Abstract
The purpose of this is study was to review pearls and pitfalls of advanced imaging, such as computed tomography perfusion and diffusion-weighed imaging and perfusion-weighted imaging in the selection of acute ischemic stroke (AIS) patients suitable for endovascular treatment (EVT) in the late time window (6–24 h from symptom onset). Advanced imaging can quantify infarct core and ischemic penumbra using specific threshold values and provides optimal selection parameters, collectively called target mismatch. More precisely, target mismatch criteria consist of core volume and/or penumbra volume and mismatch ratio (the ratio between total hypoperfusion and core volumes) with precise cut-off values. The parameters of target mismatch are automatically calculated with dedicated software packages that allow a quick and standardized interpretation of advanced imaging. However, this approach has several limitations leading to a misclassification of core and penumbra volumes. In fact, automatic software platforms are affected by technical artifacts and are not interchangeable due to a remarkable vendor-dependent variability, resulting in different estimate of target mismatch parameters. In addition, advanced imaging is not completely accurate in detecting infarct core, that can be under- or overestimated. Finally, the selection of candidates for EVT remains currently suboptimal due to the high rates of futile reperfusion and overselection caused by the use of very stringent inclusion criteria. For these reasons, some investigators recently proposed to replace advanced with conventional imaging in the selection for EVT, after the demonstration that non-contrast CT ASPECTS and computed tomography angiography collateral evaluation are not inferior to advanced images in predicting outcome in AIS patients treated with EVT. However, other authors confirmed that CTP and PWI/DWI postprocessed images are superior to conventional imaging in establishing the eligibility of patients for EVT. Therefore, the routine application of automatic assessment of advanced imaging remains a matter of debate. Recent findings suggest that the combination of conventional and advanced imaging might improving our selection criteria.
Keywords: Stroke, Reperfusion therapies, Patient selection, Advanced imaging, Automatic software (maximum 6)
Highlights
-
•
Advanced imaging are currently considered the method of choice for the selection of patients with acute ischemic stroke suitable for endovascular treatment in the late time window.
-
•
Automated software packages are increasing being adopted for the calculation of the optimal selection parameters provided by advanced imaging which are collectively called target mismatch.
-
•
Automated software platform have several limitations since are not interchangeable e can under- and overestimated infarct core volume.
-
•
Selection remains suboptimal due to high rates of futile recanalization and restricted selection criteria resulting in a overselection.
-
•
The routine use of advanced imaging for the selection of patients treated with endovascular therapy is still controversial due to some evidence that conventional imaging are not inferior to advanced imaging in establishing patients who can benefit from endovascular treatment.
-
•
The combination of results coming from both conventional and advanced imaging could be the solution for improving our selection criteria.
1. Introduction
Intravenous thrombolysis (IVT) and endovascular treatment (EVT) are the currently available reperfusion therapies for patients with acute ischemic stroke (AIS) that represents one of the leading causes of long-term disability and mortality worldwide [1], [2]. It is well-known that the target of IVT and EVT is the rescue of ischemic penumbra, the reversibly damaged brain tissue at risk for infarction, located around the irreversibly injured infarct core [3]. More precisely, while infarct core is no longer viable, always evolving towards infarct because of cytotoxic edema resulting in cellular swelling and death, ischemic penumbra is a still viable tissue due to the activation of hemodynamic and metabolic compensatory mechanisms, such leptomeningeal collaterals opening, cerebral autoregulation and increase in cellular oxygen extraction fraction [3], [4], [5]. However, in absence of reperfusion, compensatory responses gradually exhaust, and penumbra progressively transforms into infarction [4], [6]. Therefore, ischemic penumbra is a potentially salvageable tissue if circulation is restored [1], [2], [3], [4], [5], typically surviving up to 24–48 h after symptom onset [7], [8]. Based on these observations, an appropriate delineation of infarct core and ischemic penumbra regions is crucial for identifying patients who can benefit from reperfusion therapies as patients with small core and large penumbra are probably more likely to achieve a good outcome [9]. Precise quantification of core and penumbra is difficult with conventional imaging such as non-contrast CT (NCCT) and Computed Tomography Angiography (CTA). NCCT recognizes early ischemic changes (areas of weak parenchymal hypoattenuation such as focal hypodensity, obscuration of lentiform nucleus, loss of gray-white matter differentiation, loss of insular ribbon) corresponding to the infarct core and estimates their extent using the semiquantitative Alberta Stroke Program Early CT Score (ASPECTS) methodology but does not detect the ischemic penumbra [10]. CTA identifies the occlusion site and provides a detailed evaluation of collateral supply using two different techniques, single-phase CTA (sCTA) and multi-phase CTA (mCTA) but does not provide information about infarct core and ischemic penumbra [11]. Conversely, a recent randomized controlled trials (RCTs) showed benefit from reperfusion therapies in the late time window using advanced imaging [9]. In particular, the measurement of core and penumbra volumes with computed tomography perfusion (CTP) or magnetic resonance imaging (MRI) techniques, such as diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI), were proven to be a powerful tool for establishing the eligibility of AIS patients for IVT between 4.5 and 9 h and for EVT between 6 and 24 h after onset [9], [12], [13]. Of note, the calculation of core and penumbra volumes in these extended time window RCTs was performed with dedicated automatic software programs using prespecified thresholds [3], [5], [9]. Therefore, the ability to automatically elaborate CTP or PWI and DWI imaging seems crucial step to overcome the lack of standardization that is one of the most important limitations of advanced imaging [14], [15]. Consequently, automated processing of advanced imaging is currently considered an essential requirement for the selection of AIS patients suitable for reperfusion therapies in the late time window [16], [17]. However, as the routine application of automatic assessment of advanced imaging for the selection of patients with AIS candidates for reperfusion therapies remains controversial, and a detailed analysis on advantages and disadvantages of this approach is warranted to understand its value in clinical practice.
2. Automated selection with target mismatch
2.1. The mismatch concept
It is largely accepted that MRI is able to distinguish irreversibly and reversibly damaged brain tissue within the ischemic area. Cytotoxic edema characterizing infarct core is indicated by DWI lesion range, PWI lesion size corresponds to the total critically hypoperfused ischemic area infarcted and penumbral tissue is the difference between these two volumes, the so-called PWI-DWI mismatch, indicates ischemic penumbra volume (Fig. 1) [2], [5], [16], [18], [19]. However, the use of specific thresholds is needed for a correct delineation of the limits of infarct core and ischemic penumbra. Currently, critically hypoperfused tissue is recognized by time to the peak of the residual function absolute values greater than 6 s (Tmax>6 s) on PWI [20], whereas Apparent Diffusion Coefficient absolute values lower than 620 × 10-6 mm2/seconds (ADC<620 × 10-6 mm2/s) represent infarct core [21]. On the other hand, for many years the difference between CTP abnormalities observed on mean transit time (MTT) and cerebral blood volume (CBV) maps was considered the best method for identifying salvageable brain tissues [22]. This model, named MTT-CBV mismatch, was based on the assumption that MTT defect size referred to total hypoperfused area and CBV defect extent described infarct core (in CBV maps ischemic penumbra is not visible due to normal or increased CBV levels resulting from the opening of collaterals) (Fig. 2) [23]. Relative MTT greater than 145 % of the contralateral normal side (rMTT>145 %) and CBV absolute values lower than 2 mL/100gr (CBV<2 mL/100gr) were found to be the optimal cut-off values for detecting critical hypoperfused and infarcted regions, respectively [24]. Several studies reported high accuracy of CBV and MTT maps for determining infarct core and ischemic penumbra sizes [24], [25], [26], [27], [28], [29], [30], [31], [32] and a strong association between CTP MTT-CBV mismatch and functional outcome [26], [30], [33], [34], [35], [36]. Nevertheless, other publications showed that the predictive value of CBV and MTT maps for the definition of infarcted and penumbral tissues was low [37], [38], [39], [40], [41], [42]. In addition, many investigators demonstrated that the borders of infarct core and total hypoperfusion were more precisely estimated by CTP focal disturbances observed on cerebral blood flow (CBF) and Tmax maps rather than on CBV and MTT maps, suggesting that Tmax lesion extent minus CBF lesion size indicated ischemic penumbra (Fig. 3) [43], [44], [45], [46], [47], [48]. This paradigm was termed Tmax-CBF mismatch in which the best cut-off values for delineating critical hypoperfused and infarcted areas were established to be Tmax values> 6 s and relative CBF threshold values less than 30 % of normally perfused tissue (rCBF<30 %), respectively [9], [12], [16], [17]. The superiority of Tmax-CBF paradigm compared to MTT-CBV model in identifying infarct core and ischemic penumbra explains why Tmax-CBF mismatch is now considered the method of choice and therefore was used for the selection of AIS patients candidates for reperfusion therapies in all MRI and CTP-guided RCTs recently published [12], [49], [50], [51].
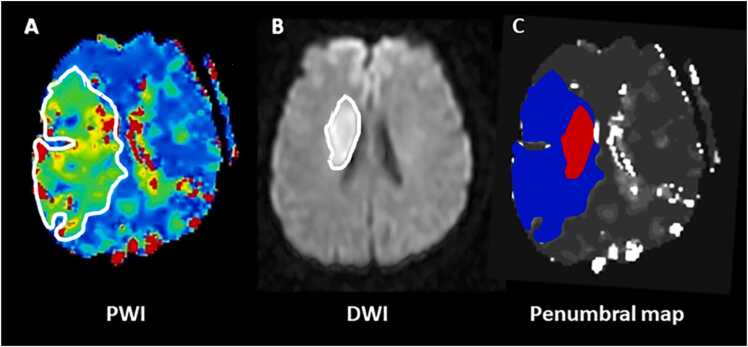
Fig. 1.
The perfusion-weighted imaging (PWI)-diffusion-weighted imaging (DWI) mismatch in a 57 year-old patient with acute ischemic stroke (AIS) and ischemic lesion in the right middle cerebral artery (MCA) territory. Panel A: Color coded PWI time to the peak of the residual function (Tmax) map indicates total critically hypoperfused tissue; Panel B: DWI shows infarct core; Panel C: penumbral map illustrates infarcted tissue (red) and ischemic penumbra (blue) obtained from the difference between PWI and DWI lesion sizes.
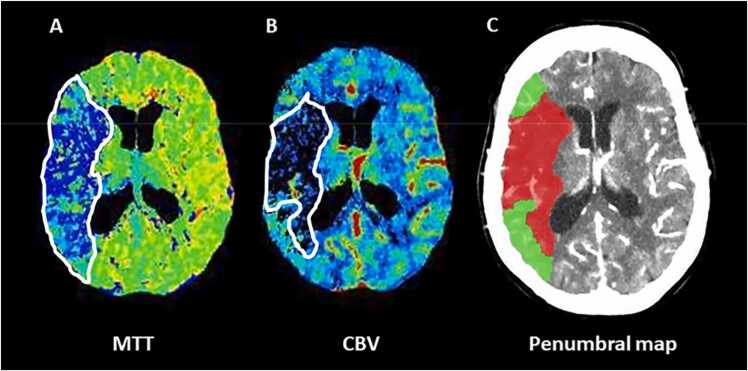
Fig. 2.
The computed tomography perfusion (CTP) mean transit time (MTT)-cerebral blood volume (CBV) mismatch in a 65 year-old patient with acute ischemic stroke (AIS) and ischemic lesion in the right middle cerebral artery (MCA) territory. Panel A: Color coded MTT map indicates total critically hypoperfused tissue; Panel B: Color coded CBV shows infarct core; Panel C: penumbral map illustrates infarcted tissue (red) and ischemic penumbra (green) obtained from the difference between MTT and CBV lesion sizes.
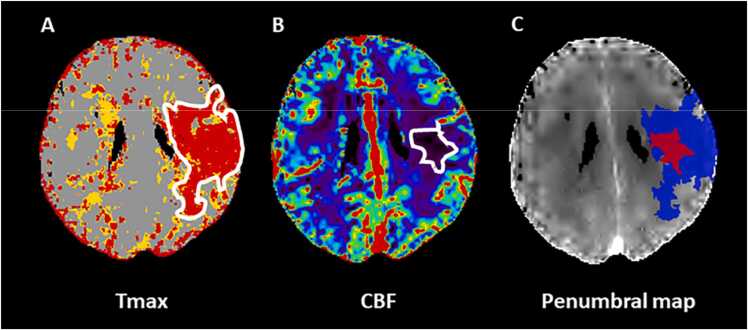
Fig. 3.
The computed tomography perfusion (CTP) time to the peak of the residual function (Tmax)-cerebral blood flow (CBF) mismatch in a 69 year-old patient with acute ischemic stroke (AIS) and ischemic lesion in the left middle cerebral artery (MCA) territory. Panel A: Color coded Tmax map indicates total critically hypoperfused tissue; Panel B: Color coded CBF shows infarct core; Panel C: penumbral map illustrates infarcted tissue (red) and ischemic penumbra (blue) obtained from the difference between Tmax and CBF lesion sizes.
2.2. Optimal parameters for the selection: the target mismatch
The identification of optimal MRI and CTP parameters to identifyAIS patients who could benefit from reperfusion therapies was considered as an essential condition for the selection of subjects suitable for IVT and EVT. Consequently, according to DEFUSE 2 (The Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2) [52], in MRI and CTP-based RCTs patients were selected for therapy if they satisfied a combination of criteria derived from PWI-DWI or Tmax-CBF mismatch that was called target mismatch [16], [49], [51]. Therefore, patients meeting target mismatch criteria were judged having a favorable MRI or CTP profile with a small core and a large penumbra (Fig. 5) [16], [48]. However, the parameters of target mismatch differed in RCTs based on advanced imaging (Table 1), such as EXTEND-IA (Extending the Time for Thrombolysis in Emergency Neurological Deficits-Intra-Arterial) [53] and SWIFT PRIME (Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment) [54] performed in the early time window for EVT within 6 h from symptom onset, DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) [55] and DAWN (Triage of Wake-up and Late Presenting Strokes Undergoing Neurointervention With Trevo) carried out in the late time window for EVT between 6 and 24 h after ictus [56] and EXTEND (Extending the Time for Thrombolysis in Emergency Neurological Deficits) realized in the late time window for IVT at 4.5–9 h after stroke [57]. More precisely, EXTEND-IA trial demonstrated the efficacy of combined EVT and IVT compared to IVT alone in patients selected within 4.5 h from symptom onset with a target mismatch including CTP parameters: infarct core volume< 70 mL, ischemic penumbra volume> 10 mL and mismatch ratio (the quotient between total hypoperfusion and core volumes)> 1.2. In SWIFT PRIME trial patients undergoing combined EVT and IVT within 6 h after stroke achieved more frequently a good outcome than controls treated with IVT alone after selection with a target mismatch characterized by MRI or CTP criteria: infarct core volume≤ 50 mL, ischemic penumbra volume≥ 15 mL and mismatch ratio> 1.8. DEFUSE 3 trial showed the association with favorable outcome in patients receiving EVT at 6–16 h after onset compared to controls treated with standard care, using as selection criteria a target mismatch consisting of MRI or CTP measures: infarct core volume< 70 mL, ischemic penumbra volume> 15 mL and mismatch ratio> 1.8. In DAWN trial patients treated with EVT at 6–24 h after stroke had better outcome compared to controls treated with standard care after selection with a target mismatch based on the mismatch between stroke severity and infarct core volume adjusted by age according to the following criteria: ≥ 80 years, National Institute of Health Stroke Scale (NIHSS)≥ 10, core volume< 21 mL; < 80 years, NIHSS≥ 10, core volume< 31 mL; < 80 years, NIHSS≥ 20; core volume< 31–51 mL. EXTEND proved the effectiveness of IVT compared to placebo in patients selected at 4.5–9 h from onset with a target mismatch including MRI or CTP parameters: infarct core volume< 70 mL, ischemic penumbra volume> 10 mL and mismatch ratio> 1.2. Among these RCTs guided by advanced imaging, only DEFUSE 3 and DAWN criteria were included in the American Heart Association/American Stroke Association (AHA/ASA) guidelines [58], [59]. EXTEND-IA and SWIFT PRIME were excluded because other concomitant RCTs demonstrated the benefit of EVT in the early time window (0–6 h) using conventional imaging. Although confirmed by a following meta-analysis [60], EXTEND criteria for IVT in extended time window (4.5–9 h) were not recommended probably because AHA/ASA guidelines were prepared before the publication of this trial. Thus, the use of advanced imaging is currently advocated only in the late time window (6–24 h) for EVT according to the target mismatch of DEFUSE 3 and DAWN. Of note, a recent study reported that DEFUSE 3 criteria were more inclusive than DAWN criteria as patients with pretreatment core infarct volume< 70 mL satisfying DEFUSE 3 criteria, but too large for inclusion according to DAWN criteria, demonstrated benefit from EVT [61]. In addition, another publication showed that DEFUSE 3 and DAWN criteria were equivalent for predicting outcome in patients receiving EVT at 6–24 h [62]. These findings suggest that the selection for EVT in the late time window could be based on DEFUSE 3 criteria only.
Fig. 5.
Two illustrative examples of computed tomography perfusion favorable and unfavorable profiles obtained with automatic calculation of target mismatch (Olea Sphere). Panel A: a 68 year-old patient with acute ischemic stroke (AIS) having an ischemic lesion in the right middle cerebral artery (MCA) territory. The patient was admitted at 10 h from onset with National Institute of Health Stroke Scale of 20, 37.95 mL of core volume, 192.68 mL of penumbra volume and 5.1 of mismatch ratio. Therefore, this patient satisfied both DEFUSE 3 and DAWN criteria representing a typical DEFUSE 3/DAWN patient. Panel B: a 75 year-old patient with acute ischemic stroke (AIS) having an ischemic lesion in the right middle cerebral artery (MCA) territory. The patient was admitted at 15 h after onset with National Institute of Health Stroke Scale of 22, 82.30 mL of core volume, 9.81 mL of penumbra volume and 1.12 of mismatch ratio. Therefore, this patient did not satisfy both DEFUSE 3 and DAWN criteria representing a typical non-DEFUSE 3/non-DAWN patient.
