Abstract
Objectives
The objective of this study was to expand the phenotypic spectrum of glutamine-fructose-6-phosphate transaminase 1 (GFPT1)–related congenital myasthenia syndrome (CMS).
Methods
A 61-year-old man with agenesis of the left pectoralis major muscle presented with progressive muscle weakness for a decade that transiently improved after exertion.
Results
His examination revealed proximal and distal muscle weakness in upper extremities and proximal muscle weakness in lower extremities. Muscle enzymes were elevated. An electromyogram revealed a myopathic pattern; however, a muscle biopsy of deltoid muscle and genetic testing for limb-girdle muscular dystrophies were nondiagnostic. A 3-Hz repetitive nerve stimulation of the spinal accessory nerve recording from trapezius muscle demonstrated a >20% drop in amplitude of the 5th compound motor action potential relative to 1st at both baseline and after 45-second exercise. Acetylcholine receptor binding, lipoprotein-related protein 4, muscle-specific kinase, and voltage-gated calcium channel P/Q antibodies were negative. Genetic testing targeting CMS revealed 2 likely pathogenic variants within GFPT1: novel c.7+2T>G (intron 1) that was predicted to result in a null allele and known c*22 C>A (exon 19) associated with reduced GFPT1 expression. His muscle strength dramatically improved after pyridostigmine initiation.
Discussion
In addition to other reported neurodevelopmental abnormalities, pectoralis major muscle agenesis (or Poland syndrome) may be a clinical manifestation of GFPT1-related CMS.
Introduction
Congenital myasthenic syndromes (CMS) are inherited and frequently treatable neuromuscular junction (NMJ) disorders that are often misdiagnosed as seronegative myasthenia or myopathy, which may delay the initiation of effective therapies.1 CMS can be caused by defective genes encoding presynaptic, synaptic, or postsynaptic NMJ components or enzymes that have an important role in the development and maintenance of the NMJ.1,2 Glutamine-fructose-6-phosphate transaminase 1 (GFPT1) is a rate-limiting enzyme in the hexosamine biosynthetic pathway responsible for the correct glycosylation of lipids and proteins of the NMJ, which is key for its successful development and maintenance.3 GFPT1-related CMS is an autosomal recessive disorder typically characterized by a limb-girdle muscle weakness distribution that frequently presents within the first 2 decades of life and is responsive to pyridostigmine.4 In this study, we present a patient with congenital agenesis of the pectoralis major muscle and GFPT1-related CMS.
Case Report
A 61-year-old athletic man reported progressive muscle weakness for a decade. He was an avid water skier, hockey player, and was able to bench press 200 lbs of weight. However, in his early 50s, he experienced increasing difficulty standing up while water skiing, a slow and weak hockey shot, and reduced grip strength. His bench press dropped to approximately 85 lbs. He also had to use his arms to lift his legs to get in and out of his car. He noticed that his muscle weakness appeared to improve after exertion.
He denied ptosis, diplopia, speech changes, dysphagia, dyspnea, episodes of dark urines, pain, numbness, or tingling. He was born without a left pectoralis major muscle for which he underwent surgery for cosmetic purposes. He had normal motor development as a child and excelled athletically. He was not taking any medications when he developed muscle weakness. His medical history included hyperlipidemia, hypertension, surgery of his cervical and lumbar spine, and thalassemia. There was no family history of consanguinity or weakness.
His examination revealed normal cognition, language, speech, and cranial nerves. He had normal muscle bulk and tone. There were no fasciculations or scapular winging. There was no action or percussion myotonia or paramyotonia. Manual muscle testing revealed the following MRC grades (R/L where applicable): neck flexion 5, neck extension 5, shoulder abduction 4/4, shoulder flexion 4+/4+, elbow flexors and extensors 5/5, finger extensors 4/4-, abductor digiti minimi 4+/4+, wrist extensors and flexors 5/5, finger flexors 5/5, hip flexors 4-/4-, hip abductors 4+/4+, hip extensors 4+/4+, knee flexors 5/5, and ankle plantar and dorsal flexors 5/5. Although one would expect lack of adduction of the left abducted arm and lack of internal rotation of the left shoulder because of the absence of sternal and clavicular head of pectoralis major muscle, respectively, we suspect that he was able to perform these actions due to the compensation of pectoralis minor muscle that was preserved. Likewise, although pectoralis major muscle is the main driver of shoulder flexion, compensation of coracobrachalis and deltoid muscles probably accounted for the lack of differences in muscle weakness between both sides. Deep tendon reflexes were 0 at the biceps, triceps, brachioradialis, and ankles bilaterally, 2+ at the left patella, and 1+ at the right patella, which may be at least partially explained by his history of cervical and lumbar spine disease. Plantar responses were flexor. There was no clonus. Coordination, sensation, and gait were normal.
