Abstract
The nucleoprotein genes of influenza virus A/Netherlands/018/94 (H3N2) and influenza virus B/Harbin/7/94 were cloned into the bacterial expression vector pMalC to yield highly purified recombinant influenza virus A and B nucleoproteins. With these recombinant influenza nucleoproteins, enzyme-linked immunosorbent assays (ELISAs) were developed for the detection of influenza virus A- and B-specific immunoglobulin A (IgA) and IgG serum antibodies. Serum samples were collected at consecutive time points after the onset of clinical symptoms from patients with confirmed influenza virus A or B infections. Nucleoprotein-specific IgA antibodies were detected in 41.2% of influenza virus A-infected patients and in 66.7% of influenza virus B-infected patients on day 6 after the onset of clinical symptoms. In serum samples taken on day 21 (influenza virus A-infected patients) or day 28 (influenza virus B-infected patients), nucleoprotein-specific IgA antibodies could be detected in 58.8 and 58.3% of influenza virus A- and B-infected patients, respectively. At the same time, IgG antibody rises were detected in 88.2% of influenza virus A-infected patients and in 95.8% of influenza virus B-infected patients. On comparison, hemagglutination inhibition assays detected antibody titer rises in 81.3 and 72.7% of patients infected with influenza viruses A and B, respectively. In contrast to the detection of nucleoprotein-specific IgG antibodies or hemagglutination-inhibiting antibodies, the detection of nucleoprotein-specific IgA antibodies does not require paired serum samples and therefore can be considered an attractive alternative for the rapid serological diagnosis of influenza.
Influenza viruses (family Orthomyxoviridae) are the causal agents of recurrent epidemics of acute respiratory disease in humans. For the laboratory diagnosis of influenza virus infections, several methods which detect either viral antigens or antigen-specific serum antibodies are used. For the quantification of influenza virus-specific serum antibodies, the hemagglutination inhibition (HI) assay and complement fixation (CF) assay are routinely used. However, these assays suffer from some disadvantages. They are laborious to perform, difficult to incorporate into automated procedures, and require a continuous source of the appropriate erythrocytes. Alternatively, enzyme-linked immunosorbent assays (ELISAs) have been used for the detection of influenza virus-specific antibodies. ELISAs measuring influenza virus-specific serum IgG antibodies have been shown to be more sensitive than the HI or the CF assay (1, 10, 11, 13–17, 22, 23). In addition, ELISAs enable the detection of antibodies of different isotypes (3, 6, 18, 19). For example, the demonstration of virus-specific IgA antibodies after influenza virus infections has been shown to be of diagnostic value (5, 6, 19). The preparation of viral antigens to be used in these ELISAs usually requires the concentration and purification of virus conventionally propagated in embryonated chicken eggs or cell culture. However, ELISAs with purified (recombinant) viral proteins have also been described (8, 9, 12, 20). In the present paper we describe the production of recombinant nucleoproteins (NPs) of influenza viruses A and B as a virtually unlimited source of viral antigen. By using highly purified recombinant NPs of influenza viruses A and B, ELISAs were developed for the detection of virus-specific immunoglobulin A (IgA) and IgG serum antibodies. With serum samples obtained from patients with confirmed influenza virus A and B infections, the value of these recombinant NP-based ELISA systems was demonstrated.
MATERIALS AND METHODS
Cloning of the NP genes of influenza viruses A and B.
