in progressive-onset MS, which is more treatment invariant and predominantly neurodegenerative, is further evidence in favor of this hypothesis. It is possible that premorbid income and education capture population variance in neurological function, such that patients who have an above-average education or vocational performance may have a higher baseline neurocognitive reserve that is more resistant to decompensated disability32,33,34,35 and symptoms.
Our results suggest that being separated or divorced prior to disease onset was a negative prognostic factor in relapse-onset MS. The results were similar among the primary progressive cohort; however, the CIs were wider and included 1. There is a large body of evidence for poor health outcomes among separated persons, and the stress and social support hypothesis for the effect of marital status on health.11,12,36 There was no observed difference in disease severity between premorbidly single and married persons. This could suggest that the worse prognosis may be attributable to the stress of separation more than the fact of being unmarried. Notably, we did not capture change in marital status post-MS onset, which may have led to the discrepancy.
Relapsing-onset MS is more common, responds more favorably to treatment, and imparts an overall better prognosis compared with progressive-onset disease. The differential associations between sociodemographic factors and disease severity between these 2 disease subtypes may be in part due to optimization of treatment factors that are not captured by our adjustment for treatment intensity. Additionally, given the relative rarity of progressive onset disease, the imprecise estimates may be due to lower statistical power.
Our findings have implications in clinical practice and health policy. Early indicators of future disease severity help patients and clinicians make early treatment decisions. We found here that those who are socioeconomically disadvantaged are at most risk of severe disease-related disability and may benefit from early commencement of high-efficacy therapy to mitigate this. From a policy perspective, our study urges equitable access to treatment and resources for health optimization, particularly in those contexts where access to these resources is highly dependent on SES.
Our study has several strengths. First, it uses highly granular data with minimal data loss due to Sweden’s mandatory real-time collection of socioeconomic data, thereby eliminating recall or ascertainment bias. The SES indices in our study were individual-level, which, to our knowledge, provides a perspective distinct from previous research using postcode-based indices.37 The universal health care context of this study and the adjustment for individual treatment exposures addresses the potential confounding effect of access to treatment. Additionally, the consistency of results at 2 index times and in secondary analyses indicates that our findings are robust.
Limitations
This study had limitations. Reverse causation, although unlikely, cannot be completely excluded in our study design, due to the unknown duration and effect of the MS prodrome.38 In other words, prodromal disease activity may have affected premorbid educational attainment, income, and marital status, and subsequent MS severity. Indeed, epidemiological studies have demonstrated that patients’ historical grade 5 school grades were linearly associated with their time to subsequent MS onset during adulthood,39 as well as differential cognitive performance in army recruits who developed MS up to 20 years later.40 Although these studies demonstrate poor cognitive performance as an indicator of MS risk and proximity to diagnosis, they did not assess the relationship to MS severity. In our study, there was little difference seen between the effect size of SES indices at 1-year and 5-years preonset, which suggests that the results may not be predominantly driven by the prodrome, or that the prodrome effect on SES does not change considerably over this time.
While the Swedish MS Registry covers 84% of the estimated prevalent patient population, it is not known whether there may be selection bias in the unregistered group that challenge the conclusions presented here, as has been demonstrated for other population registries.41 Future studies may compare the sociodemographic characteristics of registered vs unregistered patients for validation within the Swedish population. Additionally, in addition to external validation of our results outside of a Swedish context, further studies should explore the biological underpinnings of SES and its association with potential mediating factors which would together help explain our findings.
Conclusions
In this cohort study of working-age adults with MS, we found that premorbid education, income, and marital status may be associated with subsequent disability and patient-reported symptoms in a universal health care context. These results suggest that individual or contextual factors linked to sociodemographic status may inform MS severity.