Table 1.
Different parameters of target mismatch in the various RCTs based on advanced imaging.
| RCT | Year of publication | Time window | Treatment | Target mismatch |
|---|---|---|---|---|
| EXTEND-IA | 2015 | ≤ 4.5 h | EVT+ IVT vs IVT | core volume< 70 mL ischemic penumbra volume> 10 mL mismatch ratio > 1.2 |
| SWIFT PRIME | 2015 | ≤ 6 h | EVT+ IVT vs IVT | core volume≤ 50 mL ischemic penumbra volume≥ 15 mL mismatch ratio > 1.8 |
| DEFUSE 3 | 2018 | 6–16 h | EVT vs BMT | core volume< 70 mL ischemic penumbra volume> 15 mL mismatch ratio > 1.8 |
| DAWN | 2018 | 6–24 h | EVT vs BMT | ≥ 80 years, NIHSS≥ 10, core volume< 21 mL < 80 years, NIHSS≥ 10, core volume< 31 mL < 80 years, NIHSS≥ 20, core volume< 31–51 mL |
| EXTEND | 2019 | 4.5–9 h | IVT vs placebo | core volume< 70 mL ischemic penumbra volume> 10 mL mismatch ratio > 1.2 |
RTC = randomized controlled trial; EXTEND-IA = Extending the Time for Thrombolysis in Emergency Neurological Deficits-Intra-Arterial; SWIFT PRIME = Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment; DEFUSE 3 = Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke; DAWN = Triage of Wake-up and Late Presenting Strokes Undergoing Neurointervention With Trevo; EXTEND = Extending the Time for Thrombolysis in Emergency Neurological Deficits; EVT = endovascular treatment: IVT = intravenous thrombolysis; BMT = best medical therapy; mismatch ratio = the quotient between total hypoperfusion and core volumes; NIHSS = National Institute of Health Stroke Scale
2.3. Automated processing of target mismatch
Although the optimal parameters of target mismatch varied across the different RCTs [53], [54], [55], [56], [57], they were always automatically calculated with the same dedicated software (RAPID; Rapid Processing of Perfusion and Diffusion; iSchemaView, Menlo Park, CA) that is at present considered a powerful tool to reduce imaging processing time and inter-operator variability, favoring reproducibility and rapid interpretation [63]. However, other available software platforms can provide the same performances represented by the automatic calculation of infarct core, total hypoperfusion and ischemic penumbra volume, as well as mismatch ratio. Some of these software packages use the same CTP thresholds for core (rCBF<30%) and hypoperfused tissue (Tmax>6 s) utilized by RAPID, such as Syngo.via CT Neuro Perfusion (Siemens Healthineers, Erlangen, Germany) [64] and Brainomix e-CTP (Brainomix Ltd., Oxford, UK) [65]. In contrast, other software programs identify irreversible and critically damaged tissue with different CTP cut-off values compared to RAPID, such as MIStar (Apollo Medical Imaging Technology, Melbourne, Australia) [66] and Olea Sphere (Olea Medical Solutions, La Ciotat, France) [67]. In particular, MIStar determines total hypoperfusion extent with delay time (a map similar to Tmax) values more than 3 s (delay time>3 s), whereas Olea Sphere defines infarct core size with rCBF values< 40 % (Fig. 4). Interestingly, Vitrea (Vital Images, Minnetonka, Minnesota) and Olea recently developed an algorithm alternative to deconvolution for generating CTP maps based on probabilistic Bayesian approach, obtaining different threshold values for core and penumbra compared to RAPID [68], [69]. Vitrea Bayesian algorithm indicates total hypoperfusion with delay time values more than 5.7 s (delay time>5.7 s) and infarct core with rCBF values< 38 %. Olea Bayesian algorithm defines total hypoperfusion with difference in time to peak compared with healthy brain more than 5 s (dTTP>5 s) and infarct core with rCBF values< 25 %. The different algorithms and thresholds used by the various automatic software packages for recognizing core and penumbra regions are summarized in Table 2.
Fig. 4.
Automatic calculation of target mismatch obtained with Olea Sphere from computed tomography perfusion in a 72 year-old patient with acute ischemic stroke (AIS) having an ischemic lesion in the left middle cerebral artery (MCA) territory. The patient was admitted at 13 h from onset with National Institute of Health Stroke Scale of 8, 6.57 mL of core volume, 94,75 mL of penumbra volume and 15.4 of mismatch ratio. Therefore, this patient satisfied both DEFUSE 3 and DAWN criteria. Panel A: Color coded time to the peak of the residual function (Tmax) maps indicate total critically hypoperfused tissue; Panel B: Color coded CBF maps show infarct core; Panel C: penumbral maps illustrate infarcted tissue (red) and ischemic penumbra (blue) resulting from the difference between Tmax and relative CBF lesion sizes.
Table 2.
Different algorithms threshold values for automatically identifying infarct core and ischemic penumbra sizes.
| Software | Algorithm | Thresholds for total hypoperfusion | Thresholds for infarct core |
|---|---|---|---|
| RAPID (iSchemaView) | Deconvolution | Tmax > 6 s | rCBF< 30% |
| SYNGO.VIA (Siemens Healthineers) | Deconvolution | Tmax > 6 s | rCBF< 30% |
| BRAINOMIX e-CTP (Brainomix) | Deconvolution | Tmax > 6 s | rCBF< 30% |
| MISTAR (Apollo Medical Imaging Technology) | Deconvolution | delay time > 3 s | rCBF< 30% |
| OLEA SPHERE (Olea Medical Solutions) | Deconvolution | Tmax > 6 s | rCBF< 40% |
| OLEA SPHERE (Olea Medical Solutions) | Bayesian | dTTP > 5 s | rCBF< 25% |
| VITREA (Vital Images) | Bayesian | delay time > 5.7 s | rCBF< 38% |
Tmax = time to the peak of the residual function: rCBF = relative CBF of ischemic area compared to contralateral normal side; dTTP = difference in time to peak of ischemic area compared with healthy brain
3. Automated selection in the real world
3.1. The selection remains suboptimal
The introduction of PWI-DWI mismatch on MRI and CTP Tmax-rCBF with automatic assessment of target mismatch parameters significantly improved the selection of AIS patients suitable for reperfusion therapies in the late time window for EVT and IVT [70]. Nevertheless, patient selection remains a challenge as around half of late-window patients do not achieve favorable outcome despite complete recanalization [71]. Furthermore, another important shortcoming affecting patient selection is the potential exclusion of some patients who could benefit from treatment due to the current restricted criteria [72]. This overselection was well described in two recent studies suggesting that the use of more permissive inclusion criteria could increase the number of patients treated with EVT in the late time window [73], [74]. Lopez-Rivera and coworkers reported that the number of treatments with EVT in AIS patients with large vessel occlusion (LVO) admitted in both early and late time window was higher in centers with low advanced imaging utilization compared to those in which CTP was routinely used, without a significant increase in the incidence of poor outcome and hemorrhagic transformation [73]. More recently, Dittrich and colleagues showed that patients undergoing EVT at 6–24 h from onset and not satisfying DEFUSE 3 and DAWN inclusion criteria had an elevated probability to achieve a favorable outcome, without a higher frequency of symptomatic hemorrhagic transformation, compared to controls treated with best medical therapy [74]. In the attempt to further extend the patient population receiving EVT in the late time window, the opportunity of treating patients with large core were extensively explored [75]. HERMES (Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials) meta-analysis [76] and preliminary studies [77], [78] suggested the potential benefit of EVT in AIS patients with large core admitted in early and late time windows. Rates of symptomatic intracranial hemorrhage were increased compared to control group in HERMES analysis, but similar to controls in the other two studies. Large core was classified as a low Alberta Stroke Program Early CT Score (ASPECTS) of 0–4 by HERMES collaborators, a core volume> 100 mL assessed with CTP Tmax threshold values> 10 s by Nogueira et al. and a core volume> 50 mL evaluated with CTP rCBF cut-off values< 30 % by Rebello and associates. The effectiveness of EVT in patient with large core selected in early and late time windows was confirmed in subsequent publications using different definition of large core. Large core was considered as a core volume≥ 50 mL (rCBF<30%) and/or a low ASPECTS< 5 in a secondary analysis of SELECT (Selection for Endovascular Treatment in Acute Ischemic Stroke) study [79], a core volume> 50 mL (rCBF<30 % or ADC<620 × 10–6mm2/s) by Seners and coworkers [80], a low ASPECTS≤ 5 with a core volume> 70 mL (rCBF<30%) by Bouslama et al. [81] and a core volume≥ 70 mL (rCBF<30 %) by Garcia-Esperon et al. [82]. Interestingly, the last two investigations found that the association between EVT and good outcome was present only in patients with ASPECTS≤ 5 and a core volume≤ 70 mL [81] and those with a core volume 70–100 mL [82]. In addition, the benefit of EVT over controls, without an increased risk of intracerebral hemorrhage, was further highlighted by three meta-analyses in which large core was categorized as ASPECTS< 5 [83] and ASPECTS≤ 6 with a core volume≥ 50 mL [84], [85]. Only one study was not consistent with these findings showing no association with a favorable outcome and an increased frequency of symptomatic hemorrhagic transformation in patients treated with EVT in the early time window with large core represented by ASPECTS < 5 [86]. More recently, three RCTs definitively demonstrated the usefulness of EVT in the late time window for AIS patients with large core who achieved higher odds of favorable outcome than controls treated with standard medical therapy and/or IVT [87], [88], [89]. Again, the definition of large core differed across these trials since SELECT-2 used a low ASPECTS 3–5 with a core volume≥ 50 mL (rCBF<30 % or ADC<620 × 10–6mm2/s) [87], RESCUE-Japan LIMIT a low ASPECTS 3–5 [88] and ANGEL-ASPECT a low ASPECTS 3–5 with a core volume 70–100 mL (rCBF<30 % or ADC<620 × 10–6mm2/s) [89]. However, EVT for patients with extensive irreversible ischemic tissue damage remains challenging in clinical practice because of the lack of standardized methods for the definition of large core [75], [90], [91].
3.2. Accuracy of automatic software platforms
The increasing use of automatic software packages in the selection of patients suitable for EVT in the late time window focused the attention on the accuracy of these programs in correctly quantifying infarct core and ischemic penumbra volumes [92]. These investigations revealed that automated software programs had several limitations, resulting in postprocessing failure characterized by misclassification of core and penumbra volumes that occurred in about 11 % of cases [93]. First of all, automated advanced imaging outputs was disturbed by technical drawbacks, such as patient motion, poor contrast bolus due to delayed arrival or insufficient administration and too short acquisition leading to a truncation of perfusion curves [92], [94], [95]. On the other hand, the quality of postprocessed images was also reduced by imaging artifacts, such as the appearance of artifactual hypoperfused areas at the level of the skull base, posterior fossa and orbits, inducing errors in the calculation of perfusion abnormalities with overestimation of penumbra volume [94], [95]. Although these artifactual perfusion lesions were easily identified by the operators [96], their removal implicates a manual segmentation that is not available in the currently used software platforms [94]. Nevertheless, despite these limitations and the demonstration that visual assessment of perfusion maps could be not inferior to automated estimation in defining infarct core and ischemic penumbra extent [97], [98], [99], the quantitative analysis offered by automatic softwares remained more reliable than a qualitative approach [96], [100], [101]. Another important flaw affecting the automated processing of penumbral maps was the demonstration that the different software packages were not interchangeable. In fact, when the performance of the various other software solutions was compared with RAPID, significant variability across vendors was found [94], [95]. In particular, Syngo.via estimated larger or smaller infarct core, ischemic penumbra and final infarct volumes compared to RAPID [102], [103], [104], [105], although when the core thresholds were changed from rCBF< 30% to rCBF< 20% or smoothing was applied, Syngo.via results were more consistent with RAPID [106], [107], [108], [109]. A good correlation was reported between RAPID and Brainomix for determination of core and penumbra volumes with Brainomix that, however, overestimated final infarct volume [65], [104]. MIStar had a strong association with RAPID for estimation of core, penumbra and final infarct volumes, but with a trend towards the overestimation of core [103], [105], [110], [111]. Olea was less precise than RAPID in predicting core, penumbra and final infarct volumes due to its propensity to overestimate them [105], [112], [113]. In this context, the RAPID’s accuracy for identifying core, penumbra and final infarct volumes was often moderate. Indeed, RAPID underestimated infarct core compared to the Bayesian algorithm used by Vitrea and Olea [68], [114], whereas Vitrea was superior to RAPID and Olea in estimating penumbra [68], [114] but inferior to Olea in predicting core volume [114]. Nevertheless, the main limitation of automatic software packages is probably the inaccuracy in detecting infarct core volume because of the lack of consensus on the optimal cut-off values [95], [115]. The most pronounced effect of this weakness was the inability to obtain a reliable measurement of ischemic penumbra [92], [115]. An additional reason that could explain the difficulty of automated postprocessed imaging in delineating infarct core was the evidence that DWI lesions can be reversible and CTP thresholds for infarct core were time-dependent [9], [115]. Reversal of DWI lesions consisted in the disappearance on follow-up images of DWI ischemic abnormalities visible at onset [116], [117], [118], [119], [120]. DWI reversibility was reported in a variable proportion ranging from 11 % to 50 % of cases in the early time window and ascribed to early recanalization. As demonstrated in prior Positron Emission Tomography studies [121], [122], [123], these findings suggest that DWI lesions not only reflect the cytotoxic edema of the tissue irreversibly destined to infarction, but also include a portion of ischemic penumbra [116]. Concerning CTP, it was clearly demonstrated that the optimal values for defining infarct core changed over time because the amount of tissue that infarcts increased with the increasing of CT-to-recanalization time [124], [125], onset-to-imaging time [126] and onset-to-recanalization time [127], [128], [129]. Therefore, the time-dependence of CTP cut-off values for delineating infarct tissue implies that the identification of rigid CTP core thresholds can currently be considered unlikely [115] and might lead to underestimation or overestimation of infarct core size [94], [95]. The suboptimal performance of automated computer-aided detection of infarct core volume accounts for two important conditions that can be experienced in clinical practice: a CTP core underestimation referred as perfusion scotoma [130] and a CTP core overestimation termed ghost infarct [131]. The perfusion scotoma represents a mismatch between the presence of a visible hypodensity on NCCT and a smaller core volume on CTP at onset [130], [132], [133]. This situation occurred in about 25 %− 36 % of cases in the early time window (<6 h from onset) and in about 20–25 % of patients in the late time window (>6 h after onset) and was attributed to a spontaneous reperfusion of infarcted tissue due to a good collateral status [130], [133]. In this case, a possible solution could be the analysis of collaterals extent: the presence of a large core on NCCT, small core on CTP and good collaterals are highly suggestive of perfusion scotoma (Fig. 6). Conversely, the ghost infarct referred to the presence of a large infarct on pretreatment CTP with a smaller or absent final infarct volume on follow-up imaging [131], [134], [135], [136], [137]. The incidence of this phenomenon were higher in the early time window, where it ranged from 15 % (<6 h after onset) to 20 % (<4.5 h from onset) of subjects [131], [135], [136], [137], but can also occur in the late time window (>6 h after stroke) [134]. However, a pooled analysis including patients from HERMES and EXTEND-IA TNK (Tenecteplase Versus Alteplase Before Endovascular Therapy for Ischemic Stroke) datasets reported that an overestimation of infarct core by CTP was present only in 5 % of cases [138]. Poor collaterals were the major factor associated with ghost infarct (Fig. 7) [131], [137], [139], but large core volume at onset and short CT-to-recanalization time and onset-to-imaging time can favor the occurrence of ghost infarct [140]. Recent studies proposed a solution to overcome this issue, represented by the use of a more conservative rCBF threshold (<20 % instead of 30 %) that substantially reduced the number of patients with ghost infarct [141] and was mainly suitable to patients receiving EVT, but not to those treated with IVT [127]. Overall, the incorrect identification of core and penumbra sizes due to software limitations and vendor-dependent variability could justify the results emerged from a previous HERMES meta-analysis [141] and a recent study evaluating the accuracy of automated software programs in stroke diagnosis [142]. HERMES collaborators reported that patients with small infarct core volume achieved more frequently a good clinical outcome, without any significant association between infarct tissue size and the beneficial effect of EVT over controls [141]. Wardlaw and coworkers showed that the proportion of patients correctly diagnosed by automated software packages varied from 44 % to 83 % in case of ischemic patients and from 7 % to 43 % for subjects without ischemia (stroke mimics) [142].