The influenza viruses A/Netherlands/018/94 (H3N2) and B/Harbin/7/94 were obtained from the repository of the Dutch National Influenza Centre. Viral RNA was extracted from these viruses as described previously (4). A reverse transcriptase (RT) reaction was performed to obtain single-stranded DNA copies of gene segment 5, which encodes the NP. To 10 μl of viral RNA 2 μl of forward primer (10 pmol/μl) was added and the mixture was incubated at 80°C for 2 min, followed by 5 min of incubation on ice. Then, deoxynucleoside triphosphates (0.5 mM each), dithiothreitol (10 mM), RNasin (40 units), and Moloney murine leukemia virus RT (200 U) were added in a total volume of 25 μl of 1× RT buffer followed by incubation at 42°C for 45 min. The reaction was stopped by heating the mixture to 95°C for 3 min. The DNA obtained was used as a template in a PCR. Besides the DNA, the PCR mixture contained 20 pmol of forward primer and 20 pmol of reverse primer, deoxynucleoside triphosphates (0.2 mM each), and Pfu polymerase (5 units) in a total volume of 100 μl of 1× Pfu buffer. The PCR cycles consisted of 1 min at 94°C, 2 min at 52°C, and 4 min at 72°C for a total of 40 cycles. Primer sequences were based on the consensus sequence of the NP genes of recent influenza virus A and B strains obtained from the Wisconsin Sequence Analysis Package and were designed in such a way that the ultimate PCR product contained an EcoRI (influenza virus A) or XbaI (influenza virus B) restriction endonuclease recognition sequence upstream of the start codon and a SalI restriction endonuclease recognition sequence downstream of the stop codon of the NP genes. The PCR products of the NP genes of both viruses were cloned into the bacterial expression vector pMalC (New England Biolabs) in frame with the gene encoding the maltose binding protein (MBP) by using the EcoRI or XbaI and SalI sites in the multiple cloning site of this plasmid. Restriction endonuclease digestion, ligation, transformation in Escherichia coli DH5α, plasmid DNA isolation, and agarose gel electrophoresis were performed by standard procedures (21).
Production, isolation, and purification of recombinant NP.
A total of 500 ml of SOB medium (21) containing ampicillin (50 μg/ml) and supplemented with glucose (2 g/liter) was inoculated with 5 ml of an overnight culture of recombinant E. coli, and the mixture was incubated at 37°C in a shaking incubator. The optical density (OD) at a wavelength of 600 nm (OD600) was monitored, and at an OD600 value of between 0.5 and 0.6, 1 mM isopropyl β-d-thiogalactopyranoside was added to induce expression of the fusion gene. Four hours after induction, the bacteria were pelleted by centrifugation, resuspended in 25 ml of column buffer (20 mM Tris-HCl [pH 7.4], 200 mM NaCl, 1 mM EDTA) containing Pefablock protease inhibitor (Boehringer Mannheim), and lysed by sonication. The lysate was diluted to 100 ml in column buffer and was run through an amylose resin column (New England Biolabs). After extensive washing of the column, recombinant protein was eluted with column buffer containing 25 mM maltose. Peak fractions were pooled, and the purified proteins were stored at −70°C until use. Protein concentrations were determined by using the Bradford reagent (2). The procedure was carried out for recombinant E. coli carrying the pMalC plasmid without cloned sequences to obtain recombinant MBP (rMBP) and for recombinant E. coli carrying the pMalC plasmid in which the NP gene of influenza virus A or B was cloned to obtain recombinant fusion proteins consisting of MBP and influenza virus A NP (rNPA) or influenza virus B NP (rNPB), respectively.
SDS-PAGE and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting were performed by standard procedures (21). Blots were incubated with blocking buffer (2% nonfat milk powder, 0.05% Tween 20 in phosphate-buffered saline [PBS]) for 1 h, followed by 1 h of incubation with 1:100-diluted polyclonal rabbit antisera specific for influenza virus A or B. After washing of the blots with PBS, the blots were incubated for 1 h with 1:500-diluted horseradish peroxidase (HRP)-labeled swine anti-rabbit IgG antibodies (Dako, Glostrup, Denmark). Then, the blots were washed with PBS followed by incubation in diaminobenzidine-H2O2 in PBS (250 μg of diaminobenzidine/ml, 0.002% H2O2). The reaction was stopped with H2O when protein bands became visible.
Sera.
Influenza virus A- and B-specific polyclonal rabbit and ferret antisera were obtained from rabbits injected with sucrose gradient-purified influenza virus A/Hong Kong/2/68 (H3N2) or B/Harbin/7/94 and from ferrets experimentally infected with influenza virus A/Netherlands/018/94 (H3N2) or B/Harbin/7/94.
