1. Introduction
The rapid emergence of drug-resistance bacteria is a growing threat to human life which requires the development of alternative therapeutic approaches. As potential novel antibiotics, host defense peptides (HDPs) have less opportunities of inducing drug resistance due to their relatively non-specific and complex bactericidal mechanisms. In this editorial, we outline the mode of action of HDPs and highlight the recent development of a few examples of small molecules that mimic the mechanism of action of HDPs to combat antibiotic resistance.
Host defense peptides (HDPs) are short cationic amphipathic peptides which mediate a broad range of activities expressed among all complex life forms, including plants, mammalians and insect. After the identification of the first antimicrobial insect HDP, cecropin from silk moths, numerous HDPs with potential antibiotic activities were reported, such as magainin II, α-helical peptides SMAP 29 and BMAPs. Their higher activities against bacteria have made them a promising alternative strategy to combat emerging drug resistance.
Generally, most HDPs can directly act on the bacteria cell envelope depending on their positive charge and amphipathicity. As well known, the surface of a bacteria cell envelope is negatively charged, due to the presence of outer membrane components including lipopolysaccharides (Gram-negative bacteria) and lipoteichoic acids (Gram-positive bacteria). Positive charged HDPs initially approach negatively charged bacterial membranes through electrostatic attraction, and then their hydrophobic groups insert into the membranes through hydrophobic interaction, leading to the destabilization and permeabilization of cytoplasmic membrane(1). Some models to explain membrane interruption mechanism have been proposed, such as barrel-stave model, carpet model and toroidal model(2). It is worth to note that the membranes of mammalian cells are compose of amphiphilic phospholipids, which are generally neutral. This difference is one of the most important reasons why HDPs usually have selectivity toward bacterial membranes over mammalian cell membranes. Virtually, except for membrane interruption, HDPs apply complex mechanisms to eradicate bacteria, such as inhibiting cell wall synthesis or directly binding to intracellular targets(3). The complex antibacterial mechanism and broad-spectrum activity of HDPs may enable them to be less prone to eliciting resistance than conventional antibiotics(4).
Many eukaryotic HDPs have been identified as broad-spectrum antibiotics with potent bactericidal activity, however, few of them have advanced to clinical use. The main drawbacks of HDPs are their possible systemic toxicity, susceptibility to protease degradation and high production cost(2),(5). Therefore, there have been extensive efforts in the development of antibiotic agents that mimic the mode of action of HDPs, but have smaller molecular weight, better selectivity, stronger activity, and important protease-resistance. For instance, antibacterial peptidomimetics include peptoids, β-peptides, γ-AApeptides, azapeptide, oligocarbamates. These are unnatural oligomers with comparable size to HDPs which possess enhanced stability and antibacterial activity. Nonetheless, to develop antibiotic agents with more practice applications, small molecules are more preferred due to their lower production cost, better druggability, large diversity and high market share. It is encouraging that some small molecules have been developed in recent years by mimicking bactericidal mechanism of HDPs and currently are investigated in clinical trials.
Two polymyxins, colistin (polymyxin E) and polymyxin B were discovered in 1940s. They were not subjected to clinical use due to their narrow antibacterial spectrum until rapid increase in resistance to other antibiotics. Encouragingly, more potential analogies of polymyxins are being explored recently(6). Brilacidin (PMX-30063), developing by Innovation Pharmaceuticals Inc. is among the most promising HDP mimic compounds to undergo the clinical evaluation (Figure 1a). Bearing both cationic and hydrophobic group, as well as an amphipathic structure, it exhibited similar to greater antibacterial efficacy when comparing with vancomycin. The most recent phase II clinical study result has been released on January 2019, which shows that Brilacidin has high potential for preventative treatment, as evidenced by a clear reduction of Severe Oral Mucositis among patients on Brilacidin when compared to those on placebo(7). Lytixar (LTX-109) is another HDP mimic as an antibiotic drug had been studied on phase II clinical trial for skin infection caused by Gram-positive bacteria (Figure 1b), impetigo and nasally colonized with MRSA and MSSA. Although Lytix Biopharma decided not to proceed with the planned clinical LTX-109 program in diabetic foot ulcers in 2015, it is another example of HDP-mimicking small molecule advanced into clinical trials(8).
