Abstract
Background
Surgery remains a mainstay of treatment for malignant tumours; however, surgical manipulation leads to a significant systemic release of tumour cells. Whether these cells lead to metastases is largely dependent on the balance between aggressiveness of the tumour cells and resilience of the body. Surgical stress per se, anaesthetic agents and administration of opioid analgesics perioperatively can compromise immune function and might shift the balance towards progression of minimal residual disease. Regional anaesthesia techniques provide perioperative pain relief; they therefore reduce the quantity of systemic opioids and of anaesthetic agents used. Additionally, regional anaesthesia techniques are known to prevent or attenuate the surgical stress response. In recent years, the potential benefit of regional anaesthesia techniques for tumour recurrence has received major attention and has been discussed many times in the literature. In preparing this review, we aimed to summarize the current evidence systematically and comprehensively.
Objectives
To establish whether anaesthetic technique (general anaesthesia versus regional anaesthesia or a combination of the two techniques) influences the long‐term prognosis for individuals with malignant tumours.
Search methods
We searched The Cochrane Library (2013, Issue 12), PubMed (1950 to 15 December 2013), EMBASE (1974 to 15 December 2013), BIOSIS (1926 to 15 December 2013) and Web of Science (1965 to 15 December 2013). We handsearched relevant websites and conference proceedings and reference lists of cited articles. We applied no language restrictions.
Selection criteria
We included all randomized controlled trials or controlled clinical trials that investigated the effects of general versus regional anaesthesia on the risk of malignant tumour recurrence in patients undergoing resection of primary malignant tumours. Comparisons of interventions consisted of (1) general anaesthesia alone versus general anaesthesia combined with one or more regional anaesthetic techniques; (2) general anaesthesia combined with one or more regional anaesthetic techniques versus one or more regional anaesthetic techniques; and (3) general anaesthesia alone versus one or more regional anaesthetic techniques. Primary outcomes included (1) overall survival, (2) progression‐free survival and (3) time to tumour progression.
Data collection and analysis
Two review authors independently scanned the titles and abstracts of identified reports and extracted study data.
All primary outcome variables are time‐to‐event data. If the individual trial report provided summary statistics with odds ratios, relative risks or Kaplan‐Meier curves, extracted data enabled us to calculate the hazard ratio using the hazard ratio calculating spreadsheet. To assess risk of bias, we used the standard methodological procedures expected by The Cochrane Collaboration.
Main results
We included four studies with a total of 746 participants. All studies included adult patients undergoing surgery for primary tumour resection. Two studies enrolled male and female participants undergoing major abdominal surgery for cancer. One study enrolled male participants undergoing surgery for prostate cancer, and one study male participants undergoing surgery for colon cancer. Follow‐up time ranged from nine to 17 years. All four studies compared general anaesthesia alone versus general anaesthesia combined with epidural anaesthesia and analgesia. All four studies are secondary data analyses of previously conducted prospective randomized controlled trials.
Of the four included studies, only three contributed to the outcome of overall survival, and two each to the outcomes of progression‐free survival and time to tumour progression. In our meta‐analysis, we could not find an advantage for either study group for the outcomes of overall survival (hazard ratio (HR) 1.03, 95% confidence interval (CI) 0.86 to 1.24) and progression‐free survival (HR 0.88, 95% CI 0.56 to 1.38). For progression‐free survival, the level of inconsistency was high. Pooled data for time to tumour progression showed a slightly favourable outcome for the control group (general anaesthesia alone) compared with the intervention group (epidural and general anaesthesia) (HR 1.50, 95% CI 1.00 to 2.25).
Quality of evidence was graded low for overall survival and very low for progression‐free survival and time to tumour progression. The outcome of overall survival was downgraded for serious imprecision and serious indirectness. The outcomes of progression‐free survival and time to tumour progression were also downgraded for serious inconsistency and serious risk of bias, respectively.
Reporting of adverse events was sparse, and data could not be analysed.
Authors' conclusions
Currently, evidence for the benefit of regional anaesthesia techniques on tumour recurrence is inadequate. An encouraging number of prospective randomized controlled trials are ongoing, and it is hoped that their results, when reported, will add evidence for this topic in the near future.
Plain language summary
Anaesthetic techniques for risk of malignant tumour recurrence
Background
Surgery remains a mainstay of treatment for patients with many types of cancer. However, surgical stress and certain anaesthesia and pain medications commonly given during anaesthesia for cancer surgery are known to suppress body defences. Therefore, surgery and anaesthesia might contribute to long‐term cancer recurrence. Different types of anaesthesia are available. General anaesthesia indicates that the patient goes to sleep for his or her surgery, regional anaesthesia means that the part of the body that is operated on is numbed by a numbing medication (local anaesthetic), or a combination of the two techniques can be used. Regional anaesthesia has the potential to reduce the use of certain anaesthesia and pain medications that are injected into the vein or inhaled into the lung, as well as to attenuate surgical stress. Therefore, previous research has suggested that regional anaesthesia might reduce the risk of long‐term cancer recurrence.
Research question
We aimed to discover whether different types of anaesthesia used during cancer surgery could influence long‐term survival or the rate of tumour recurrence in patients undergoing cancer surgery.
Search date
Evidence is current to December 2013.
Study characteristics
We found four studies with a total of 746 adult men and women undergoing abdominal surgery for removal of cancer. All studies were reanalyses of previously conducted trials, which means that none of the included studies was actually designed to investigate tumour recurrence. All patients underwent primary cancer surgery, which means that surgery on cancer metastases was not included. A total of 354 participants received general anaesthesia and 392 participants received a general anaesthesia along with an epidural anaesthesia. Epidural anaesthesia is a certain type of regional anaesthesia by which a numbing medication is injected continuously via a catheter into the epidural space. The epidural space serves as the outermost surrounding of the spinal cord. Numbing medication injected into the epidural space causes certain parts of the belly area to go numb and be insensitive to pain. Study participants were followed for at least 7.8 years after they had undergone cancer surgery.
Key results
We did not find a benefit for either study group on cancer recurrence or survival. Because of incomplete reporting and the low number of reported adverse events, we cannot estimate possible differences in adverse effects between the different anaesthesia techniques used.
Quality of the evidence
The quality of the evidence for outcomes was graded low for overall survival and very low for progression‐free survival and time to tumour progression. The main limitations of the evidence we identified were that the results could have been influenced by the background treatments given to people who participated in the trials.
Summary of findings
Summary of findings for the main comparison. Epidural anaesthesia in addition to general anaesthesia compared with general anaesthesia alone for patients undergoing primary tumour surgery.
| Epidural anaesthesia in addition to general anaesthesia compared with general anaesthesia alone for patients undergoing primary tumour surgery | |||||
| Patient or population: patients undergoing primary tumour surgery Settings: Intervention: epidural anaesthesia and analgesia in addition to general anaesthesia Comparison: general anaesthesia alone | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect† (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| General anaesthesia alone (control) | Epidural anaesthesia in addition to general anaesthesia (intervention) | ||||
|
Death from all causes
Range of follow‐up timesa: 7.8‐14.8 years (Myles) 8.3‐10.75 years (Christopherson) |
Study population | HR 1.03 (0.86 to 1.24) | 647 (3 studies) | ⊕⊕⊝⊝ lowb,c | |
| 805 per 1000a | 815 per 1000 (755 to 868) | ||||
|
Tumour progression or death from all causes
Range of follow‐up times: 7.8‐14.8 yearsd |
Study population | HR 0.88 (0.56 to 1.38) | 535 (2 studies) | ⊕⊝⊝⊝ very lowb,c,e | |
| 944 per 1000d | 921 per 1000 (802 to 981) | ||||
|
Tumour progression
Median follow‐up: 4.5 yearsf |
Study population | HR 1.50 (1 to 2.25) | 545 (2 studies) | ⊕⊝⊝⊝ very lowb,c,h | |
| 360 per 1000g | 488 per 1000 (360 to 634) | ||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: Hazard ratio. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
HR = hazard ratio, defined as intervention/control.
†HR < 1 denotes advantage for the intervention group, HR > 1 denotes advantage for the control group.
aThe assumed risk and the range of follow‐up times are based on data reported by Myles and Christophersen. Data on absolute events per group were not reported by Binczak. bSerious indirectness (‐1): Regional anaesthesia techniques are a surrogate for reduced or absent immunosuppression mediated by opioids and volatile anaesthetics, both of which are not controlled for in the included studies. cSerious imprecision (‐1): Combined sample sizes are deemed too small to show an effect. dThe assumed risk and the range of follow‐up times are based on data from Myles only. Data on absolute risk for tumour progression and death from all causes are not reported by Binczak. eSerious inconsistency (‐1): substantial unexplained heterogeneity. fThe median follow‐up time is based on data from Tsui. gThe assumed risk is based on data from Tsui only. Data on the absolute risk for TTP are not reported by Myles. hSerious risk of bias (‐1): 1 study with unclear risk of selective reporting and other bias.
Background
Cancer is the second most common cause of death in the United States and Europe (Centers for Disease Control and Prevention 2013; World Health Organization 2012). Cancer might be tumour forming (malignant tumour) or not (such as leukaemia). The most common cancers contributing to mortality are malignant tumours of lung, prostate and breast and colorectal malignant tumours (Jemal 2010). For these malignant tumours, surgery remains a mainstay of treatment. Surgery may be curative in the early stages, and it may at least prolong life in late stages.
Description of the condition
Metastatic disease is the most important cause of cancer‐related death in patients after malignant tumour surgery (Snyder 2010). Surgical manipulation leads to a significant systemic release of tumour cells (Eschwege 1995; Wang 2006; Yamaguchi 2000; Yamashita 2000). Whether these cells lead to metastases is largely dependent on the balance between aggressiveness of the tumour cells and resilience of the body. At least three perioperative factors shift the balance towards progression of minimal residual disease.
Surgery per se induces a stress response that can decrease host defences and promote tumour growth. Innate immunity and especially natural killer (NK) cells are known to play a major role in elimination of circulating tumour cells (Shakhar 2003; Whiteside 1995). Several studies have demonstrated decreased postoperative NK cell activity and an inverse correlation of NK cell activity with tumour stage and metastatic growth (Konjevic 1993; Lennard 1985; Mafune 2000; Pollock 1991; Tarle 1993). Additionally, increased postoperative concentrations of pro‐angiogenic factors such as vascular endothelial growth factor were found in humans (Ikeda 2002; Maniwa 1998). In animal models, surgical removal of the primary tumour significantly reduces concentrations of tumour‐related antiangiogenic factors (e.g. angiostatin, endostatin) and promotes tumour growth (Holmgren 1995).
Anaesthetic agents might impair numerous immune functions, including those of neutrophils, macrophages, dendritic cells, T cells and NK cells. Numerous in vitro and animal studies were able to show the immunosuppressive effects of anaesthetic agents such as halothane, isoflurane, sevoflurane, ketamine and thiopental (Kurosawa 2008; Melamed 2003; Mitsuhata 1995; Moudgil 1997). More recently, the immunosuppressive effects of the volatile anaesthetics isoflurane and sevoflurane were confirmed in humans undergoing surgery (Inada 2004; Schneemilch 2005; Zhang 2014).
Opioid analgesics inhibit both cellular and humoral immune function in humans (Beilin 1996; Sacerdote 2000; Vallejo 2004; Yardeni 2008; Yeager 1995). Moreover, in a human cell culture model, morphine increased angiogenesis and promoted breast tumour growth in a mouse model (Gupta 2002).
Other perioperative interventions or medications may influence the patient's immune response as well. In recent years, perioperative intravenous lidocaine infusion was introduced into clinical practice to improve pain management after major surgery. Randomized controlled trials in humans suggest that continuous administration of perioperative low‐dose lidocaine reduces postoperative opioid consumption, attenuates postoperative pain scores and reduces surgery‐induced alterations of immunity (Koppert 2004; Yardeni 2009).
Description of the intervention
Regional anaesthetic techniques include neuraxial techniques, such as spinal anaesthesia and epidural anaesthesia; nerve block techniques, such as intercostal or paravertebral nerve blocks; and an intravenous regional anaesthesia technique. Local anaesthetic techniques, such as wound infiltration by a single shot or continuously via a catheter, might also be considered as a type of regional anaesthesia. All these techniques provide pain relief during, as well as after, surgical procedures; they therefore reduce the quantity of systemic opioids needed perioperatively. Additionally, regional anaesthesia techniques are known to prevent or attenuate the surgical stress response by blocking afferent neuronal transmission, which prevents noxious afferent input from reaching the central nervous system (Deegan 2009; O'Riain 2005).
How the intervention might work
Regional anaesthetic techniques provide excellent pain relief during and after surgical interventions. A working regional anaesthesia technique implies that:
in many cases, general anaesthesia can be replaced by regional anaesthetic techniques, and the potential immunosuppressive effects of anaesthetic agents such as volatile anaesthetics can be avoided;
the quantity of intraoperative and postoperative opioids needed for intraoperative and postoperative pain management can at least be significantly reduced without compromising adequate pain relief; and
the surgical stress response is at least attenuated by regional anaesthetic techniques; therefore the immunosuppressive effect of surgical stress might be attenuated as well.
Why it is important to do this review
Based on available basic research data as outlined above, the hypothesis was stated that perioperative immunosuppression caused by surgical stress, anaesthetics and opioids might promote the progression of minimal residual disease in patients undergoing surgical resection of malignant tumours. Clinical researchers started to investigate the long‐term outcomes of patients with cancer after tumour surgery based on the anaesthetic technique used both intraoperatively and postoperatively. However, these data seem to be inconsistent until today. Therefore, the aim of this Cochrane review is to provide the clinician with an up‐to‐date and comprehensive summary of the best available evidence on whether anaesthetic techniques may influence malignant tumour recurrence.
Objectives
To establish whether anaesthetic technique (general anaesthesia versus regional anaesthesia or a combination of the two techniques) influences the long‐term prognosis for individuals with malignant tumours.
Methods
Criteria for considering studies for this review
Types of studies
We considered any randomized controlled trials (RCTs) or controlled clinical trials (CCTs) that investigated the effect of the anaesthetic technique on the risk of malignant tumour recurrence in study participants undergoing resection of primary malignant tumours. We did not include non‐randomized studies in the meta‐analysis, but we provided a narrative summary of non‐randomized studies in the discussion. To obtain the widest range of studies, we did not limit date of publication or language.
Types of participants
We considered all studies that included participants having surgery for primary malignant tumour resection. Adult and paediatric participant populations were eligible for inclusion. We defined paediatric patients as children younger than 18 years of age.
Types of interventions
Interventions of interest include different anaesthetic techniques used during the surgical procedure for primary malignant tumour resection. General anaesthesia included inhalational and intravenous techniques of drug administration. Regional anaesthesia included peripheral regional anaesthesia; neuraxial regional anaesthesia, that is, spinal anaesthesia and epidural anaesthesia; and local anaesthesia including continuous wound infiltration techniques. Comparisons of interventions consist of:
general anaesthesia alone versus general anaesthesia combined with one or more regional anaesthetic techniques;
general anaesthesia combined with one or more regional anaesthetic techniques versus one or a combination of regional anaesthetic techniques; and
general anaesthesia alone versus one or more regional anaesthetic techniques.
Types of outcome measures
Primary outcomes
Overall survival (OS): the time elapsed between surgery and death from any cause.
Progression‐free survival (PFS): the time elapsed between surgery and tumour progression or death from any cause.
Time to tumour progression (TTP): the time elapsed between surgery and tumour progression.
Secondary outcomes
Postoperative adverse events including failed epidural catheter placement, postoperative nausea and vomiting (PONV), postoperative respiratory complications and postoperative cardiac complications.
Search methods for identification of studies
Electronic searches
We searched The Cochrane Library (2013, Issue 12), PubMed (1950 to 15 December 2013), EMBASE (1974 to 15 December 2013), BIOSIS (1926 to 15 December 2013) and Web of Science (1965 to 15 December 2013). We developed a specific search strategy for each database based on that developed for PubMed (Appendix 1). We combined the PubMed search strategy with the Cochrane highly sensitive search strategy for identifying RCTs (Higgins 2011a).
Searching other resources
We identified trials by manually searching abstracts of relevant conference proceedings, such as Annual Meetings of the American Society of Anesthesiologists and the European Society of Anaesthesiologists, as well as the National Cancer Research Institute Cancer Conference.