Human sera were obtained from adult patients with acute influenza virus B (n = 24) and influenza virus A (H1N1; n = 2, H3N2; n = 15) infection; the patients were enrolled in clinical studies during the respiratory season in March 1995 and December 1995, respectively. Influenza virus infection was confirmed by an immunofluorescence test or by virus isolation from cell culture. Sera were collected on the day of onset of clinical symptoms (day 1) and at several time points thereafter. For the influenza virus A-infected patients, additional serum samples collected on days 6, 21, and 60 were available. For the patients with influenza virus B infection, additional serum samples collected on days 6 and 28 were available. Sera were stored at −20°C until use.
HI assay.
One volume of serum was mixed with 5 volumes of cholera filtrate, and the mixture was incubated at 37°C for approximately 16 h, followed by 1 h of incubation at 56°C. To 50 μl of twofold dilution series of serum in PBS, 25 μl of a solution of influenza virus A/Singapore/6/86 (H1N1), A/Johannesburg/33/94 (H3N2), or B/Harbin/7/94 containing 4 hemagglutinating units was added, and the mixture was incubated at 37°C for 30 min. Then, 25 μl of a 1% turkey erythrocyte suspension in PBS was added, followed by 1 h of incubation at 4°C. Subsequently, the hemagglutination pattern was examined and was expressed as the reciprocal value of the highest serum dilution inhibiting hemagglutination. A fourfold titer rise for paired serum samples was considered indicative of a recent influenza virus infection.
ELISA. (i) ELISA for detection of IgA serum antibodies (capture IgA NP ELISA).
Ninety-six-well plates coated with rabbit anti-human IgA antibodies (Meddens Diagnostics, Brummen, The Netherlands) were washed with demineralized H2O containing 0.05% Tween 80, followed by incubation with patient sera diluted 1:100 in ELISA buffer (Meddens Diagnostics). After 1 h of incubation at 37°C, the plates were washed and incubated with rNPA or rNPB, which were conjugated with HRP by previously described methods (24). Following 1 h of incubation at 37°C, the plates were washed again and incubated with tetramethylbenzidine substrate (Meddens Diagnostics) for 10 min. The reaction was stopped with 2 M H2SO4, and the OD was measured at 450 nm. NP-specific reactivities were expressed as the following ratio: OD450 for patient serum/OD450 for negative control serum. The negative control serum consisted of a pool of sera negative for influenza virus A- and B-specific IgA antibodies. Ratios greater than 2.0 were considered positive.
(ii) ELISA for detection of IgG serum antibodies (indirect IgG NP ELISA).
For rabbit and ferret sera, 96-well plates were coated overnight at room temperature with 50 ng of rNPA or rNPB in 100 μl of 0.1 M sodium carbonate buffer (pH 9.6). The plates were washed with demineralized H2O containing 0.05% Tween 80. Influenza virus A- and B-specific rabbit and ferret antisera were twofold diluted from 1:100 to 1:6,400 in ELISA buffer. A total of 50 μl of each dilution was incubated in the recombinant NP-coated plates for 1 h at 37°C. After washing of the plates, 50 μl of 1:500-diluted goat anti-ferret IgG antibodies (Kirkegaard & Perry) or 1:500-diluted swine anti-rabbit IgG antibodies (Dako, Glostrup, Denmark) conjugated with HRP was added, and the mixture was incubated for 1 h at 37°C. The plates were washed again and were incubated with 50 μl of tetramethylbenzidine substrate for 10 min. The reaction was stopped by adding 50 μl of 2 M H2SO4, and the OD450 was measured.
For human sera, ELISA was performed as described above. Human sera were diluted 1:100,000, and IgG antibodies were detected with 1:5,000-diluted HRP-labeled goat anti-human IgG antibodies (Biosource Europe, Fleurus, Belgium). In addition to reactivities with rNPA and rNPB, the reactivity of human sera with rMBP was also measured. NP-specific reactivities were expressed as the following ratio: OD450 measured with rNPA or rNPB/OD450 measured with rMBP. An increase in this ratio for paired serum samples of at least a factor 2.0 was considered indicative of a recent influenza virus infection.
RESULTS
Recombinant NPs of influenza viruses A and B.