Ancillary investigations included creatine kinase 732–1262 IU/L (ref = 30–194 IU/L), aldolase 11.1 U/L (ref = <8.1 IU/L), negative antinuclear, 3-hydroxy-3-methylglutaryl-CoA reductase, acetylcholine receptor–binding and voltage-gated calcium channel P/Q antibodies, normal activity of alpha-glucosidase, and a normal MRI of the right thigh muscles. An electromyogram revealed early recruitment of small motor unit potentials in the right biceps, deltoid, infraspinatus, and iliopsoas muscles without abnormal spontaneous activity. A muscle biopsy of the left deltoid muscle showed mild fiber size variation and scattered atrophic fibers as the only findings (Figure 1). He also underwent genetic testing for limb-girdle muscular dystrophies that showed a variant of uncertain significance in COL12A1 (exon 18, c.3593>G, p.Ala1198Gly) that was unlikely to account for his muscle weakness.
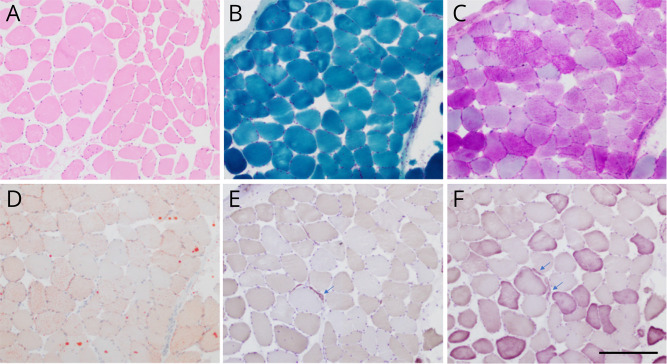
Figure 1. Left Deltoid Muscle Biopsy.
Mild variation in fiber size and rare atrophic fibers (arrows) were seen in sections stained with hematoxylin and eosin (A), ATPase at pH 4.3 (E), and ATPase at pH 9.4 (F). Tubular aggregates were not seen on trichrome (B) or electron microscopy (not shown). Glycogen and lipid content in muscle fibers were normal on periodic acid Schiff (C) and Oil Red O (D), respectively. Scale bar, 250 μm.
At first evaluation with us, we considered the possibility of an NMJ disorder based on the fluctuation of muscle weakness with reported improvement in muscle strength after physical activity. We then performed a 3-Hz repetitive nerve stimulation (RNS) study of the right spinal accessory nerve recording from trapezius muscle that demonstrated a significant decrement (>20%) in the amplitude of the fifth compound motor action potential (CMAP) relative to the first CMAP at both baseline and after a 45 second (postexhaustion) exercise. Electrical facilitation after a 10-second exercise was not observed. A 3-Hz RNS of the right ulnar nerve recording from the abductor digiti minimi muscle was normal (Figure 2). In view of this electrical postsynaptic dysfunction pattern, repeated acetylcholine receptor–binding antibodies and lipoprotein-related protein 4 (LRP4) and muscle-specific kinase (MUSK) antibodies were tested and showed negative results. We then performed sequencing and deletion/duplication assay of 19 genes known to cause CMS, which revealed 2 likely pathogenic variants within GFPT1 gene: a novel c.7+2T>G (intron 1) canonical splice site variant that was predicted to result in a null allele and a known c*22 C>A in exon 19 (rs199678034) that had been associated with reduced GFPT1 expression via an aberrant microRNA binding site.3-5 Both patient’s parents were deceased and segregation of these variants could not be investigated. We reviewed his deltoid muscle biopsy, but tubular aggregates on electron microscopy were not seen.
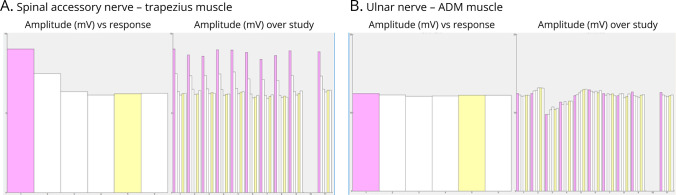
Figure 2. Repetitive Nerve Stimulation Studies (RNS).