We checked the reference lists of relevant articles and contacted relevant trial authors to identify additional or ongoing studies. We also searched for relevant trials by searching specific websites.
http://www.science.gov/index.html.
We applied no language or publication date restrictions.
Data collection and analysis
Selection of studies
We merged results identified by the described variety of search strategies using literature manager software (Reference Manager). Two review authors (OSC, KK) independently scanned the titles and abstracts of identified reports. We retrieved and evaluated potentially relevant studies chosen by at least one review author in the full‐text version. We identified multiple reports of the same study. Two review authors (OSC and KK) independently assessed the congruence of the remaining trials with the review's inclusion criteria, using a checklist that had been designed in advance (study eligibility screening form) (Appendix 2). A third review author (NLP) resolved disagreements.
Data extraction and management
Two review authors (OSC, KK) independently extracted data using a data extraction form (Appendix 3) that was based on the Cochrane Anaesthesia Review Group data extraction form (CARG 2007; Jüni 2001). For each of the outcome variables (OS, PFS, TTP), the review authors used the data extraction tables suggested by Tierney 2007. If the individual trial report provided summary statistics with odds ratios, risk ratios or Kaplan‐Meier curves, the extracted data enabled us to calculate the hazard ratio (HR) using the HR calculating spreadsheet (Tierney 2007). We resolved disagreements through consultation with a third review author (NLP).
Assessment of risk of bias in included studies
We judged the study quality using the The Cochrane Collaboration's tool for assessing risk of bias—a two‐part tool that addresses the six specific domains of random sequence generation; allocation concealment; blinding of participants, personnel and outcome assessors; incomplete outcome data; selective reporting; and other sources of bias (Higgins 2011b). The first part describes the risk of bias, and the second part provides criteria for making judgements about risk of bias based on each of the six domains in the tool (Appendix 4). Based on this tool, we completed the 'Risk of bias' table—enclosed in the RevMan 5.2 software—for each included study. Risk of bias was assessed by two review authors (OSC, KK). We resolved disagreements through consultation with a third review author (NLP). We created a 'Risk of bias' graph and a 'Risk of bias' summary figure using RevMan 5.2 software to display the results. We present the risk of bias in the Results section and provide summary assessments of the risk of bias for each outcome within and across studies.
Measures of treatment effect
All primary outcome variables are time‐to‐event data. The treatment effect was the log hazard ratio for general anaesthesia versus regional anaesthesia or a combination of the two for the primary outcomes of OS, PFS and TTP. Treatment effects for the dichotomous secondary outcomes (adverse events) were planned to be expressed as the risk ratio .
Unit of analysis issues
We found no studies with non‐standard design, including no non‐randomized controlled trials.
Dealing with missing data
When necessary, we contacted the authors of included studies regarding missing data. When data were found to be missing and the study authors could not be contacted, we calculated missing statistics from other quoted statistics, if possible. When data were still missing, we performed an available case analysis, excluding data from which outcome information was unavailable. An intention‐to‐treat analysis was attempted to address missing data resulting from participant dropout.
Assessment of heterogeneity
We assessed statistical heterogeneity using the Chi2 test. We increased the significance level from 0.05 to 0.10 to adjust for the fact that a small number of studies and studies with small sample sizes were included. We assessed the level of inconsistency across studies using the I2 statistic, where I2 > 50 % indicates significant inconsistency. We evaluated clinical heterogeneity by comparing clinical characteristics of the included studies. If present, we explored and discussed possible reasons for heterogeneity and inconsistency (Higgins 2011a).
Assessment of reporting biases
We assessed reporting biases through careful attention to quality assessment, particularly of study methodology. A thorough search for unpublished studies through contact with known experts in the field also assisted in reducing the risk of publication bias. We deferred funnel plot analysis to examine publication bias because of the low number of studies included in the review.
Data synthesis
The effect measure for comparing interventions for survival outcomes was the log HR and the standard error (SEHR). We defined HR as intervention group/control group so that HR < 1 denotes advantage for the intervention group and HR > 1 denotes advantage for the control group. We adjusted the HR derived from individual trials accordingly as appropriate. We report HRs with 95% confidence intervals (CIs) on a non‐log scale. For trials providing the HR but not providing individual participant data and not reporting the SEHR, we used the methods of Parmar 1998 to estimate variance from the reported CI (Parmar 1998). For trials that did not report the HR, we used the approximation methods of Parmar 1998 and Williamson 2002 to estimate HR and variance from cumulative survival rates (Kaplan‐Meier plots), observed and expected event tallies, logrank statistics or the Mantel‐Haenszel test (Parmar 1998; Williamson 2002). Estimation of the summary HR across trials was attained by the generic inverse variance method with a fixed‐effect model, using the statistical software Review Manager. To meet concerns about judgement of clinical heterogeneity, we additionally used a random‐effects model to analyse the data.
The pooled treatment effect for the risk ratio was planned using an inverse variance approach. Because data on adverse events were lacking, the analysis was deferred.
Subgroup analysis and investigation of heterogeneity
Because data were few, we did not perform subgroup analysis.
Sensitivity analysis
Because data were few, planned sensitivity analyses were deferred (see Differences between protocol and review) .
'Summary of findings' table
The primary outcomes of OS, PFS and TTP were incorporated into a 'Summary of findings' table. The treatment effect for these three primary outcomes is the HR of time‐to‐event data, incorporating both beneficial and adverse effects. Because data were lacking, we did not include secondary outcomes (adverse events) into the 'Summary of findings' table.
Based on the content of the included studies and the 'Risk of bias' tables, the quality of evidence is presented using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach, with particular attention to limitations of study design and heterogeneity of results.
Results
Description of studies
Results of the search
Results of the database searches are displayed in the study flow diagram (Figure 1). The manual search of conference proceedings and specific websites, as well as handsearching of reference lists, did not reveal additional eligible studies. Handsearching yielded nine ongoing clinical trials possibly meeting the inclusion criteria, all registered on ClinicalTrials.gov (http://www.clinicaltrials.gov/).
1.
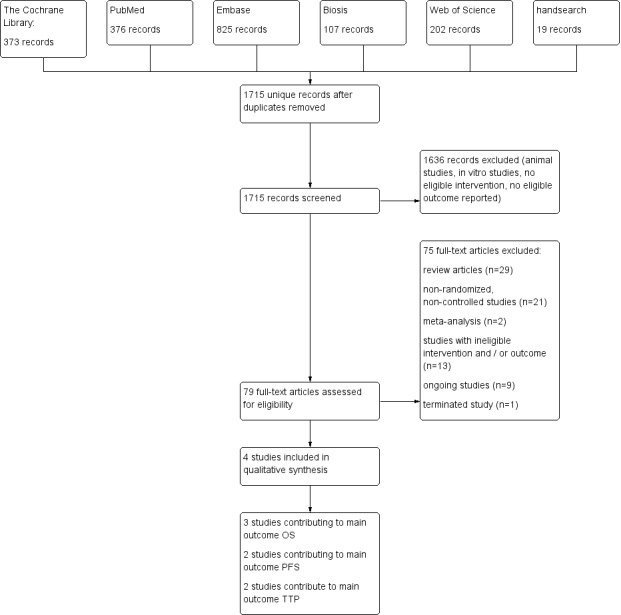
Study flow diagram.
Included studies
We included four studies with a total of 746 participants (Binczak 2013; Christopherson 2008; Myles 2011; Tsui 2010). All four studies are secondary data analyses of previously published prospective RCTs (Jayr 1993; O'Connor 2006; Park 2001; Rigg 2000; Rigg 2002). The subset of the included patient population is described in Characteristics of included studies/notes). All studies included adult participants undergoing surgery for primary tumour resection. Two studies enrolled participants undergoing major abdominal surgery for cancer. Major abdominal surgery included oesophagectomy, gastrectomy, hepatectomy, pancreatectomy, colectomy, nephrectomy, cystectomy, radical hysterectomy and open prostatectomy in one trial (Myles 2011), and surgery for colorectal cancer, gastric cancer, pancreatic cancer, bladder cancer, bile duct carcinoma, small intestine cancer, adenopathy and peritoneal gelatinous disease in the other trial (Binczak 2013). One study enrolled participants undergoing surgery for prostate cancer (Tsui 2010), and one study for colon cancer (Christopherson 2008). Two studies included male participants only (Christopherson 2008; Tsui 2010), and the remaining two studies enrolled male and female participants. Follow‐up time ranged from nine years to 17 years postoperatively. Two studies (Christopherson 2008; Myles 2011) reported results of multi‐centre trials, and the two remaining studies were single‐centre studies. Demographics and perioperative data are displayed in Table 2.
1. Demographic, perioperative and study design characteristics.
| Number of participants | Recruitment site(s) | Age (years) | Male sex | ASA | Type of surgery | Outcome data derived from | |
| Christopherson 2008 | 112 | USA; multi‐centre | Control group: 69.1 ± 7.8 Epidural group: 68.6 ± 7.7 |
Male only | IIIa | Elective surgery for colon cancer | Veterans Affairs Beneficiary Information and Records Locator System (VA BIRLS) |
| Myles 2011 | 446 | Australia, East Asia, Middle East; multi‐centre (MASTERS trial) | Control group: 70 ± 11 Epidural group: 71 ± 9.5 |
Control group: 53% Epidural group: 60% |
'High risk patients'b | Major abdominal surgery for cancer | 1. Medical hospital record 2. Contact with participant's general practitioner 3. State‐based cancer registry or National Health Index 4. Participant contact 5. Contact with next of kin |
| Tsui 2010 | 99 | Canada; single‐centre | Control group: 63.9 ± 6.1 Epidural group: 63.0 ± 5.5 |
Male only | ASA I‐III | Radical prostatectomy and bilateral pelvic lymphadenectomy | Participant's hospital charts and medical records |
| Binczak 2013 | 89 | France; single‐centre | Not reported for subcohort (mean for full cohort 58 years) |
Not reported for subcohort (full cohort includes > 62% male) | Not reported | Major abdominal surgery for cancer | 1. Hospital intern cancer registry 2. Participant contact 3. French National Registry |
ASA = American Society of Anesthesiologists physical status classification.
USA = United States of America.
aThe study by Christopherson 2008 reports that ASA I‐III patients were included. However, the original trial included only ASA III patients (Park 2001).
bAccording to the inclusion criteria noted in the original study (Rigg 2002), 'high risk' translates to ASA II‐III.
All four studies compared general anaesthesia alone versus general anaesthesia combined with epidural anaesthesia and analgesia. General anaesthesia was a balanced anaesthesia in all four studies. Three studies used isoflurane to maintain anaesthesia, and one study did not specify the type of volatile anaesthetic used (Myles 2011). Intraoperative and early postoperative analgesia was mainly archived with participant‐ or physician‐controlled administration of opioids. We summarize the data on epidural and intravenous analgesia in the table "Intraoperative and early postoperative analgesia" (Table 3).
2. Intraoperative and early postoperative analgesia.
| GA maintenance | Epidural catheter level | Time placed | Duration | LA used | Epidural medications intraoperatively | Epidural medications postoperatively | Intraoperative IV opioids | Postoperative IV opioids | |
| Christopherson 2008 | Isoflurane 0.9% (mean) + N2O | Thoracic or lumbar epidural catheter | Preoperatively | "as long as needed" | Bupivacaine 0.5% | 3‐6 mg morphine; 25‐50 mg boluses bupivacaine/3‐5 hours as needed; epinephrine |
25‐50 mg boluses bupivacaine/3‐5 hours as needed; morphine 3‐6 mg/12‐24hours as needed |
Fentanyl for both groups | Morphine, meperidine as needed (IV in epidural group, IV or IM in control group) |
| Myles 2011 | Balanced anaesthesia (volatile anaesthetic not specified), N2O use not specified or recorded, but usual practice was to include it | At discretion of the anaesthesiologist "With the exception of some pelvic operations, all epidural catheters were inserted in the thoracic region" |
Preoperatively | 3 days after surgery | Bupivacaine or ropivacaine | Bupivacaine or ropivacaine | Continuous infusion of ropivacaine or bupivacaine, supplemented with fentanyl or pethidine | Fentanyl pethidine |
Postoperative opioids, mostly PCA in control group (fentanyl, pethidine) |
| Tsui 2010 | Isoflurane 1‐2% + N2O 60% | Low thoracic or high lumbar epidural catheter | Preoperatively | Not reported | Ropivacaine | Ropivacaine bolus + continuous infusion; fentanyl |
Not reported | Morphine for control group | Not reported |
| Binczak 2013 | Isoflurane 1‐2% + N2O 70% | Thoracic 7‐11 | Preoperatively | Until 5th postoperative day | Bupivacaine | 50 mg bupivacaine as needed; epinephrine |
12.5 mg/h bupivacaine; 0.25 mg/h morphine |
Fentanyl for both groups | Epidural group: morphine boluses SC as needed; control group: 2.5 mg/h morphine SC via catheter |
GA = general anaesthesia.
LA = local anaesthetic.
IV = intravenous.
IM = intramuscular.
SC = subcutaneous.
We did not identify any studies comparing general anaesthesia plus regional anaesthesia versus regional anaesthesia alone or general anaesthesia alone versus regional anaesthesia alone.
Three studies with 647 participants reported OS (Binczak 2013; Christopherson 2008; Myles 2011), two studies with 535 participants reported PFS (Binczak 2013; Myles 2011) and two studies with 545 participants reported TTP (Myles 2011; Tsui 2010). Investigators from only one study commented on postoperative adverse events (secondary outcomes) (Tsui 2010).
We summarized the included studies in the Characteristics of included studies table. We developed Table 4 to display additional results reported in each included study.
3. Additional results reported from included studies.
| Tumour stage (TNM) | Clinical vs pathologic staging | Median overall survival (95% CI) | Median progression‐free survival | Median time to tumour progression | 5‐Year survival | Follow‐up time | Statistical test used (uni‐ vs. multivariable) | |
| Christopherson 2008 | All T, N0, M0 | Pathological | 6.14 (5.22 to 7.99) | Not reported | Not reported | Not reported | Up to 9 years | Data extracted from Kaplan‐Meier curve; HR and SEHR calculated according to Tierney (Tierney 2007) |
| Myles 2011 | All T, all N, no distant metastasis (M0) 'complete surgical excision' |
Not reported | Epidural group: 3.3 (95% CI 2.1 to 4.5) Control group: 3.7 (95% 2.0 to 5.4) |
Epidural group: 2.6 (IQR 0.7 to 8.7) Control group: 2.8 (IQR 0.7 to 8.7) |
Epidural group: 1.1 (95% CI 0.7 to 1.6) Control group: 1.4 (95% CI 0.6 to 2.3) |
Epidural group: 42% Control group: 44% |
Up to 12 years | Univariable testing, log‐rank statistics, intention‐to‐treat analysis |
| Tsui 2010 | All T, all N, M not reported | Pathological | Not reported | Not reported | 1644 days | Not reported | Up to 3403 days (˜9.3 years) | Unadjusted Cox model, no intention‐to‐treat analysis |
| Binczak 2013 | Primary tumour resection (all stages) with or without residual disease postoperatively | Not reported | Not reported | Not reported | Not reported | Not reported | Up to 17 years | Unadjusted HR (reported by the contact author through personal communication) |
IQR = interquartile range.
TNM classification of malignant tumours: T = tumour size, N = lymph node involvement, M = distant metastasis.
HR = hazard ratio.
SEHR = standard error of hazard ratio.
CI = confidence interval.
We contacted the corresponding authors of three included studies via email to clarify reported results or to ask for additional data. Two study authors replied and provided precise data clarification (Binczak 2013; Myles 2011). One study author did not respond to our inquiry (Tsui 2010).
Assessment of clinical heterogeneity
| Commonality | Differences |
| Adult participant population | Different types of tumours |
| Abdominal surgery for primary tumour resection | Different or unknown opioid regimens |
| Comparison: general anaesthesia versus general anaesthesia plus epidural analgesia | |
| Intervention: epidural catheter | |
| Epidural catheter placed before surgery and run intraoperatively | |
| Intraoperative and postoperative epidural opioids administered | |
| Balanced anaesthesia for maintenance during surgery |
Although the type of cancer broadly varies among and within the studies, all participants underwent abdominal surgery for tumour removal. This might indicate that the invasiveness of the surgical procedure was very similar. Given the clinical commonalities of the included studies, we deemed it appropriate to perform meta‐analyses.