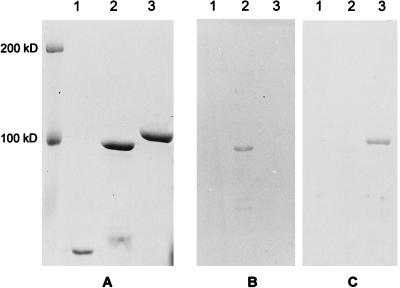
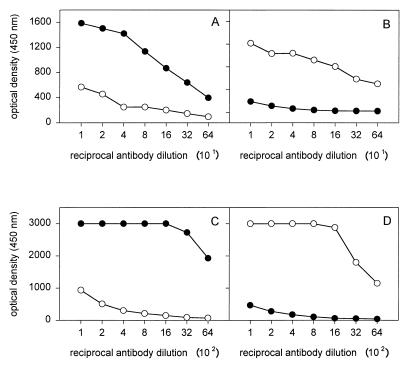
After induction of expression and purification by affinity chromatography, the recombinant proteins rNPA and rNPB were analyzed by SDS-PAGE. As shown in Fig. 1A, highly purified protein preparations with molecular masses of 100 kDa for rNPA and 107 kDa for rNPB (including one of 40 kDa for MBP) were obtained. The difference in the molecular masses between rNPA and rNPB is in accordance with the difference in the lengths of the coding sequences for both proteins (1,494 bp for the NP of influenza virus A and 1,680 bp for the NP of influenza virus B). The identities of rNPA and rNPB were confirmed by Western blot analysis. Rabbit antiserum raised against an influenza virus A reacted only with rNPA and not with rNPB, whereas a rabbit antiserum directed against an influenza virus B showed reactivity with rNPB but not with rNPA (Fig. 1B and C, respectively). The identities of the recombinant proteins were further confirmed in indirect ELISAs with rabbit and ferret antisera raised against influenza viruses A and B which showed reactivity only with the homologous rNPA and rNPB, respectively (Fig. 2).
FIG. 1.
Analysis of rNPA and rNPB by SDS-PAGE and Western blotting. rMBP (lane 1), rNPA (lane 2), and rNPB (lane 3) were separated on an SDS–10% polyacrylamide gel and stained with Coomassie brilliant blue (A) or transferred to nitrocellulose membranes which were incubated with rabbit serum specific for influenza virus A (B) or influenza virus B (C).
FIG. 2.
Confirmation of the identity of rNPA and rNPB in indirect IgG NP ELISAs. Plates were coated with rNPA (A and C) or rNPB (B and D) and incubated with ferret (A and B) or rabbit (C and D) antisera raised against influenza virus A (solid circles) or influenza virus B (open circles).
Detection of IgA antibodies in patient sera by capture IgA NP ELISA.
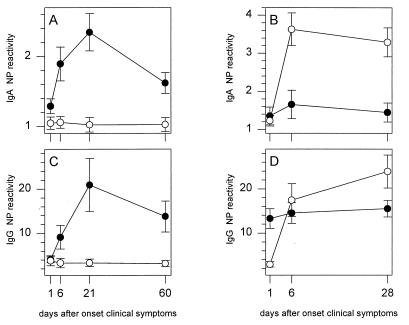
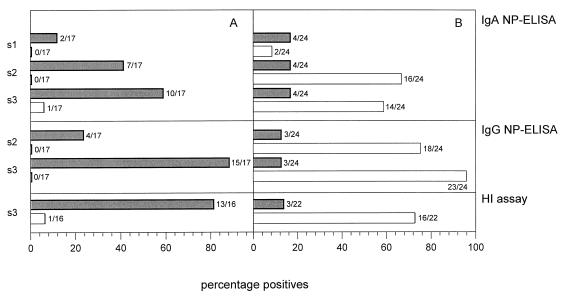
Serum samples collected at consecutive time points from patients with confirmed influenza virus A and B infections were analyzed for the presence of NP-specific IgA antibodies. For the group of patients infected with influenza virus A, an IgA response against the NP of influenza virus A but not that of influenza virus B was measured (Fig. 3A). The IgA response peaked at day 21 and subsequently declined. Sera from 10 of 17 patients (58.8%) showed reactivity with rNPA at day 21, while serum from only 1 patient (5.9%) showed reactivity with rNPB at this time point (Fig. 4A). For the group of patients infected with influenza virus B, a type-specific IgA response against NP was observed. The response showed a peak 6 days after the onset of clinical symptoms and slowly declined by day 28 (Fig. 3B). For this group of patients, sera from two patients (8.3%) showed reactivity with rNPB on day 1 (Fig. 4B). This number increased until sera from 16 patients (66.7%) showed reactivity by day 6. Sera from four patients (16.7%) showed reactivity with rNPA on day 1, but this number did not increase during the course of infection.