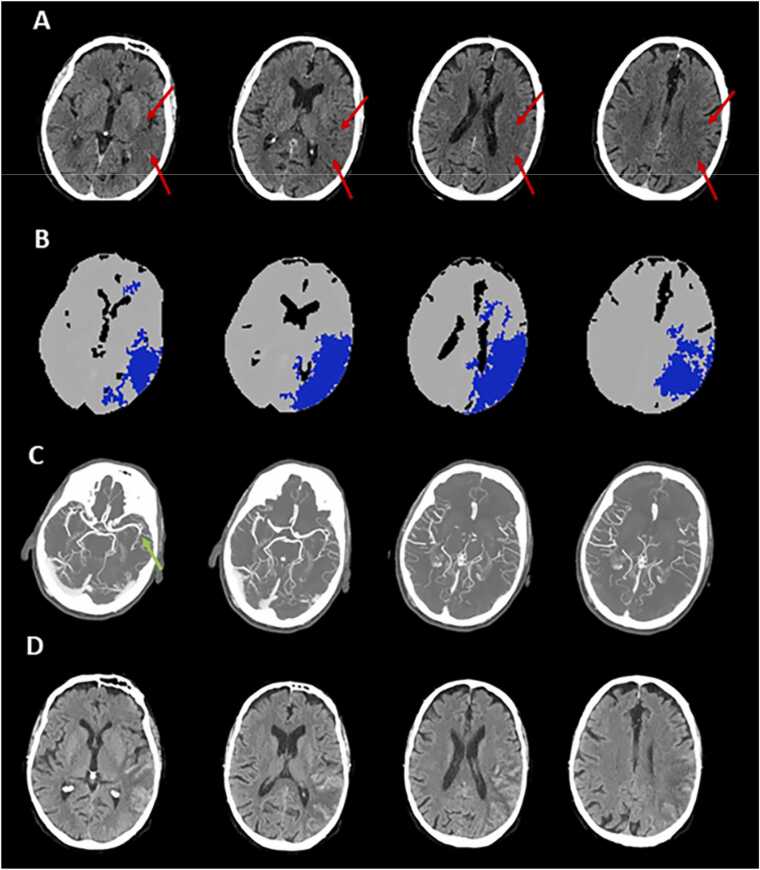
Fig. 6.
An illustrative case of computed tomography perfusion (CTP) scotoma obtained with automatic calculation of target mismatch (Olea Sphere). A 59 year-old patient with acute ischemic stroke (AIS) having an ischemic lesion in the left middle cerebral artery (MCA) territory due to the occlusion of M2 segment admitted at 6 h after onset. A visible hypodensity on non-contrast computed tomography (NCCT) in ASPECTS regions I, M2, M3 M5 and M6 resulting in ASPECTS = 5 (Panel A), without detection of infarct core on CTP (Panel B), and with good collaterals on multiphase computed tomography angiography (Panel C). Follow-up NCCT performed at 24 h indicates a final infarct volume overlapping the hypoattenuated NCCT lesion observed at admission with hemorrhagic transformation (Panel D).
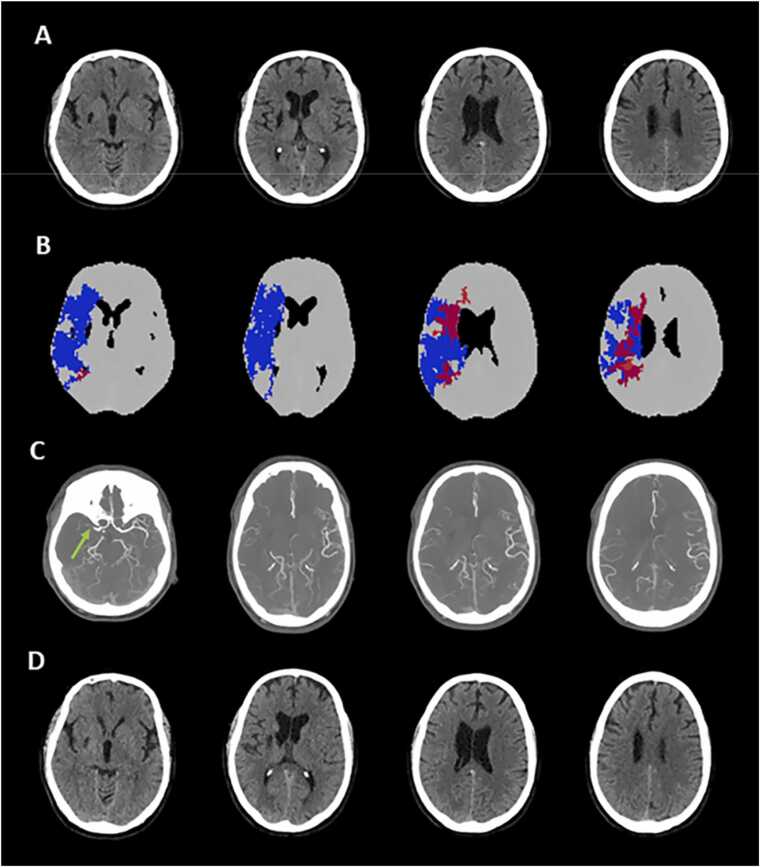
Fig. 7.
An illustrative case of computed tomography perfusion (CTP) ghost infarct obtained with automatic calculation of target mismatch (Olea Sphere). A 63 year-old patient with acute ischemic stroke (AIS) having an ischemic lesion in the right middle cerebral artery (MCA) territory due to the occlusion of M1 segment admitted at 1.5 h after onset. The absence of hypodense lesion on non-contrast computed tomography (NCCT) with ASPECTS = 10 (Panel A), in presence of infarct core on CTP (Panel B), and with poor collaterals on multiphase computed tomography angiography (Panel C). Follow-up NCCT performed at 24 h did not indicate any final infarct volume (Panel D).
4. Imaging modalities for the selecion: Conventional versus advanced
4.1. Evidence that conventional imaging is enough for patients’ selection
The potential suboptimal accuracy of advanced imaging prompted some investigators to explore the ability of conventional imaging to identify AIS patients with LVO who can benefit from EVT in the late time window. Bouslama et al. reported an identical association with clinical outcome for AIS patients treated with EVT after selection with NCCT ASPECTS ≥ 6 or advanced imaging according to DEFUSE 3 and DAWN criteria [143]. In addition, a publication from Endovascular treatment for Acute ischemic stroke in the Netherlands (MR CLEAN) registry found that the selection with favorable NCCT ASPECT profile combined to good collateral on sCTA did not result in a high proportion of poor outcome [144]. These findings were extended in a multicenter study showing no differences in functional outcome, incidence of hemorrhagic transformation and mortality between patients selected with NCCT ASPECTS ≥ 5–7 and advanced imaging following DEFUSE 3 and DAWN criteria [145]. These results were confirmed by Porto and colleagues in a study from the Stroke Thrombectomy and Aneurysm Registry (STAR) database [146]. The equivalence between NCCT ASPECTS and CTP criteria for patient’s selection was also confirmed in the early time window [147], [148], [149]. Next, Bouslama and colleagues demonstrated that ischemic core volume automatically calculated with Brainomix software and CTP DEFUSE 3 target mismatch measured with RAPID were equivalent in predicting final infarct volume [150]. However, an editorial associated to this publication pointed out that automated evaluation of infarcted tissue with NCCT and CTP overestimated and underestimated final infarct volume, respectively, suggesting that the correct delineation of infarct core with imaging modalities remains a matter of debate [151]. On the other hand, the Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE) trial demonstrated the utility of collateral assessment with mCTA in the selection of AIS patients undergoing EVT within 12 h from onset as patients with good collaterals achieved more frequently a favorable outcome than controls treated with standard medical therapy [152]. Therefore, some authors tested the potential of mCTA as selection tool for patients treated with EVT in comparison to advanced imaging in the late time window. Kim et al. were the first to report that the incidence of favorable outcome was similar in patients who were considered eligible for EVT according to these criteria (good collaterals) or CTP DEFUSE 3 and DAWN target mismatch [153]. A similar effect on functional outcome was obtained in other three studies comparing patient selection with mCTA evaluation of collateral filling and CTP DAWN and/or DEFUSE 3 criteria [154], [155]. Furthermore, combined NCCT ASPECTS and collateral-guided selection was comparable to CTP-guided selection in determining final infarct volume and clinical outcome in a pooled analysis of data coming from DAWN, DEFUSE-3 and ESCAPE-NA1 trials [156]. The predictive value for final infarct volume of mCTA collateral assessment and CTP-aided parameters did not differ in another publication as well [157]. Next, a pooled analysis from Selection Of Late-window Stroke for Thrombectomy by Imaging Collateral Extent (SOLSTICE) Consortium established that the selection with sCTA or mCTA collateral score and with combined collateral and perfusion imaging led to similar functional outcome [158]. More important, the role of collateral grading in determining the eligibility of candidates for EVT in the late time window (6–24 h after onset) was more recently validated in MR CLEAN-LATE trial in which patients were selected for EVT if having visible collaterals on sCTA or mCTA and clinical outcome was better in subjects receiving EVT than in controls not treated with EVT [159]. Collectively, these findings were consistent with previous studies proving that predictive value of advanced imaging for final infarct volume, as well as penumbral salvage and infarct growth were collateral dependent. In fact, infarct core was smaller than predicted in presence of good collaterals and larger than predicted when collaterals were poor [160] and penumbral rescue and infarct expansion were associated with collateral extend and not with time [161], [162]. In addition, there is cumulative evidence indicating that conventional imaging is not inferior to advanced imaging for identifying AIS patients suitable for EVT in the extended time window, suggesting that conventional imaging could replace advanced imaging for determining patient eligibility. This was the reason why some researchers recently proposed to adopt more simplified imaging selection criteria for these patients based on NCCT and CTA and not on MRI or CTP [163], [164].
4.2. Evidence that advanced imaging is necessary for patients’ selection
In contrast with the above-mentioned publications, other studies showed that advanced imaging remained superior to conventional imaging in the selection of AIS patients receiving EVT. Albers analyzed the recent RCTs and reported that the treatment effect was larger in the late than in early time windows, the so-called late window paradox, due to the use of advanced imaging with automatically calculated target mismatch in the extended time window [165]. Moreover, the treatment effect was greater also in the early time window when advanced imaging was used for the selection [165]. Consistent with this view, CT Perfusion to Predict Response to Recanalization in Ischemic Stroke Project (CRISP) investigators [166] and an analysis of ESCAPE trial dataset [167] showed that a successful recanalization was associated with a good functional outcome in patients treated with EVT having a favorable CTP profile according to DEFUSE 3 criteria in both early and late time window. In addition, automatically calculated CTP ischemic core volume predicted clinical outcome better than NCCT ASPECTS within 18 h from onset [168], whereas a large penumbra extent assessed according to EXTEND criteria was a strong predictor of favorable outcome in the early time window [169]. Next, while infarct core volume and total hypoperfusion volumes, as measured by DEFUSE 3 criteria, predicted final infarct volume in the delayed time window with good accuracy [170], only a moderate correlation between NCCT ASPECTS and automatically estimated CTP infarct core volume was demonstrated at 6–24 after stroke [171]. A meta-analysis confirmed that the use of advanced imaging for EVT selection was associated with an improved functional outcome compared to conventional imaging in both early and late time window [172]. Furthermore, an association between pretreatment CTP EXTEND-IA, SWIFT PRIME, DEFUSE 3 and DAWN inclusion criteria and clinical outcome was a more recently found in the early time window (<6 h after onset), independent of time of onset [173]. Of note, a following study revealed that part of the hypondensity observed on NCCT did not represent infarct core, but ischemic penumbra, making it difficult to recognize irreversibly damaged tissue with this technique [174]. On the other hand, CTP-based selection criteria predicted clinical outcome better than mCTA classification of collaterals [175]. Finally, as reported in two studies, functional outcome of patients treated with EVT in both early and delayed time windows was not dependent from onset time, but rather correlated with salvageable penumbra volume that only advanced imaging can quantify [176], [177]. Therefore, as some authors assumed that time of onset was not a sufficient reason to exclude patients from EVT, a change in the selection paradigm was proposed suggesting a switch from time-based to tissue or imaging-based models for defining patients who can be eligible for EVT [178], [179], [180].
4.3. An interpretation of current evidence
The conflicting results obtained by the above discussed studies might be explained by an accurate analysis of some pivotal publications. SELECT group conducted a study on patients successfully treated with EVT both in the early (0–6 h from onset) and extended (6–24 h after onset) windows [181]. A good outcome was achieved in 58 % of subjects with favorable NCCT ASPECTS (ASPECTS≥6) and favorable CTP (core volume<70 mL, ischemic penumbra volume>10 mL and mismatch ratio>1.2) profiles, in 46.4% of cases with unfavorable NCCT ASPECTS (ASPECTS<6) and favorable CTP patterns, 23.5 % of cases with favorable NCCT ASPECTS and unfavorable CTP patterns in and in 0 % of patients when both NCCT ASPECTS and CTP profiles were unfavorable. Ospel and associates observed a population of AIS patients receiving EVT within 12 h of symptom onset on behalf of The Precise and Rapid Assessment of Collaterals Multi-phase CTA in the Triage of Patients With Acute Ischemic Stroke for IA Therapy (Prove-IT) investigators [182]. Patients eligible for endovascular treatment by combined CTP (core volume<70 mL, ischemic penumbra volume>15 mL and mismatch ratio>1.8) and mCTA (good collaterals) criteria had favorable outcome in 62–87 % of cases, depending on CTP thresholds used for core definition (rCBF<30 %, CBF<7 mL/100 g/min or CBV<2 mL/100 g). mCTA eligibility criteria selected more patients (91 %) than CTP eligibility criteria (7–36 %), but with lower good outcome rates (53–57 %). A good outcome was found in 51–62 % patients who were not eligible by either mCTA or CTP criteria. Dhillon and coworkers reported an association between EVT and functional outcome in a large population of patients admitted in the late time window and selected only with NCCT ASPECTS and without advanced imaging [183]. However, the same group also showed that, always in a large cohort of AIS patients, the use of perfusion images improved functional independence compared to conventional images in both early and delayed time windows, although CTP definition of core and penumbra was obtained with variable methods including automated evaluation and visual assessment [184]. This last observation was in line with some recent studies showing that the efficacy of EVT in the late time window was similar to RCTs when patients were selected with visual inspection of classical CTP MTT-CBV mismatch using an infarct core size < 50 % of total hypoperfusion extent, approximately corresponding toto a mismatch ratio> 1.8, as alternative method for automatic calculation of core and penumbra [185], [186]. Finally, in a more recent meta-analysis CTP-aided selection did not improve clinical outcome in patients undergoing EVT compared to NCCT selection in the extended time window, but it was associated with lower mortality [187]. Taken together, these findings suggest that the selection of patients suitable for EVT based on conventional imaging results in an inclusion of a larger population of AIS patients, but with lower rates of good outcome compared to selection guided by advanced imaging. In addition, these results indicate that higher rates of good outcome are achieved when conventional and advanced images are used in combination and confirm that our selection criteria are currently suboptimal.
5. Current views and future directions
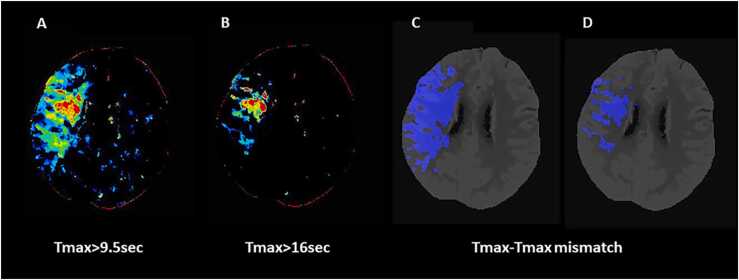
The growing and controversial body of evidence accumulating in these last years on the selection of AIS patients for reperfusion therapies suggests that the eligibility criteria based on advanced imaging have limitations, but they can be currently still considered as the method of choice for identifying patients which can benefit from EVT. However, the selection can also be performed with conventional imaging alone obtaining the inclusion of a high number of patients but with an expected decreased incidence of favorable outcome. Another suggestion emerged from our analysis is that advanced imaging could be used to determine the eligibility of patients for EVT in the early time window. Anyway, the main take home message from the examined studies seems that a selection paradigm based on the integration between conventional and advanced images might lead to higher rates of good outcome. Nevertheless, selection criteria have to be improved as the number of futile recanalization remains too high. Following this direction, a recent study [188] explored the impact on outcomes of the previously published Tmax-Tmax mismatch [124] in patients admitted within 24 h and receiving EVT. In this model, in which core and penumbra were identified using only one CTP map, critically hypoperfused tissue was indicated by Tmax with threshold value > 9.5 s, irreversibly injured tissue was delineated by Tmax with threshold value > 16 s and ischemic penumbra was defined by Tmax value > 9.5 s minus Tmax > 16 lesions (Fig. 8). All the volumes of these Tmax lesions were automatically calculated and strongly correlated with final infarct volume and functional independence at 3 months. In addition, a total hyperfusion volume ≤ 111.6 mL, a core volume ≤ 67.0 mL, a penumbra volume ≤ 58.3 mL and mismatch ratio > 2.5 were recognized as the optimal parameters predicting good outcome and then represented the Tmax target mismatch. However, these new criteria need to be validated in future studies demonstrating the non-inferiority or the superiority of Tmax target mismatch to DEFUSE 3 and DAWN criteria in the selection of patients suitable for EVT. Interestingly, two recent publications proposed a dedicated software that was able to automatically generate tissue maps directly from mCTA acquisition [189], [190]. These mCTA-derived delay maps were very similar to Tmax maps and could be useful to reduce the radiation dose and acquisition time of CT protocol. On the other hand, the development of this technology could allow a more rapid and combined analysis of mCTA and CTP data. Therefore, further studies are justified to understand whether this novel approach based on Tmax maps with an integration between mCTA and Tmax findings can contribute to improve our ability to recognize AIS patients who can benefit from EVT. In this case, this novel paradigm could be incorporated in the CT workflow adopted in clinical practice.
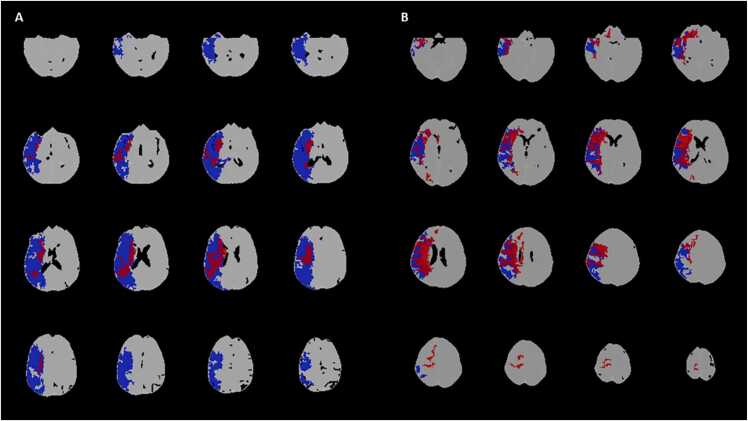
Fig. 8.