eTable 1. Effect of 1-Year Premorbid Socioeconomic Status on EDSS and MSIS
eTable 2. Effect of 5-Year Premorbid Socioeconomic Status on EDSS and MSIS
eTable 3. Effect of 1-Year Premorbid Socioeconomic Status on EDSS and MSIS, Adjusted for Comorbidity
eTable 4. Estimated Effect of 1-Year Premorbid Sociodemographic Factors on Multiple Sclerosis Severity, Unadjusted Analyses
eTable 5. Estimated Effect of 5-Year Premorbid Sociodemographic Factors on Multiple Sclerosis Severity, Unadjusted Analyses
Data Sharing Statement
References
- 1.Dobson R, Rice DR, D’hooghe M, et al. Social determinants of health in multiple sclerosis. Nat Rev Neurol. 2022;18(12):723-734. doi: 10.1038/s41582-022-00735-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amezcua L, Rivera VM, Vazquez TC, Baezconde-Garbanati L, Langer-Gould A. Health disparities, inequities, and social determinants of health in multiple sclerosis and related disorders in the US: a review. JAMA Neurol. 2021;78(12):1515-1524. doi: 10.1001/jamaneurol.2021.3416 [DOI] [PubMed] [Google Scholar]
- 3.Boorgu DSSK, Venkatesh S, Lakhani CM, et al. The impact of socioeconomic status on subsequent neurological outcomes in multiple sclerosis. Mult Scler Relat Disord. 2022;65(March):103994. doi: 10.1016/j.msard.2022.103994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calocer F, Dejardin O, Kwiatkowski A, et al. Socioeconomic deprivation increases the risk of disability in multiple sclerosis patients. Mult Scler Relat Disord. 2020;40:101930. doi: 10.1016/j.msard.2020.101930 [DOI] [PubMed] [Google Scholar]
- 5.Harding KE, Wardle M, Carruthers R, et al. Socioeconomic status and disability progression in multiple sclerosis: a multinational study. Neurology. 2019;92(13):e1497-e1506. doi: 10.1212/WNL.0000000000007190 [DOI] [PubMed] [Google Scholar]
- 6.Marmot M, Allen J, Bell R, Bloomer E, Goldblatt P; Consortium for the European Review of Social Determinants of Health and the Health Divide . WHO European review of social determinants of health and the health divide. Lancet. 2012;380(9846):1011-1029. doi: 10.1016/S0140-6736(12)61228-8 [DOI] [PubMed] [Google Scholar]
- 7.Solar O, Irwin A. A conceptual framework for action on the social determinants of health. Published online 2010:79. https://www.who.int/sdhconference/resources/ConceptualframeworkforactiononSDH_eng.pdf
- 8.Riley AR. Advancing the study of health inequality: fundamental causes as systems of exposure. SSM Popul Health. 2020;10:100555. doi: 10.1016/j.ssmph.2020.100555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diderichsen F, Andersen I, Manuel C, et al. ; Working Group of Danish Review on Social Determinants of Health . Health inequality–determinants and policies. Scand J Public Health. 2012;40(8)(suppl):12-105. doi: 10.1177/1403494812457734 [DOI] [PubMed] [Google Scholar]
- 10.Thoits PA. Mechanisms linking social ties and support to physical and mental health. J Health Soc Behav. 2011;52(2):145-161. doi: 10.1177/0022146510395592 [DOI] [PubMed] [Google Scholar]
- 11.Verbrugge LM. Marital status and health. J Marriage Fam. 1979;41(2):267. doi: 10.2307/351696 [DOI] [Google Scholar]
- 12.Burman B, Margolin G. Analysis of the association between marital relationships and health problems: an interactional perspective. Psychol Bull. 1992;112(1):39-63. doi: 10.1037/0033-2909.112.1.39 [DOI] [PubMed] [Google Scholar]
- 13.Hillert J, Stawiarz L. The Swedish MS registry – clinical support tool and scientific resource. Acta Neurol Scand. 2015;132(199):11-19. doi: 10.1111/ane.12425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. doi: 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 15.Meyer-Moock S, Feng YS, Maeurer M, Dippel FW, Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14(1):58. doi: 10.1186/1471-2377-14-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain. 2001;124(Pt 5):962-973. doi: 10.1093/brain/124.5.962 [DOI] [PubMed] [Google Scholar]
- 17.Tilling K, Lawton M, Robertson N, et al. Modelling disease progression in relapsing-remitting onset multiple sclerosis using multilevel models applied to longitudinal data from two natural history cohorts and one treated cohort. Health Technol Assess. 2016;20(81):1-48. doi: 10.3310/hta20810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rae-Grant A, Day GS, Marrie RA, et al. Comprehensive systematic review summary: disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):789-800. doi: 10.1212/WNL.0000000000005345 [DOI] [PubMed] [Google Scholar]
- 19.R Development Core Team R . R: A language and environment for statistical computing. Published online 2022. Accessed August 29, 2023. https://www.r-project.org/index.html
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 21.Goulden R, Ibrahim T, Wolfson C. Is high socioeconomic status a risk factor for multiple sclerosis? a systematic review. Eur J Neurol. 2015;22(6):899-911. doi: 10.1111/ene.12586 [DOI] [PubMed] [Google Scholar]
- 22.Alfredsson L, Hillert J, Olsson T, Hedström AK. Observed associations between indicators of socioeconomic status and risk of multiple sclerosis in Sweden are explained by a few lifestyle-related factors. Eur J Neurol. 2023;30(4):1001-1013. doi: 10.1111/ene.15705 [DOI] [PubMed] [Google Scholar]