FIG. 3.
IgA (A and B) and IgG (C and D) responses in influenza virus A-infected (A and C) or influenza virus B-infected (B and D) patients as measured by the capture IgA NP ELISAs and indirect IgG NP ELISAs with rNPA (solid circles) and rNPB (open circles). In the capture IgA NP ELISAs, the reactivities of sera with rNPA and rNPB were measured, and NP-specific reactivities were expressed as the ratio OD450 for patient serum/OD450 for negative control serum. In the indirect IgG NP ELISAs, the reactivities of sera with rMBP, rNPA, and rNPB were measured, and NP-specific reactivities were expressed as the ratio OD450 measured for rNPA or rNPB/OD450 measured for rMBP. The mean ± standard error of the mean values for the influenza virus A-infected (n = 17) and influenza virus B-infected (n = 24) patients at each time point are presented.
FIG. 4.
Percentages of influenza virus A-infected (A) or influenza virus B-infected (B) patients showing NP-specific antibody responses as measured by the capture IgA NP ELISAs and indirect IgG NP ELISAs and percentages of infected patients showing titer rises by the HI assay. The results of the capture IgA NP ELISAs for serum samples s1 (day 1), s2 (day 6), and s3 (day 21 for influenza virus A-infected patients and day 28 for influenza virus B-infected patients) are shown. For the indirect IgG NP ELISAs, results for paired samples (serum samples s2 and s3 compared to serum sample s1) are shown. In the HI assays, serum sample s3 was compared to serum sample s1. The sera were tested for influenza virus type A (solid bars) and influenza virus type B (open bars).
Detection of IgG antibodies in patient sera by indirect IgG NP ELISA.
The same serum samples were also analyzed for NP-specific IgG antibodies. For the group of patients infected with influenza virus A, a type-specific IgG response against NP was observed (Fig. 3C). No reactivity was measured with the heterotypic rNPB. The NP-specific IgG response reached a maximum at 21 days after the onset of clinical symptoms and subsequently declined. For sera from 15 of 17 patients (88.2%) an increase in reactivity with rNPA was observed on day 21, whereas sera from none of these patients showed reactivity with rNPB (Fig. 4A). In the influenza virus B-infected patients, a strong IgG response against the homologous NP was observed, and this response increased at least until day 28 after the onset of clinical symptoms (Fig. 3D). For this group, sera from 23 of 24 patients (95.8%) showed an increase in reactivity with rNPB on day 28, while sera from only 3 patients (12.5%) showed an increase in reactivity with rNPA (Fig. 4B). In addition to rNPA and rNPB, the reactivities of sera with rMBP were also measured. The reactivity with rMBP did not change during the time course of influenza virus A or B infection (data not shown).
Comparison of the indirect IgG NP ELISA and the HI assay.
In the HI assay, 13 of 16 (81.3%) patients infected with influenza virus A showed a fourfold rise in serum antibody titer (between day 1 and day 21) against influenza virus A, while 88.2% showed an NP-specific IgG response (Fig. 4A). Three patients who showed NP-specific IgG responses did not show a titer rise in the HI assay, whereas in two patients a rise in the HI assay titer was observed but no NP-specific IgG response was observed. None of the influenza virus A-infected patients showed an influenza virus B NP-specific IgG response, whereas by the HI assay the serum of one patient showed a rise in titer against influenza virus B. Among the patients infected with influenza virus B, the sera of 16 of 22 (72.7%) patients showed rises in titers against influenza virus B (between day 1 and day 28) by the HI assay, while the sera of 95.8% showed NP-specific IgG responses (Fig. 4B). For five patients, IgG responses against NP were observed in the absence of at least fourfold rises in titer by the HI assay. The serum of one patient did not show an NP-specific IgG response and no rise in titer by the HI assay. The sera of three patients showed influenza virus A and B NP-specific IgG responses. Serum from one of those three patients and sera from another two patients showed rises in titer to influenza viruses A and B by the HI assay.