Ongoing studies
We identified nine ongoing clinical trials registered at clinicaltrials.gov that potentially met inclusion criteria. Three trials investigated breast cancer recurrence, two trials each colon cancer and lung cancer recurrence and one trial each malignant melanoma recurrence and pancreatic cancer recurrence. Characteristics of ongoing trials are summarized under Characteristics of ongoing studies and in Table 5.
4. Characteristics of ongoing studies.
| Study PI | Start date (clinical trials.gov) | Population | Sample size | Intervention | Control group |
| Sessler 2007 | 2007 | Female patients 18‐85 years of age, diagnosed with primary breast cancer without known extension beyond the breast and axillary nodes, scheduled for unilateral or bilateral mastectomy with or without implant or isolated "lumpectomy" with axillary node dissection (anticipated removal of at least 5 nodes) | 1100 | Regional anaesthesia and analgesia (epidural or paravertebral), combined with deep sedation or general anaesthesia (sevoflurane) | General anaesthesia (sevoflurane) followed by opioid administration |
| Kurz 2008 | 2008 | Patients scheduled for open, laparoscopic or laparoscopic‐assisted surgery for colon cancer without known extension beyond colon | 2500 | Intraoperative and postoperative regional anaesthesia and analgesia (epidural or paravertebral anaesthesia) plus intraoperative general anaesthesia | General anaesthesia followed by postoperative opioid analgesia |
| Chang 2009 | 2009 | Female patients 21‐75 years of age, ASA I‐II, diagnosed with biopsy‐proven breast cancer, scheduled for mastectomy and axillary node dissection in a single procedure | 40 | Local anaesthesia + sedation | General anaesthesia |
| Kurz 2010 | 2010 | Male and female patients 18‐85 years of age, diagnosed with primary non‐small cell lung cancer and scheduled for potentially curative tumour resection | 1532 | Intraoperative and postoperative general anaesthesia + epidural anaesthesia and analgesia | General anaesthesia and postoperative intravenous analgesia |
| Ilfeld 2010 | 2010 | Female patients 18 years of age and older, undergoing unilateral or bilateral mastectomy | 60 | Postoperative paravertebral catheter analgesia with ropivacaine | Placebo (normal saline) |
| Gupta 2011a | 2011 | Male and female patients 40‐80 years of age, ASA I‐III, undergoing elective surgery for colorectal cancer | 300 | Epidural analgesia with ropivacaine and opioid | PCA with morphine |
| Lee 2011 | 2011 | Male and female patients 25‐80 years of age, diagnosed with non‐small cell lung cancer with clinical staging of I or II for whom thoracoscopic lobectomy (VATS) is feasible | 100 | Intraoperative thoracic epidural anaesthesia | Intraoperative general anaesthesia |
| Van Aken 2012 | 2012 | Patients scheduled for inguinal lymph node dissection because of malignant melanoma of the lower limb | 230 | Spinal anaesthesia | General anaesthesia |
| Chan 2013 | 2013 | Male and female patients 20‐85 years of age with pancreatic cancer, expected to receive curative Whipple operation | 150 | Epidural analgesia with ropivacaine and opioid | PCA with opioid |
VATS = video‐assisted thoracic surgery.
ASA = American Society of Anesthesiologists physical status classification.
PCA = patient‐controlled analgesia.
Risk of bias in included studies
See Figure 2, Figure 3 and Characteristics of included studies.
2.
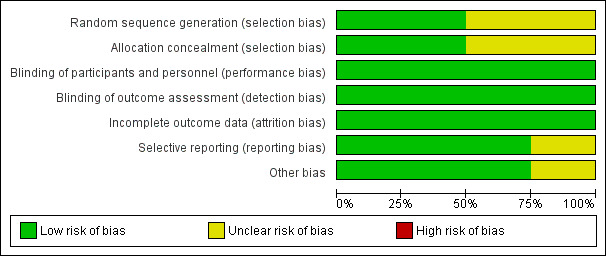
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
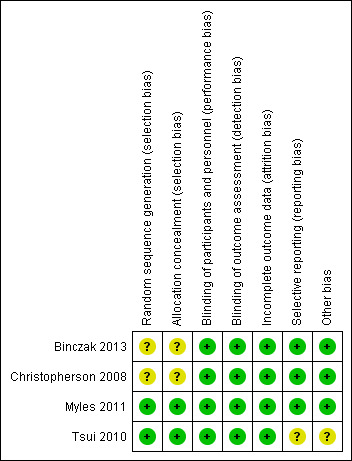
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two studies describe a proper, concealed randomization and allocation process (Myles 2011; Tsui 2010). One study used an adaptive randomization scheme, but concealment of allocation was not described (Christopherson 2008). The authors of another study described the study as randomized without further elaboration (Binczak 2013).
One study described the randomization process for the full analysed cohort (Tsui 2010). The remaining three studies are secondary analyses of subgroups of previously published randomized controlled trials. Therefore, it can only be assumed that the analysed subgroups were evenly distributed despite proper randomization of the original trial. One study reported that the distribution of demographic and perioperative characteristics of the analysed subgroups was comparable, and it was judged as having low risk of selection bias, accordingly (Myles 2011). Two studies did not provide information on the distribution of perioperative characteristics within the analysed subgroups, and we deemed the risk of selection bias for these as unclear (Binczak 2013; Christopherson 2008).
Blinding
Blinding of participants and personnel was attempted in one trial with the placement of a subcutaneous sham catheter at the site where an epidural catheter could be found (Binczak 2013). Three other studies were not blinded to participants and personnel.
One of the included studies reported an attempt to blind the outcome assessment process by temporary removal of the treatment allocation from the data set (Tsui 2010). The three remaining studies did not comment on the blinding of the outcome assessor.
Given the well‐defined end points (OS, PFS, TTP), we deemed the risk low that lack of blinding could influence the outcome measurement.
Incomplete outcome data
All four studies described excluded participants appropriately. One study used intention‐to‐treat analysis (Myles 2011), and one study explicitly did not (Tsui 2010). The two remaining studies did not comment on intention‐to‐treat.
Selective reporting
For none of the four studies was a study protocol available. Two studies reported outcomes in accordance with their methods description (Binczak 2013; Christopherson 2008). One study reported a secondary outcome that was not mentioned and defined in the methods section (Myles 2011). Another study did not define its outcome variable without ambiguity (Tsui 2010). The outcome was named 'survival', 'disease‐free survival' and 'recurrence', and it remained unclear whether these terms were used interchangeably, or if only one of these outcomes was reported. Therefore, we judged the risk of reporting bias for this study as unclear.
Other potential sources of bias
We did not identify other sources of potential bias in two studies (Binczak 2013; Christopherson 2008). In two other studies, we noted a mismatch in the reported numbers of included participants across the published articles. We deemed this unlikely to introduce bias for one study because of the very small difference (Myles 2011) and judged the risk of bias as unclear for the other study (Tsui 2010).
In addition, the outcome definition of one study (Tsui 2010) did not exactly match the outcome definitions of our review. However, given a small actual difference, we did not expect this to add bias.
Effects of interventions
See: Table 1
Intervention 1: general anaesthesia alone versus general anaesthesia combined with one or more regional anaesthetic techniques (Table 1).
Primary outcomes
Overall survival (OS)
Three studies with a total of 647 participants reported OS (Binczak 2013; Christopherson 2008; Myles 2011). None of the single studies showed a difference between study groups. Pooled results of these three studies did not show a survival benefit for either study group in a fixed‐effect model (HR 1.03, 95% CI 0.86 to 1.24; Figure 4). Statistical heterogeneity did not reach the significance level (P value 0.21), and the level of inconsistency across studies was low (I2 = 36%). To address concerns on clinical heterogeneity, we repeated the analysis using a random‐effects model. The results changed only marginally (HR 1.02, 95% CI 0.78 to 1.34).
4.
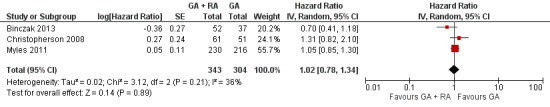
Forest plot of comparison: 1 general anaesthesia + regional anaesthesia (GA + RA) vs general anaesthesia (GA), outcome: 1.1 overall survival.
Progression‐free survival (PFS)
Two studies with a total of 535 participants reported PFS (Binczak 2013; Myles 2011). None of the single studies showed a difference between study groups. Pooled results of these studies showed a high level of heterogeneity and inconsistency (I2 = 64%, P value 0.10). We therefore pooled the results in a random‐effects model. The analysis did not show a survival benefit for either study group (HR 0.88, 95% CI 0.56 to 1.38; Figure 5). With only two studies included and no individual participant data available, subgroup analysis to further evaluate the source of heterogeneity was not feasible.
5.
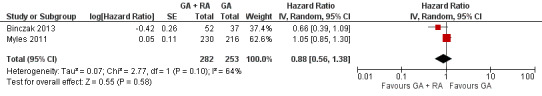
Forest plot of comparison: 1 general anaesthesia + regional anaesthesia (GA + RA) vs general anaesthesia (GA), outcome: 1.2 progression‐free survival.
Time to tumour progression (TTP)
Two studies with a total of 545 participants reported TTP (Myles 2011; Tsui 2010). None of the single studies showed a difference between study groups. Pooled results of these studies just reached the significance level in favour of the control group in a fixed‐effect model (HR 1.50, 95% CI 1.00 to 2.25; Figure 6). Statistical heterogeneity did not reach the significance level (P value 0.70), and the level of inconsistency across studies was I2 = 0%. The results did not change in a random‐effects model analysis (HR 1.50, 95% CI 1.00 to 2.25).
6.
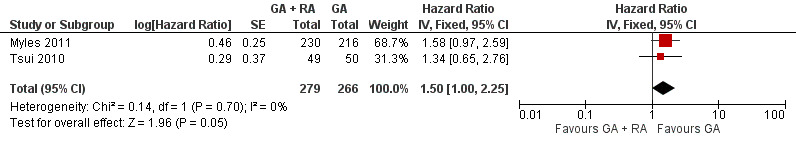
Forest plot of comparison: 1 general anaesthesia + regional anaesthesia (GA + RA) vs general anaesthesia (GA), outcome: 1.3 time to tumour progression.
Intervention 2: general anaesthesia combined with one or more regional anaesthetic techniques versus one or a combination of regional anaesthetic techniques.
We identified no trials investigating this comparison.
Intervention 3: general anaesthesia alone versus one or more regional anaesthetic techniques.
We identified no trials investigating this comparison.
Secondary outcomes
Postoperative adverse events including failed epidural catheter placement, PONV, postoperative respiratory complications and postoperative cardiac complications were reported only sparsely. In one study, epidural placement failed in two of 51 participants assigned to the epidural group. Both were excluded from the study and from the analysis (Tsui 2010). The same study reported one participant with postdural puncture headache postoperatively and one participant with postoperative ST depressions in the epidural group. Another study noted that epidural catheter placement was not always possible, but the study did not provide numbers on the failure rate (Myles 2011). Because of lack of data, no further analysis was performed on secondary outcomes.
Discussion
Summary of main results
Only three of four included studies contributed to the outcome of overall survival (OS) (Binczak 2013; Christopherson 2008; Myles 2011), and two each to the outcomes of progression‐free survival (PFS) (Binczak 2013; Myles 2011) and time to tumour progression (TTP) (Myles 2011; Tsui 2010). In our meta‐analysis, we could find no advantage for either study group for the outcomes of OS and PFS. Pooled results for the outcome of PFS showed a high level of inconsistency and heterogeneity. One possible explanation could be the interaction of risk factors that was not controlled for in the RCTs (i.e. opioid administration regimen and/or type of tumour).
Pooled data for TTP showed a slightly favourable outcome for the control group (general anaesthesia alone) compared with the intervention group (epidural and general anaesthesia). However, only two studies are included, and confidence intervals are wide. We therefore interpret these results very cautiously and would not derive clinical recommendations from these data at this point.
Overall completeness and applicability of evidence
All four identified studies are secondary data analyses of previous randomized controlled trials. Although we judged the quality of all included randomized trials and the following secondary data analyses as moderate, this study design has important limitations. All studies indeed compared regional anaesthesia techniques versus general anaesthesia in accordance with our inclusion criteria. However, regional anaesthesia techniques are meant to be only a surrogate for three important factors that might influence long‐term outcomes after cancer surgery: (1) reduction in or avoidance of anaesthetics, especially volatile anaesthetics; (2) reduction in or avoidance of opioid analgesics; and (3) reduction in or avoidance of surgical stress. In all four trials, both study groups received volatile anaesthetics in a comparable fashion, most often isoflurane, and the study design allowed for opioid administration in both study groups. The study reports do suggest that the total quantity of opioids was less in the regional anaesthesia group, but no study actually reported real‐time numbers on opioid consumption and comparisons between study groups (Table 3; Myles 2011). Moreover, the protocol of all four studies allowed for epidural opioid administration in the intervention group perioperatively. Preliminary retrospective data in patients undergoing surgery for pancreatic cancer suggest the possibility that large amounts of epidural opioids might worsen long‐term survival (Alexander 2009; Kienbaum 2010).
In addition, type of cancer was considerably different within as well as between studies. Characteristics of different types of cancer such as aggressiveness, natural course and affected patient population might be so different that the fusion of those into a single set of data might blur the results significantly.
We therefore established that currently available data prove only lack of evidence. RCTs are sparse, and the study designs of available RCTs are not ideal for illuminating the underlying hypothesis.
Quality of the evidence
The GRADE approach considers the domains risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias to assess the quality of evidence. We graded the quality of evidence as low for the outcome OS because of serious imprecision and serious indirectness. For PFS we graded the quality of evidence as very low because of serious imprecision, serious indirectness and serious inconsistency and for TTP we graded the quality of evidence as very low because of serious imprecision, serious indirectness and serious risk of bias (Summary of findings table 1).
We downgraded all three outcomes in the domain of precision because of a small sample size in relation to the expected effect size and also in the domain of directness because the proposed surrogate measure regional anaesthetic techniques did not in fact truly reflect the three possible pathways of how the intervention might work (see How the intervention might work). In detail, all study patients received volatile anaesthetics and none of the included studies reported complete data on perioperative opioid administration in each study group. In addition, we downgraded PFS for serious inconsistency based on the unexplained heterogeneity, and TTP for serious risk of bias (see Risk of bias in included studies).
Potential biases in the review process
The type of cancer was not specified in two of the four studies, and the remaining studies included participants with colorectal cancer or prostate cancer. Different types of cancer can have a very different biology and natural course. Therefore, a possible effect of anaesthetic technique on tumour recurrence is better investigated with stratification according to type of tumour and tumour stage. Current data do not allow for subgroup analysis according to type of cancer.
Data on perioperative opioid management were not available for any of the included studies. However, all four studies administered opioids to both study groups. Given the immunosuppressive effects of opioids (see Background section), the quantity of opioids administered might be a highly relevant factor influencing tumour recurrence. We based the rationale of this review on the assumption that a regional anaesthetic technique would reduce the amount of administered opioid considerably. However, this assumption could not be quantified with the current data. Moreover, in all studies, both study groups received a balanced general anaesthesia with comparable administration of volatile anaesthetics. Consequently, the immunosuppressive effects of volatile anaesthetics (see How the intervention might work) cannot be investigated using this study design. Therefore, the results of this review are based on incomplete data and might only provide direction for further research rather than clinical recommendations.
Agreements and disagreements with other studies or reviews
The effect of the anaesthetic technique on tumour recurrence has been discussed intensely in the literature over recent years. The hypothesis that the anaesthesiologist could influence long‐term outcomes after cancer surgery seemed obvious based on the scientific findings of in vitro and animal studies. Although the first encouraging clinical reports date back to the 1990s (Schlagenhauff 2000; Seebacher 1990), the retrospective analysis performed by Exadaktylos and colleagues in 2006 received major attention and, as of 2013, was cited more than 160 times in the literature (Exadaktylos 2006). Our comprehensive literature search until December 2013 revealed no prospective RCTs with the primary outcome of tumour recurrence at the date the study was performed. We identified four secondary data analyses of RCTs previously conducted on other outcomes.