(A) A 3-Hz RNS of the right spinal accessory nerve recording from trapezius muscle demonstrated a 31% decrement in the amplitude of the fifth CMAP relative to the first at baseline (left and train 1 on the right) that remained present after a 45-second exercise (trains 3 to 11 on the right). No electrical facilitation was observed after a 10-second exercise (train 2 on the right). (B) A 3-Hz RNS of the right ulnar nerve recording from abductor digiti minimi (ADM) muscle was normal.
He was started on 60 mg of pyridostigmine 3 times a day after which he reported dramatic improvement in his muscle strength. He was able to shoot a hockey puck out of the rink, his bench press increased up to 150 lbs, and he no longer required the use of his arms to lift his legs.
Discussion
We describe a patient with late-onset GFPT1-related CMS who was born with an absent left pectoralis major muscle (Poland syndrome). Although an incidental coexistence of both conditions is plausible, their rarity prompts consideration of a common pathogenic mechanism; impaired glycosylation due to a defective GFPT1 (with one of the likely pathogenic variants being novel and predicted to cause a null allele) may have contributed to the lack of appropriate pectoralis major development.
Although GFPT1-related CMS has been associated with neurodevelopmental abnormalities such as cranial synostosis, camptodactyly, and leukoencephalopathy,4,6-8 skeletal muscle agenesis has not been reported to date. On the contrary, agenesis of unilateral pectoralis major muscle is a cardinal feature mandatory for the diagnosis of Poland syndrome.9 The cause of Poland syndrome is unknown; a vascular insult during early embryologic stages and/or as yet unidentified genetic defects have been postulated as potential etiologies. Whereas pectoralis major agenesis may be the only clinical feature of Poland syndrome (as in this patient), other congenital anomalies involving the ipsilateral thoracic wall and upper limb may occur (i.e., hypoplastic hand, symbrachydactyly, and high scapula).9,10
Exercise is known to contribute to NMJ integrity and induce increased calcium influx to the presynaptic motor neuron that results in increased acetylcholine release to the synaptic cleft, after which, postsynaptic muscle membrane depolarization occurs.11 This effect of exercise on the NMJ likely accounts for the transient improvement in strength that the patient reported. It is plausible that this patient could not have become symptomatic if he had had a sedentary life. On the contrary, and although rare, a late-onset GFPT1-associated CMS has been previously described (Table)6,12-15 and whether the level of patients' physical activity affects the age at symptom onset is uncertain. Fortunately, he responded well to pyridostigmine, which reduces the clearance of acetylcholine from the synaptic cleft and is the treatment of choice in this CMS form.4 Other agents such as 3,4 diaminopyridine and salbutamol have also been tried in patients reported in literature.6,8,13,15
Table.
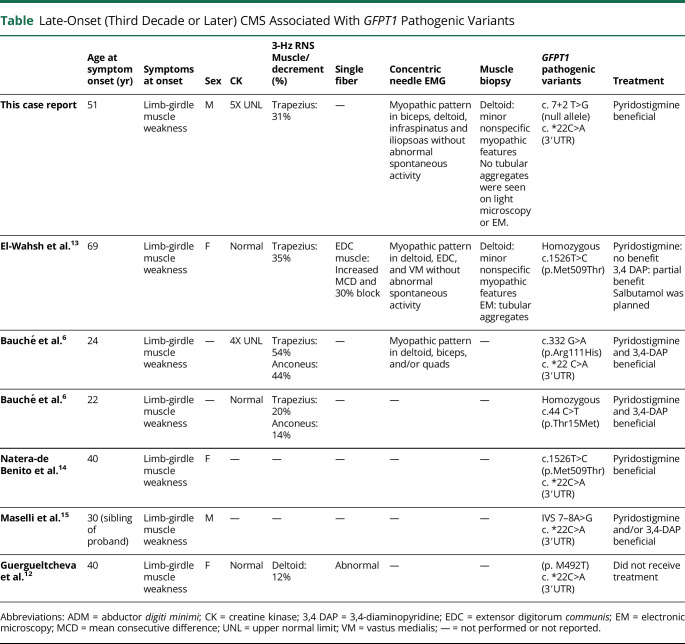
Late-Onset (Third Decade or Later) CMS Associated With GFPT1 Pathogenic Variants
| Age at symptom onset (yr) | Symptoms at onset | Sex | CK | 3-Hz RNS Muscle/decrement (%) |
Single fiber | Concentric needle EMG | Muscle biopsy | GFPT1 pathogenic variants | Treatment | |
| This case report | 51 | Limb-girdle muscle weakness | M | 5X UNL | Trapezius: 31% | — | Myopathic pattern in biceps, deltoid, infraspinatus and iliopsoas without abnormal spontaneous activity | Deltoid: minor nonspecific myopathic features No tubular aggregates were seen on light microscopy or EM. |