The computed tomography perfusion (CTP) Tmax-Tmax mismatch in a 58 year-old patient with acute ischemic stroke (AIS) and ischemic lesion in the right middle cerebral artery (MCA) territory. Panel A: Color coded Tmax map with threshold value > 9.5 s indicates total critically hypoperfused tissue; Panel B: Color coded Tmax map with threshold value > 916 s shows infarct core; Panel C: Tmax > 9.5 s volume automatically segmented on CTP averaged images. Panel D: Tmax > 16 s volume automatically segmented on CTP averaged images. The difference between Tmax > 9.5 s and Tmax > 16 s volumes represents Tmax-Tmax mismatch. Tmax = time to the peak of the residual function.
Funding
None.
CRediT authorship contribution statement
Morotti Andrea: Writing – review & editing, Writing – original draft, Supervision. Fainardi Enrico: Writing – review & editing, Writing – original draft, Visualization, Project administration, Conceptualization. Busto Giorgio: Writing – review & editing, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Campbell B.C.V., Khatri P. Stroke. Lancet. 2020;396:129–142. doi: 10.1016/S0140-6736(20)31179-X. [DOI] [PubMed] [Google Scholar]
- 2.Powers W.J. Acute ischemic stroke. N. Engl. J. Med. 2020;383:252–260. doi: 10.1056/NEJMcp1917030. [DOI] [PubMed] [Google Scholar]
- 3.Ermine C.M., Bivard A., Parsons M.W., Baron J.C. The ischemic penumbra: from concept to reality. Int. J. Stroke. 2021;16:497–509. doi: 10.1177/1747493020975229. [DOI] [PubMed] [Google Scholar]
- 4.Baron J.C. Protecting the ischaemic penumbra as an adjunct to thrombectomy for acute stroke. Nat. Rev. Neurol. 2018;14:325–337. doi: 10.1038/s41582-018-0002-2. [DOI] [PubMed] [Google Scholar]
- 5.Heiss W.-D., Zaro-Weber O. Extension of therapeutic window in ischemic stroke by selective mismatch imaging. Int. J. Stroke. 2019;14:351–358. doi: 10.1177/1747493019840936. [DOI] [PubMed] [Google Scholar]
- 6.Markus H.S. Cerebral perfusion and stroke. J. Neurol. Neurosurg. Psychiatry. 2004;75:353–361. doi: 10.1136/jnnp.2003.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Copen W.A., Rezai Gharai L., Barak E.R., Schwamm L.H., Wu O., Kamalian S., Gonzalez R.G., Schaefer P.W. Existence of the diffusion-perfusion mismatch within 24 h after onset of acute stroke: dependence on proximal arterial occlusion. Radiology. 2009;250:878–886. doi: 10.1148/radiol.2503080811. [DOI] [PubMed] [Google Scholar]
- 8.Ma H., Wright P., Allport L., Phan T.G., Churilov L., Ly J., Zavala J.A., Arakawa S., Campbell B., Davis S.M., Donnan G.A. Salvage of the PWI/DWI mismatch up to 48h from stroke onset leads to favorable clinical outcome. Int. J. Stroke. 2015;10:565–570. doi: 10.1111/ijs.12203. [DOI] [PubMed] [Google Scholar]
- 9.Abdalkader M., Siegler J.E., Lee J.S., Yaghi S., Qiu Z., Huo X., Miao Z., Campbell B.C.V., Nguyen T.N. Neuroimaging of acute ischemic stroke: multimodal imaging approach for acute endovascular therapy. J. Stroke. 2023;25:55–71. doi: 10.5853/jos.2022.03286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puetz V., Dzialowski I., Hill M.D., Demchuk A.M. The Alberta stroke program early CT score in clinical practice: what have we learned? Int. J. Stroke. 2009;4:354–364. doi: 10.1111/j.1747-4949.2009.00337.x. [DOI] [PubMed] [Google Scholar]
- 11.Busto G., Morotti A., Carlesi E., Fiorenza A., Di Pasquale F., Mancini S., Lombardo I., Scola E., Gadda D., Moretti M., Miele V., Fainardi E. Pivotal role of multiphase computed tomography angiography for collateral assessment in patients with acute ischemic stroke. Radiol. Med. 2023;128:944–959. doi: 10.1007/s11547-023-01668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shafie M., Yu W. Recanalization therapy for acute ischemic stroke with large vessel occlusion: where we are and what comes next? Transl. Stroke Res. 2021;12:369–381. doi: 10.1007/s12975-020-00879-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsivgoulis G., Katsanos A.H., Sandset E.C., Turc G., Nguyen T.N., Bivard A., Fischer U., Khatri P. Thrombolysis for acute ischaemic stroke: current status and future perspectives. Lancet Neurol. 2023;22:418–429. doi: 10.1016/S1474-4422(22)00519-1. [DOI] [PubMed] [Google Scholar]
- 14.Kudo K., Sasaki M., Yamada K., Momoshima S., Utsunomiya H., Shirato H., Ogasawara K. Differences in CT perfusion maps generated by different commercial software: quantitative analysis by using identical source data of acute stroke patients. Radiology. 2010;254:200–209. doi: 10.1148/radiol.254082000. [DOI] [PubMed] [Google Scholar]
- 15.Dani K.A., Thomas R.G.R., Chappell F.M., Shuler K., MacLeod M.J., Muir K.W., Wardlaw J.M., Translational Medicine Research Collaboration Multicentre Acute Stroke Imaging Study Computed tomography and magnetic resonance perfusion imaging in ischemic stroke: definitions and thresholds. Ann. Neurol. 2011;70:384–401. doi: 10.1002/ana.22500. [DOI] [PubMed] [Google Scholar]
- 16.Campbell B.C.V., Parsons M.W. Imaging selection for acute stroke intervention. Int. J. Stroke. 2018;13:554–567. doi: 10.1177/1747493018765235. [DOI] [PubMed] [Google Scholar]
- 17.Campbell B.C.V., Lansberg M.G., Broderick J.P., Derdeyn C.P., Khatri P., Sarraj A., Saver J.L., Vagal A., Albers G.W. STAIR XI Consortium, Acute stroke imaging research roadmap IV: imaging selection and outcomes in acute stroke clinical trials and practice. Stroke. 2021;52:2723–2733. doi: 10.1161/STROKEAHA.121.035132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kidwell C.S., Alger J.R., Saver J.L. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–2735. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- 19.Yang S.-H., Liu R. Four decades of ischemic penumbra and its implication for ischemic stroke. Transl. Stroke Res. 2021;12:937–945. doi: 10.1007/s12975-021-00916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olivot J.-M., Mlynash M., Thijs V.N., Kemp S., Lansberg M.G., Wechsler Le, Bammer R., Marks M.P., Albers G.W. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. 2009;40:469–475. doi: 10.1161/STROKEAHA.108.526954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purushotham A., Campbell B.C.V., Straka M., Mlynash M., Olivot J.-M., Bammer R., Kemp S.M., Albers G.W., Lansberg M.G. Apparent diffusion coefficient threshold for delineation of ischemic core. Int. J. Stroke. 2015;10:348–353. doi: 10.1111/ijs.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsons M.W. Perfusion CT: is it clinically useful? Int. J. Stroke. 2008;3:41–50. doi: 10.1111/j.1747-4949.2008.00175.x. [DOI] [PubMed] [Google Scholar]
- 23.Konstas A.A., Goldmakher G.V., Lee T.-Y., Lev M.H. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 2: technical implementations. AJNR Am. J. Neuroradiol. 2009;30:885–892. doi: 10.3174/ajnr.A1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wintermark M., Flanders A.E., Velthuis B., Meuli R., van Leeuwen M., Goldsher D., Pineda C., Serena J., van der Schaaf I., Waaijer A., Anderson J., Nesbit G., Gabriely I., Medina V., Quiles A., Pohlman S., Quist M., Schnyder P., Bogousslavsky J., Dillon W.P., Pedraza S. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37:979–985. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- 25.Wintermark 1 M., Reichhart M., Thiran J.-P., Maeder P., Chalaron M., Schnyder P., Bogousslavsky J., Meuli R. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann. Neurol. 2002;51:417–432. doi: 10.1002/ana.10136. [DOI] [PubMed] [Google Scholar]
- 26.Parsons M.W., Pepper E.M., Chan V., Siddique S., Rajaratnam S., Bateman G.A., Levi C.R. Perfusion computed tomography: prediction of final infarct extent and stroke outcome. Ann. Neurol. 2005;58:672–679. doi: 10.1002/ana.20638. [DOI] [PubMed] [Google Scholar]
- 27.Murphy B.D., Fox A.J., Lee D.H., Sahlas D.J., Black S.E., Hogan M.J., Coutts S.B., Demchuk A.M., Goyal M., Aviv R.I., Symons S., Gulka I.B., Beletsky V., Pelz D., Hachinski V., Chan R., Lee T.-Y. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 2006;37:1771–7177. doi: 10.1161/01.STR.0000227243.96808.53. [DOI] [PubMed] [Google Scholar]
- 28.Tan J.C., Dillon W.P., Liu S., Adler F., Smith W.S., Wintermark M. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann. Neurol. 2007;61:533–543. doi: 10.1002/ana.21130. [DOI] [PubMed] [Google Scholar]
- 29.Aviv R.I., Mandelcorn J., Chakraborty S., Gladstone D., Malham S., Tomlinson G., Fox A.J., Symons S. Alberta stroke program early CT scoring of CT perfusion in early stroke visualization and assessment. AJNR Am. J. Neuroradiol. 2007;28:1975–1980. doi: 10.3174/ajnr.A0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lum C., Ahmed M.E., Patro S., Thornhill R., Hogan M., Iancu D., Lesiuk H., Dos Santos M., Dowlatshahi D., Ottawa Stroke Research Group (OSRG) Computed tomographic angiography and cerebral blood volume can predict final infarct volume and outcome after recanalization. Stroke. 2014;45:2683–2688. doi: 10.1161/STROKEAHA.114.006163. [DOI] [PubMed] [Google Scholar]
- 31.Padroni M., Bernardoni A., Tamborino C., Roversi G., Borrelli M., Saletti A., De Vito A., Azzini C., Borgatti L., Marcello O., d'Esterre C., Ceruti S., Casetta I., Lee T.-Y., Fainardi E. Cerebral blood volume ASPECTS is the best predictor of clinical outcome in acute ischemic stroke: a retrospective, combined semi-quantitative and quantitative assessment. PLOS One. 2016;11 doi: 10.1371/journal.pone.0147910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Consoli A., Andersson T., Holmberg A., Verganti L., Saletti A., Vallone S., Zini A., Cerase A., Romano D., Bracco S., Lorenzano S., Fainardi E., Mangiafico S., CAPRI Collaborative Group CT perfusion and angiographic assessment of pial collateral reperfusion in acute ischemic stroke: the CAPRI study. J. Neurointerv. Surg. 2016;8:1211–1216. doi: 10.1136/neurintsurg-2015-012155. [DOI] [PubMed] [Google Scholar]
- 33.Psychogios M.-N., Schramm P., Frölich A.M., Kallenberg K., Wasser K., Reinhardt L., Kreusch A.S., Jung K., Knauth M. Alberta Stroke Program Early CT Scale evaluation of multimodal computed tomography in predicting clinical outcomes of stroke patients treated with aspiration thrombectomy. Stroke. 2013;44:2188–2193. doi: 10.1161/STROKEAHA.113.001068. [DOI] [PubMed] [Google Scholar]
- 34.Turk A.S., Magarick J.A., Frei D., Fargen K.M., Chaudry I., Holmstedt C.A., Nicholas J., Mocco J., Turner R.D., Huddle D., Loy D., Bellon R., Dooley G., Adams R., Whaley M., Fanale C., Jauch E. CT perfusion-guided patient selection for endovascular recanalization in acute ischemic stroke: a multicenter study. J. Neurointerv. Surg. 2013;5:523–527. doi: 10.1136/neurintsurg-2012-010491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prabhakaran S., Soltanolkotabi M., Honarmand A.R., Bernstein R.A., Lee V.H., Conners J.J., Dehkordi-Vakil F., Shaibani A., Hurley M.C., Ansari S.A. Perfusion-based selection for endovascular reperfusion therapy in anterior circulation acute ischemic stroke. AJNR Am. J. Neuroradiol. 2014;35:1303–1308. doi: 10.3174/ajnr.A3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mokin M., Morr S., Fanous A.A., Shallwani H., Natarajan S.K., Levy E.I., Snyder K.V., Siddiqui A.H. Correlation between cerebral blood volume values and outcomes in endovascular therapy for acute ischemic stroke. J. Neurointerv. Surg. 2015;7:705–708. doi: 10.1136/neurintsurg-2014-011279. [DOI] [PubMed] [Google Scholar]
- 37.Silvennoinen H.M., Hamberg L.M., Lindsberg P.J., Valanne L., Hunter G.J. CT perfusion identifies increased salvage of tissue in patients receiving intravenous recombinant tissue plasminogen activator within 3 h of stroke onset. AJNR Am. J. Neuroradiol. 2008;29:1118–1123. doi: 10.3174/ajnr.A1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamalian S., Kamalian S., Konstas A.A., Maas M.B., Payabvash S., Pomerantz S.R., Schaefer P.W., Furie K.L., González R.G., Lev M.H. CT perfusion mean transit time maps optimally distinguish benign oligemia from true "at-risk" ischemic penumbra, but thresholds vary by postprocessing technique. AJNR Am. J. Neuroradiol. 2012;33:545–549. doi: 10.3174/ajnr.A2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deipolyi A.R., Wu O., Macklin E.A., Schaefer P.W., Schwamm L.H., Gonzalez R.G., Copen W.A. Reliability of cerebral blood volume maps as a substitute for diffusion-weighted imaging in acute ischemic stroke. J. Magn. Reason. Imaging. 2012;36:1083–1087. doi: 10.1002/jmri.23740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaefer P.W., Souza L., Kamalian S., Hirsch J.A., Yoo A.J., Kamalian 2 S., Gonzalez R.G., Lev M.H. Limited reliability of computed tomographic perfusion acute infarct volume measurements compared with diffusion-weighted imaging in anterior circulation stroke. Stroke. 2015;46:419–424. doi: 10.1161/STROKEAHA.114.007117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.d'Esterre C.D., Roversi G., Padroni M., Bernardoni A., Tamborino C., De Vito A., Azzini C., Marcello O., Saletti A., Ceruti S., Lee T.-Y., Fainardi E. CT perfusion cerebral blood volume does not always predict infarct core in acute ischemic stroke. Neurol. Sci. 2015;36:1777–1783. doi: 10.1007/s10072-015-2244-8. [DOI] [PubMed] [Google Scholar]
- 42.Copen W.A., Yoo A.J., Rost N.S., Morais L.T., Schaefer P.W., González R.G., Wu O. In patients with suspected acute stroke, CT perfusion-based cerebral blood flow maps cannot substitute for DWI in measuring the ischemic core. PLOS One. 2017;12 doi: 10.1371/journal.pone.0188891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bivard A., McElduff P., Spratt N., Levi C., Parsons M. Defining the extent of irreversible brain ischemia using perfusion computed tomography. Cerebrovasc. Dis. 2011;31:238–245. doi: 10.1159/000321897. [DOI] [PubMed] [Google Scholar]
- 44.Bivard A., Spratt P.N., Levi C., Parsons M. Perfusion computer tomography: imaging and clinical validation in acute ischaemic stroke. Brain. 2011;134:3408–3416. doi: 10.1093/brain/awr257. [DOI] [PubMed] [Google Scholar]
- 45.Campbell B.C.V., Christensen S., Levi C.R., Desmond P.M., Donnan G.A., Davis S.M., Parsons M.W. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke. 2011;42:3435–3440. doi: 10.1161/STROKEAHA.111.618355. [DOI] [PubMed] [Google Scholar]
- 46.Campbell B.C.V., Christensen S., Levi C.R., Desmond P.M., Donnan G.A., Davis S.M., Parsons M.W. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke. 2012;43:2648–2653. doi: 10.1161/STROKEAHA.112.660548. [DOI] [PubMed] [Google Scholar]