In addition, our search yielded 21 non‐randomized retrospective studies. Type of cancer, type of surgery, type of intervention(s), outcome measures and definitions, as well as statistical analysis, vary broadly, and so do the results. We summarize characteristics of the non‐randomized studies in Table 6. Overall, 10 non‐randomized studies report some positive effects of regional anaesthesia techniques on tumour recurrence, often only for a subgroup of the participant population or for one of two or more outcome measures. Three studies report negative effects of regional anaesthesia techniques on tumour recurrence, and eight studies found no significant correlation of anaesthesia techniques and tumour recurrence.
5. Characteristics of non‐randomized studies.
| Author year | Type of cancer | Type of surgery | Intervention 1 (n) | Intervention 2 (n) | Control (n) | Endpoint | Statistical method | Result* | Date of surgery | Follow‐up until |
| Exadaktylos 2006 | Breast CA | Mastectomy + LND | GA + paravertebral catheter (50) |
‐ | GA (79) | Time to tumour recurrence (local or metastasis) | Adjusted Cox regression | HR 0.21 (0.06‐0.71) | 2001‐2002 | 2005 |
| Ismail 2010 | Cervical CA | First brachytherapy (of several) | SPA or EC (63) |
‐ | GA (69) | 1. Time to tumour recurrence 2. Overall survival |
Adjusted Cox regression | 1. HR 0.95 (0.54‐1.67) 2. HR 1.46 (0.81‐2.61) |
1996‐2003 | nr |
| Gupta 2011b | Colon CA | Colorectal cancer surgery (open) | GA + EC preop (302) |
‐ | GA (58) | Overall mortality | Adjusted Cox regression with stratification on propensity score | HR 0.82 (0.30‐2.19) | 2004‐2009 | 2009 |
| Rectal CA | Colorectal cancer surgery (open) | GA + EC preop (260) |
‐ | GA (35) | Overall mortality | Adjusted Cox regression with stratification on propensity score | HR 0.45 (0.22‐0.90) | 2004‐2009 | 2009 | |
| Vogelaar 2012 (abstract) | Colon CA | Surgery for colon CA | EC 'perioperative' (407) |
‐ | GA (198) | Overall survival | Adjusted Cox regression | HR 0.93 (0.93‐0.98) | 1995‐2003 | 2011 |
|
Luo 2010 (abstract) |
Colon CA | Primary colon surgery | GA + EC (182) |
‐ | GA (931) | Tumour recurrence | Univariable | HR 1.33 (0.94‐1.87) | 2001‐2006 | 2009 |
| Gottschalk 2010 | Colorectal CA | Colorectal cancer surgery | GA + EC preop (256) |
‐ | GA (253) | Time to tumour recurrence | Adjusted Cox model with stratification on propensity score quintiles | HR 0.74 (0.45‐1.22) | 2000‐2007 | 2008 |
| Cummings 2012 | Colorectal CA w/no metastases | Open colectomy | EC (Medicare code) (9670) |
‐ | No EC (Medicare code) (32481) |
1. Overall survival 2. 4‐Year tumour recurrence |
1. Adjusted marginal Cox model with propensity score as co‐variate 2. Adjusted logistic regression |
1. HR 0.92 (0.88‐0.96) 2. OR 1.05 (0.95‐1.15) |
1996‐2005 | 2009 |
| Day 2012 | Colorectal CA | Laparoscopic resection | EC preop (107) |
SPA (144) |
GA + PCA (173) |
1. Overall survival 2. Disease‐free survival |
KM estimate, log‐rank test | 1. P value 0.622 2. P value 0.490 |
2003‐2010 | |
| Lai 2012 | Hepatocellular CA | Percutaneous radiofrequency ablation | GA + EC preop (62) |
‐ | GA (117) |
1. Recurrence‐free survival 2. Overall survival |
Adjusted Cox model with propensity score as co‐variate |
1. 3.66 (2.59‐5.15) 2. 0.77 (0.50‐1.18) |
1999‐2008 | 2011 |
| Gottschalk 2012 | Malignant melanoma | Lymph node dissection | SPA (52) | ‐ | GA (221) | Long‐term survival | Mean survival (months) of matched pairs (52 pairs) |
95.9 (81.2‐110.5) SPA 70.4 (53.6‐87.1) GA P value 0.087 |
1998‐2005 | 2009 |
| Seebacher 1990 | Malignant melanoma | Melanoma resection | Local anaesthesia (376) |
‐ | GA (190) | Survival | KM estimate, log‐rank test | P value 0.51 (stage pT1/2, n = 237) P value 0.006 (stage pT3a, n = 195) in favour of local anaesthesia P value 0.47 (stage pT3b/4, n = 134) |
Control: 1972‐1980 Intervention: 1981‐88 |
1988 |
| Schlagenhauff 2000 | Malignant melanoma w/no metastases | Primary melanoma excision | Local anaesthesia (2185) | ‐ | GA (2136) | Survival | Log‐rank test on matched pairs (1501 pairs) | P value < 0.01 in favour of local anaesthesia | 1976‐1986 | nr |
| De Oliveira 2011 | Ovarian CA | Surgery for ovarian cancer | GA + EC preop (26) |
GA intraop/EC postop (29) |
GA (127) |
1. Overall survival 2. Time to recurrence |
1. Median survival time (months), log‐rank test 2. Adjusted Cox model |
1. 71 m (62‐80) for GA 96 m (84‐109) for EC intraop 70 m (58‐83) for EC postop P value 0.01 for GA vs EC intraop (favours EC intraop) 2. HR 0.37 (0.19‐0.73) for intraop EC HR 0.86 (0.52‐1.41) for postop EC |
2000‐2006 | 2009 |
| Lin 2011 | Ovarian CA | Surgery for ovarian cancer | EC only preop (106) |
‐ | GA (37) | Survival time | Adjusted Cox regression on propensity matched pairs (29 pairs) | HR 0.83 (0.67‐0.99) | 1994‐2006 | 2008 |
|
Koensgen 2013 (abstract) |
Ovarian CA | Primary radical tumour debulking | EC preop + GA (72) | GA (33) |
1. Recurrence‐free survival 2. Overall survival |
KM estimate, log‐rank test |
1. HR 1.52 (1.4‐1.56), P value 0.008 2. nr |
2003‐2010 | nr | |
| Lacassie 2013 | Ovarian cancer (Figo IIIc‐IV) |
Exploratory laparotomy | EC preop or postop + GA (37) | GA (43) | 1. Time to recurrence 2. Cancer‐specific survival |
Adjusted Cox regression with propensity score weighting | 1. HR 0.65 (0.40‐1.08) 2. HR 0.59 (0.32‐1.08) |
2000‐2011 | nr | |
| Kienbaum 2010/Alexander 2009 (abstracts) | Pancreatic CA | Radical pancreatic tumour resection | GA + EC (71) |
‐ | GA (29) | Overall survival | Log‐rank | P value 0.05 (P value 0.025 in favour of control for participants receiving high‐dose epidural opioids) |
2005‐2008 | nr |
| Biki 2008 | Prostate CA | Open radical prostatectomy | GA + EC preop (102) | ‐ | GA (123) | BCR‐free survival | Univariable Cox regression on propensity matched pairs (71 pairs) | HR 0.48 (0.23‐1.00) | 1994‐2003 | 2006 |
| Forget 2011 | Prostate CA w/no metastasis | Radical prostatectomy | GA + EC preop (578) |
‐ | GA (533) | BCR‐free survival | Adjusted Cox model | HR 0.84 (0.52‐1.17) | 1993‐2006 | 2006 |
| Wuethrich 2010 | Prostate CA (all stages) | Open radical retropubic prostatectomy w/LND | GA + EC preop (103) |
‐ | GA (158) | 1. BCR‐free survival 2. Clinical progression‐free survival 3. Cancer‐specific survival 4. Overall survival |
Adjusted Cox model with propensity score as co‐variate | 1. HR 0.82 (0.50‐1.34) 2. HR 0.40 (0.20‐0.79) 3. HR 0.95 (0.36‐2.47) 4. HR 1.01 (0.44‐2.32) |
Intervention: 1994‐1997 Control: 1997‐2000 |
nr |
| Wuethrich 2013 | Prostate CA (pT3/4) | Retropubic radical prostatectomy w/LND | GA + EC preop (67) |
‐ | GA (81) | 1. BCR‐free survival 2. Local recurrence‐free survival 3. Distant recurrence‐free survival 4. Cancer‐specific survival 5. Overall survival |
Univariable Cox regression on matched pairs (67 pairs) | 1. HR 1.00 (0.69‐1.47) 2. HR 1.16 (0.41‐3.29) 3. HR 0.56 (0.26‐1.25) 4. HR 0.96 (0.45‐2.05) 5. HR 1.17 (0.63‐2.17) |
1994‐2000 | nr |
Several statistical methods were used in most studies. We weighted reported results in the following descending order: adjusted regression with propensity score or matched pairs, adjusted regression, univariable analysis. Only the highest weighted analysis is reported in the table.
HR = hazard ratio, defined as intervention/control.
*HR < 1 denotes advantage for the intervention group, HR > 1 denotes advantage for the control group. We adjusted the HR derived from individual trials accordingly, as needed.
bold font denotes significant results in favour of the intervention group (EC).
italic font denotes significant results in favour of the control group (GA).
CA = cancer.
pT = pathological tumour staging.
EC = epidural catheter.
SPA = spinal anaesthesia.
GA = general anaesthesia.
LND = lymph node dissection.
preop = preoperatively.
postop = postoperatively.
n = number of participants.
OR = odds ratio.
n.s. = non‐significant.
BCR = biochemical recurrence.
nr = not reported.
m = months.
We identified two meta‐analyses on the effects of anaesthesia technique on the risk of tumour recurrence (Chang 2011; Chen 2013). Both meta‐analyses pooled randomized and non‐randomized data; one included seven studies, and the other 14 studies. The meta‐analysis by Chang and colleagues (Chang 2011) did not find a significant difference overall between the effects of general anaesthesia versus general and epidural anaesthesia on tumour recurrence. The meta‐analysis by Chen and colleagues (Chen 2013) reported significant benefit of general and epidural anaesthesia versus general anaesthesia on overall survival but not on recurrence‐free survival. Further evaluation using subgroup analysis by study design showed that the benefit of regional anaesthesia for overall survival was evident in non‐randomized studies only, but no effect could be shown for randomized studies. This result is in accordance with those of our meta‐analysis on RCTs.
Authors' conclusions
Implications for practice.
Although bench data and retrospective studies have provided a promising picture of the possible influence of anaesthetic technique on the risk of tumour recurrence, current evidence from RCTs is inadequate to show whether regional anaesthesia might influence tumour recurrence. Clinical decisions should not be made on these grounds until additional high‐level evidence data become available.
Implications for research.
This review illustrates the current lack of evidence for an effect of regional anaesthesia techniques on long‐term outcomes after cancer surgery. Well‐designed randomized trials are needed to further investigate this highly relevant topic. Specifically, studies are needed that minimize opioid administration in the intervention group while at the same time documenting and reporting on opioid consumption perioperatively in both study groups. Epidural, intrathecal or peripheral opioid injections might be a relevant confounder, and this should be taken into account when procedures for the intervention group are standardized. Moreover, studies avoiding general anaesthesia in the intervention group, which means that no potentially immunosuppressive anaesthetics will be administered, should be designed. In addition, the outcome measure should be well defined and—if possible—consistent across studies to allow for comparison and summary of the results, and investigations should be stratified according to tumour type and stage.
We identified several apparently well‐designed ongoing RCTs that will allow further insight once their results become available.
What's new
| Date | Event | Description |
|---|---|---|
| 28 November 2014 | Amended | Typo corrected |
History
Protocol first published: Issue 12, 2010 Review first published: Issue 11, 2014
| Date | Event | Description |
|---|---|---|
| 23 February 2012 | Amended | Contact details updated. |
Acknowledgements
We wish to thank Gloria Won, MLIS (librarian, HM, Fishbon Memorial Library, UCSF Medical Center at Mount Zion) for invaluable assistance in developing the search strategies for this review.
We would like to thank Mathew Zacharias (content editor); Donal Buggy, George Shorten and Richard L Nelson (peer reviewers); and Sai Janani (representative of the Cochrane Consumer Network) for help and editorial advice during preparation of the protocol for the systematic review.
We would like to thank Stephan Kettner (content editor); Cathal Walsh (statistical editor); and Kate Leslie, Gabriel L Snyder, Kwok M Ho and Richard L Nelson (peer reviewers) for help and editorial advice provided during preparation of this systematic review.
Appendices
Appendix 1. Search strategies
Search strategy for PubMed (1950 to present)
| #12 | Search #9 AND #11 |
| #11 | Search randomized controlled trial OR randomized controlled trials OR controlled clinical trial OR controlled clinical trials OR random* OR trial OR trials OR groups OR double blind method OR double blind methods OR single blind method OR single blind methods OR clinical trial OR clinical trials OR research design OR controlled study OR controlled studies OR "clinical study" OR "clinical studies" OR control OR controlled OR controls |
| #10 | Search #8 AND (animals[mh] NOT humans[mh]) |
| #9 | Search #8 NOT (animals[mh] NOT humans[mh]) |
| #8 | Search #7 NOT (editorial[pt] OR letter[pt] OR case reports[pt] OR news[pt] OR newspaper article[pt]) |
| #7 | Search #3 OR #5 OR #6 |
| #6 | Search #4 AND (neoplasm*[ti] OR tumor*[ti] OR tumour*[ti] OR cancer*[ti]) AND (recur*[ti] OR risk*[ti] OR metasta*[ti]) |
| #5 | Search #2 AND #4 AND neoplasm[mh] AND adverse effects[sh] |
| #4 | Search opioid* OR opiate* OR morphine* OR alfentanil OR alphadolone OR alphaxalone OR benoxinate OR benzocaine OR "benzyl alcohol" OR bumecain OR bupivacaine OR butamben OR carbizocaine OR carticaine OR chloralose OR chloroprocaine OR cyclopropane OR desflurane OR diazepam OR dibucaine OR diphenhydramine OR dyclonine OR emla OR enflurane OR entonox OR etidocaine OR etomidate OR ether OR fentanyl OR halothane OR heptacaine OR innovar OR isoflurane OR ketamine OR levobupivacaine OR lidocaine OR lignocaine OR "magnesium sulfate" OR mepivacaine OR methohexital OR methoxyflurane OR methyleugenol OR midazolam OR minaxolone OR "nitrous oxide" OR norflurane OR pentacaine OR phenoxyethanol OR pregnanolone OR prilocaine OR procaine OR propanidid OR propisomide OR propofol OR propoxycaine OR proxymetacaine OR remifentanil OR romifidine OR ropivacaine OR sevoflurane OR "sodium oxybate" OR sufentanil OR "tec solution" OR tetracaine OR tetrahydrodeoxycorticosterone OR tetrodotoxin OR thiamylal OR thiopental OR tiletamine OR tribromoethanol OR tricaine OR trichloroethylene OR trimecaine OR urethane OR anesthe*[ti] OR anaesthe*[ti] OR analges*[ti] |
| #3 | Search #1 AND #2 |
| #2 | Search neoplasm recurrence, local[mh] OR neoplasm invasiveness[mh] OR neoplasm metastasis[mh] OR cocarcinogenesis[mh] |
| #1 | Search "anesthesia and analgesia"[mh:noexp] OR anesthesia[mh] OR analgesia[mh:noexp] OR analgesia, epidural[mh] OR analgesia, patient‐controlled[mh] OR anesthetics[majr] OR anesthetics/adverse effects OR anesthetics/immunology OR anesthetics/pharmacology OR analgesics[majr] OR analgesics/adverse effects OR analgesics/immunology OR analgesics/pharmacology OR adjuvants, anesthesia[mh] |
The PubMed search will use a combination of Medical Subject Headings and Keyword terms.