DISCUSSION
In the present paper, recombinant NPs of influenza viruses A and B were used for the development of ELISA systems which can detect virus-specific IgA and IgG serum antibodies. By using serum samples from laboratory animals experimentally immunized with influenza viruses A and B and from humans with confirmed influenza virus A or B infections, the specificities of these ELISAs were confirmed. In the majority of the patients with influenza, virus type-specific antibodies were detected, demonstrating the diagnostic value of these recombinant NP-based ELISAs. These assays may replace the commonly used HI and CF assays for the serodiagnosis of influenza virus infections and can be performed when respiratory specimens are not available or to confirm results obtained by culture procedures with respiratory specimens.
Capture IgA NP ELISAs were developed for the detection of influenza virus A and B NP-specific IgA serum antibodies with virus type-specific recombinant NP directly labeled with HRP. IgA responses were detected within 21 days after the onset of clinical symptoms in 58.8% of the influenza virus A-infected patients and 66.7% of the influenza virus B-infected patients. These percentages are comparable to the percentages of patients with virus-specific IgA responses reported in other studies (3, 6, 18). The sera of four patients with confirmed influenza virus B infections had IgA antibodies directed to influenza virus A from the first day of clinical onset onward. These patients may have suffered from a recent infection with an influenza A virus. Since influenza viruses of type A have also circulated in the 1994 and 1995 influenza season, this is a likely explanation. Since the level of preexisting influenza virus NP-specific IgA antibody levels is low, the capture IgA NP ELISAs do not require paired serum samples and, therefore, allow rapid serodiagnosis of influenza virus infections. This ELISA can be considered an alternative to assays that measure IgG serum antibodies when only one serum sample is available.
In addition to the capture IgA NP ELISAs, virus type-specific recombinant NP was also used for the detection of influenza virus A and B NP-specific IgG serum antibodies in indirect IgG NP ELISAs. By these ELISAs, IgG antibody rises could be detected in almost all of the influenza virus A- and B-infected patients. Although in these ELISAs serum antibodies were measured against influenza virus NP, while in the HI assay serum antibodies directed against the hemagglutinin were measured, the results of both assays were compared to evaluate the diagnostic value of the IgG NP ELISAs. The results of the IgG NP ELISAs for the detection of influenza virus A-specific antibodies compared well with the results obtained by the HI assay. The IgG NP ELISA for the measurement of influenza virus B-specific antibodies, however, detected a higher percentage of patients with increased antibody titers than the HI assay, which is in agreement with the results of earlier studies (2, 11, 14, 16, 17, 22, 23).
In contrast to antibodies of the IgA and IgG isotypes, the diagnostic value of IgM antibodies in influenza virus infection seems to be limited. Although the measurement of IgM responses has been shown to be of diagnostic value in primary influenza virus infection (3, 18), IgA and IgG antibody responses predominate in influenza virus-infected patients (5, 7, 11). Therefore, the measurement of IgG and IgA antibody responses is preferred for the serologic confirmation of influenza virus infections.
The division of influenza A and B viruses is based on antigenic differences in the NPs and matrix proteins. Indeed, no rises in influenza virus B NP-specific IgG antibody titers were measured in the influenza virus A-infected patients. However, rises in influenza virus A and B NP-specific IgG antibody titers were measured in 12.5% of the influenza virus B-infected patients. A recent influenza virus A infection or simultaneous infection with influenza viruses A and B may explain this observation.
Since the NP is well conserved within the influenza A viruses, the IgG NP ELISA enables the detection of antibodies induced by influenza A viruses of both circulating subtypes (H1N1 and H3N2). Furthermore, this assay does not require the annual adjustment of the viral antigen preparations, in contrast to the HI assay, which measures antibodies against the highly variable hemagglutinin. Two of the influenza virus A-infected patients seemed to be infected with an H1N1 virus since in the HI assay only rises in titer against an H1N1 virus were measured (data not shown). Rises in NP-specific IgG antibody levels could be measured in these individuals, even though the recombinant NP used in the IgG NP ELISA was derived from an H3N2 virus.