- 47.Bivard A., Levi C., Krishnamurthy V., McElduff P., Miteff 2 F., Spratt N.J., Bateman G., Donnan G., Davis S., Parsons M. Perfusion computed tomography to assist decision making for stroke thrombolysis. Brain. 2015;138:1919–1931. doi: 10.1093/brain/awv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin L., Bivard A., Parsons M.W. Perfusion patterns of ischemic stroke on computed tomography perfusion. J. Stroke. 2013;15:164–173. doi: 10.5853/jos.2013.15.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heit J.J., Zaharchuk G., Wintermark M. Advanced neuroimaging of acute ischemic stroke: penumbra and collateral assessment. Neuroimaging Clin. N. Am. 2018;28:585–597. doi: 10.1016/j.nic.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Puig J., Shankar J., Liebeskind D., Terceño M., Nael K., Demchuk A.M., Menon B., Dowlatshahi D., Leiva-Salinas C., Wintermark M., Thomalla G., Silva Y., Serena J., Pedraza S., Essig M. From "time is brain" to "imaging is brain": a paradigm shift in the management of acute ischemic Stroke. J. Neuroimaging. 2020;30:562–571. doi: 10.1111/jon.12693. [DOI] [PubMed] [Google Scholar]
- 51.Geisbush T.R., Snyder S.J., Heit J.J. Neuroimaging in patient selection for thrombectomy, AJR. Am. J. Roentgenol. 2023;220:630–640. doi: 10.2214/AJR.22.28608. [DOI] [PubMed] [Google Scholar]
- 52.Lansberg M.G., Straka M., Kemp S., Mlynash M., Wechsler L.R., Jovin T.G., Wilder M.J., Lutsep H.L., Czartoski T.J., Bernstein R.A., Chang C.W.J., Warach S., Fazekas F., Inoue M., Tipirneni A., Hamilton S.A., Zaharchuk G., Marks M.P., Bammer R., Albers G.W., DEFUSE 2 study investigators MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell B.C.V., Mitchell P.J., Kleinig T.J., Dewey H.M., Churilov L., Yassi N., Yan B., Dowling R.J., Parsons M.W., Oxley T.J., Wu T.Y., Brooks M., Simpson M.A., Miteff F., Levi C.R., Krause M., Harrington T.J., Faulder K.C., Steinfort B.S., Priglinger M., Ang T., Scroop R., Barber P.A., McGuinness B., Wijeratne T., Phan T.G., Chong W., Chandra R.V., Bladin C.F., Badve M., Rice H., de Villiers L., Ma H., Desmond P.M., Donnan G.A., Davis S.M., EXTEND-IA Investigators Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 54.Saver J.L., Goyal M., Bonafe A., Diener H.-C., Levy E.I., Pereira V.M., Albers G.W., Cognard C., Cohen D.J., Hacke W., Jansen O., Jovin T.G., Mattle H.P., Nogueira R.G., Siddiqui A.H., Yavagal D.R., Baxter B.W., Devlin T.G., Lopes D.K., Reddy V.K., du Mesnil de Rochemont R., Singer O.C., Jahan R., SWIFT PRIME Investigators Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 55.Nogueira R.G., Jadhav A.P., Haussen D.C., Bonafe A., Budzik R.F., Bhuva P., Yavagal D.R., Ribo M., Cognard C., Hanel R.A., Sila C.A., Hassan aE., Millan M., Levy E.I., Mitchell P., Chen M., English J.D., Shah Q.A., Silver F.L., Pereira V.M., Mehta B.P., Baxter B.W., Abraham M.G., Cardona P., Veznedaroglu E., Hellinger F.R., Feng L., Kirmani J.F., Lopes D.K., Jankowitz B.T., Frankel M.R., Costalat V., Vora N.A., Yoo A.J., Malik A.M., Furlan A.J., Rubiera M., Aghaebrahim A., Olivot J.-M., Tekle W.G., Shields R., Graves T., Lewis R.J., Smith W.S., Liebeskind D.S., Saver J.L., Jovin T.G., DAWN Trial Investigators Thrombectomy 6 to 24 h after stroke with a mismatch between deficit and infarct. N. Engl. J. Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 56.Albers G.W., Marks M.P., Kemp S., Christensen S., Tsai J.P., Ortega-Gutierrez S., McTaggart R.A., Torbey M.T., Kim-Tenser M., Leslie-Mazwi T., Sarraj A., Kasner S.E., Ansari S.A., Yeatts S.D., Hamilton S., Mlynash M., Heit J.J., Zaharchuk G., Kim S., Carrozzella J., Palesch Y.Y., Demchuk A.M., Bammer 1 R., Lavori P.W., Broderick J.P., Lansberg 1 M.G., DEFUSE 3 Investigators Thrombectomy for stroke at 6 to 16 h with selection by perfusion imaging. N. Engl. J. Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma H., Campbell B.C.V., Parsons M.W., Churilov L., Levi C.R., Hsu C., Kleinig T.J., Wijeratne T., Curtze S., Dewey H.M., Miteff F., Tsai C.-H., Lee J.-T., Phan T.G., Mahant1 N., Sun M.-C., Krause M., Sturm J., Grimley R., Chen C.-H., Hu C.-J., Wong A.A., Field D., Sun Y., Barber P.A., Sabet A., Jannes J., Jeng J.-S., Clissold B., Markus R., Lin C.-H., Lien L.-M., Bladin C.F., Christensen S., Yassi N., Sharma G., Bivard A., Desmond 1 P.M., Yan B., Mitchell P.J., Thijs V., Carey L., Meretoja A., Davis S.M., Donnan 1 G.A., EXTEND Investigators Thrombolysis guided by perfusion imaging up to 9 h after onset of stroke. N. Engl. J. Med. 2019;380:1795–1803. doi: 10.1056/NEJMoa1813046. [DOI] [PubMed] [Google Scholar]
- 58.Powers W.J., Rabinstein A.A., Ackerson T., Adeoye O.M., Bambakidis N.C., Becker K., Biller J., Brown M., Demaerschalk B.M., Hoh B., Jauch E.C., Kidwell C.S., Leslie-Mazwi T.M., Ovbiagele B., Scott P.A., Sheth K.N., Southerland A.M., Summers D.V., Tirschwell D.L., on behalf of the American Heart Association Stroke Council, American Heart Association Stroke Council 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 59.Powers W.J., Rabinstein A.A., Ackerson T., Adeoye O.M., Bambakidis N.C., Becker K., Biller J., Brown M., Demaerschalk B.M., Hoh B., Jauch E.C., Kidwell C.S., Leslie-Mazwi T.M., Ovbiagele B., Scott P.A., Sheth K.N., Southerland A.M., Summers D.V., Tirschwell D.L., on behalf of the American Heart Association Stroke Council Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 60.Campbell B.C.V., Ma H., Ringleb P.A., Parsons M.W., Churilov L., Bendszus M., Levi C.R., Hsu C., Kleinig T.J., Fatar M., Leys D., Molina C., Wijeratne T., Curtze S., Dewey H.,M., Barber P.A., Butcher K.S., De Silva D.A., Bladin C.F., Yassi N., Pfaff J.A.R., Sharma G., Bivard A., Desmond P.M., Schwab S., Schellinger P.D., Yan B., Mitchell P.J., Serena J., Toni D., Thijs V., Hacke W., Davis S.M., Donnan G.A., EXTEND, ECASS-4, and EPITHET Investigators Extending thrombolysis to 4·5–9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet. 2019;394:139–147. doi: 10.1016/S0140-6736(19)31053-0. [DOI] [PubMed] [Google Scholar]
- 61.Leslie-Mazwi T.M., Hamilton S., Mlynash M., Patel A.B., Schwamm L.H., Lansberg M.G., Marks M., Hirsch J.A., Albers G.W. DEFUSE 3 Non-DAWN Patients. A closer look at late window thrombectomy selection. Stroke. 2019;50:618–625. doi: 10.1161/STROKEAHA.118.023310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Albers G.W., Lansberg M.G., Brown S., Jadhav A.P., Haussen D.C., Martins S.O., Rebello L.C., Demchuk A.M., Goyal M., Ribo M., Turk A.S., Liebeskind D.S., Heit J.J., Marks M.P., Jovin T.G., Nogueira R.G., AURORA Investigators Assessment of optimal patient selection for endovascular thrombectomy beyond 6 h after symptom onset: a pooled analysis of the AURORA database. JAMA Neurol. 2021;78:1064–1071. doi: 10.1001/jamaneurol.2021.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campbell B.C.V., Yassi N., Ma H., Sharma G., Salinas S., Churilov L., Meretoja A., Parsons M.W., Desmond P.M., Lansberg M.G., Donnan G.A., Davis S.M. Imaging selection in ischemic stroke: feasibility of automated CT-perfusion analysis. Int. J. Stroke. 2015;10:51–54. doi: 10.1111/ijs.12381. [DOI] [PubMed] [Google Scholar]
- 64.Bathla G., Limaye K., Policeni B., Klotz E., Juergens M., Derdeyn C. Achieving comparable perfusion results across vendors. The next step in standardizing stroke care: a technical report. J. Neurointerv. Surg. 2019;11:1257–1260. doi: 10.1136/neurintsurg-2019-014810. [DOI] [PubMed] [Google Scholar]
- 65.Mallon D.H., Taylor E.J.R., Vittay O.I., Sheeka A., Doig D., Lobotesis K. Comparison of automated ASPECTS, large vessel occlusion detection and CTP analysis provided by Brainomix and RapidAI in patients with suspected ischaemic stroke. J. Stroke Cerebrovasc. Dis. 2022;31 doi: 10.1016/j.jstrokecerebrovasdis.2022.106702. [DOI] [PubMed] [Google Scholar]
- 66.Lin L., Bivard A., Krishnamurthy V., Levi C.R., Parsons M.W. Whole-Brain CT perfusion to quantify acute ischemic penumbra and core. Radiology. 2016;279:876–887. doi: 10.1148/radiol.2015150319. [DOI] [PubMed] [Google Scholar]
- 67.Xiong Y., Huang C.C., Fisher M., Hackney D.B., Bhadelia R.A., Selim M.H. Comparison of automated CT perfusion softwares in evaluation of acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2019;28 doi: 10.1016/j.jstrokecerebrovasdis.2019.104392. [DOI] [PubMed] [Google Scholar]
- 68.Rava R.A., Snyder K.V., Mokin M., Waqas M., Allman A.B., Senko J.L., Podgorsak A.R., Shiraz Bhurwani M.M., Hoi Y., Siddiqui A.H., Davies J.M., Levy E.I., Ionita C.N. Assessment of a bayesian Vitrea CT perfusion analysis to predict final infarct and penumbra volumes in patients with acute ischemic stroke: a comparison with RAPID. AJNR Am. J. Neuroradiol. 2020;41:206–212. doi: 10.3174/ajnr.A6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rava R.A., Snyder K.V., Mokin M., Waqas M., Zhang X., Podgorsak A.R., Allman A.B., Senko J., Bhurwani M.M.S., Hoi Y., Davies J.M., Levy E.I., Siddiqui A.H., Ionita C.N. Assessment of computed tomography perfusion software in predicting spatial location and volume of infarct in acute ischemic stroke patients: a comparison of Sphere, Vitrea, and RAPID. J. Neurointerv. Surg. 2021;13:130–135. doi: 10.1136/neurintsurg-2020-015966. [DOI] [PubMed] [Google Scholar]
- 70.Campbell B.C.V. Optimal imaging at the primary stroke center. Stroke. 2020;51:1932–1940. doi: 10.1161/STROKEAHA.119.026734. [DOI] [PubMed] [Google Scholar]
- 71.Goyal M., Menon B.K., van Zwam W.H., Dippel D.W.J., Mitchell P.J., Demchuk A.M., Dávalos A., Majoie C.B.L.M., van der Lugt A., de Miquel M.A., Donnan G.A., Roos Y.B.W.E.M., Bonafe A., Jahan R., Diener H.-C., van den Berg L.A., Levy E.I., Berkhemer O.A., Pereira V.M., Rempel J., Millán M., Davis S.M., Roy D., Thornton J., Román L.S., Ribó M., Beumer D., Stouch B., Brown S., Campbell B.C.V., van Oostenbrugge R.J., Saver J.L., Hill M.D., Jovin T.G., HERMES collaborators Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 72.Nogueira R.G., Ribó M. Endovascular treatment of acute stroke. A call for individualized patient selection. Stroke. 2019;50:2612–2618. doi: 10.1161/STROKEAHA.119.023811. [DOI] [PubMed] [Google Scholar]
- 73.Lopez-Rivera V., Abdelkhaleq R., Yamal J.-Ml, Singh N., Savitz S.I., Czap A.L., Alderazi Y., Chen P.R., Grotta J.C., Blackburn S., Spiegel G., Dannenbaum M.J., Wu T.-C., Yoo A.J., McCullough L.D., Sheth S.A. Impact of initial imaging protocol on likelihood of endovascular stroke therapy. Stroke. 2020;51:3055–3063. doi: 10.1161/STROKEAHA.120.030122. [DOI] [PubMed] [Google Scholar]
- 74.Dittrich T.D., Sporns P.B., Kriemler L.F., Rudin S., Nguyen A., Zietz 1 Annaelle, Polymeris A.A., Tränka C., Thilemann S., Wagner B., Altersberger V.L., Piot I., Barinka F., Müller S., Hänsel M., Gensicke H., Engelter S.T., Lyrer P.A., Sutter R., Nickel C.H., Katan M., Peters N., Kulcsár Z., Karwacki G.M., Pileggi M., Cereda C., Wegener S., Bonati L.H., Fischer U., Psychogios M., De Marchis G.M. Mechanical thrombectomy versus best medical treatment in the late time window in non-DEFUSE-non-DAWN patients: a multicenter cohort study. Stroke. 2023;54:722–730. doi: 10.1161/STROKEAHA.122.039793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ren Z., Huo X., Kumar J., Jadhav A.P., Costalat V., Fiehler J., Bendszus M., Yoshimura S., Ma G., Tong X., Zhang X., Zaidat O.O., Jovin T.G., Liebeskind D.S., Pereira V.M., Miao Z. Review of current large core volume stroke thrombectomy clinical trials: controversies and progress. Stroke Vasc., Interv. Neurol. 2022;2 [Google Scholar]
- 76.Román L.S., Menon B.K., Blasco J., Hernández-Pérez M M., Dávalos A., Majoie C.B.L.M., Campbell B.C.V., Guillemin F., Lingsma H., Anxionnat R., Epstein J., Saver J.L., Marquering H., Wong J.H., Lopes D., Reimann G., Desal H., Dippel D.W.J., Coutts S., du Mesnil de Rochemont R., Yavagal D., Ferre J.C., Roos Y.B.W.E.M., Liebeskind D.S., Lenthall R., Molina C., Ajlan Al.F.S., Reddy V., Dowlatshahi D., Sourour N.A., Oppenheim C., Mitha A.P., Davis S.M., Weimar C., van Oostenbrugge R.J., Cobo E., Kleinig T.J., Donnan G.A., van der Lugt A., Demchuk A.M., Berkhemer O.A., Boers A.M.M., Ford G.A., Muir K.W., Brown B.S., Jovin T T., van Zwam W.H., Mitchell P.J., Hill M.D., White P., Bracard S., Goyal M. HERMES collaborators Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018;17:895–904. doi: 10.1016/S1474-4422(18)30242-4. [DOI] [PubMed] [Google Scholar]
- 77.Nogueira R.G., Haussen D.C., Dehkharghani S., Rebello L.C., Lima A., Bowen M., Belagaje S., Anderson A., Frankel M. Large volumes of critically hypoperfused penumbral tissue do not preclude good outcomes after complete endovascular reperfusion: redefining malignant profile. Stroke. 2016;47:94–98. doi: 10.1161/STROKEAHA.115.011360. [DOI] [PubMed] [Google Scholar]
- 78.Rebello L.C., Bouslama M., Haussen D.C., Dehkharghani S., Grossberg J.A., Belagaje S., Frankel M.R., Nogueira R.G. Endovascular treatment for patients with acute stroke who have a large ischemic core and large mismatch imaging profile. JAMA Neurol. 2017;74:34–40. doi: 10.1001/jamaneurol.2016.3954. [DOI] [PubMed] [Google Scholar]
- 79.Sarraj A., Hassan A.E., Savitz S., Sitton C., Grotta J., Chen P., Cai C., Cutter G., Imam B., Reddy S., Parsha K., Pujara D., Riascos R., Vora N., Abraham M., Kamal H., Haussen D.C., Barreto A.D., Lansberg M., Gupta R., Albers G.W. Outcomes of endovascular thrombectomy vs medical management alone in patients with large ischemic cores: a secondary analysis of the optimizing patient's selection for endovascular treatment in acute ischemic stroke (SELECT) study. JAMA Neurol. 2019;76:1147–1156. doi: 10.1001/jamaneurol.2019.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seners P., Oppenheim C., Turc G., Albucher J.-F., Guenego A., Raposo N., Christensen S., Calvière L., Viguier A., Darcourt J., Januel A.-C., Mlynash M., Sommet A., Thalamas C., Sibon I., Rousseau V., Tourdias T., Menegon P., Bonneville F., Mazighi M., Charron S., Legrand L., Cognard C., Albers G.W., Baron J.-C., Olivot J.-M., FRAME investigators Perfusion imaging and clinical outcome in acute ischemic stroke with large core. Ann. Neurol. 2021;90:417–427. doi: 10.1002/ana.26152. [DOI] [PubMed] [Google Scholar]
- 81.Bouslama M., Barreira C.M., Haussen D.C., Rodrigues G.M., Pisani L., Frankel M.R., Nogueira R.G. Endovascular reperfusion outcomes in patients with a stroke and low ASPECTS is highly dependent on baseline infarct volumes. J. Neurointerv. Surg. 2022;14:117–121. doi: 10.1136/neurintsurg-2020-017184. [DOI] [PubMed] [Google Scholar]
- 82.Garcia-Esperon C., Bivard A., Johns H., Chen C., Churilov L., Lin L., Butcher K., Kleinig T.J., Choi P.M.C., Cheng X., Dong Q., Aviv R.I., Miteff F., Spratt N.J., Levi C.R., Parsons M.W., INSPIRE Study group Association of endovascular thrombectomy with functional outcome in patients with acute stroke with a large ischemic core. Neurology. 2022;99:e1345–e1355. doi: 10.1212/WNL.0000000000200908. [DOI] [PubMed] [Google Scholar]
- 83.Cagnazzo F., Derraz I., Dargazanli C., Lefevre P.-H., Gascou G., Riquelme C., Bonafe A., Costalat V. Mechanical thrombectomy in patients with acute ischemic stroke and ASPECTS ≤6: a meta-analysis. J. Neurointerv. Surg. 2020;12:350–355. doi: 10.1136/neurintsurg-2019-015237. [DOI] [PubMed] [Google Scholar]