Search strategy for EMBASE (1974 to present)
| #14 | #11 AND #13 |
| #13 | 'randomized controlled trial' OR 'randomized controlled trials' OR 'controlled clinical trial' OR 'controlled clinical trials' OR random*:ab,ti OR 'double blind procedure' OR 'double blind procedures' OR 'single blind procedure' OR 'single blind procedures' OR 'clinical trial' OR 'clinical trials' OR 'controlled study' OR 'controlled studies' OR 'clinical study'/de OR 'major clinical study'/exp |
| #12 | #10 AND [animals]/lim NOT [humans]/lim |
| #11 | #10 NOT ([animals]/lim NOT [humans]/lim) |
| #10 | #9 NOT ('editorial'/de OR 'letter'/de OR 'case report'/de) |
| #9 | #3 OR #6 OR #7 OR #8 |
| #8 | anesthe*:ti OR anaesthe*:ti OR analges*:ti AND metasta*:ti |
| #7 | anesthe*:ti OR anaesthe*:ti OR analges*:ti AND (neoplasm*:ti OR tumor*:ti OR tumour*:ti OR cancer*:ti) AND (recur*:ti OR risk*:ti OR metasta*:ti) |
| #6 | #4 AND #5 |
| #5 | 'cancer recurrence'/exp OR 'recurrent cancer'/exp OR 'tumor recurrence'/exp OR 'metastasis'/de OR 'cocarcinogenesis'/de OR 'cancer invasion'/exp |
| #4 | 'anesthesiological techniques'/exp/mj OR 'anesthetic agent'/exp/mj OR 'analgesic agent'/exp/mj OR 'local anesthetic agent'/exp/mj OR 'anesthesia complication'/exp/mj |
| #3 | #1 AND #2 |
| #2 | 'cancer recurrence'/exp/mj OR 'recurrent cancer'/exp/mj OR 'tumor recurrence'/exp/mj OR 'metastasis'/mj OR 'cocarcinogenesis'/mj OR 'cancer invasion'/exp/mj |
| #1 | 'anesthesiological techniques'/exp OR 'anesthetic agent'/exp OR 'analgesic agent'/exp OR 'local anesthetic agent'/exp OR 'anesthesia complication'/exp |
The EMBASE search will use EMTREE subject headings and select Title Word terms.
Search strategy for ISI Web of Science (1965 to present)
| #9 | #8 AND #7 Databases=SCI‐EXPANDED, SSCI, A&HCI Timespan=All years |
| #8 | Topic=(random* OR "controlled clinical trial" OR "controlled clinical trials" OR "double blind method" OR "double blind methods" OR "single blind method" OR "single blind methods" OR "clinical trial" OR "clinical trials" OR "research design" OR "controlled study" OR "controlled studies" OR "clinical study" OR "clinical studies") Databases=SCI‐EXPANDED, SSCI, A&HCI Timespan=All years |
| #7 | #5 OR #6 Databases=SCI‐EXPANDED, SSCI, A&HCI Timespan=All years |
| #6 | Title=(anesthe* or anaesthe* or analges*) AND Title=(metasta*) Databases=SCI‐EXPANDED, SSCI, A&HCI Timespan=All years |
| #5 | #3 and #4 Databases=SCI‐EXPANDED, SSCI, A&HCI Timespan=All years |
| #4 | Topic=(neoplasm* or tumor* or tumour* or cancer*) AND Topic=(recur* or risk* or metasta*) Databases=SCI‐EXPANDED, SSCI, A&HCI Timespan=All years |
| #3 | #1 OR #2 Databases=SCI‐EXPANDED, SSCI, A&HCI Timespan=All years |
| #2 | Title=("magnesium sulfate" OR mepivacaine OR methohexital OR methoxyflurane OR methyleugenol OR midazolam OR minaxolone OR "nitrous oxide" OR norflurane OR pentacaine OR phenoxyethanol OR pregnanolone OR prilocaine OR procaine OR propanidid OR propisomide OR propofol OR propoxycaine OR proxymetacaine OR remifentanil OR romifidine OR ropivacaine OR sevoflurane OR "sodium oxybate" OR sufentanil OR "tec solution" OR tetracaine OR tetrahydrodeoxycorticosterone OR tetrodotoxin OR thiamylal OR thiopental OR tiletamine OR tribromoethanol OR tricaine OR trichloroethylene OR trimecaine OR urethane) Databases=SCI‐EXPANDED, SSCI, A&HCI Timespan=All years |
| #1 | Title=(anesthe* OR anaesthe* OR analges* OR opioid* OR opiate* OR morphine* OR alfentanil OR alphadolone OR alphaxalone OR benoxinate OR benzocaine OR "benzyl
alcohol" OR bumecain OR bupivacaine OR butamben OR carbizocaine OR carticaine OR chloralose OR chloroprocaine OR cyclopropane OR desflurane OR diazepam OR dibucaine OR diphenhydramine OR dyclonine OR emla OR enflurane OR entonox OR etidocaine OR etomidate OR ether OR fentanyl OR halothane OR heptacaine OR innovar OR isoflurane OR ketamine OR levobupivacaine OR lidocaine OR lignocaine) Databases=SCI‐EXPANDED, SSCI, A&HCI Timespan=All years |
Search strategy for BIOSIS (1926 to present)
| # 11 | #10 AND Document Type=(Article OR Meeting OR Meeting Paper) AND Taxa Notes=(Humans) Databases=PREVIEWS Timespan=All Years |
| # 10 | #9 AND Document Type=(Article OR Meeting OR Meeting Paper) Databases=PREVIEWS Timespan=All Years |
| # 9 | #8 AND #7 Databases=PREVIEWS Timespan=All Years |
| # 8 | Topic=(random* OR "controlled clinical trial" OR "controlled clinical trials" OR "double blind method" OR "double blind methods" OR "single blind method" OR "single blind methods" OR "clinical trial" OR "clinical trials" OR "research design" OR "controlled study" OR "controlled studies" OR "clinical study" OR "clinical studies") Databases=PREVIEWS Timespan=All Years |
| # 7 | #6 OR #5 Databases=PREVIEWS Timespan=All Years |
| # 6 | Title=(anesthe* or anaesthe* or analges*) AND Title=(metasta*) Databases=PREVIEWS Timespan=All Years |
| # 5 | #3 and #4 Databases=PREVIEWS Timespan=All Years |
| # 4 | Topic=(neoplasm* or tumor* or tumour* or cancer*) AND Topic=(recur* or risk* or metasta*) Databases=PREVIEWS Timespan=All Years |
| # 3 | #1 OR #2 Databases=PREVIEWS Timespan=All Years |
| # 2 | Title=("magnesium sulfate" OR mepivacaine OR methohexital OR methoxyflurane OR methyleugenol OR midazolam OR minaxolone OR "nitrous oxide" OR norflurane OR pentacaine OR phenoxyethanol OR pregnanolone OR prilocaine OR procaine OR propanidid OR propisomide OR propofol OR propoxycaine OR proxymetacaine OR remifentanil OR romifidine OR ropivacaine OR sevoflurane OR "sodium oxybate" OR sufentanil OR "tec solution" OR tetracaine OR tetrahydrodeoxycorticosterone OR tetrodotoxin OR thiamylal OR thiopental OR tiletamine OR tribromoethanol OR tricaine OR trichloroethylene OR trimecaine OR urethane) Databases=PREVIEWS Timespan=All Years |
| # 1 | Title=(anesthe* OR anaesthe* OR analges* OR opioid* OR opiate* OR morphine* OR alfentanil OR alphadolone OR alphaxalone OR benoxinate OR benzocaine OR "benzyl alcohol" OR bumecain OR bupivacaine OR butamben OR carbizocaine OR carticaine OR chloralose OR chloroprocaine OR cyclopropane OR desflurane OR diazepam OR dibucaine OR diphenhydramine OR dyclonine OR emla OR enflurane OR entonox OR etidocaine OR etomidate OR ether OR fentanyl OR halothane OR heptacaine OR innovar OR isoflurane OR ketamine OR levobupivacaine OR lidocaine OR lignocaine) Databases=PREVIEWS Timespan=All Years |
The Biosis search will use a simplified RCT strategy.
Search strategy for The Cochrane Library
#1 MeSH descriptor Anesthesia and Analgesia explode all trees #2 MeSH descriptor Analgesia, this term only #3 MeSH descriptor Analgesia, Patient‐Controlled explode all trees #4 MeSH descriptor Analgesia, Epidural explode all trees #5 MeSH descriptor Anesthetics, this term only #6 MeSH descriptor Analgesics explode all trees #7 MeSH descriptor Adjuvants, Anesthesia explode all trees #8 MeSH descriptor Anesthesia, Epidural explode all trees #9 MeSH descriptor Anesthesia, Spinal explode all trees #10 MeSH descriptor Anesthesia, Local explode all trees #11 MeSH descriptor Anesthesia, Conduction explode all trees #12 MeSH descriptor Nerve Block explode all trees #13 (opioid* or opiate* or morphin* or alfentanil or alphadolone or alphaxalone or benoxinate or benzocaine or "benzyl alcohol" or bumecain or bupivacaine or butamben or carbizocaine or carticaine or chloralose or chloroprocaine or cyclopropane or desflurane or diazepam or dibucaine or diphenhydramine or dyclonine or emla or enflurane or entonox or etidocaine or etomidate or ether or fentanyl or halothane or heptacaine or innovar or isoflurane or ketamine or levobupivacaine or lidocaine or lignocaine or "magnesium sulfate" or mepivacaine or methohexital or methoxyflurane or methyleugenol or midazolam or minaxolone or "nitrous oxide" or norflurane or pentacaine or phenoxyethanol or pregnanolone or prilocaine or procaine or propanidid or propisomide or propofol or propoxycaine or proxymetacaine or remifentanil or romifidine or ropivacaine or sevoflurane or "sodium oxybate" or sufentanil or "tec solution" or tetracaine or tetrahydrodeoxycorticosterone or tetrodotoxin or thiamylal or thiopental or tiletamine or tribromoethanol or tricaine or trichloroethylene or trimecaine or urethane):ti,ab #14 (ane?sthe* or analges*):ti #15 ((an?esth* or analg* or neuraxial or nerve block*) near (technique* or method*)) #16 ((intercostal or paravertebral) near nerve block*) #17 ((an?esth* or analg*) near complicat*) #18 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17) #19 MeSH descriptor Neoplasm Recurrence, Local explode all trees #20 MeSH descriptor Neoplasm Invasiveness explode all trees #21 MeSH descriptor Neoplasm Metastasis explode all trees #22 MeSH descriptor Cocarcinogenesis explode all trees #23 ((neoplasm* or tumo?r* or cancer* or malignant*) near (recur* or risk* or metasta* or growth* or intensif* or escalat* or develop* or invasion)) #24 carcinogenesis or metastas*:ti,ab #25 (#19 OR #20 OR #21 OR #22 OR #23 OR #24) #26 (#18 AND #25)
Appendix 2. Study eligibility screening form
Study eligibility screening form
| Study title | Screener | |
| OSC | KK | |
| First study author | Source (e.g. journal, abstract) | Publication year |
Study eligibility
| Inclusion criteria | Yes | No | Unknown | |
| Study design | RCT, CCT? | |||
| Participants | Tumour surgery in adults and/or children? |
|||
| Intervention | General anaesthesia (GA) vs regional anaesthesia (RA) or vs combination (GA + RA)? or opioid anaesthesia vs opioid‐free anaesthesia? |
|||
| Outcome | Mortality and/or tumour recurrence? |
If you answer any of the questions above ‘NO,’
Exclude the study
Provide a reason for exclusion
| Reason for exclusion |
If you answer all questions above with ‘YES’ or ‘UNKNOWN,’ proceed with the data abstraction form.
Appendix 3. Data extraction form
Data extraction form
| First study author | Year | |
| Study title: | Initials of review author: | |
| Source (Journal, Abstract…): | ||
| Study design: | RCT | CCT |
| Participants | Group 1 (control) |
Group 2 (intervention) |
| N per group | ||
| Age (mean) | ||
| Paediatric patients (n) | ||
| Male/female (n) | ||
| Type of cancer, site | ||
| Type of cancer, histology | ||
| TNM clinical | ||
| TNM pathological | ||
| Stage (0–IV) | ||
| Type(s) of surgery | ||
| Resection of the primary tumour (yes/no) | ||
| Preceding chemotherapy (n) | ||
| Preceding radiation (n) | ||
| Following chemotherapy (n) | ||
| Following radiation (n) | ||
| Additional information/notes: | ||
| Intervention | |
| Type of intervention (RA or combination GA + RA) | |
| Type of RA (block technique) (name all that apply) | |
| Single shot or catheter technique? | |
| Local anaesthetic (LA) used for RA | |
| Dose of LA (% mL) (mean) used for RA | |
| Time RA administered (preop/postop) | |
| Duration of RA (for catheter techniques) (mean per group) | |
| Control group GA? | |
| Type of GA (TIVA, BAL) | |
| If BAL: type of volatile anaesthetic used | |
| Amount of opioid used perioperatively per group (mean) | |
| Continuous IV lidocaine infusion used perioperatively? If yes: give duration and dosage |
|
| Any opioids administered intrathecally, epidurally or peripherally? If yes: give route of administration, dose and time |
|
| Outcome: overall survival | Group 1 (control) |
Group 2 (intervention) |
total |
| Randomization ratio | |||
| Participants randomly assigned (n) | |||
| Participants analysed (n) | |||
| Observed events (n) | |||
| Log‐rank expected events (n) | |||
| Hazard ratio, CI, level (e.g. 95%) | |||
| Log‐rank variance | |||
| Log‐rank observed—expected events | |||
| Hazard ratio (+CI/level or standard error or variance) from adjusted or unadjusted Cox |
|||
| Test statistics, 2‐sided P value, test used (e.g. log‐rank, Mantel‐Haenszel, Cox) |
|||
| Advantage for intervention or control? | |||
| Actuarial or Kaplan‐Meier curves reported? | |||
| Number at risk reported | |||
| Follow‐up: Minimum Maximum Median Time period of recruitment |
|||
| Interval censoring method | |||
| Outcome: progression‐free survival | Group 1 (control) |
Group 2 (intervention) |
total |
| Randomization ratio | |||
| Participants randomly assigned (n) | |||
| Participants analysed (n) | |||
| Observed events (n) | |||
| Log‐rank expected events (n) | |||
| Hazard ratio, CI, level (e.g. 95%) | |||
| Log‐rank variance | |||
| Log‐rank observed—expected events | |||
| Hazard ratio (+CI/level or standard error or variance) from adjusted or unadjusted Cox |
|||
| Test statistics, 2‐sided P value, test used (e.g. log‐rank, Mantel‐Haensel, Cox) |
|||
| Advantage for intervention or control? | |||
| Actuarial or Kaplan‐Meier curves reported? | |||
| Number at risk reported | |||
| Follow‐up: Minimum Maximum Median Time period of recruitment |
|||
| Interval censoring method | |||
| Outcome: time to tumour progression | Group 1 (control) |
Group 2 (intervention) |
total |
| Randomization ratio | |||
| Participants randomly assigned (n) | |||
| Participants analysed (n) | |||
| Observed events (n) | |||
| Log‐rank expected events (n) | |||
| Hazard ratio, CI, level (e.g. 95%) | |||
| Log‐rank variance | |||
| Log‐rank observed—expected events | |||
| Hazard ratio (+CI/level or standard error or variance) from adjusted or unadjusted Cox |
|||
| Test statistics, 2‐sided P value, test used (e.g. log‐rank, Mantel‐Haensel, Cox) |
|||
| Advantage for intervention or control? | |||
| Actuarial or Kaplan‐Meier curves reported? | |||
| Number at risk reported | |||
| Follow‐up (months): Minimum Maximum Median Time period of recruitment |
|||
| Interval censoring method | |||
| Adverse events reported (in‐hospital) | Group 1 (control) |
Group 2 (intervention) |
| PONV | ||
| Postoperative respiratory complications (i.e. pneumonia, respiratory insufficiency, aspiration, pulmonary embolism) |
||
| Postoperative cardiovascular events (i.e. myocardial ischaemia, myocardial infarction, heart failure, cardiac arrest) |
| Trial characteristics | ||
| Single‐centre/multi‐centre | ||
| Country/Countries | ||
| How was participant eligibility defined? | ||
| Was the outcome of interest (tumour recurrence) defined as a primary or secondary outcome in the original protocol? | ||
| If 'NO': | When was the decision made to assess tumour recurrence? | |
| Was there a formal study protocol amendment? If 'YES': When? What was the amendment? |
||
| How was tumour recurrence assessed? ‐ Follow‐up visits were part of the original study design and were used to assess tumour recurrence ‐ Assessment of tumour recurrence was extracted from cancer registry ‐ Assessment of tumour recurrence was extracted from hospital records |
||
| Additional information/notes: | ||
| Methodological quality: | Adequate (random) |
Inadequate (e.g. alternate) | Unclear |
| Allocation of intervention | |||
| Describe method: | |||
| Concealment of allocation | |||
| Describe method: | |||
| Blinding | Yes | No | Unclear |
| Caregiver | |||
| Participant | |||
| Outcome assessor | |||
| Intention‐to‐treat | |||
| All participants entering trial | |||
| 15% or fewer excluded | |||
| More than 15% excluded | |||
| Not analysed as intention‐to‐treat |
|||
| Unclear | |||
| Withdrawals described? | |||
| Additional notes: | |||
Appendix 4. The Cochrane Collaboration's tool for assessing risk of bias
Description
| Domain | Description | Review authors’ judgement |
| Sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups | Was the allocation sequence adequately generated? |
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment | Was allocation adequately concealed? |
| Blinding of participants, personnel and outcome assessorsAssessments should be made for each main outcome (or class of outcomes) | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information regarding whether the intended blinding was effective | Was knowledge of the allocated intervention adequately prevented during the study? |
| Incomplete outcome dataAssessments should be made for each main outcome (or class of outcomes) | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomly assigned participants), reasons for attrition/exclusions when reported and any re‐inclusions in analyses performed by the review authors | Were incomplete outcome data adequately addressed? |
| Selective outcome reporting | State how the possibility of selective outcome reporting was examined by the review authors, and describe what was found | Are reports of the study free of suggestion of selective outcome reporting? |
| Other sources of bias | State any important concerns about bias not addressed in the other domains in the tool If particular questions/entries were prespecified in the review protocol, responses should be provided for each question/entry |
Was the study apparently free of other problems that could put it at high risk of bias? |