- 23.Vasileiou ES, Filippatou AG, Pimentel Maldonado D, et al. Socioeconomic disparity is associated with faster retinal neurodegeneration in multiple sclerosis. Brain. 2021;144(12):3664-3673. doi: 10.1093/brain/awab342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boorgu DSSK, Venkatesh S, Lakhani CM, et al. The impact of socioeconomic status on subsequent neurological outcomes in multiple sclerosis. Mult Scler Relat Disord. 2022;65(June):103994. doi: 10.1016/j.msard.2022.103994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marmot M, Allen JJ. Social determinants of health equity. Am J Public Health. 2014;104(Suppl 4)(suppl 4):S517-S519. doi: 10.2105/AJPH.2014.302200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eloranta S, Lambert PC, Cavalli-Bjorkman N, Andersson TML, Glimelius B, Dickman PW. Does socioeconomic status influence the prospect of cure from colon cancer–a population-based study in Sweden 1965-2000. Eur J Cancer. 2010;46(16):2965-2972. doi: 10.1016/j.ejca.2010.05.028 [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald KC, Salter A, Tyry T, Fox RJ, Cutter G, Marrie RA. Measures of general and abdominal obesity and disability severity in a large population of people with multiple sclerosis. Mult Scler. 2020;26(8):976-986. doi: 10.1177/1352458519845836 [DOI] [PubMed] [Google Scholar]
- 28.Rosso M, Chitnis T. Association between cigarette smoking and multiple sclerosis: a review. JAMA Neurol. 2020;77(2):245-253. doi: 10.1001/jamaneurol.2019.4271 [DOI] [PubMed] [Google Scholar]
- 29.Harroud A, Stridh P, McCauley JL, et al. ; International Multiple Sclerosis Genetics Consortium; MultipleMS Consortium . Locus for severity implicates CNS resilience in progression of multiple sclerosis. Nature. 2023;619(7969):323-331. doi: 10.1038/s41586-023-06250-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flemmen HØ, Simonsen CS, Broch L, et al. Maternal education has significant influence on progression in multiple sclerosis. Mult Scler Relat Disord. 2021;53:103052. doi: 10.1016/j.msard.2021.103052 [DOI] [PubMed] [Google Scholar]
- 31.Baranzini SE, Sawcer S; International Multiple Sclerosis Genetics Consortium MMC . Genetic analysis of multiple sclerosis severity identifies a novel locus and implicates CNS resilience as a major determinant of outcome. Res Sq. Preprint posted online June 10, 2022. doi: 10.21203/rs.3.rs-1723574/v1 [DOI]
- 32.Sumowski JF, Wylie GR, Deluca J, Chiaravalloti N. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: functional magnetic resonance imaging evidence for cognitive reserve. Brain. 2010;133(Pt 2):362-374. doi: 10.1093/brain/awp307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vollmer TL. Nair KV, Williams IM, Alvarez E. Multiple sclerosis phenotypes as a continuum. Neurol Clin Pract. 2021;11(4):342-351. doi: 10.1212/CPJ.0000000000001045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandroff BM, Schwartz CE, DeLuca J. Measurement and maintenance of reserve in multiple sclerosis. J Neurol. 2016;263(11):2158-2169. doi: 10.1007/s00415-016-8104-5 [DOI] [PubMed] [Google Scholar]
- 35.Ghaffar O, Fiati M, Feinstein A. Occupational attainment as a marker of cognitive reserve in multiple sclerosis. PLoS One. 2012;7(10):e47206. doi: 10.1371/journal.pone.0047206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Booysen F, Botha F, Wouters E. Conceptual causal models of socioeconomic status, family structure, family functioning and their role in public health. BMC Public Health. 2021;21(1):191. doi: 10.1186/s12889-021-10214-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCartney G, Hoggett R, Walsh D, Lee D. How well do area-based deprivation indices identify income- and employment-deprived individuals across Great Britain today? Public Health. 2023;217:22-25. doi: 10.1016/j.puhe.2023.01.020 [DOI] [PubMed] [Google Scholar]
- 38.Makhani N, Tremlett H. The multiple sclerosis prodrome. Nat Rev Neurol. 2021;17(8):515-521. doi: 10.1038/s41582-021-00519-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinay V, Perez Akly M, Zanga G, Ciardi C, Racosta JM. School performance as a marker of cognitive decline prior to diagnosis of multiple sclerosis. Mult Scler. 2015;21(7):945-952. doi: 10.1177/1352458514554054 [DOI] [PubMed] [Google Scholar]
- 40.Cortese M, Riise T, Bjørnevik K, et al. Preclinical disease activity in multiple sclerosis: a prospective study of cognitive performance prior to first symptom. Ann Neurol. 2016;80(4):616-624. doi: 10.1002/ana.24769 [DOI] [PubMed] [Google Scholar]
- 41.McKay KA, Tremlett H, Zhu F, Kastrukoff L, Marrie RA, Kingwell E. A population-based study comparing multiple sclerosis clinic users and non-users in British Columbia, Canada. Eur J Neurol. 2016;23(6):1093-1100. doi: 10.1111/ene.12990 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Effect of 1-Year Premorbid Socioeconomic Status on EDSS and MSIS
eTable 2. Effect of 5-Year Premorbid Socioeconomic Status on EDSS and MSIS
eTable 3. Effect of 1-Year Premorbid Socioeconomic Status on EDSS and MSIS, Adjusted for Comorbidity
eTable 4. Estimated Effect of 1-Year Premorbid Sociodemographic Factors on Multiple Sclerosis Severity, Unadjusted Analyses
eTable 5. Estimated Effect of 5-Year Premorbid Sociodemographic Factors on Multiple Sclerosis Severity, Unadjusted Analyses
Data Sharing Statement