- 84.Sarraj A., Grotta J.C., Pujara D.K., Shaker F., Tsivgoulis G. Triage imaging and outcome measures for large core stroke thrombectomy – a systematic review and meta-analysis. J. Neurointerv. Surg. 2020;12:1172–1179. doi: 10.1136/neurintsurg-2019-015509. [DOI] [PubMed] [Google Scholar]
- 85.Kerleroux B., Janot K., Hak J.F., Kaesmacher J., Hassen W.B., Benzakoun J., Oppenheim C., Herbreteau D., Ifergan H., Bricout N., Henon H., Yoshimoto T., Inoue M., Consoli A., Costalat V., Naggara O., Lapergue B., Cagnazzo F., Boulouis G. Mechanical thrombectomy in patients with a large ischemic volume at presentation: systematic review and meta-analysis. J. Stroke. 2021;23:358–366. doi: 10.5853/jos.2021.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meyer L., Bechstein M., Bester M., Hanning U., Brekenfeld C., Flottmann F., Kniep H., van Horn N., Deb-Chatterji M., Thomalla G., Sporns P., Yeo L.L., Tan B.Y.-Q., Gopinathan Anil, Kastrup A., Politi M., Papanagiotou P., Kemmling A., Fiehler J., Broocks G., German Stroke Registry–Endovascular Treatment (GSR-ET) Thrombectomy in extensive stroke may not be beneficial and is associated with increased risk for hemorrhage. Stroke. 2021;52:3109–3117. doi: 10.1161/STROKEAHA.120.033101. [DOI] [PubMed] [Google Scholar]
- 87.Sarraj A., Hassan A.E., Abraham M.G., Ortega-Gutierrez S., Kasner S.E., Hussain M.S., Chen M., Blackburn S., Sitton C.W., Churilov L., Sundararajan S., Hu Y.C., Herial N.A., Jabbour P., Gibson D., Wallace A.N., Arenillas J.F., Tsai J.P., Budzik R.F., Hicks W.J., Kozak O., Yan B., Cordato D.J., Manning N.W., Parsons M.W., Hanel 1 R.A., Aghaebrahim A.N., Wu T.Y., Cardona-Portela P., Pérez de la Ossa N., Schaafsma J.D., Blasco J., Sangha N., Warach S., Gandhi C.D., Kleinig T.J., Sahlein Daniel, Elijovich L., Tekle W., Samaniego E.A., Maali L., Abdulrazzak M.A., Psychogios M.N., Shuaib A., Pujara D.K., Shaker F., Johns H., Sharma G., Yogendrakumar V., Ng F.C., Rahbar M.H., Cai C., Lavori P., Hamilton S., Nguyen T., Fifi J.T., Davis S., Wechsler L., Pereira V.M., Lansberg M.G., Hill M.D., Grotta J.C., Ribo M., Campbell B.C., Albers G.W., SELECT2 Investigators Trial of endovascular thrombectomy for large ischemic strokes. N. Engl. J. Med. 2023;388:1259–1271. doi: 10.1056/NEJMoa2214403. [DOI] [PubMed] [Google Scholar]
- 88.Yoshimura S., Sakai N., Yamagami H., Uchida K., Beppu M., Toyoda K., Matsumaru Y., Matsumoto Y., Kimura K., Takeuchi M., Yazawa Y., Kimura N., Shigeta K., Imamura H., Suzuki I., Enomoto Y., Tokunaga S., Morita K., Sakakibara F., Kinjo N., Saito Takuya, Ishikura R., Inoue M., Morimoto T. Endovascular therapy for acute stroke with a large ischemic region. N. Engl. J. Med. 2022;386:1303–1313. doi: 10.1056/NEJMoa2118191. [DOI] [PubMed] [Google Scholar]
- 89.Huo X., Ma G., Tong X., Zhang X., Pan Y., Nguyen T.N., Yuan G., Han H., Chen W., Wei M., Zhang J., Zhou Z., Yao Xi, Wang G., Song W., Cai X., Nan G., Li D., Wang A.Y.-C., Ling W., Cai C., Wen C., Wang E., Zhang L., Jiang C., Liu Y., Liao G., Chen X., Li T., Liu S., Li J., Gao F., Ma N., Mo D., Song L., Sun X., Li X., Deng Y., Luo G., Lv M., He H., Liu A., Zhang J., Mu S., Liu L., Jing J., Nie X., Ding Z., Du W., Zhao X., Yang P., Liu L., Wang Y., Liebeskind D.S., Pereira V.M., Ren Z., Wang Y., Miao Z., ANGEL-ASPECT Investigators Trial of endovascular therapy for acute ischemic stroke with large infarct. N. Engl. J. Med. 2023;388:1272–1283. doi: 10.1056/NEJMoa2213379. [DOI] [PubMed] [Google Scholar]
- 90.Leslie-Mazwi T.M., Altschul D., Simonsen C.Z. Thrombectomy for patients with large infarct core in practice: where should the pendulum swing? Stroke. 2021;52:3118–3120. doi: 10.1161/STROKEAHA.121.034754. [DOI] [PubMed] [Google Scholar]
- 91.Jadhav A.P., Hacke W., Dippel D.W.J., Simonsen C.Z., Costalat V., Fiehler J., Thomalla G., Bendszus M., Andersson T., Mattle H.P., Leslie-Mazwi T.M., Mokin M., Yoo A.J., Zaidat O.O., Sheth S.A., Jovin T.G., Liebeskind David. Select wisely: the ethical challenge of defining large core with perfusion in the early time window. J. Neurointerv. Surg. 2021;13:497–499. doi: 10.1136/neurintsurg-2021-017386. [DOI] [PubMed] [Google Scholar]
- 92.Chung C.Y., Hu R., Peterson R.B., Allen J.W. Automated processing of head CT perfusion imaging for ischemic stroke triage: a practical guide to quality assurance and interpretation. AJR Am. J. Roentgenol. 2021;217:1401–1416. doi: 10.2214/AJR.21.26139. [DOI] [PubMed] [Google Scholar]
- 93.Kauw F., Heit J.J., Martin B.W., van Ommen F., Kappelle L.J., Velthuis B.K., de Jong H.W.A.M., Dankbaar J.W., Wintermark M. Computed tomography perfusion data for acute ischemic stroke evaluation using rapid software: pitfalls of automated postprocessing. J. Comput. Assist. Tomogr. 2020;44:75–77. doi: 10.1097/RCT.0000000000000946. [DOI] [PubMed] [Google Scholar]
- 94.Vagal A., Wintermark M., Nael K., Bivard A., Parsons M., Grossman A.W., Khatri P. Automated CT perfusion imaging for acute ischemic stroke: pearls and pitfalls for real-world use. Neurology. 2019;93:888–898. doi: 10.1212/WNL.0000000000008481. [DOI] [PubMed] [Google Scholar]
- 95.Nicolas-Jilwan M., Wintermark M. Automated brain perfusion imaging in acute ischemic stroke: interpretation pearls and pitfalls. Stroke. 2021;52:3728–3738. doi: 10.1161/STROKEAHA.121.035049. [DOI] [PubMed] [Google Scholar]
- 96.Campbell B.C.V., Yassi N., Ma H., Sharma G., Salinas S., Churilov L., Meretoja A., Parsons M.W., Desmond P.M., Lansberg M.G., Donnan G.A., Davis S.M. Imaging selection in ischemic stroke: feasibility of automated CT-perfusion analysis. Int. J. Stroke. 2015;10:51–54. doi: 10.1111/ijs.12381. [DOI] [PubMed] [Google Scholar]
- 97.Cimflova P., Volny O., Mikulik P.R., Tyshchenko B., Belaskova S., Vinklarek J., Cervenak V., Krivka T., Vanicek A.P.J., Krajina P.A. Detection of ischemic changes on baseline multimodal computed tomography: expert reading vs. Brainomix and RAPID software. J. Stroke Cerebrovasc. Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.104978. [DOI] [PubMed] [Google Scholar]
- 98.Psychogios M.-N., Sporns P.B., Ospel J., Katsanos A.H., Kabiri R., Flottmann F.A., Menon B.K., Horn M., Liebeskind D.S., Honda T., Ribo M., Ruiz M.R., Kabbasch C., Lichtenstein T., Maurer C.J., Berlis A., Hellstern V., Henkes H., Möhlenbruch M.A., Seker F., Ernst M.S., Liman J., Tsivgoulis G., Brehm A. Automated perfusion calculations vs. visual scoring of collaterals and CBV-ASPECTS: has the machine surpassed the eye? Clin. Neuroradiol. 2021;31:499–506. doi: 10.1007/s00062-020-00974-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rigler I., Gspan T., Avsenik J., Milošević Z., Pretnar Oblak J. Independent significance of visual assessment of perfusion CT maps in anterior circulation stroke patients treated with mechanical thrombectomy. Clin. Neuroradiol. 2022;32:829–837. doi: 10.1007/s00062-022-01140-7. [DOI] [PubMed] [Google Scholar]
- 100.Dehkharghani S., Bammer R., Straka M., Albin L.S., Kass-Hout O., Allen J.W., Rangaraju S., Qiu D., Winningham M.J., Nahab F. Performance and predictive value of a user-independent platform for CT perfusion analysis: threshold-derived automated systems outperform examiner-driven approaches in outcome prediction of acute ischemic stroke. AJNR Am. J. Neuroradiol. 2015;36:1419–1425. doi: 10.3174/ajnr.A4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khaw A.V., Angermaier A., Michel P., Kirsch M., Kessler C., Langner S. Inter-rater agreement in three perfusion-computed tomography evaluation methods before endovascular therapy for acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2016;25:960–968. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 102.Austein F., Riedel C., Kerby T., Meyne J., Binder A., Lindner T., Huhndorf M., Wodarg F., Jansen O. Comparison of perfusion CT software to predict the final infarct volume after thrombectomy. Stroke. 2016;47:2311–2317. doi: 10.1161/STROKEAHA.116.013147. [DOI] [PubMed] [Google Scholar]
- 103.Lu Q., Fu J., Lv K., Han Y., Pan Y., Xu Y., Zhang J., Geng D. Agreement of three CT perfusion software packages in patients with acute ischemic stroke: a comparison with RAPID. Eur. J. Radiol. 2022;156 doi: 10.1016/j.ejrad.2022.110500. [DOI] [PubMed] [Google Scholar]
- 104.Muehlen I., Borutta M., Siedler G., Engelhorn T., Hock S., Knott M., Hoelter P., Volbers B., Schwab S., Doerfler A. Prognostic accuracy of CTP summary maps in patients with large vessel occlusive stroke and poor revascularization after mechanical thrombectomy-comparison of three automated perfusion software applications. Tomography. 2022;8:1350–1362. doi: 10.3390/tomography8030109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suomalainen O.P., Martinez-Majander N., Sibolt G., Bäcklund K., Järveläinen J., Korvenoja A., Tiainen M., Forss N., Curtze S. Comparative analysis of core and perfusion lesion volumes between commercially available computed tomography perfusion software. Eur. Stroke J. 2023;8:259–267. doi: 10.1177/23969873221135915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bathla G., Limaye K., Policeni B., Klotz E., Juergens M., Derdeyn C. Achieving comparable perfusion results across vendors. The next step in standardizing stroke care: a technical report. J. Neurointerv. Surg. 2019;11:1257–1260. doi: 10.1136/neurintsurg-2019-014810. [DOI] [PubMed] [Google Scholar]
- 107.Koopman M.S., Berkhemer O.A., Geuskens R.R.E.G., Emmer 1 B.J., van Walderveen Marianne A.A., Jenniskens S.F.M., van Zwam W.H., van Oostenbrugge R.J., van der Lugt A., Dippel D.W.J., Beenen L.F., Roos Y.B.W.E.M., Marquering H.A., Majoie C.B.L.M., MR CLEAN Trial Investigators Comparison of three commonly used CT perfusion software packages in patients with acute ischemic stroke. J. Neurointerv. Surg. 2019;11:1249–1256. doi: 10.1136/neurintsurg-2019-014822. [DOI] [PubMed] [Google Scholar]
- 108.Bathla G., Ortega-Gutierrez S., Klotz E., Juergens M., Zevallos C.B., Ansari S., Ward C.E., Policeni B., Samaniego E., Derdeyn C. Comparing the outcomes of two independent computed tomography perfusion softwares and their impact on therapeutic decisions in acute ischemic stroke. J. Neurointerv. Surg. 2020;12:1028–1032. doi: 10.1136/neurintsurg-2020-015827. [DOI] [PubMed] [Google Scholar]
- 109.Muehlen I., Sprügel M., Hoelter P., Hock S., Knott M., Huttner H.B., Schwab S., Kallmünzer B., Doerfler A. Comparison of two automated computed tomography perfusion applications to predict the final nfarct volume after thrombolysis in cerebral infarction 3 recanalization. Stroke. 2022;53:1657–1664. doi: 10.1161/STROKEAHA.121.035626. [DOI] [PubMed] [Google Scholar]
- 110.Gunasekera L., Churilov L., Mitchell P., Bivard A., Sharma G., Parsons M.W., Yan B. Automated estimation of ischemic core prior to thrombectomy: comparison of two current algorithms. Neuroradiology. 2021;63:1645–1649. doi: 10.1007/s00234-021-02651-9. [DOI] [PubMed] [Google Scholar]
- 111.Bivard A., Churilov L., Ma H., Levi C., Campbell B., Yassi N., Meretoja A., Zhao H., Sharma G., Chen C., Davis S., Donnan G., Yan B., Parsons M., EXTEND investigators Does variability in automated perfusion software outputs for acute ischemic stroke matter? Reanalysis of EXTEND perfusion imaging. CNS Neurosci. Ther. 2022;28:139–144. doi: 10.1111/cns.13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xiong Y., Huang C.C., Fisher M., Hackney D.B., Bhadelia R.A., Selim M.H. Comparison of automated CT perfusion softwares in evaluation of acute ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2019;28 doi: 10.1016/j.jstrokecerebrovasdis.2019.104392. [DOI] [PubMed] [Google Scholar]
- 113.Bani-Sadr A., Cho T.-H., Cappucci M., Hermier M., Ameli R., Filip A., Riva R., Derex Lt, De Bourguignon C., Mechtouff L., Eker O.F., Nighoghossian N., Berthezene Y. Assessment of three MR perfusion software packages in predicting final infarct volume after mechanical thrombectomy. J. Neurointerv. Surg. 2023;15:393–398. doi: 10.1136/neurintsurg-2022-018674. [DOI] [PubMed] [Google Scholar]
- 114.Rava R.A., Snyder K.V., Mokin M., Waqas M., Zhang X., Podgorsak A.R., Allman A.B., Senko J., Shiraz Bhurwani M.M., Hoi Y., Davies J.M., Levy E.I., Siddiqui A.H., Ionita C.N. Assessment of computed tomography perfusion software in predicting spatial location and volume of infarct in acute ischemic stroke patients: a comparison of Sphere, Vitrea, and RAPID. J. Neurointerv. Surg. 2021;13:130–135. doi: 10.1136/neurintsurg-2020-015966. [DOI] [PubMed] [Google Scholar]
- 115.Goyal M., Ospel J.M., Menon B., Almekhlafi M., Jayaraman M., Fiehler J., Psychogios M., Chapot R., van der Lugt A., Liu J., Yang P., Agid R., Hacke W., Walker M., Fischer U., Asdaghi N., McTaggart R., Srivastava P., Nogueira R.G., Moret J., Saver J.L., Hill M.D., Dippel D., Fisher M. Challenging the ischemic core concept in acute ischemic stroke imaging. Stroke. 2020;51:3147–3155. doi: 10.1161/STROKEAHA.120.030620. [DOI] [PubMed] [Google Scholar]
- 116.Kidwell C.S., Saver J.L., Mattiello J., Starkman S., Vinuela F., Duckwiler G., Gobin Y.P., Jahan R., Vespa P., Kalafut M., Alger J.R. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann. Neurol. 2000;47:462–469. [PubMed] [Google Scholar]
- 117.Kranz P.G., Eastwood J.D. Does diffusion-weighted imaging represent the ischemic core? An evidence-based systematic review. AJNR Am. J. Neuroradiol. 2009;30:1206–1212. doi: 10.3174/ajnr.A1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Olivot J.-M., Mlynash M., Thijs V.N., Purushotham A., Kemp S., Lansberg M.G., Wechsler L., Bammer R., Marks M.P., Albers G.W. Relationships between cerebral perfusion and reversibility of acute diffusion lesions in DEFUSE: insights from RADAR. Stroke. 2009;40:1692–1697. doi: 10.1161/STROKEAHA.108.538082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Labeyrie M.-A., Turc G., Hess A., Hervo P., Mas J.-L., Meder J.-F., Baron J.-C., Touzé E., Oppenheim C. Diffusion lesion reversal after thrombolysis: a MR correlate of early neurological improvement. Stroke. 2012;43:2986–2991. doi: 10.1161/STROKEAHA.112.661009. [DOI] [PubMed] [Google Scholar]
- 120.Campbell B.C.V., Purushotham A., Christensen S., Desmond P.M., Nagakane Y., Parsons M.W., Lansberg M.G., Mlynash M., Straka M., De Silva D.A., Olivot J.-M., Bammer R., Albers G.W., Donnan G.A., Davis S.M., Investigators E.P.I.T.H.E.T.-D.E.F.U.S.E. The infarct core is well represented by the acute diffusion lesion: sustained reversal is infrequent. J. Cereb. Blood Flow. Metab. 2012;32:50–56. doi: 10.1038/jcbfm.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guadagno J.V., Warburton E.A., Aigbirhio F.I., Smielewski P., Fryer T.D., Harding S., Price C.J., Gillard J.H., Carpenter T.A., Baron J.-C. Does the acute diffusion-weighted imaging lesion represent penumbra as well as core? A combined quantitative PET/MRI voxel-based study. J. Cereb. Blood Flow. Metab. 2004;24:1249–1254. doi: 10.1097/01.WCB.0000141557.32867.6B. [DOI] [PubMed] [Google Scholar]