Figure 1.
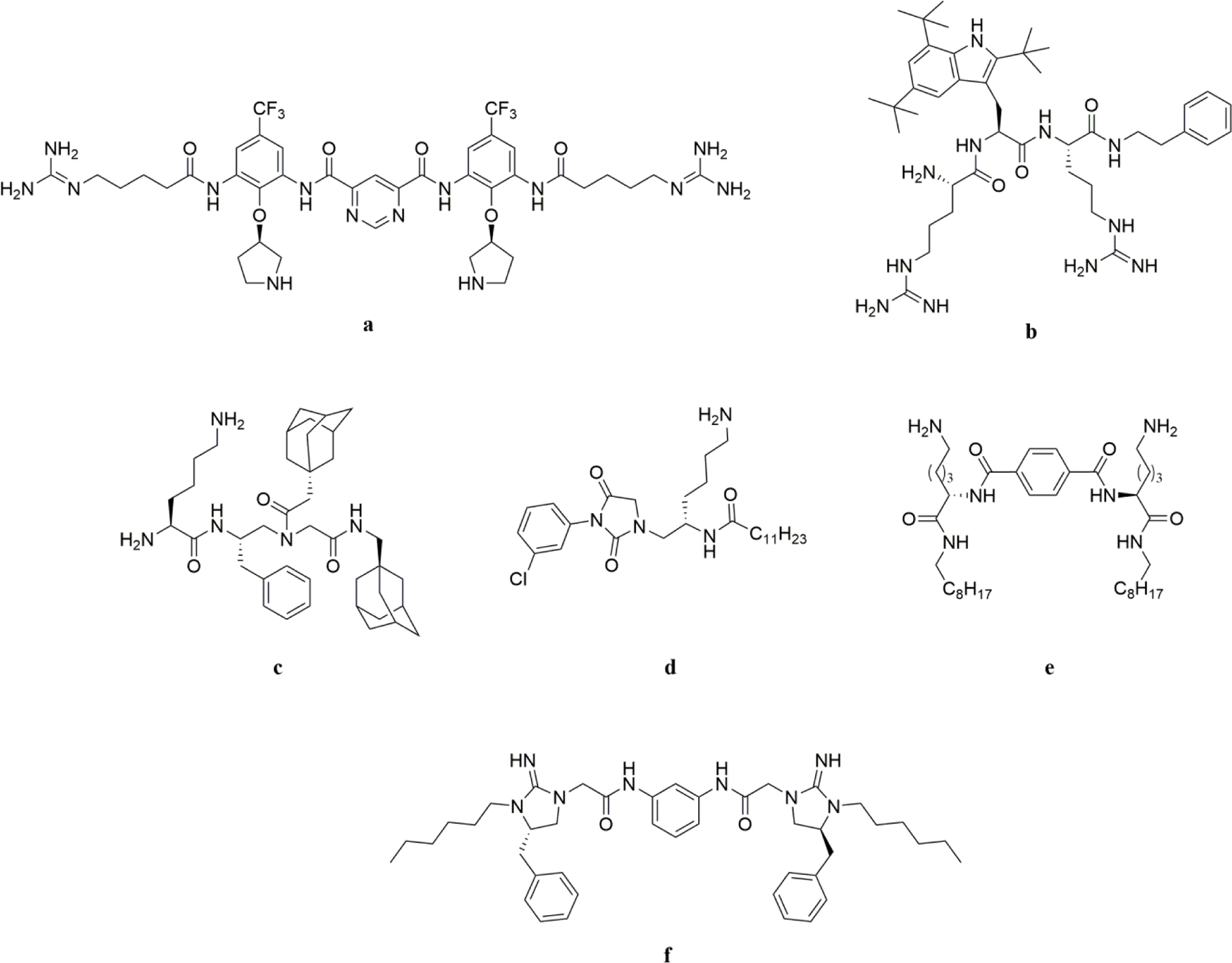
Structures of small-molecule antibiotics.