Although the reactivities with MBP differed between patients, none of them showed increases in MBP-specific IgG antibody levels during the time course after the infection. Thus, it does not seem to be necessary to measure MBP antibody titers separately to be able to detect rises in the levels of influenza virus-induced antibodies in the IgG NP ELISAs.
In conclusion, recombinant influenza virus NPs were produced in virtually unlimited quantities and were purified to a high degree. These bacterially expressed viral antigens proved to be valuable reagents for the development of ELISA systems for the detection of virus-specific IgA and IgG antibodies which can be used for the serodiagnosis of influenza virus type A and B infections. Especially for the detection of NP-specific IgA antibodies, the IgA NP ELISA proved to be valuable since it allows early diagnosis and does not require paired serum samples.
ACKNOWLEDGMENTS
Part of this work was supported by the Foundation for Respiratory Virus Infections, notably Influenza (SRVI).
We thank Glaxo Wellcome for kindly providing us with serum samples. We also thank Ger van der Water for continuous support and Cedrick Copra for technical assistance.
REFERENCES
- 1.Bishai F R, Galli R. Enzyme-linked immunosorbent assay for detection of antibodies to influenza A and B and parainfluenza type 1 in sera of patients. J Clin Microbiol. 1978;8:648–656. doi: 10.1128/jcm.8.6.648-656.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Burlington D B, Clements M L, Meiklejohn G, Phelan M, Murphy B R. Hemagglutinin-specific antibody responses in immunoglobulin G, A, and M isotypes as measured by enzyme-linked immunosorbent assay after primary or secondary infection of humans with influenza A virus. Infect Immun. 1983;41:540–545. doi: 10.1128/iai.41.2.540-545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claas E C J, Sprenger M J W, Kleter G E M, van Beek R, Quint W G W, Masurel N. Type-specific identification of influenza A, B and C by the polymerase chain reaction. J Virol Methods. 1992;39:1–13. doi: 10.1016/0166-0934(92)90120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doller G, Doller P C, Gerth H J. Diagnostic significance of influenza subtype-specific IgG, IgA, and IgM antibodies. J Biol Stand. 1986;14:163–175. doi: 10.1016/0092-1157(86)90001-6. [DOI] [PubMed] [Google Scholar]
- 6.Groen J, Claas E C J, Balentien E, Osterhaus A D M E. High influenza morbidity and mortality in unvaccinated elderly people in Curacao. J Infect. 1998;36:241–242. doi: 10.1016/s0163-4453(98)80026-1. [DOI] [PubMed] [Google Scholar]
- 7.Harmon M W, Phillips D J, Reimer C B, Kendal A P. Isotype-specific enzyme immunoassay for influenza antibody with monoclonal antibodies to human immunoglobulins. J Clin Microbiol. 1986;24:913–916. doi: 10.1128/jcm.24.6.913-916.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harmon M W, Jones I, Shaw M, Keitel W, Reimer C B, Halonen P, Kendal A P. Immunoassay for serological diagnosis of influenza type A using recombinant DNA produced nucleoprotein antigen and monoclonal antibody to human IgG. J Med Virol. 1989;27:25–30. doi: 10.1002/jmv.1890270106. [DOI] [PubMed] [Google Scholar]
- 9.Joassin L, Reginster M, Vaira D. Anti M-protein antibody responses to type A or B natural influenza detected by solid phase enzyme linked immunosorbent assay and by complement fixation. Arch Virol. 1983;76:15–23. doi: 10.1007/BF01315700. [DOI] [PubMed] [Google Scholar]
- 10.Julkunen I, Kleemola M, Hovi T. Serological diagnosis of influenza A and B infections by enzyme immunoassay. Comparison with the complement fixation test. J Virol Methods. 1984;9:7–14. doi: 10.1016/0166-0934(84)90078-8. [DOI] [PubMed] [Google Scholar]