- 122.Guadagno J.V., Warburton E.A., Jones P.S., Fryer T.D., Day D.J., Gillard J.H., Carpenter T.A., Aigbirhio F.I., Price C.J., Baron J.-C. The diffusion-weighted lesion in acute stroke: heterogeneous patterns of flow/metabolism uncoupling as assessed by quantitative positron emission tomography. Cerebrovasc. Dis. 2005;19:239–246. doi: 10.1159/000084087. [DOI] [PubMed] [Google Scholar]
- 123.Guadagno J.V., Warburton E.A., Jones P.S., Day D.J., Aigbirhio F.I., Fryer T.D., Harding S., Price C.J., Green H.A., Barret O., Gillard J.H., Baron J.-C. How affected is oxygen metabolism in DWI lesions? A combined acute stroke PET-MR study. Neurology. 2006;67:824–829. doi: 10.1212/01.wnl.0000233984.66907.db. [DOI] [PubMed] [Google Scholar]
- 124.d'Esterre C.D., Boesen M.E., Ahn S.H., Pordeli P., Najm M., Minhas P., Davari P., Fainardi E., Rubiera M., Khaw A.V., Zini A., Frayne R., Hill M.D., Demchuk A.M., Sajobi T.T., Forkert N.D., Goyal 1 M., Lee 1 T.Y., Menon B.K. Time-dependent computed tomographic perfusion thresholds for patients with acute ischemic stroke. Stroke. 2015;46:3390–3397. doi: 10.1161/STROKEAHA.115.009250. [DOI] [PubMed] [Google Scholar]
- 125.Qiu W., Kuang H., Lee T.Y., Boers A.M., Brown S., Muir K., Majoie C.B.L.M., Dippel D.W.J., White P., Guillemin F., Mitchell P.J., Dávalos A., Bracard S., Campbell B.C., Saver J.L., Jovin T.G., Hill M.D., Demchuk A.M., Goyal M., Menon B.K. Confirmatory study of time-dependent computed tomographic perfusion thresholds for use in acute ischemic stroke. Stroke. 2019;50:3269–3273. doi: 10.1161/STROKEAHA.119.026281. [DOI] [PubMed] [Google Scholar]
- 126.Yoshie T., Yu Y., Jiang H., Honda T., Trieu H., Scalzo F., Saver J.L., Liebeskind D.S., UCLA Reperfusion Therapy Investigators Perfusion parameter thresholds that discriminate ischemic core vary with time from onset in acute ischemic stroke. AJNR Am. J. Neuroradiol. 2020;41:1809–1815. doi: 10.3174/ajnr.A6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bivard A., Kleinig T., Miteff F., Butcher K., Lin L., Levi C., Parsons M. Ischemic core thresholds change with time to reperfusion: a case control study. Ann. Neurol. 2017;82:995–1003. doi: 10.1002/ana.25109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Laredo C., Renú A., Tudela R., Lopez-Rueda A., Urra X., Llull L., Macías N.G., Rudilosso S., Obach V., Amaro S., Chamorro Á. The accuracy of ischemic core perfusion thresholds varies according to time to recanalization in stroke patients treated with mechanical thrombectomy: a comprehensive whole-brain computed tomography perfusion study. J. Cereb. Blood Flow. Metab. 2020;40:966–977. doi: 10.1177/0271678X19855885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rotem S.-H., Mor S., Chen B., Firas S., Elliot S., Ayelet E., Eitan A., T. Gregory Infarct core reliability by CT perfusion is a time-dependent phenomenon. J. Neuroimaging. 2020;30:240–245. doi: 10.1111/jon.12692. [DOI] [PubMed] [Google Scholar]
- 130.Abrams K., Dabus G. Perfusion scotoma: a potential core underestimation in CT perfusion in the delayed time window in patients with acute ischemic stroke. AJNR Am. J. Neuroradiol. 2022;43:813–816. doi: 10.3174/ajnr.A7524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.García-Tornel Á., Campos D., Rubiera M., Boned S., Olivé-Gadea M., Requena M., Ciolli L., Muchada M., Pagola J., Rodriguez-Luna D., Deck M., Juega J., Rodríguez-Villatoro N., Sanjuan E., Tomasello A., Piñana C., Hernández D., Álvarez-Sabin J., Molina C.A., Ribó M. Ischemic core overestimation on computed tomography perfusion. Stroke. 2021;52:1751–1760. doi: 10.1161/STROKEAHA.120.031800. [DOI] [PubMed] [Google Scholar]
- 132.Siegler J.E., Messé S.R., Sucharew H., Kasner S.E., Mehta T., Arora N., Starosciak A.K., De Los Rios La Rosa F., Barnhill N.R., Mistry A.M., Patel K., Assad S., Tarboosh A., Dakay K., Wagner J., Bennett A., Jagadeesan B., Streib C., Weber S.A., Chitale R., Volpi J.J., Mayer S.A., Yaghi S., Jayaraman M.V., Khatri P., Mistry E.A. Noncontrast CT versus perfusion-based core estimation in large vessel occlusion: the blood pressure after endovascular stroke therapy study. J. Neuroimaging. 2020;30:219–226. doi: 10.1111/jon.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bouslama M., Ravindran K., Rodrigues G.M., Pisani L., Haussen D.C., Frankel M.R., Nogueira R.G. Falsely normal CT perfusion ischemic core readings are common and often associated with deep infarcts. J. Neurointerv. Surg. 2023;15:183–187. doi: 10.1136/neurintsurg-2021-018490. [DOI] [PubMed] [Google Scholar]
- 134.Haussen D.C., Dehkharghani S., Rangaraju S., Rebello L.C., Bouslama M., Grossberg J.A., Anderson A., Belagaje S., Frankel M., Nogueira R.G. Automated CT perfusion ischemic core volume and noncontrast CT ASPECTS (Alberta Stroke Program Early CT Score): correlation and clinical outcome prediction in large vessel stroke. Stroke. 2016;47:2318–2322. doi: 10.1161/STROKEAHA.116.014117. [DOI] [PubMed] [Google Scholar]
- 135.Martins N., Aires A., Mendez B., Boned S., Rubiera M., Tomasello A., Coscojuela P., Hernandez D., Muchada M., Rodríguez-Luna D., Rodríguez N., Juega J.M., Pagola J., Molina C.A., Ribó M. Ghost infarct core and admission computed tomography perfusion: redefining the role of neuroimaging in acute ischemic stroke. Interv. Neurol. 2018;7:513–521. doi: 10.1159/000490117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tsang A.C.O., Lenck S., Hilditch C., Nicholson P., Brinjikji W., Krings T., Pereira V.M., Silver F.L., Schaafsma J.D. Automated CT perfusion imaging versus non-contrast CT for ischemic core assessment in large vessel occlusion. Clin. Neuroradiol. 2020;30:109–114. doi: 10.1007/s00062-018-0745-6. [DOI] [PubMed] [Google Scholar]
- 137.Rodrigues G.M., Mohammaden M.H., Haussen D.C., Bouslama M., Ravindran K., Pisani L., Prater A., Frankel M.R., Nogueira R.G. Ghost infarct core following endovascular reperfusion: A risk for computed tomography perfusion misguided selection in stroke. Int. J. Stroke. 2021;19 doi: 10.1177/17474930211056228. [DOI] [PubMed] [Google Scholar]
- 138.Hoving J.W., Marquering H.A., Majoie C.B.L.M., Yassi N., Sharma G., Liebeskind D.S., van der Lugt A., Roos Y.B., van Zwam W., van Oostenbrugge R.J., Goyal M., Saver J.L., Jovin T.G., Albers G.W., Davalos A., Hill M.D., Demchuk A.M., Bracard S., Guillemin F., Muir K.W., White P., Mitchell P.J., Donnan G.A., Davis S.M., Campbell B.C.V. Volumetric and spatial accuracy of computed tomography perfusion estimated ischemic core volume in patients with acute ischemic stroke. Stroke. 2018;49:2368–2375. doi: 10.1161/STROKEAHA.118.020846. [DOI] [PubMed] [Google Scholar]
- 139.He G., Wei L., Lu H., Deng J., Wang F., Zhu Y. Core overestimation of CT perfusion in patients with cardiac insufficiency who had a stroke is mediated by impaired collaterals. J. Neurointerv. Surg. 2023 doi: 10.1136/jnis-2023-020096. [DOI] [PubMed] [Google Scholar]
- 140.Xu X.-Q., Ma G., Lu S.-S., Shen G.-C., Cao Y.-Z., Liu S., Shi H.-B., Wu F.-Y. Predictors of ghost infarct core on baseline computed tomography perfusion in stroke patients with successful recanalization after mechanical thrombectomy. Eur. Radiol. 2023;33:1792–1800. doi: 10.1007/s00330-022-09189-1. [DOI] [PubMed] [Google Scholar]
- 141.Campbell B.C.V., Majoie C.B.L.M., Albers G.W., Menon B.K., Yassi N., Sharma G., van Zwam W.H., van Oostenbrugge R.J., Demchuk A.M., Guillemin F., White P., Dávalos A., van der Lugt A., Butcher K.S., Cherifi A., Marquering H.A., Cloud G., Macho Fernández J.M., Madigan J., Oppenheim C., Donnan G.A., Roos Y.B.W.E.M., Shankar J., Lingsma H., Bonafé A., Raoult H., Hernández-Pérez M., Bharatha A., Jahan R., Jansen O., Richard S., Levy E.I., Berkhemer O.A., Soudant M., Aja L., Davis S.M., Krings T., Tisserand M., Román L.S., Tomasello A., Beumer D., Brown S., Liebeskind D.S., Bracard S., Muir K.W., Dippel D.W.J., Goyal M., Saver 42 J.L., Jovin T.G., Hill M.D., Mitchell 44 P.J. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. 2019;18:46–55. doi: 10.1016/S1474-4422(18)30314-4. (HERMES collaborators) [DOI] [PubMed] [Google Scholar]
- 142.Wardlaw J.M., Mair Gt, von Kummer R., Williams M.C., Li W., Storkey A.J., Trucco E., Liebeskind D.S., Farrall A., Bath P.M., White P. Accuracy of automated computer-aided diagnosis for stroke imaging: a critical evaluation of current evidence. Stroke. 2022;53:2393–2403. doi: 10.1161/STROKEAHA.121.036204. [DOI] [PubMed] [Google Scholar]
- 143.Bouslama M., Haussen D.C., Rodrigues G., Barreira C., Frankel M., Nogueira R.G. Novel selection paradigms for endovascular stroke treatment in the extended time window. J. Neurol. Neurosurg. Psychiatry. 2021;92:1152–1157. doi: 10.1136/jnnp-2020-325284. [DOI] [PubMed] [Google Scholar]
- 144.Dekker L., Venema E., Pirson F.A.V., Majoie C.B.L.M., Emmer B.J., Jansen I.G.H., Mulder M.J.H.L., Lemmens R., Goldhoorn R.-J.B., Wermer M.J.H., Boiten J., Nijeholt G.J.L.À., Roos Y.W.E.M., van Es A.C.G.M., Lingsma H.F., Dippel D.W.J., van Zwam W.H., van Oostenbrugge R.J., van den Wijngaard I.R., MR CLEAN Registry investigators Endovascular treatment in anterior circulation stroke beyond 6.5 h after onset or time last seen well: results from the MR CLEAN Registry. Stroke Vasc. Neurol. 2021;6:572–580. doi: 10.1136/svn-2020-000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nguyen T.N., Abdalkader M., Nagel S., Qureshi M.M., Ribo M., Caparros F., Haussen D.C., Mohammaden M.H., Sheth S.A., Ortega-Gutierrez S., Siegler J.E., Zaidi S., Olive-Gadea M., Henon H., Möhlenbruch M.A., Castonguay A.C., Nannoni S., Kaesmacher J., Puri A.S., Seker F., Farooqui M., Salazar-Marioni S., Kuhn A.L., Kaliaev A., Farzin B., Boisseau W., Masoud H.E., Lopez C.Y., Rana A., Kareem S.A., Sathya A., Klein P., Kassem M.W., Ringleb P.A., Cordonnier C., Gralla J., Fischer U., Michel P., Jovin T.G., Raymond J., Zaidat O.O., Nogueira R.G. Noncontrast computed tomography vs computed tomography perfusion or magnetic resonance imaging selection in late presentation of stroke with large-vessel occlusion. JAMA Neurol. 2022;79:22–31. doi: 10.1001/jamaneurol.2021.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Porto G.B.F., Chen C.-J., Al Kasab S., Essibayi M.A., Almallouhi E., Hubbard Z., Chalhoub R., Alawieh A., Maier I., Psychogios M.-N., Wolfe S.Q., Jabbour P., Rai A., Starke R.M., Shaban A., A.Arthur J.-T., Kim S., Yoshimura J., Grossberg P., Kan I., Fragata A., Polifka J., Osbun J., Mascitelli M.R., Levitt, Williamson R., Jr, Romano D.G., Crosa R., Gory B., Mokin M., Limaye K.S., Casagrande W., Moss M., Grandhi R., Yoo A., Spiotta 1 A.M., Park M.S., Stroke Thrombectomy and Aneurysm Registry (STAR) Collaborators Association of noncontrast computed tomography and perfusion modalities with outcomes in patients undergoing late-window stroke thrombectomy. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.41291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Demeestere J., Garcia-Esperon C., Garcia-Bermejo P., Ombelet F., McElduff P., Bivard A., Parsons M., Levi C. Evaluation of hyperacute infarct volume using ASPECTS and brain CT perfusion core volume. Neurology. 2017;88:2248–2253. doi: 10.1212/WNL.0000000000004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jadhav A.P., Goyal M., Ospel J., Campbell B.C., Majoie C.B.L.M., Dippel D.W., White P., Bracard S., Guillemin F., Davalos A., Hill M.D., Demchuk A.M., Brown S., Saver J.L., Muir K.W., Mitchell P., Desai S.M., Jovin T.G. Thrombectomy with and without computed tomography perfusion imaging in the early time window: a pooled analysis of patient-level data. Stroke. 2022;53:1348–1353. doi: 10.1161/STROKEAHA.121.034331. [DOI] [PubMed] [Google Scholar]
- 149.Hoving J.W., van Voorst H., Peerlings D., Daems J.D., Koopman M.S., Wouters A., Kappelhof M., LeCouffe N.E., Treurniet K.M., Bruggeman A.A.E., Rinkel L.A., van den Wijngaard I.R., Coutinho J.M., van der Lugt A., Marquering H.A., Roos Y.B.W.E.M., Majoie C.B.L.M., Emmer 3 B.J., MR CLEAN-NO IV Investigators Association between computed tomography perfusion and the effect of intravenous alteplase prior to endovascular treatment in acute ischemic stroke. Neuroradiology. 2023;65:1053–1061. doi: 10.1007/s00234-023-03139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bouslama M., Ravindran K., Harston G., Rodrigues G.M., Pisani L., Haussen 1 D.C., Frankel 1 M.R., Nogueira R.G. Noncontrast computed tomography e-stroke infarct volume is similar to RAPID computed tomography perfusion in estimating postreperfusion infarct volumes. Stroke. 2021;52:634–641. doi: 10.1161/STROKEAHA.120.031651. [DOI] [PubMed] [Google Scholar]
- 151.Parsons M.W. Automated measurement of computed tomography acute ischemic core in stroke: does the emperor have no clothes? Stroke. 2021;52:642–644. doi: 10.1161/STROKEAHA.120.032998. [DOI] [PubMed] [Google Scholar]
- 152.Goyal M., Demchuk A.M., Menon B.K., Eesa M., Rempel J.L., Thornton J., Roy D D., Jovin T.G., Willinsky R.A., Sapkota B.L., Dowlatshahi D., Frei D.F., Kamal N.R., Montanera W.J., Poppe A.Y., Ryckborst K.J., Silver F.L., Shuaib A., Tampieri D., Williams D., Bang O.Y., Baxter B.W., Burns P.A., Choe H., Heo J.H., Holmstedt C.A., Jankowitz B., Kelly M., Linares G., Mandzia J.L., Shankar J., Sohn S.I., Swartz R.H., Barber P.A., Coutts S.B., Smith E.E., Morrish W.F., Weill A., Subramaniam S., Mitha A.P., Wong J.H., Lowerison M.W., Sajobi T.T., Hill M.D., ESCAPE Trial Investigators Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 153.Kim B., Jung C., Nam H.S., Kim B.M., Kim Y.D., Heo J.H., Kim D.J., Kim J.-H., Han K., Kim J.H., Kim B.J. Comparison between perfusion- and collateral-based triage for endovascular thrombectomy in a late time window. Stroke. 2019;50:3465–3470. doi: 10.1161/STROKEAHA.119.027216. [DOI] [PubMed] [Google Scholar]
- 154.Almekhlafi M.A., Kunz W.G., McTaggart R.A., Jayaraman M.V., Najm M., Ahn S.H., Fainardi E., Rubiera M., Khaw A.V., Zini A.M.D., Hill A.M., Demchuk M., Goyal M., Menon B.K. Imaging triage of patients with late-window (6–24 h) acute ischemic stroke: a comparative study using multiphase CT angiography versus CT perfusion. AJNR Am. J. Neuroradiol. 2020;41:129–133. doi: 10.3174/ajnr.A6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang Z., Xie J., Tang T.-Y., Zeng C.-H., Zhang Y., Zhao Z., Zhao D.-L., Geng L.-Y., Deng G., Zhang Z.-J., Ju S.-H., Teng G.-J. Collateral status at single-phase and multiphase CT angiography versus CT perfusion for outcome prediction in anterior circulation acute ischemic stroke. Radiology. 2020;296:393–400. doi: 10.1148/radiol.2020192029. [DOI] [PubMed] [Google Scholar]
- 156.Menon B.K., Ospel J.M., McTaggart R.A., Nogueira R.G., Demchuk A.M., Poppe A., Rempel J.L., Zerna C., Joshi M., M.A, Field T.S., Dowlatshahi D., van Adel B.A., Sauvageau E., Tarpley J., Moreira T., Bang O.Y., Heck D., Psychogios M.N., Tymianski M., Hill M.D., Goyal M., ESCAPE-NA1 investigators Imaging criteria across pivotal randomized controlled trials for late window thrombectomy patient selection. J. Neurointerv. Surg. 2020 doi: 10.1136/neurintsurg-2020-016902. [DOI] [PubMed] [Google Scholar]