Judgement
| SEQUENCE GENERATION Was the allocation sequence adequately generated? [Short form: Adequate sequence generation?] | |
| Criteria for a judgement of ‘YES’ (i.e. low risk of bias) | Investigators describe a random component in the sequence generation process such as: · Referring to a random number table; · Using a computer random number generator; · Coin tossing; · Shuffling of cards or envelopes; · Throwing of dice; · Drawing of lots; · Minimization*. *Minimization may be implemented without a random element, and this is considered to be equivalent to being random. |
| Criteria for a judgement of ‘NO’ (i.e. high risk of bias) | Investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: · Sequence generated by odd or even date of birth; · Sequence generated by some rule based on date (or day) of admission; · Sequence generated by some rule based on hospital or clinic record number. Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorization of participants, for example: · Allocation by judgement of the clinician; · Allocation by preference of the participant; · Allocation based on the results of a laboratory test or a series of tests; · Allocation by availability of the intervention. |
| Criteria for a judgement of ‘UNCLEAR’ (uncertain risk of bias) | Insufficient information about the sequence generation process to permit judgement of ‘Yes’ or ‘No’ |
| ALLOCATION CONCEALMENT Was allocation adequately concealed? [Short form: Allocation concealment?] | |
| Criteria for a judgement of ‘YES’ (i.e. low risk of bias) | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: · Central allocation (including telephone, web‐based and pharmacy‐controlled, randomization); · Sequentially numbered drug containers of identical appearance; · Sequentially numbered, opaque, sealed envelopes. |
| Criteria for a judgement of ‘NO’ (i.e. high risk of bias) | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: · Use of an open random allocation schedule (e.g. a list of random numbers); · Assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or were not sequentially numbered); · Alternation or rotation; · Date of birth; · Case record number; · Any other explicitly unconcealed procedure. |
| Criteria for a judgement of ‘UNCLEAR’ (uncertain risk of bias) | Insufficient information to permit judgement of ‘Yes’ or ‘No.’ This is usually the case if the method of concealment is not described or is not described in sufficient detail to allow a definitive judgement—for example, if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed |
| BLINDING OF PARTICIPANTS, PERSONNEL AND OUTCOME ASSESSORS Was knowledge of the allocated interventions adequately prevented during the study? [Short form: Blinding?] | |
| Criteria for a judgement of ‘YES’ (i.e. low risk of bias) | Any one of the following: · No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding; · Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken; · Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others is unlikely to introduce bias. |
| Criteria for a judgement of ‘NO’ (i.e. high risk of bias) | Any one of the following: · No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding; · Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken; · Either participants or some key study personnel were not blinded, and the non‐blinding of others is likely to introduce bias. |
| Criteria for a judgement of ‘UNCLEAR’ (uncertain risk of bias) | Any one of the following: · Insufficient information to permit judgement of ‘Yes’ or ‘No’; · The study did not address this outcome. |
| INCOMPLETE OUTCOME DATA Were incomplete outcome data adequately addressed? [Short form: Incomplete outcome data addressed?] | |
| Criteria for a judgement of ‘YES’ (i.e. low risk of bias) | Any one of the following: · No missing outcome data; · Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); · Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; · For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; · For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; · Missing data have been imputed using appropriate methods. |
| Criteria for a judgement of ‘NO’ (i.e. high risk of bias) | Any one of the following: · Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; · For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; · For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; · ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomization; · Potentially inappropriate application of simple imputation. |
| Criteria for a judgement of ‘UNCLEAR’ (uncertain risk of bias) | Any one of the following: · Insufficient reporting of attrition/exclusions to permit judgement of ‘Yes’ or ‘No’ (e.g. number randomly assigned not stated, no reasons for missing data provided); · The study did not address this outcome. |
| SELECTIVE OUTCOME REPORTING Are reports of the study free of suggestion of selective outcome reporting? [Short form: Free of selective reporting?] | |
| Criteria for a judgement of ‘YES’ (i.e. low risk of bias) | Any of the following: · The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way; · The study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). |
| Criteria for a judgement of ‘NO’ (i.e. high risk of bias) | Any one of the following: · Not all of the study’s prespecified primary outcomes have been reported; · One or more primary outcomes are reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified; · One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); · One or more outcomes of interest in the review are reported incompletely, so that they cannot be entered into a meta‐analysis; · The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
| Criteria for a judgement of ‘UNCLEAR’ (uncertain risk of bias) | Insufficient information to permit judgement of ‘Yes’ or ‘No.’ It is likely that most studies will fall into this category |
| OTHER POTENTIAL THREATS TO VALIDITY Was the study apparently free of other problems that could put it at risk of bias? [Short form: Free of other bias?] | |
| Criteria for a judgement of ‘YES’ (i.e. low risk of bias) | The study appears to be free of other sources of bias |
| Criteria for a judgement of ‘NO’ (i.e. high risk of bias) | There is at least one important risk of bias. For example, the study: · Had a potential source of bias related to the specific study design used; or · Stopped early because of some data‐dependent process (including a formal‐stopping rule); or · Had extreme baseline imbalance; or · Has been claimed to have been fraudulent; or · Had some other problem. |
| Criteria for a judgement of ‘UNCLEAR’ (uncertain risk of bias) | There may be a risk of bias, but there is either: · Insufficient information to assess whether an important risk of bias exists; or · Insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. GA + RA versus GA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 3 | 647 | Hazard Ratio (Random, 95% CI) | 1.02 [0.78, 1.34] |
| 2 Progression‐free survival | 2 | 535 | Hazard Ratio (Random, 95% CI) | 0.88 [0.56, 1.38] |
| 3 Time to tumour progression | 2 | 545 | Hazard Ratio (Fixed, 95% CI) | 1.50 [1.00, 2.25] |
1.1. Analysis.
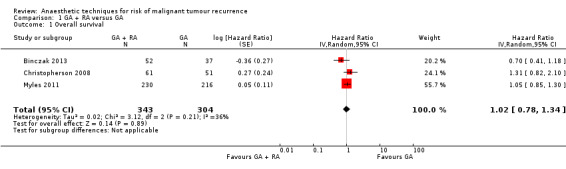
Comparison 1 GA + RA versus GA, Outcome 1 Overall survival.
1.2. Analysis.
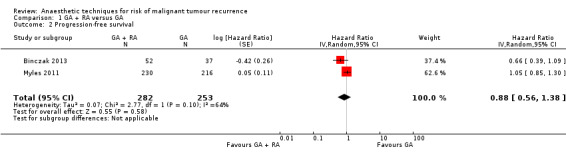
Comparison 1 GA + RA versus GA, Outcome 2 Progression‐free survival.
1.3. Analysis.
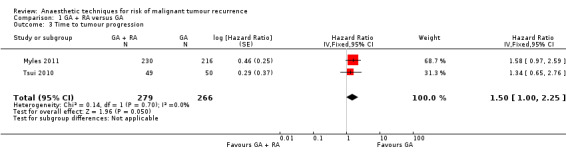
Comparison 1 GA + RA versus GA, Outcome 3 Time to tumour progression.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Binczak 2013.
| Methods | Secondary data analysis of a single‐centre double‐blinded RCT | |
| Participants | 89 adult patients scheduled for major abdominal surgery for cancer | |
| Interventions | Intraoperative and postoperative epidural analgesia vs general anaesthesia alone (IV opioids) | |
| Outcomes | Overall survival; recurrence‐free survival | |
| Notes | 163 participants were randomly assigned in the prospective trial; 153 completed the study, 132 of those had malignancy and 89 of those underwent primary tumour resection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The original trial was described as randomized, but no information on the randomization process was given. Analysed subgroup might not be perfectly balanced |
| Allocation concealment (selection bias) | Unclear risk | No information was given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants was attempted with sham SC catheter, but placement of epidural took place preoperatively in awake participants, while SC catheter was placed postoperatively while participant was anaesthetised. Blinding of caregivers was not reported. Incomplete blinding likely did not influence the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not reported. However, it was judged unlikely that outcome assessment was influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was properly described Missing outcome data are unlikely to be related to survival outcomes |
| Selective reporting (reporting bias) | Low risk | No study protocol was available. Outcomes are reported in accordance with the methods section of the published study |
| Other bias | Low risk | Appears to be free of other sources of bias |
Christopherson 2008.
| Methods | Secondary data analysis of multi‐centre RCT (subgroup) | |
| Participants | 112 male adult ASA III patients undergoing surgery for colon cancer | |
| Interventions | Intraoperative and postoperative epidural analgesia with bupivacaine, epinephrine and morphine vs general anaesthesia alone (IV or IM opioids) | |
| Outcomes | Overall survival | |
| Notes | 1021 participants randomly assigned in prospective multi‐centre trial; 982 completed the study; of those 177 with colon cancer and available pathology staging data; of those, 112 without metastasis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Original study: adaptive randomization scheme within each site (balanced variables: type of surgery, age, Goldman index). However, even distribution of subgroup analysed is not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were not blinded. However, it was judged unlikely that the outcome was influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not reported. However, it was judged unlikely that the outcome assessment was influenced by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Pathological staging data could not be obtained for 70 participants and were excluded from the analysis. The survival experience for these 70 participants was similar to that for the 177 participants for whom staging data were available Missing outcome data are unlikely to be related to survival outcomes |
| Selective reporting (reporting bias) | Low risk | No study protocol was available. Outcomes are reported in accordance with the methods section of the published study |
| Other bias | Low risk | Appears to be free of other sources of bias |
Myles 2011.
| Methods | Secondary data analysis of a multi‐centre RCT (subgroup) | |
| Participants | 446 adult patients scheduled for major abdominal surgery for primary cancer without metastasis | |
| Interventions | Intraoperative and postoperative epidural analgesia vs general anaesthesia alone (IV opioids) | |
| Outcomes | Primary endpoint: progression‐free survival; secondary endpoint: overall survival, time to tumour progression | |
| Notes | 915 participants were included in the prospective multi‐centre trial; 506 of those had undergone surgery for cancer, and 3 were unclassified/excluded. 31 participants were excluded because of metastasis at the time of surgery. 26 additional participants were excluded because they were lost to follow‐up or refused to provide consent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization for the original study was based on use of random permuted blocks within each institution, and was maintained and allocated to each institution by a central trial secretariat at the Department of Public Health. Baseline characteristics of the analysed subgroup are reported and comparable |
| Allocation concealment (selection bias) | Low risk | Random permuted blocks used, assigned by central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and caregivers were not blinded. Likely no influence on outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was not reported. However, it was judged unlikely that outcome assessment was influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In all, 26 cases (14 participants in the study group and 12 in the control group) were lost to follow‐up and were not included in the data analysis. Characteristics of this group have not been reported Only 4 (< 1%) of the participants had incomplete (censored) data within the first 5 years after surgery Missing outcome data are unlikely to be related to survival outcomes |
| Selective reporting (reporting bias) | Low risk | No study protocol was available. One secondary outcome (TTR) was not specified or defined in the methods section of the published article but was reported in the results section. Primary outcome was reported in accordance with the methods section of the published study |
| Other bias | Low risk | The number of included participants varies between 445 (forest plot), 446 (text) and 447 (flow chart), most likely as the result of calculation error. However, as the difference is only 1 among more than 450 participants, we find that this most likely does not introduce bias |
Tsui 2010.
| Methods | Secondary data analysis of a single‐centre RCT | |
| Participants | 99 adult male patients undergoing radical prostatectomy and bilateral lymphadenectomy for adenocarcinoma of the prostate | |
| Interventions | Intraoperative general anaesthesia + epidural analgesia vs general anaesthesia alone (IV morphine) | |
| Outcomes | Clinical evidence or biochemical recurrence of prostate cancer (defined as PSA > 0.2 ng/mL) | |
| Notes | 102 participants randomly assigned in prospective single‐centre trial; 99 completed the protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on a computer‐generated table of random numbers, participants were block randomly assigned (block size = 10) |
| Allocation concealment (selection bias) | Low risk | Blinded study envelopes, which were opened immediately before surgery. Then, treatment allocation is predictable towards the end of a block of 10 if block size is known |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and caregivers were not blinded. Likely no influence on outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treatment allocation was temporarily removed from the data set during the censoring process in an attempt to make it as non‐informative as possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 102 participants were randomly assigned, 51 to each group. It was not possible to site the epidural catheter for two participants in the epidural group, and one participant with renal failure was recruited to the control group in violation of protocol. These 3 participants were excluded from the study (no data available) In 22 cases (14 participants in the study group and eight in the control group), outcome data (PSA) were not available after hospital discharge. These participants were effectively removed from the analysis by right‐censoring on the day of hospital discharge Missing outcome data are unlikely to be related to survival outcomes |
| Selective reporting (reporting bias) | Unclear risk | Published article uses 'survival,' 'disease‐free survival' and 'recurrence' to describe outcomes. It remains unclear whether these terms are used interchangeably or if 'survival' as used in the methods section was a predefined outcome that then was not reported |
| Other bias | Unclear risk | Progress of participants through the trial flow chart (Figure 1) is inconsistent in terms of numbers of included patients. The breakdown of the general anaesthesia group (n = 50) calculates to 54 participants, and it is unclear how this mismatch might influence data analysis Outcome definition does not match any of the standardized outcomes used for this review. The wording appears to come closest to our outcome of 'time to tumour progression (TTP)' = time elapsed between surgery and tumour progression, with the difference that prostate cancer–related deaths were considered to show tumour progression. However, only one participant in each group died of prostate cancer and was included in the calculation of TTP |
RCT = randomized controlled trial.
ASA = American Society of Anesthesiologists physical status classification.
IV = intravenous.
IM = intramuscular.
PSA = prostate‐specific antigen.
TTP = time to progression.