- 11.Julkunen I, Pyhala R, Hovi T. Enzyme immunoassay, complement fixation and hemagglutination inhibition tests in the diagnosis of influenza A and B virus infections. Purified hemagglutinin in subtype-specific diagnosis. J Virol Methods. 1985;10:75–84. doi: 10.1016/0166-0934(85)90091-6. [DOI] [PubMed] [Google Scholar]
- 12.Khan M W, Bucher D J, Koul A K, Kalish G, Smith H, Kilbourne E D. Detection of antibodies to influenza virus M protein by an enzyme-linked immunosorbent assay. J Clin Microbiol. 1982;16:813–820. doi: 10.1128/jcm.16.5.813-820.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koskinen P, Vuorinen T, Meurman O. Influenza A and B virus IgG and IgM serology by enzyme immunoassays. Epidemiol Infect. 1987;99:55–64. doi: 10.1017/s0950268800066863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madore H P, Reichman R C, Dolin R. Serum antibody responses in naturally occurring influenza A virus infection determined by enzyme-linked immunosorbent assay, hemagglutination inhibition, and complement fixation. J Clin Microbiol. 1983;18:1345–1350. doi: 10.1128/jcm.18.6.1345-1350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masihi K N, Lange W. Enzyme-linked immunosorbent assay for the detection of influenza type-specific antibodies. J Immunol Methods. 1980;36:173–179. doi: 10.1016/0022-1759(80)90041-1. [DOI] [PubMed] [Google Scholar]
- 16.Murphy B R, Tierney E L, Barbour B A, Yolken R H, Alling D W, Holley H P, Mayner R E, Chanock R M. Use of the enzyme-linked immunosorbent assay to detect serum antibody responses of volunteers who received attenuated influenza A virus vaccines. Infect Immun. 1980;29:342–347. doi: 10.1128/iai.29.2.342-347.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy B R, Phelan M A, Nelson D L, Yarchoan R, Tierney E L, Alling D W, Chanock R M. Hemagglutinin-specific enzyme-linked immunosorbent assay for antibodies to influenza A and B viruses. J Clin Microbiol. 1981;13:554–560. doi: 10.1128/jcm.13.3.554-560.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy B R, Nelson D L, Wright P F, Tierney E L, Phelan M A, Chanock R M. Secretory and systemic immunological response in children infected with live attenuated influenza A virus vaccines. Infect Immun. 1982;36:1102–1108. doi: 10.1128/iai.36.3.1102-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimmelzwaan G F, Baars M, van Beek R, van Amerongen G, Lovrgen-Bengtsson K, Claas E C J, Osterhaus A D M E. Induction of protective immunity against influenza virus in a macaque model: comparison of conventional and iscom vaccines. J Gen Virol. 1997;78:757–765. doi: 10.1099/0022-1317-78-4-757. [DOI] [PubMed] [Google Scholar]
- 20.Rota P A, Black R A, De B K, Harmon M W, Kendal A P. Expression of influenza A and B virus nucleoprotein antigens in baculovirus. J Gen Virol. 1990;71:1545–1554. doi: 10.1099/0022-1317-71-7-1545. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 22.Turner R, Lathey J L, Van Voris L P, Belshe R B. Serological diagnosis of influenza B virus infection: comparison of an enzyme-linked immunosorbent assay and the haemagglutination inhibition test. J Clin Microbiol. 1982;15:824–829. doi: 10.1128/jcm.15.5.824-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Voris L P, Betts R F, Menegus M A, Murphy B R, Roth F K, Douglas R G., Jr Serological diagnosis of influenza A/USSR/77 H1N1 infection: value of ELISA compared to other antibody techniques. J Med Virol. 1985;16:315–320. doi: 10.1002/jmv.1890160403. [DOI] [PubMed] [Google Scholar]
- 24.Wilson M B, Nakane P K. Recent developments in the periodate method of conjugating horseradish peroxidase (HRPO) to antibodies. In: Knapp W, editor. Immunofluorescense and related staining techniques. Amsterdam, The Netherlands: Elsevier; 1978. pp. 215–224. [Google Scholar]