c. 7+2 T>G (null allele) c. *22C>A (3′UTR) |
Pyridostigmine beneficial |
| El-Wahsh et al.13 | 69 | Limb-girdle muscle weakness | F | Normal | Trapezius: 35% | EDC muscle: Increased MCD and 30% block | Myopathic pattern in deltoid, EDC, and VM without abnormal spontaneous activity | Deltoid: minor nonspecific myopathic features EM: tubular aggregates |
Homozygous c.1526T>C (p.Met509Thr) | Pyridostigmine: no benefit 3,4 DAP: partial benefit Salbutamol was planned |
| Bauché et al.6 | 24 | Limb-girdle muscle weakness | — | 4X UNL | Trapezius: 54% Anconeus: 44% |
— | Myopathic pattern in deltoid, biceps, and/or quads | — | c.332 G>A (p.Arg111His) c. *22 C>A (3′UTR) |
Pyridostigmine and 3,4-DAP beneficial |
| Bauché et al.6 | 22 | Limb-girdle muscle weakness | — | Normal | Trapezius: 20% Anconeus: 14% |
— | — | — | Homozygous c.44 C>T (p.Thr15Met) | Pyridostigmine and 3,4-DAP beneficial |
| Natera-de Benito et al.14 | 40 | Limb-girdle muscle weakness | F | — | — | — | — | — | c.1526T>C (p.Met509Thr) c. *22C>A (3′UTR) |
Pyridostigmine beneficial |
| Maselli et al.15 | 30 (sibling of proband) | Limb-girdle muscle weakness | M | — | — | — | — | — | IVS 7–8A>G c. *22C>A (3′UTR) |
Pyridostigmine and/or 3,4-DAP beneficial |
| Guergueltcheva et al.12 | 40 | Limb-girdle muscle weakness | F | Normal | Deltoid: 12% | Abnormal | — | — | (p. M492T) c. *22C>A (3′UTR) |
Did not receive treatment |
Abbreviations: ADM = abductor digiti minimi; CK = creatine kinase; 3,4 DAP = 3,4-diaminopyridine; EDC = extensor digitorum communis; EM = electronic microscopy; MCD = mean consecutive difference; UNL = upper normal limit; VM = vastus medialis; — = not performed or not reported.
RNS of spinal accessory nerve suggested a postsynaptic NMJ disorder. Although mainly considered postsynaptic, an impairment of the presynaptic NMJ components has been described in GFPT1 mouse models.16,17 Furthermore, a superimposed myopathy is not uncommon in patients with CMS; elevated muscle enzymes and myopathic pattern on needle electromyogram (as seen in this patient) and myopathologic features in biopsy (i.e., tubular aggregates) can be seen in GFPT1-related CMS.8,12,18 It is plausible that eventual involvement of presynaptic and skeletal muscle components contributed to the occurrence of patient's symptoms later in life. Prompt CMS recognition might help defining disease-modifying effects that available treatments may have if early initiated. Thus, it is possible that development of extrasynaptic pathology accounts for resistance to acetylcholinesterase inhibitor therapy as previously suggested.8
Agenesia of skeletal muscles in patients with muscle weakness should prompt suspicion for CMS. Furthermore, GFPT1 should be considered a candidate gene to screen in patients with Poland syndrome.
Appendix. Authors
| Name | Location | Contribution |
| Erika K. Williams, MD, PhD | Department of Neurology, Massachusetts General Hospital; Department of Neurology, Brigham Women's Hospital, Harvard Medical School, Boston, MA | Major role in the acquisition of data; analysis or interpretation of data |
| Cristina Shea, MD | Department of Neurology, Massachusetts General Hospital; Department of Neurology, Brigham Women's Hospital, Harvard Medical School, Boston, MA | Major role in the acquisition of data; analysis or interpretation of data |
| Paloma Gonzalez-Perez, MD, PhD | Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA | Major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
Study Funding
Dr. Gonzalez-Perez is funded by the NIH/NINDS (K23NS118048).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NG for full disclosures.