TTR = time to recurrence
Characteristics of ongoing studies [ordered by study ID]
Chan 2013.
| Trial name or title | Perioperative Epidural Analgesia for Short‐term and Long‐term Outcomes of Pancreatic Cancer Surgery—Randomized Trial |
| Methods | Randomized open‐label controlled trial |
| Participants | Male and female patients 20‐85 years of age with pancreatic cancer, scheduled for curative Whipple procedure Estimated enrolment: 150 participants |
| Interventions | Epidural patient‐controlled analgesia (PCEA) with bupivacaine + fentanyl vs intravenous patient‐controlled analgesia (PCA) with morphine for postoperative pain control |
| Outcomes | 1‐year survival rate (secondary outcome) |
| Starting date | 2012 |
| Contact information | National Taiwan University Hospital, Department of Anesthesiology. PI: Kuang Cheng Chan, MD |
| Notes |
Chang 2009.
| Trial name or title | Comparing Local Anesthesia With General Anesthesia for Breast Cancer Surgery |
| Methods | Randomized single‐blinded trial |
| Participants | Female patients 21‐75 years of age, ASA I‐II, diagnosed with biopsy‐proven breast cancer, scheduled for mastectomy and axillary node dissection in a single procedure Estimated enrolment: 40 participants |
| Interventions | Local anaesthesia + sedation vs general anaesthesia |
| Outcomes | Disease‐free survival up until 5 years after surgery |
| Starting date | 2008 |
| Contact information | Mackay Memorial Hospital, Taipei/Taiwan. PI: Yuan‐Ching Chang, MD |
| Notes |
Gupta 2011a.
| Trial name or title | Epidural Versus Patient‐Controlled Analgesia for Reduction in Long‐term Mortality Following Colorectal Cancer Surgery (EPICOL) |
| Methods | Randomized open‐label controlled trial |
| Participants | Male and female patients 40‐80 years of age, ASA I‐III, undergoing elective surgery for colorectal cancer Estimated enrolment: 300 participants |
| Interventions | Epidural analgesia with ropivacaine and opioid vs PCA with morphine |
| Outcomes | All‐cause mortality and cancer recurrence up until 5 years after surgery |
| Starting date | 2011 |
| Contact information | Örebro University, Sweden. PI: Anil Gupta |
| Notes |
Ilfeld 2010.
| Trial name or title | Prevention of Post‐Mastectomy Breast Pain Using Ambulatory Continuous Paravertebral Blocks |
| Methods | Randomized double‐blind controlled trial |
| Participants | Female patients 18 years of age and older, undergoing unilateral or bilateral mastectomy Estimated enrolment: 60 participants |
| Interventions | Postoperative paravertebral catheter analgesia with ropivacaine vs placebo (normal saline) |
| Outcomes | Cancer recurrence up until 3 years after surgery |
| Starting date | 2010 |
| Contact information | University of California, San Diego. PI: Brian Ilfeld, MD, MS |
| Notes |
Kurz 2008.
| Trial name or title | Regional Anesthesia in Patients Undergoing Colon‐Rectal Surgery |
| Methods | Randomized controlled double‐blind clinical trial |
| Participants | Patients scheduled for open laparoscopic or laparoscopic assisted surgery for colon cancer Estimated enrolment: 2500 participants |
| Interventions | General anaesthesia followed by postoperative opioid analgesia vs intraoperative and postoperative regional anaesthesia and analgesia (epidural or paravertebral anaesthesia) plus intraoperative general anaesthesia |
| Outcomes | Cancer recurrence up to 5 years after surgery |
| Starting date | 2007 |
| Contact information | The Cleveland Clinic, Outcomes Research Consortium. PI: Andrea Kurz, MD |
| Notes | Multi‐centre study |
Kurz 2010.
| Trial name or title | The Effect of Adding Intraoperative Regional Anesthesia on Cancer Recurrence in Patients Undergoing Lung Cancer Resection |
| Methods | Randomized double‐blinded controlled clinical trial |
| Participants | Male and female patients 18‐85 years of age diagnosed with primary non‐small cell lung cancer and scheduled for potentially curative tumour resection Estimated enrolment: 1532 participants |
| Interventions | Intraoperative and postoperative general anaesthesia + epidural anaesthesia and analgesia vs general anaesthesia and postoperative intravenous analgesia |
| Outcomes | Disease‐free survival up to 5 years after surgery |
| Starting date | 2010 |
| Contact information | The Cleveland Clinic, Outcomes Research Consortium. PI: Andrea Kurz, MD |
| Notes |
Lee 2011.
| Trial name or title | Thoracoscopic Lobectomy Using Thoracic Epidural Anesthesia Versus General Anesthesia for Lung Cancer Patients |
| Methods | Randomized open‐label controlled trial |
| Participants | Male and female patients 25‐80 years of age diagnosed with non‐small cell lung cancer with clinical staging of I or II for whom thoracoscopic lobectomy (VATS) is feasible Estimated enrolment: 100 participants |
| Interventions | Intraoperative general anaesthesia vs intraoperative thoracic epidural anaesthesia |
| Outcomes | Overall survival up until 5 years after surgery |
| Starting date | 2010 |
| Contact information | National Taiwan University Hospital. PI: Yung‐Chie Lee, MD, PhD |
| Notes |
Sessler 2007.
| Trial name or title | Regional Anesthesia and Breast Cancer Recurrence |
| Methods | Randomized controlled trial |
| Participants | Female participants 18‐85 years of age diagnosed with primary breast cancer without known extension beyond the breast and with axillary nodes scheduled for unilateral or bilateral mastectomy with or without implant or isolated "lumpectomy" with axillary node dissection (anticipated removal of at least 5 nodes) Estimated enrolment: 1100 participants |
| Interventions | Regional anaesthesia and analgesia (epidural or paravertebral), combined with deep sedation or general anaesthesia (sevoflurane) vsgeneral anaesthesia (sevoflurane) followed by opioid administration |
| Outcomes | Cancer recurrence rate up until 10 years after surgery |
| Starting date | 2007 |
| Contact information | The Cleveland Clinic, Outcomes Research Consortium. PI: Daniel I. Sessler, MD |
| Notes | Multi‐centre study |
Van Aken 2012.
| Trial name or title | Anesthesia and Cancer Recurrence im Malignant Melanoma |
| Methods | Randomized single blinded (outcome assessor) clinical trial |
| Participants | Patients scheduled for inguinal lymph node dissection because of malignant melanoma of the lower limb Estimated enrolment: 230 participants |
| Interventions | Spinal anaesthesia vs general anaesthesia |
| Outcomes | Overall survival up to 5 years after surgery |
| Starting date | 2012 |
| Contact information | University Hospital Muenster, Department of Anesthesia, Intensive Care and Pain Therapy. Study Chair: Hugo K. van Aken, MD, PhD |
| Notes |
ASA = American Society of Anesthesiologists.
PCA = patient‐controlled analgesia.
VATS = video‐assisted thoracic surgery.
PCEA = epidural patient‐controlled analgesia
Differences between protocol and review
We made the following changes to the published protocol (Apfel 2010).
Background/Description of the condition
We added the volatile anaesthetic sevoflurane to the list of intraoperative medications that might cause immunosuppression and added two references to support this.
Search methods
The search in CENTRAL was expanded to a search of the full Cochrane Library.
A spelling error in the CENTRAL search strategy was corrected (#23 intensif*).
Our institution no longer has a subscription to SCOPUS, and we are unable to search this database.
The WOS search strategy was refined.
The link to the New York Academy of Medicine (NYAM) Library was updated.
We are using Review Manager software version 5.2. The 'Risk of bias' table was created within RevMan 2.0 software, rather than by creating a 'Risk of bias' worksheet. Appendix 5 was consequently removed.
Contribution of review authors was adjusted.
Types of outcome measures/primary outcomes were modified
We removed censoring from the description of outcome measures (OS, PFS, TTP) to avoid potential confusion about different definitions of 'lost to follow‐up.'
Assessment of reporting biases
We deferred funnel plot analysis, as fewer than 10 studies were included.
Subgroup analysis and investigation of heterogeneity
No subgroup analysis was performed because data were lacking.
Types of outcome measures
Failed epidural placement was added as a secondary outcome.
Assessment of heterogeneity/data synthesis
We added information on assessment of clinical heterogeneity and on statistical heterogeneity.
We added a definition of HR and adjustment of individual trial HRs if necessary.
Sensitivity analysis
We planned to perform sensitivity analysis to explore the consistency of effect size measures within the domains of the risk of bias. We planned to perform sensitivity analysis using different definitions of progression‐free survival. We deferred sensitivity analysis because data were lacking.
Subgroup analysis
We deferred subgroup analysis because of lack of data.
Contributions of authors
Conceiving of the review: Christian C Apfel (CCA), Ozlem S Cakmakkaya (OSC).
Co‐ordinating the review: Kerstin Kolodzie (KK).
Undertaking manual searches: OSC, KK.
Screening search results: OSC, KK.
Organizing retrieval of papers: OSC, KK.
Screening retrieved papers against inclusion criteria: OSC, KK.
Appraising quality of papers: OSC, KK.
Abstracting data from papers: OSC, KK.
Writing to authors of papers to ask for additional information: KK.
Providing additional data about papers: KK, Nathan Leon Pace (NLP).
Obtaining and screening data on unpublished studies: OSC, KK.
Managing data for the review: KK.
Entering data into Review Manager (5.2): OSC, KK.
Analysing RevMan statistical data: NLP, OSC, KK.
Performing other statistical analyses not using RevMan: NLP, OSC, KK.
Interpreting data: CCA, NLP, OSC, KK.
Making statistical inferences: NLP, CCA, OSC, KK.
Writing the review: KK, OSC.
Securing funding for the review:
Performing previous work that served as the foundation of the present study:
Serving as guarantor for the review (one review author): OSC.
Taking responsibility for reading and checking the review before submission: OSC, KK.
The first (OSC) and second (KK) listed review authors contributed equally to the review and should be considered equal first review authors.
Sources of support
Internal sources
Department of Anesthesia & Perioperative Care, University of California San Francisco, CA, USA.
Department of Anesthesiology and Reanimation, University of Istanbul, Cerrahpasa Medical School, Istanbul, Turkey.
University of Utah, Salt Lake City, UT, USA.
External sources
No sources of support supplied
Declarations of interest
Ozlem S Cakmakkaya: none known.
Kerstin Kolodzie: none known.
Christian C Apfel: none known.
Nathan Leon Pace: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Binczak 2013 {published data only}
- Binczak M, Tournay E, Billard V, Rey A, Jayr C. Major abdominal surgery for cancer: Does epidural analgesia have a long‐term effect on recurrence‐free and overall survival?. Annales Françaises d'Anesthèsie et de Rèanimation 2013;32(5):e81‐8. [PUBMED: 23618609] [DOI] [PubMed] [Google Scholar]
Christopherson 2008 {published data only}
- Christopherson R, James KE, Tableman M, Marshall P, Johnson FE. Long‐term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesthesia and Analgesia 2008;107(1):325‐32. [PUBMED: 18635504] [DOI] [PubMed] [Google Scholar]
Myles 2011 {published data only}
- Myles PS, Peyton P, Silbert B, Hunt J, Rigg JR, Sessler DI. Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence‐free survival: randomised trial. BMJ 2011;342:d1491. [PUBMED: 21447587] [DOI] [PubMed] [Google Scholar]
Tsui 2010 {published data only}
- Tsui BC, Rashiq S, Schopflocher D, Murtha A, Broemling S, Pillay J, et al. Epidural anesthesia and cancer recurrence rates after radical prostatectomy. Canadian Journal of Anaesthesia 2010;57(2):107‐12. [PUBMED: 19911247] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Chan 2013 {published data only}
- Perioperative Epidural Analgesia for Short‐term and Long‐term Outcomes of Pancreatic Cancer Surgery—Randomized Trial. Ongoing study 2012.
Chang 2009 {published data only}
- Comparing Local Anesthesia With General Anesthesia for Breast Cancer Surgery. Ongoing study 2008.
Gupta 2011a {published data only}
- Epidural Versus Patient‐Controlled Analgesia for Reduction in Long‐term Mortality Following Colorectal Cancer Surgery (EPICOL). Ongoing study 2011.
Ilfeld 2010 {published data only}
- Prevention of Post‐Mastectomy Breast Pain Using Ambulatory Continuous Paravertebral Blocks. Ongoing study 2010.
Kurz 2008 {published data only}
- Regional Anesthesia in Patients Undergoing Colon‐Rectal Surgery. Ongoing study 2007.
Kurz 2010 {published data only}
- The Effect of Adding Intraoperative Regional Anesthesia on Cancer Recurrence in Patients Undergoing Lung Cancer Resection. Ongoing study 2010.
Lee 2011 {published data only}
- Thoracoscopic Lobectomy Using Thoracic Epidural Anesthesia Versus General Anesthesia for Lung Cancer Patients. Ongoing study 2010.
Sessler 2007 {published data only}
- Regional Anesthesia and Breast Cancer Recurrence. Ongoing study 2007.
Van Aken 2012 {published data only}
- Anesthesia and Cancer Recurrence im Malignant Melanoma. Ongoing study 2012.
Additional references
Alexander 2009
- Alexander A, Unzeitig S, Knoefel WT, Rehders A, Riediger R, Eisenberger CF, et al. Can perioperative peridural analgesia improve the prognosis of pancreatic cancer?. Pancreas 2009;38(8):980‐1. [Google Scholar]
Beilin 1996
- Beilin B, Shavit Y, Hart J, Mordashov B, Cohn S, Notti I, et al. Effects of anesthesia based on large versus small doses of fentanyl on natural killer cell cytotoxicity in the perioperative period. Anesthesia and Analgesia 1996;82(3):492‐7. [PUBMED: 8623949] [DOI] [PubMed] [Google Scholar]
Biki 2008
- Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology 2008;109:180‐7. [PUBMED: 18648226] [DOI] [PubMed] [Google Scholar]
CARG 2007
- Study Selection, Quality Assessment and Data Extraction Form. 2007, Version 3. Cochrane Anaesthesia Review Group (CARG).
Centers for Disease Control and Prevention 2013
- Center for Disease Control and Prevention. Leading causes of death 2010. http://www.cdc.gov/nchs/fastats/lcod.htm, (last accessed December 2013).
Chang 2011
- Chang XL, Zhu D, Ren XL, Lu HW. Influence of combined general and epidural anesthesia on cancer prognosis: a meta‐analysis (Provisional abstract). Chinese Journal of Evidence Based Medicine 2011;11(8):954‐9. [PUBMED: 23437162] 23437162 [Google Scholar]
Chen 2013
- Chen WK, Miao CH. The effect of anesthetic technique on survival in human cancers: a meta‐analysis of retrospective and prospective studies. PLoS One 2013;8(2):e56540. [PUBMED: 23437162] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cummings 2012
- Cummings KC III, Xu F, Cummings LC, Cooper GS. A comparison of epidural analgesia and traditional pain management effects on survival and cancer recurrence after colectomy: a population‐based study. Anesthesiology 2012;116(4):797‐806. [PUBMED: 22273991] [DOI] [PubMed] [Google Scholar]
Day 2012
- Day A, Smith R, Jourdan I, Fawcett W, Scott M, Rockall T. Retrospective analysis of the effect of postoperative analgesia on survival in patients after laparoscopic resection of colorectal cancer. British Journal of Anaesthesia 2012;109(2):185‐90. [PUBMED: 22525284] [DOI] [PubMed] [Google Scholar]
De Oliveira 2011
- Oliveira GS Jr, Ahmad S, Schink JC, Singh DK, Fitzgerald PC, et al. Intraoperative neuraxial anesthesia but not postoperative neuraxial analgesia is associated with increased relapse‐free survival in ovarian cancer patients after primary cytoreductive surgery. Regional Anesthesia and Pain Medicine 2011;36(3):271‐7. [PUBMED: 21519312] [DOI] [PubMed] [Google Scholar]
Deegan 2009
- Deegan CA, Murray D, Doran P, Ecimovic P, Moriarty DC, Buggy DJ. Effect of anaesthetic technique on oestrogen receptor‐negative breast cancer cell function in vitro. British Journal of Anaesthesia 2009;103(5):685‐90. [PUBMED: 19776028] [DOI] [PubMed] [Google Scholar]
Eschwege 1995
- Eschwege P, Dumas F, Blanchet P, Maire V, Benoit G, Jardin A, et al. Haematogenous dissemination of prostatic epithelial cells during radical prostatectomy. Lancet 1995;346(8989):1528‐30. [PUBMED: 7491049] [DOI] [PubMed] [Google Scholar]
Exadaktylos 2006
- Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha, E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis?. Anesthesiology 2006;105(4):660‐4. [PUBMED: 17006061] [DOI] [PMC free article] [PubMed] [Google Scholar]
Forget 2011
- Forget P, Tombal B, Scholtes JL, Nzimbala J, Meulders C, Legrand C, et al. Do intraoperative analgesics influence oncological outcomes after radical prostatectomy for prostate cancer?. European Journal of Anaesthesiology 2011;28(12):830‐5. [PUBMED: 21946823] [DOI] [PubMed] [Google Scholar]
Gottschalk 2010
- Gottschalk A, Ford JG, Regelin CC, You J, Mascha EJ, Sessler DI, et al. Association between epidural analgesia and cancer recurrence after colorectal cancer surgery. Anesthesiology 2010;113(1):27‐34. [PUBMED: 20508494] [DOI] [PubMed] [Google Scholar]
Gottschalk 2012
- Gottschalk A, Brodner G, Aken HK, Ellger B, Althaus S, Schulze HJ. Can regional anaesthesia for lymph‐node dissection improve the prognosis in malignant melanoma?. British Journal of Anaesthesia 2012;109(2):253‐9. [PUBMED: 22705968] [DOI] [PubMed] [Google Scholar]
Gupta 2002
- Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival‐promoting signaling and promotes breast tumor growth. Cancer Research 2002;62(15):4491‐8. [PUBMED: 12154060] [PubMed] [Google Scholar]
Gupta 2011b
- Gupta A, Bjornsson A, Fredriksson M, Hallbook O, Eintrei C. Reduction in mortality after epidural anaesthesia and analgesia in patients undergoing rectal but not colonic cancer surgery: a retrospective analysis of data from 655 patients in central Sweden. British Journal of Anaesthesia 2011;107(2):164‐70. [PUBMED: 21586443] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org 2011.