- 157.Qiu W., Kuang H., Ospel J.M., Hill M.D., Demchuk A.M., Goyal M., Menon B.K. Automated prediction of ischemic brain tissue fate from multiphase computed tomographic angiography in patients with acute ischemic stroke using machine learning. J. Stroke. 2021;23:234–243. doi: 10.5853/jos.2020.05064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Almekhlafi M.A., Thornton J., Casetta I., Goyal M., Nannoni S., Herlihy D., Fainardi E., Power S., Saia V., Hegarty A., Pracucci G., Demchuk A., Mangiafico S., Boyle K., Michel P., Bala F., Gill R., Kuczynski A., Ademola A., Hill M.D., Toni D., Murphy S., Kim B.J., Menon B.K. Selection of late-window stroke for thrombectomy by imaging collateral extent (SOLSTICE) consortium. Stroke imaging prior to thrombectomy in the late window results from a pooled multicentre analysis. J. Neurol. Neurosurg. Psychiatry. 2022;93:468–474. doi: 10.1136/jnnp-2021-327959. [DOI] [PubMed] [Google Scholar]
- 159.Olthuis S.G.H., Pirson F.A.V., Pinckaers F.M.E., Hinsenveld W.H., Nieboer D., Ceulemans A., Knapen R.R.M.M., Robbe M.M.Q., Berkhemer O.A., van Walderveen M.A.A., Lycklama G.J., Nijeholt À., Uyttenboogaart M., Schonewille W.J., van der Sluijs P.M., Wolff L., van Voorst H., Postma A.A., Roosendaal S.D., van der Hoorn A., Emmer B.J., Krietemeijer M.G.M., van Doormaal P.-J., Roozenbeek B., Goldhoorn R.-J.B., Staals J., de Ridder I.R., van der Leij C., Coutinho J.M., van der Worp H.B., Lo R.T.H., Bokkers R.P.H., van Dijk E.I., Boogaarts H.D., Wermer M.J.H., Adriaan C.G.M. van Es, van Tuijl J.H., Kortman H.G.J., Gons R.A.R., Yo L.S.F., Vos J.-A., de Laat K.F., van Dijk L.C., van den Wijngaard I.R., Hofmeijer J., Martens J.M., Brouwers P.J.A.M., Bulut T., Remmers M.J.M., de Jong T.E.A.M., den Hertog H.M., van Hasselt B.A.A.M., Rozeman A.D., Elgersma O.E.H., van der Veen B., Sudiono D.R., Lingsma H.F., Roos Y.B.W.E.M., Majoie C.B.L.M., van der Lugt A., Dippel D.W.J., van Zwam W.H., van Oostenbrugge R.J., MR CLEAN-LATE investigators Endovascular treatment versus no endovascular treatment after 6–24 h in patients with ischaemic stroke and collateral flow on CT angiography (MR CLEAN-LATE) in the Netherlands: a multicentre, open-label, blinded-endpoint, randomised, controlled, phase 3 trial. Lancet. 2023;401:1371–1380. doi: 10.1016/S0140-6736(23)00575-5. [DOI] [PubMed] [Google Scholar]
- 160.Rao V.L., Mlynash M., Christensen S., Yennu A., Kemp S., Zaharchuk G., Heit J.J., Marks M.P., Lansberg M.G., Albers G.W. Collateral status contributes to differences between observed and predicted 24-h infarct volumes in DEFUSE 3. J. Cereb. Blood Flow. Metab. 2020;40:1966–1974. doi: 10.1177/0271678X20918816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vagal A., Aviv R., Sucharew H., Reddy M., Hou Q., Michel P., Jovin T., Tomsick T., Wintermark M., Khatri P. Collateral clock is more Important than time clock for tissue fate. A natural history study of acute ischemic strokes. Stroke. 2018;49:2102–2107. doi: 10.1161/STROKEAHA.118.021484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Agarwal S., Bivard A., Warburton E., Parsons M., Levi C. Collateral response modulates the time-penumbra relationship in proximal arterial occlusions. Neurology. 2018;90:e316–e322. doi: 10.1212/WNL.0000000000004858. [DOI] [PubMed] [Google Scholar]
- 163.Fladt J., d'Esterre C.D., Joundi R., McDougall C., Gensicke H., Barber P. Acute stroke imaging selection for mechanical thrombectomy in the extended time window: is it time to go back to basics? A review of current evidence. J. Neurol. Neurosurg. Psychiatry. 2022;93:238–245. doi: 10.1136/jnnp-2021-328000. [DOI] [PubMed] [Google Scholar]
- 164.Dong Z., Deng S., Zhang J., Chen S., Ye Z., Zhang L., Hu R., Zhong C., Liu X., Qin C. Simplified stroke imaging selection modality for endovascular thrombectomy in the extended time window: systematic review and meta-analysis. J. Neurointerv Surg. Dec. 2022;7 doi: 10.1136/jnis-2022-019556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Albers G.W. Late window paradox. Stroke. 2018;49:768–771. doi: 10.1161/STROKEAHA.117.020200. [DOI] [PubMed] [Google Scholar]
- 166.Lansberg M.G., Christensen S., Kemp S., Mlynash M., Mishra N., Federau C., Tsai J.P., Kim S., Nogueria R.G., Jovin T., Devlin T.G., Akhtar N., Yavagal D.R., Haussen D., Dehkharghani S., Bammer R., Straka M., Zaharchuk G., Marks M.P., Albers G.W., CT Perfusion to Predict Response to Recanalization in Ischemic Stroke Project (CRISP) Investigators Computed tomographic perfusion to predict response to recanalization in ischemic stroke. Ann. Neurol. 2017;81:849–856. doi: 10.1002/ana.24953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wannamaker R., Guinand T., Menon B.K., Demchuk A., Goyal M., Frei D., Bharatha A., Jovin T.G., Shankar J., Krings T., Baxter B., Holmstedt C., Swartz R., Dowlatshahi D., Chan R., Tampieri D., Choe H., Burns P., Gentile N., Rempel J., Shuaib A., Buck B., Bivard A., Hill M., Butcher K. Computed tomographic perfusion predicts poor outcomes in a randomized trial of endovascular therapy. Stroke. 2018;49:1426–1433. doi: 10.1161/STROKEAHA.117.019806. [DOI] [PubMed] [Google Scholar]
- 168.Demeestere J., Scheldeman L., Cornelissen S.A., Heye S., Wouters A., Dupont P., Christensen S., Mlynash M., Albers G.W., Lansberg M., Lemmens R. Alberta stroke program early CT Score versus computed tomographic perfusion to predict functional outcome after successful reperfusion in acute ischemic stroke. Stroke. 2018;49:2361–2367. doi: 10.1161/STROKEAHA.118.021961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Olivot J.-M., Albucher J.-F., Guenego A., Thalamas C., Mlynash M., Rousseau V., Drif A., Christensen S., Sommet A., Viguier A., Darcourt J., Calvière L., Menegon P., Raposo N., Januel A.-C., Bonneville F., Tourdias T., Mazighi M., Sibon I., Albers G.W., Cognard C., FRAME Investigators Mismatch profile influences outcome after mechanical thrombectomy. Stroke. 2021;52:232–240. doi: 10.1161/STROKEAHA.120.031929. [DOI] [PubMed] [Google Scholar]
- 170.Rao V., Christensen S., Yennu A., Mlynash M., Zaharchuk G., Heit J., Marks M.P., Lansberg M.G., Albers G.W. Ischemic core and hypoperfusion volumes correlate with infarct size 24 h after randomization in DEFUSE 3. Stroke. 2019;50:626–631. doi: 10.1161/STROKEAHA.118.023177. [DOI] [PubMed] [Google Scholar]
- 171.Voleti S., Vidovich J., Corcoran B., Zhang B., Khandwala V., Mistry E.A., Khatri P., Tomsick T., Vagal A. Correlation of Alberta stroke program early computed tomography score with computed tomography perfusion core in large vessel occlusion in delayed time windows. Stroke. 2021;52:498–504. doi: 10.1161/STROKEAHA.120.030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tsivgoulis G., Katsanos A.H., Schellinger P.D., Köhrmann M., Caso V., Palaiodimou L., Magoufis G., Arthur A., Fischer U., Alexandrov A.V. Advanced neuroimaging in stroke patient selection for mechanical thrombectomy. A systematic review and meta-analysis. Stroke. 2018;49:3067–3070. doi: 10.1161/STROKEAHA.118.022540. [DOI] [PubMed] [Google Scholar]
- 173.Schwarz G., Agostoni E.C., Saliou G., Hajdu S.D., Salerno A., Dunet V., Michel P., Strambo D. Perfusion imaging mismatch profiles in the early thrombectomy window: a single-center analysis. Stroke. 2023;54:1182–1191. doi: 10.1161/STROKEAHA.122.041981. [DOI] [PubMed] [Google Scholar]
- 174.Alzahrani A., Zhang X., Albukhari A., Wardlaw J.M., Mair G. Assessing brain tissue viability on nonenhanced computed tomography after ischemic stroke. Stroke. 2023;54:558–566. doi: 10.1161/STROKEAHA.122.041241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tan Z., Parsons M., Bivard A., Sharma G., Mitchell P., Dowling R., Bush S., Churilov L., Xu A., Yan B. Comparison of computed tomography perfusion and multiphase computed tomography angiogram in predicting clinical outcomes in endovascular thrombectomy. Stroke. 2022;53:2926–2934. doi: 10.1161/STROKEAHA.122.038576. [DOI] [PubMed] [Google Scholar]
- 176.Olivot J.-M., Heit J.J., Mazighi M., Raposo N., Albucher J.F., Rousseau V., Guenego A., Thalamas C., Mlynash M., Drif A., Christensen S., Sommet A., Viguier A., Darcourt J., Januel A.-C., Calviere Ll, Menegon P., Caparros F., Bonneville F., Tourdias T., Sibon I., Albers G.W., Cognard C., FRAME Investigators What predicts poor outcome after successful thrombectomy in early time window? J. Neurointerv. Surg. 2022;14:1051–1055. doi: 10.1136/neurintsurg-2021-017946. [DOI] [PubMed] [Google Scholar]
- 177.Lansberg M.G., Cereda C.W., Mlynash M., Mishra N.K., Inoue M., Kemp S., Christensen S., Straka M., Zaharchuk G., Marks M.P., Bammer R., Albers G.W., Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2 (DEFUSE 2) Study Investigators Response to endovascular reperfusion is not time-dependent in patients with salvageable tissue. Neurology. 2015;85:708–714. doi: 10.1212/WNL.0000000000001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bivard A., Parsons M. Tissue is more important than time: insights into acute ischemic stroke from modern brain imaging. Curr. Opin. Neurol. 2018;31:23–27. doi: 10.1097/WCO.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 179.von Kummer R. Treatment of ischemic stroke beyond 3 h: is time really brain? Neuroradiology. 2019;61:115–117. doi: 10.1007/s00234-018-2122-1. [DOI] [PubMed] [Google Scholar]
- 180.Puig J., Shankar J., Liebeskind D., Terceño M., Nael K., Demchuk A.M., Menon B., Dowlatshahi D., Leiva-Salinas C., Wintermark M., Thomalla G., Silva Y., Serena J., Pedraza S., Essig M. From "time is brain" to "imaging is brain": a paradigm shift in the management of acute schemic stroke. J. Neuroimaging. 2020;30:562–571. doi: 10.1111/jon.12693. [DOI] [PubMed] [Google Scholar]
- 181.Sarraj A., Hassan A.E., Grotta J., Sitton C., Cutter G., Cai C., Chen P.R., Imam B., Pujara D., Arora A., Reddy S., Parsha K., Riascos R.F., Vora N., Abraham M., Edgell R., Hellinger F., Haussen D.C., Blackburn S., Kamal H., Barreto A.D., Martin-Schild S., Lansberg M., Gupta R., Savitz S., Albers G.W. Optimizing patient selection for endovascular treatment in acute ischemic stroke (SELECT): a prospective, multicenter cohort study of imaging selection. Ann. Neurol. 2020;87:419–433. doi: 10.1002/ana.25669. [DOI] [PubMed] [Google Scholar]
- 182.Ospel J.M., Volny O., Qiu W., Najm M., Hafeez M., Abdalrahman S., Fainardi E., Rubiera M., Khaw A., Shankar J.J., Hill M.D., Almekhlafi M.A., Demchuk A.M., Goyal M., Menon B.K. Impact of multiphase computed tomography angiography for endovascular treatment decision-making on outcomes in patients with acute ischemic stroke. J. Stroke. 2021;23:377–387. doi: 10.5853/jos.2021.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dhillon P.S., Butt W., Podlasek A., McConachie N., Lenthall R., Nair S., Malik L., Bhogal P., Makalanda H.L.D., Spooner O., Krishnan K., Sprigg N., Mortimer A., Booth T.C., Lobotesis K., White P., James M.A., Bath P., Dineen R.A., England T.J. Association between time to treatment and clinical outcomes in endovascular thrombectomy beyond 6 h without advanced imaging selection. J. Neurointerv. Surg. 2023;15:336–342. doi: 10.1136/neurintsurg-2021-018564. [DOI] [PubMed] [Google Scholar]
- 184.Dhillon P.S., Butt W., Podlasek A., McConachie N., Lenthall R., Nair S., Malik L., Booth T.C., Bhogal P., Makalanda H.L.D., Spooner O., Mortimer A., Lamin S., Chavda S., Chew H.S., Nader K., Al-Ali S., Butler B., Rajapakse D., Appleton J.P., Krishnan K., Sprigg N., Smith A., Lobotesis K., White P., James M.A., Bath P.M., Dineen R.A., England T.J. Perfusion imaging for endovascular thrombectomy in acute ischemic stroke is associated with improved functional outcomes in the early and late time windows. Stroke. 2022;53:2770–2778. doi: 10.1161/STROKEAHA.121.038010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mocco J., Siddiqui A.H., Fiorella D., Alexander M.J., Arthur A.S., Baxter B.W., Budzik R.F., Froehler M.T., Hanel R.A., Lena J., Persaud S., Puri A.S., Rai A.T., Wintermark M., Woodward K., Zhang X., Turk A. POSITIVE: perfusion imaging selection of ischemic stroke patients for endovascular therapy. J. Neurointerv. Surg. 2022;14:126–132. doi: 10.1136/neurintsurg-2021-017315. [DOI] [PubMed] [Google Scholar]
- 186.Casetta I., Fainardi E., Saia V., Pracucci G., Padroni M., Renieri L., Nencini P., Inzitari D., Morosetti D., Sallustio F., Vallone S., Bigliardi G., Zini A., Longo M., Francalanza I., Bracco S., Vallone I.M., Tassi R., Bergui M., Naldi A., Saletti A., De Vito A., Gasparotti R., Magoni M., Castellan L., Serrati C., Menozzi R., Scoditti U., Causin F., Pieroni A., Puglielli E., Casalena A., Sanna A., Ruggiero M., Cordici F., Di Maggio L., Duc E., Cosottini M., Giannini N., Sanfilippo G., Zappoli F., Cavallini A., Cavasin N., Critelli A., Ciceri E., Plebani M., Cappellari M., Chiumarulo L., Petruzzellis M., Terrana A., Cariddi L.P., Burdi N., Tinelli A., Auteri W., Silvagni U., Biraschi F., Nicolini E., Padolecchia R., Tassinari T., Filauri P., Sacco S., Pavia M., Invernizzi P., Nuzzi N.P., Marcheselli S., Amistà P., Russo M., Gallesio I., Craparo G., Mannino M., Mangiafico S., Toni D. Endovascular thrombectomy for acute ischemic stroke beyond 6 h from onset: a real-world experience. Stroke. 2020;51:2051–2057. doi: 10.1161/STROKEAHA.119.027974. (Italian registry of endovascular treatment in acute stroke) [DOI] [PubMed] [Google Scholar]
- 187.Kobeissi H., Ghozy S., Adusumilli G., Bilgin C., Tolba H., Amoukhteh M., Kadirvel R., Brinjikji W., Heit J.J., Rabinstein A.A., Kallmes D.F. CT Perfusion vs noncontrast CT for late window stroke thrombectomy: a systematic review and meta-analysis. Neurol. Mar. 2023;29 doi: 10.1212/WNL.0000000000207262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fainardi E., Busto G., Rosi A., Scola E., Casetta I., Bernardoni A., Saletti A., Arba F., Nencini P., Limbucci N., Mangiafico S., Demchuk A., Almekhlafi M.A., Goyal M., Lee T.Y., Menon B.K., Morotti A. Tmax volumes predict final infarct size and functional outcome in ischemic stroke patients receiving endovascular treatment. Ann. Neurol. 2022;91:878–888. doi: 10.1002/ana.26354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.McDonough R.V., Qiu W., Ospel J.M., Menon B.K., Cimflova P., Goyal M. Multiphase CTA-derived tissue maps aid in detection of medium vessel occlusions. Neuroradiology. 2022;64:887–896. doi: 10.1007/s00234-021-02830-8. [DOI] [PubMed] [Google Scholar]
- 190.Qiu W., Kuang H., Ospel J.M., Hill M.D., Demchuk A.M., Goyal M., Menon B.K. Automated prediction of ischemic brain tissue fate from multiphase computed tomographic angiography in patients with acute ischemic stroke using machine learning. J. Stroke. 2021;23:234–243. doi: 10.5853/jos.2020.05064. [DOI] [PMC free article] [PubMed] [Google Scholar]