References
- 1.Kao JC, Milone M, Selcen D, Shen X-M, Engel AG, Liewluck T. Congenital myasthenic syndromes in adult neurology clinic: a long road to diagnosis and therapy. Neurology. 2018;91(19):e1770-e1777. doi: 10.1212/wnl.0000000000006478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engel AG, Shen X-M, Selcen D, Sine SM. Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol. 2015;14(4):420-434. doi: 10.1016/s1474-4422(14)70201-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senderek J, Müller JS, Dusl M, et al. Hexosamine biosynthetic pathway mutations cause neuromuscular transmission defect. Am J Hum Genet. 2011;88(2):162-172. doi: 10.1016/j.ajhg.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selcen D, Shen X-M, Milone M, et al. GFPT1-myasthenia: clinical, structural, and electrophysiologic heterogeneity. Neurology. 2013;81(4):370-378. doi: 10.1212/wnl.0b013e31829c5e9c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dusl M, Senderek J, Müller JS, et al. A 3’-UTR mutation creates a microRNA target site in the GFPT1 gene of patients with congenital myasthenic syndrome. Hum Mol Genet. 2015;24(12):3418-3426. doi: 10.1093/hmg/ddv090 [DOI] [PubMed] [Google Scholar]
- 6.Bauché S, Vellieux G, Sternberg D, et al. Mutations in GFPT1-related congenital myasthenic syndromes are associated with synaptic morphological defects and underlie a tubular aggregate myopathy with synaptopathy. J Neurol. 2017;264(8):1791-1803. doi: 10.1007/s00415-017-8569-x [DOI] [PubMed] [Google Scholar]
- 7.Helman G, Sharma S, Crawford J, et al. Leukoencephalopathy due to variants in GFPT1-associated congenital myasthenic syndrome. Neurology. 2019;92(6):e587-e593. doi: 10.1212/wnl.0000000000006886 [DOI] [PubMed] [Google Scholar]
- 8.Jiang K, Zheng Y, Lin J, et al. Diverse myopathological features in the congenital myasthenia syndrome with GFPT1 mutation. Brain Behav. 2022;12(2):e2469. doi: 10.1002/brb3.2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldelli I, Baccarani A, Barone C, et al. Consensus based recommendations for diagnosis and medical management of Poland syndrome (sequence). Orphanet J Rare Dis. 2020;15(1):201. doi: 10.1186/s13023-020-01481-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romanini MV, Calevo MG, Puliti A, et al. Poland syndrome: a proposed classification system and perspectives on diagnosis and treatment. Semin Pediatr Surg. 2018;27(3):189-199. doi: 10.1053/j.sempedsurg.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 11.Nishimune H, Stanford JA, Mori Y. Role of exercise in maintaining the integrity of the neuromuscular junction. Muscle Nerve. 2014;49(3):315-324. doi: 10.1002/mus.24095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guergueltcheva V, Müller JS, Dusl M, et al. Congenital myasthenic syndrome with tubular aggregates caused by GFPT1 mutations. J Neurol. 2012;259(5):838-850. doi: 10.1007/s00415-011-6262-z [DOI] [PubMed] [Google Scholar]
- 13.El-Wahsh S, Wijesinghe R, Qiu J, Heard R, Stoll M, Reddel S. Very late-onset limb-girdle congenital myasthenic syndrome due to GFPT1 mutation. Muscle Nerve. 2023;68(3):E32-E34. doi: 10.1002/mus.27842 [DOI] [PubMed] [Google Scholar]
- 14.Natera-de Benito D, Töpf A, Vilchez JJ, et al. Molecular characterization of congenital myasthenic syndromes in Spain. Neuromuscul Disord. 2017;27(12):1087-1098. doi: 10.1016/j.nmd.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 15.Maselli RA, Arredondo J, Nguyen J, et al. Exome sequencing detection of two untranslated GFPT1 mutations in a family with limb-girdle myasthenia. Clin Genet. 2014;85(2):166-171. doi: 10.1111/cge.12118 [DOI] [PubMed] [Google Scholar]
- 16.Zoltowska K, Webster R, Finlayson S, et al. Mutations in GFPT1 that underlie limb-girdle congenital myasthenic syndrome result in reduced cell-surface expression of muscle AChR. Hum Mol Genet. 2013;22(14):2905-2913. doi: 10.1093/hmg/ddt145 [DOI] [PubMed] [Google Scholar]
- 17.Issop Y, Hathazi D, Khan MM, et al. GFPT1 deficiency in muscle leads to myasthenia and myopathy in mice. Hum Mol Genet. 2018;27(18):3218-3232. doi: 10.1093/hmg/ddy225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo H-Y, Zhao L, Mao C-Y, et al. Novel compound heterozygous GFPT1 mutations in a family with limb-girdle myasthenia with tubular aggregates. Neuromuscul Disord. 2019;29(7):549-553. doi: 10.1016/j.nmd.2019.05.008 [DOI] [PubMed] [Google Scholar]