Other small molecules being explored in preclinical phase are also quite meaningful(9). Teng et al. reported a series of small antibacterial compounds based on the acylated reduced amide scaffold, which has excellent broad-spectrum bactericidal activity(5). They built their HDPs mimics through systematically adjusting cationic parts such as amino or guanidino group, and hydrophobic portion including adamantyl group, phenyl group, biphenyl group and naphthyl group. The best compound (Figure 1c) showed good and rapid bactericidal activity against both Gram-positive and Gram-negative strains with high selectivity. Su et al. greatly improved the antibacterial activity of hydantoin derivates through combining the hydantoin core and the characteristics of HDPs (Figure 1d), which exhibited much better activity than nitrofurantoin. They applied a panel of alkyl lipid tail as hydrophobic domain and used versatile short alkyl chains or bulky groups to modify the hydantoin core(10).
It is well known that dimerization is widely applied during the drug development. Niu et al. synthesized dimeric lysine N- alkylamides as HDPs mimics(11). Antibacterial assays revealed their strong, rapid bacterial killing activity and high selectivity between mammalian cells and bacterial cells. The design and synthesis of these structures are straightforward. Cationic groups were provided by amino in lysine amino acid and hydrophobic groups were all alkyl chain with different carbon numbers. Among them, the structure with C8 alkyl chain showed the strongest and broad-spectrum antibiotic activity (Figure 1e) and did not elicited drug resistance readily. The dimerization strategy has also been adopted to develop bis-cyclic guanidines (Figure 1f) by Teng et al. to kill a panel of Gram-positive and Gram-negative strains such as MRSA and E.coli(12). This class of molecules also exhibited high potency against Clostridium difficile both in vitro and in vivo.
2. Conclusion
Overall, small molecular HDP mimics have already achieved substantial progress in the past decades. These mimics cannot only reach parallel or better antibacterial activity when comparing conventional antibiotics, but also have great potential to overcome drug resistance, hemolysis and cytotoxicity. Moreover, these small molecular HDP mimics have several advantages over HDPs and other antibiotics with large molecular weight, such as synthetic flexibility, enhanced stability and higher tolerability. A better understanding and further exploration of HDP mimics will potentially facilitate the widespread clinical use of this new antibacterial strategy.
3. Expert opinion
The emergent antibiotic resistance leads to the rapid development of small molecule based HDP mimics with excellent and broad-spectrum activity against both Gram-positive and Gram-negative bacteria, which provided significantly more promising candidates for clinical research. Unlike conventional antibiotics, these small molecules are not only drug-like, but also possess membrane-active activity analogues to that of HDPs, and therefore are expected to have less probability to induce resistance in bacteria. Current success exemplified their promising therapeutic potential. The main challenge preventing the development of small molecule HDP mimics are the indeterminate design principles and complex bacterial-killing mechanism. However, the past research findings already shed light on the future design. For instance, Choi et al. delineated the importance of rigidity of molecular scaffold for HDP mimicking, as seen for their hydrogen-bonded (N‒H···S, N‒H···O) arylamide template to limit the flexibility of compounds(13) However, Thaker et al. developed a series of triaryl scaffolds which led to their conclusion that the overall hydrophobicity exhibited more significant impact than conformational stiffness(14). Similarly, Ivankin et al. also believed that conformational preorganization is not obligatory(15). Indeed, as shown in Figure 1, except bralacidin (a), none of the other molecules has a very rigid scaffold. These findings may provide rationale for the design of new antibiotic agents based on small molecules to mimic HDPs. For instance, as rigidity is not critical, more molecular scaffolds could be adopted to develop antibiotic agents. One may just have to explore the ratio and nature of hydrophobic and cationic groups in order to improve their activity and selectivity. The recent experience suggested that even a known antibiotic molecular scaffold (Figure 1d) could be used for the development of HDP mimetics, which could lead to antibiotics not only retaining their original antibacterial activity, e.g., an intracellular target, but also bearing membrane-disruptive activity akin to HDPs. The dual or multi- antibacterial mechanisms may be the advantage of new generation of antibiotics with therapeutic potential to combat drug resistance.
Acknowledgements
The authors thank the support from NIH 9R01AI152416-06.