Higgins 2011b
- Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Chapter 8: Assessing risk of bias in included studies. www.cochrane‐handbook.org.
Holmgren 1995
- Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nature Medicine 1995;1(2):117‐8. [PUBMED: 7585012] [DOI] [PubMed] [Google Scholar]
Ikeda 2002
- Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, et al. Surgery for gastric cancer increases plasma levels of vascular endothelial growth factor and von Willebrand factor. Gastric Cancer 2002;5(3):137‐41. [PUBMED: 12378339] [DOI] [PubMed] [Google Scholar]
Inada 2004
- Inada T, Yamanouchi Y, Jomura S, Sakamoto S, Takahashi M, Kambara T, et al. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia 2004;59(10):954‐9. [PUBMED: 15488052] [DOI] [PubMed] [Google Scholar]
Ismail 2010
- Ismail H, Ho KM, Narayan K, Kondalsamy‐Chennakesavan S. Effect of neuraxial anaesthesia on tumour progression in cervical cancer patients treated with brachytherapy: a retrospective cohort study. British Journal of Anaesthesia 2010;105(2):145‐9. [DOI] [PubMed] [Google Scholar]
Jayr 1993
- Jayr C, Thomas H, Rey A, Farhat F, Lasser P, Bourgain JL. Postoperative pulmonary complications. Epidural analgesia using bupivacaine and opioids versus parenteral opioids. Anesthesiology 1993;78(4):666‐76. [PUBMED: 8466067] [DOI] [PubMed] [Google Scholar]
Jemal 2010
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA ‐ A Cancer Journal for Clinicians 2010;60(5):277‐300. [PUBMED: 20610543] [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [PUBMED: 11440947] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kienbaum 2010
- Kienbaum P, Unzeitig S, Alexander A, Knoefel W. Can anesthetic technique influence cancer recurrence in patients with pancreatic carcinoma scheduled for radical tumor resection?. European Journal of Anaesthesiology 2010;27(Suppl 47):8A, 4‐7. [Google Scholar]
Koensgen 2013
- Koensgen D, Mustea A, Rosanowski B, Leutzow B, Hesse T, Patrzyk M, et al. Perioperative epidural analgesia and recurrence‐free survival for ovarian cancer surgery: a retrospective analysis. ASCO Meeting Abstracts 2013;31(15 Suppl):e16534. [Google Scholar]
Konjevic 1993
- Konjevic G, Spuzic I. Stage dependence of NK cell activity and its modulation by interleukin 2 in patients with breast cancer. Neoplasm 1993;40(2):81‐5. [PUBMED: 8350959] [PubMed] [Google Scholar]
Koppert 2004
- Koppert W, Weigand M, Neumann F, Sittl R, Schuettler J, Schmelz M, et al. Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesthesia and Analgesia 2004;98(4):1050‐5. [PUBMED: 15041597] [DOI] [PubMed] [Google Scholar]
Kurosawa 2008
- Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. Journal of Anesthesia 2008;22:263‐77. [PUBMED: 18685933] [DOI] [PubMed] [Google Scholar]
Lacassie 2013
- Lacassie HJ, Cartagena J, Branes J, Assel M, Echevarria GC. The relationship between neuraxial anesthesia and advanced ovarian cancer‐related outcomes in the Chilean population. Anesthesia and Analgesia 2013;117(3):653‐60. [PUBMED: 23868889] [DOI] [PubMed] [Google Scholar]
Lai 2012
- Lai R, Peng Z, Chen D, Wang X, Xing W, Zeng W, et al. The effects of anesthetic technique on cancer recurrence in percutaneous radiofrequency ablation of small hepatocellular carcinoma. Anesthesia and Analgesia 2012;114(2):290‐6. [DOI] [PubMed] [Google Scholar]
Lennard 1985
- Lennard TWJ, Shenton BK, Borzotta A, Donnelly PK, White M, Gerrie LM, et al. The influence of surgical operations on components of the human immune system. The British Journal of Surgery 1985;72:771‐6. [PUBMED: 2412626] [DOI] [PubMed] [Google Scholar]
Lin 2011
- Lin L, Liu C, Tan H, Ouyang H, Zhang Y, Zeng W. Anaesthetic technique may affect prognosis for ovarian serous adenocarcinoma: a retrospective analysis. British Journal of Anaesthesia 2011;106(6):814‐22. [PUBMED: 21436156] [DOI] [PubMed] [Google Scholar]
Luo 2010
- Luo CF, Su B. Analgesic technique for primary colon cancer surgery doesn't affect cancer recurrence. European Journal of Anaesthesiology 2010;27(47):212. [Google Scholar]
Mafune 2000
- Mafune K, Tanaka Y. Influence of multimodality therapy on the cellular immunity of patients with esophageal cancer. Annals of Surgical Oncology 2000;7(8):609‐16. [PUBMED: 11005560] [DOI] [PubMed] [Google Scholar]
Maniwa 1998
- Maniwa Y, Okada M, Ishii N, Kiyooka K. Vascular endothelial growth factor increased by pulmonary surgery accelerates the growth of micrometastases in metastatic lung cancer. Chest 1998;114(6):1668‐75. [PUBMED: 9872204] [DOI] [PubMed] [Google Scholar]
Melamed 2003
- Melamed R, Bar‐Yosef S, Shakhar G, Shakhar K, Ben‐Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesthesia and Analgesia 2003;97(5):1331‐9. [PUBMED: 14570648] [DOI] [PubMed] [Google Scholar]
Mitsuhata 1995
- Mitsuhata H, Shimizu R, Yokoyama MM. Suppressive effects of volatile anesthetics on cytokine release in human peripheral blood mononuclear cells. International Journal of Immunopharmacology 1995;17(6):529‐34. [PUBMED: 7499031] [DOI] [PubMed] [Google Scholar]
Moudgil 1997
- Moudgil GC, Singal DP. Halothane and isoflurane enhance melanoma tumour metastasis in mice. Canadian Journal of Anaesthesia 1997;44(1):90‐4. [PUBMED: 8988831] [DOI] [PubMed] [Google Scholar]
O'Connor 2006
- O'Connor PJ, Hanson J, Finucane BT. Induced hypotension with epidural/general anesthesia reduces transfusion in radical prostate surgery. Canadian Journal of Anaesthesia 2006;53(9):873‐80. [PUBMED: 16960264] [DOI] [PubMed] [Google Scholar]
O'Riain 2005
- O'Riain SC, Buggy DJ, Kerin MJ, Watson RW, Moriarty DC. Inhibition of the stress response to breast cancer surgery by regional anesthesia and analgesia does not affect vascular endothelial growth factor and prostaglandin E2. Anesthesia and Analgesia 2005;100(1):244‐9. [PUBMED: 15616085] [DOI] [PubMed] [Google Scholar]
Park 2001
- Park WY, Thompson JS, Lee KK. Effect of epidural anesthesia and analgesia on perioperative outcome: a randomized, controlled Veterans Affairs cooperative study. Annals of Surgery 2001;234(4):560‐9. [PUBMED: 11573049] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [PUBMED: 9921604] [DOI] [PubMed] [Google Scholar]
Pollock 1991
- Pollock R, Lotzova E, Stanford S. Mechanism of surgical stress impairment of human perioperative natural killer cell cytotoxicity. Archives of Surgery 1991;126(3):338‐42. [PUBMED: 1825598] [DOI] [PubMed] [Google Scholar]
Reference Manager [Computer program]
- Thomson. Reference Manager version 11.0. Thomson, 2005.
RevMan 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rigg 2000
- Rigg JR, Jamrozik K, Myles PS, Silbert B, Peyton P, Parsons RW, et al. Design of the multicenter Australian study of epidural anesthesia and analgesia in major surgery: the MASTER trial. Controlled Clinical Trials 2000;21(3):244‐56. [PUBMED: 10822122] [DOI] [PubMed] [Google Scholar]
Rigg 2002
- Rigg JR, Jamrozik K, Myles PS, Silbert BS, Peyton PJ, Parsons RW, et al. MASTER Anaethesia Trial Study Group. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. The Lancet 2002;359(9314):1276‐82. [PUBMED: 11965272] [DOI] [PubMed] [Google Scholar]
Sacerdote 2000
- Sacerdote P, Bianchi M, Gaspani L, Manfredi B, Maucione A, Terno G, et al. The effects of tramadol and morphine on immune responses and pain after surgery in cancer patients. Anesthesia and Analgesia 2000;90(6):1411‐4. [PUBMED: 10825330] [DOI] [PubMed] [Google Scholar]
Schlagenhauff 2000
- Schlagenhauff B, Ellwanger U, Breuninger H, Stroebel W, Rassner G, Garbe C. Prognostic impact of the type of anaesthesia used during the excision of primary cutaneous melanoma. Melanoma Research 2000;10:165‐9. [PUBMED: 10803717] [PubMed] [Google Scholar]
Schneemilch 2005
- Schneemilch CE, Ittenson A, Ansorge S, Hachenberg T, Bank U. Effect of 2 anesthetic techniques on the postoperative proinflammatory and anti‐inflammatory cytokine response and cellular immune function to minor surgery. Journal of Clinical Anesthesia 2005;17(7):517‐27. [PUBMED: 16297751] [DOI] [PubMed] [Google Scholar]
Seebacher 1990
- Seebacher C, Heubaum F, Küster P, Steinert W, Koch R. Comparative analysis of narcosis and local anesthesia in surgery of malignant melanoma of the skin [Vergleichende Analyse in Narkose und Lokalanaesthesie operierter Melanome der Haut]. Hautarzt 1990;41:137‐41. [PUBMED: 2345097] [PubMed] [Google Scholar]
Shakhar 2003
- Shakhar G, Ben‐Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients?. Annals of Surgical Oncology 2003;10(8):972–92. [PUBMED: 14527919] [DOI] [PubMed] [Google Scholar]
Snyder 2010
- Snyder GL, Greenberg S. Effect of anesthetic technique and other perioperative factors on cancer recurrence. British Journal of Anaesthesia 2010;105(2):106‐15. [DOI: 10.1093/bja/aeq164] [DOI] [PubMed] [Google Scholar]
Tarle 1993
- Tarle M, Kovacic K, Kastelan M. Correlation of cell proliferation marker (TPS), natural killer (NK) activity and tumor load serotest (PSA) in untreated and treated prostatic tumors. Anticancer Research 1993;13(1):215‐8. [PUBMED: 7682800] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;7(8):16. [PUBMED: 17555582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vallejo 2004
- Vallejo R, Leon‐Casasola O, Benyamin R. Opioid therapy and immunosuppression. American Journal of Therapeutics 2004;11:354‐65. [PUBMED: 15356431] [DOI] [PubMed] [Google Scholar]
Vogelaar 2012
- Vogelaar FJ, Bogerd A, Lemmens VE, Linden JC, Cornelisse HGJM, Dorsten FRC, et al. Epidural analgesia—Associated with survival in colon cancer?. European Journal of Surgical Oncology 2012;38(9):755. [Google Scholar]
Wang 2006
- Wang JY, Wu CH, Lu CY, Hsieh JS, Wu DC, Huang SY, et al. Molecular detection of circulating tumor cells in the peripheral blood of patients with colorectal cancer using RT‐PCR: significance of the prediction of postoperative metastasis. World Journal of Surgery 2006;30(6):1007‐13. [PUBMED: 16736329] [DOI] [PubMed] [Google Scholar]
Whiteside 1995
- Whiteside T, Herberman R. The role of natural killer cells in immune surveillance of cancer. Current Opinion in Immunology 1995;7:704‐10. [PUBMED: 8573315] [DOI] [PubMed] [Google Scholar]
Williamson 2002
- Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Statistics in Medicine 2002;21(22):3337‐51. [PUBMED: 12407676] [DOI] [PubMed] [Google Scholar]
World Health Organization 2012
- World Health Organization, Regional Office for Europe. Leading causes of death in Europe: fact sheet. http://www.euro.who.int/__data/assets/pdf_file/0004/185215/Leading‐causes‐of‐death‐in‐Europe‐Fact‐Sheet.pdf 2012.
Wuethrich 2010
- Wuethrich PY, Hsu Schmitz SF, Kessler TM, Thalmann GN, Studer UE, Stueber F, et al. Potential influence of the anesthetic technique used during open radical prostatectomy on prostate cancer‐related outcome: a retrospective study. Anesthesiology 2010;113(3):570‐6. [PUBMED: 20683253] [DOI] [PubMed] [Google Scholar]
Wuethrich 2013
- Wuethrich PY, Thalmann GN, Studer UE, Burkhard FC. Epidural analgesia during open radical prostatectomy does not improve long‐term cancer‐related outcome: a retrospective study in patients with advanced prostate cancer. PLoS One 2013;8(8):e72873. [PUBMED: 23977366] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamaguchi 2000
- Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S. Significant detection of circulating cancer cells in the blood by reverse transcriptase‐polymerase chain reaction during colorectal cancer resection. Annals of Surgery 2000;232(1):58‐65. [PUBMED: 10862196] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamashita 2000
- Yamashita JI, Kurusu Y, Fujino N, Saisyoji T, Ogawa M. Detection of circulating tumor cells in patients with non‐small cell lung cancer undergoing lobectomy by video‐assisted thoracic surgery: a potential hazard for intraoperative hematogenous tumor cell dissemination. Journal of Thoracic and Cardiovascular Surgery 2000;119(5):899‐905. [PUBMED: 10788810] [DOI] [PubMed] [Google Scholar]
Yardeni 2008
- Yardeni IZ, Beilin B, Mayburd E, Alcalay Y, Bessler H. Relationship between fentanyl dosage and immune function in the postoperative period. Journal of Opioid Management 2008;4(1):27‐33. [PUBMED: 18444445] [DOI] [PubMed] [Google Scholar]
Yardeni 2009
- Yardeni IZ, Beilin B, Mayburd E, Levinson Y, Bessler H. The effect of perioperative intravenous lidocaine on postoperative pain and immune function. Anesthesia and Analgesia 2009;109(5):1464‐9. [PUBMED: 19843784] [DOI] [PubMed] [Google Scholar]
Yeager 1995
- Yeager MP, Colacchio TA, Yu CT, Hildebrandt L, Howell AL, Weiss J, et al. Morphine inhibits spontaneous and cytokine‐enhanced natural killer cell cytotoxicity in volunteers. Anesthesiology 1995;83(3):500‐8. [PUBMED: 7661350] [DOI] [PubMed] [Google Scholar]
Zhang 2014
- Zhang T1, Fan Y, Liu K, Wang Y. Effects of different general anaesthetic techniques on immune responses in patients undergoing surgery for tongue cancer. Anaesthesia and Intensive Care 2014;42(2):220‐7. [PUBMED: 24580388] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Apfel 2010
- Apfel CC, Cakmakkaya OS, Kolodzie K, Pace NL. Anaesthetic techniques for risk of malignant tumour recurrence. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008877] [DOI] [PMC free article] [PubMed] [Google Scholar]