Funding
This paper was not funded.
Footnotes
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A peer review on this manuscript has disclosed that they have more than 60 patents on antimicrobial peptides and have started 3 companies in this space although the companies highlighted in this review are not direct competitors. All other peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Fjell CD, Hiss JA, Hancock REW, et al. Designing antimicrobial peptides: form follows function. Nat. Rev. Drug Discov 2012;11(1):37–51. 2012;11(1):37–51. [DOI] [PubMed] [Google Scholar]
- 2. Hancock REW, Sahl H-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol 2006;24(12):1551–7. **This is a very comprehensive article which included the basic knowledge of Host defense peptides (HDPs), clinical experience of HDP mimics, antibacterial mechanism, limitations and tools to develop potential mimics. It’s very useful to get an outline of HDPs field.
- 3.Kumar P, Kizhakkedathu JN, Straus SK. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018;8(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson DI, Hughes D, Kubicek-Sutherland JZ. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resistance Updates 2016;26:43–57. [DOI] [PubMed] [Google Scholar]
- 5. Teng P, Huo D, Nimmagadda A, et al. Small Antimicrobial Agents Based on Acylated Reduced Amide Scaffold. J Med Chem 2016;59(17):7877–87. *Teng et al. explored a promising cationic peptidomimetic with effective in vivo activity. The design strategy is a good guide for the design of small molecular antibiotics for other researchers.
- 6.Akhoundsadegh N, Belanger CR, Hancock REW. Outer Membrane Interaction Kinetics of New Polymyxin B Analogs in Gram-Negative Bacilli. Antimicrob. Agents Chemother 2019;63(10):e00935–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brilacidin [Internet]. Wakefield: INNOVATION; [cited 2020 Feb 29]. Available from: http://www.ipharminc.com/brilacidin-1.
- 8.Lytix Biopharma shelves clinical trial with LTX-109 in diabetic foot ulcers 2015 [Internet] Oslo: Lytix Biopharma; [cited 2020 Feb 29]. Available from: http://www.lytixbiopharma.com/news/317/130/Lytix-Biopharma-shelves-clinical-trial-with-LTX-109-in-diabetic-foot-ulcers.html. [Google Scholar]
- 9.Varney KM, Bonvin AMJJ, Pazgier M, Malin J, Yu W, Ateh E, et al. Turning defense into offense: defensin mimetics as novel antibiotics targeting lipid II. PLoS Pathog 2013;9(11):e1003732-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Su M, Xia D, Teng P, et al. Membrane-Active Hydantoin Derivatives as Antibiotic Agents. J Med Chem 2017;60(20):8456–65. *Su et al. developed a series of antibacterial structures from hydantoin derivatives. Cost-effective synthesis, straightforward structures and good activity could be a good inspiration for future design.
- 11. Niu Y, Wang M, Cao Y, et al. Rational Design of Dimeric Lysine N-Alkylamides as Potent and Broad-Spectrum Antibacterial Agents. J Med Chem 2018;61(7):2865–74. *Youhong et al. applied lysin amino acid to generate several simple structures with broad spectrum antibacterial activity. The dimeric design could be used to develop small molecular antibiotics.
- 12.Teng P, Nimmagadda A, Su M, et al. Novel bis-cyclic guanidines as potent membrane-active antibacterial agents with therapeutic potential. Chem Commun 2017;53(87):11948–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi S, Isaacs A, Clements D, Liu D, Kim H, Scott RW, et al. De novo design and in vivo activity of conformationally restrained antimicrobial arylamide foldamers. Proc. Natl. Acad. Sci 2009;106(17):6968–73. *Sungwook et al. put forward an important factor for the design of antimicrobial agents. Rigid conformation influences the activity of antibiotics in some situations.
- 14.Thaker HD, Sgolastra F, Clements D, et al. Synthetic Mimics of Antimicrobial Peptides from Triaryl Scaffolds. J Med Chem 2011;54(7):2241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivankin A, Livne L, Mor A, et al. Role of the Conformational Rigidity in the Design of Biomimetic Antimicrobial Compounds. Angew. Chem 2010;49(45):8462–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


