Abstract
The Abbott LCx Neisseria gonorrhoeae assay (Abbott Laboratories, Abbott Park, Ill.) uses a ligase chain reaction (LCR) amplification in the LCx probe system for detection of a specific nucleotide sequence in the Opa-encoding gene of N. gonorrhoeae. We evaluated the LCx assay in a comparison with conventional culture employing modified Thayer-Martin media for the detection of N. gonorrhoeae from female endocervical specimens obtained from patients attending a sexually transmitted disease clinic. Discordantly LCR-positive and culture-negative specimens were further evaluated by testing with another LCR assay which used an N. gonorrhoeae-specific pilin probe. Specimens positive by both LCR assays were considered confirmed LCx-positive specimens. A specimen was considered to contain N. gonorrhoeae when it was either culture positive or culture negative and confirmed LCx positive. A total of 403 female endocervical specimens were evaluated. The prevalence of N. gonorrhoeae in this population was 8.7%. The sensitivity and specificity of the LCx assay were 94.3 and 99.4%, and those of culture were 77.1 and 100%, respectively. The Abbott LCx assay is a rapid, sensitive method for detection of N. gonorrhoeae in female endocervical specimens.
Traditionally, culture of female endocervical specimens using various types of modified Thayer-Martin media has been the standard for diagnosis of females infected with Neisseria gonorrhoeae. Nonculture methods, such as enzyme immunoassays and DNA probes, have become available in recent years. The advantages to nonculture methods include rapid turnaround time, batching of tests, and the ability to detect nonviable N. gonorrhoeae. The enzyme immunoassay methods have reported sensitivities ranging from 60.8% (12) to 92.4% (5) when tested on female endocervical specimens and 62.5% (11) when tested on female urine specimens. The DNA probe assay has a reported sensitivity ranging from 96.3% (7) to 100% (10) and a specificity of greater than 99% (6, 7, 10, 13). In several studies comparing culture to DNA probe assays, reported culture sensitivities have ranged from 88.9% (7) to 90.6% (10). This suggests that molecular methods may be an improvement over traditional culture.
The Abbott LCx assay is a nonculture method which employs ligase chain reaction (LCR) amplification in the LCx probe system for detection of N. gonorrhoeae. We compared traditional culture of female endocervical specimens to the LCx assay to determine if the LCx assay is an acceptable alternative to culture.
(This study was presented in part at the 98th General Meeting of the American Society for Microbiology [7a].)
MATERIALS AND METHODS
Specimens.
Specimens were obtained from 408 females attending the City of Milwaukee Health Department’s sexually transmitted disease clinic. The study protocol was approved by the Medical College of Wisconsin Human Research Review Committee, and informed consent was obtained prior to specimen collection. The LCx Uriprobe Swab Specimen Collection and Transport Kit (Abbott Laboratories, Abbott Park, Ill.) was used to collect endocervical specimens from 408 consecutive women seen at the clinic. The large-tipped cleaning swab was used to remove excess cervical mucus. The small-tipped swab was inserted into the endocervix and rotated for 15 to 30 s. This swab was inserted into the transport tube. A second swab was also inserted into the endocervix and rotated for 15 to 30 s. This swab was used to inoculate a modified Thayer-Martin agar plate at the bedside. The order of swab collection was varied so as not to introduce bias into the data. The LCx transport tubes were stored at 2 to 8°C for up to 4 days or at −20°C until testing was performed.
Culture.
Inoculated modified-Thayer-Martin agar plates (DIMed, St. Paul, Minn.) were incubated at 35 ± 2°C in 5% CO2 for up to 72 h. Quality control of the media was performed in accordance with National Committee for Clinical Laboratory Standards document M22-A2 (9). Cultures were examined daily. Oxidase-positive, gram-negative diplococci were identified as presumptive N. gonorrhoeae. Organisms were definitively identified based on the ability to utilize carbohydrates in Cysteine Trypticase Agar medium (Becton Dickinson, Cockeysville, Md.). Organisms consistent with N. gonorrhoeae but not identified as such by biochemical testing were resolved by using a monoclonal antibody coagglutination test (GonoGen; Becton Dickinson).
LCx assay.
The LCx assay was performed in accordance with the manufacturer’s recommendations. Processed specimens were amplified by using the LCx Thermal Cycler. Amplified products were detected by using the LCx Analyzer. A positive control of N. gonorrhoeae (ATCC 27631) prepared by following the manufacturer’s recommendations was included in each run to monitor the entire assay procedure, including specimen processing. Negative controls and calibrators were run in duplicate and included with each run as provided by the manufacturer. A ratio of the sample rate (S) to the cutoff value (CO) was calculated. Specimens were considered positive when the S/CO ratio was ≥1.20. Specimens with S/CO ratios between 0.80 and 1.20 were considered equivocal and retested. If the repeat test ratio was less than 1.00, the test was considered negative.
Analysis of discordant specimens.
Specimens which were culture negative and LCx positive or culture positive and LCx negative were frozen at −20°C. These specimens were then tested at Abbott Laboratories by using a second LCR assay which employed the pilin probe set. This pilin probe set is specific for N. gonorrhoeae (2).
Statistical methods.
Sensitivity and specificity calculations were performed by standard methods (8). The McNemar test was used to determine the difference between the two methods when matched specimens were used (1a).
RESULTS
Of the specimens collected from 408 women seen at the Milwaukee Health Department, the cultures of four patients were not inoculated and the incorrect LCx swab was submitted for one patient. A total of 403 endocervical specimens were tested by both the LCx assay and culture. Of those, 365 were negative for N. gonorrhoeae by both the LCx assay and culture. Twenty-five specimens (6.2%) were positive for N. gonorrhoeae by both the LCx assay and culture. Two specimens were equivocal on initial LCx testing. One of the specimens was determined to be negative on repeat testing, and this correlated with the culture results. The second specimen was also determined to be negative on repeat testing; however, this did not correlate with the positive culture. After repeat testing of these equivocal specimens, there were a total of 13 specimens whose LCx results did not correlate with the culture results. These discordant specimens were retested by using the pilin LCR assay. Two of these were LCx negative, culture positive, and negative by the pilin assay. Eleven were LCx positive and culture negative; of these, three were negative and eight were positive by the pilin assay (Table 1). There was no association between swab collection order and results. After resolution of the discrepant results, the sensitivity and specificity of the LCx assay based upon the initial LCx assay result were 94.3 and 99.4%, respectively. The sensitivity and specificity of culture were 77.1 and 100%, respectively.
TABLE 1.
Comparison of methods for detection of N. gonorrhoeae
| Results obtained with culture/LCx assay | Resolved status | No. of specimens |
|---|---|---|
| Positive/positive | Positive | 25 |
| Positive/negative | Positive | 1 |
| Presumptively positive/negative | Positive | 1 |
| Negative/positive | Positive | 8 |
| Negative/positive | Negative | 3 |
| Negative/negative | Negative | 365 |
DISCUSSION
There were two LCx-negative, culture-positive specimens. One specimen was equivocal on initial testing (S/CO = 0.98), negative on repeat testing (S/CO = 0.13), and pilin LCR assay negative. This was considered a false-negative LCx assay result. The specimen was not tested to determine if this could be due to the presence of an inhibitor. However, in other studies in which specimens were diluted and retested, these specimens did become positive (4), suggesting that an inhibitor was present. A second specimen was also culture positive yet negative by the LCx and pilin assays. The positive culture report had been based on Gram staining and oxidase production of a colony on modified Thayer-Martin medium. No organism could be propagated for definitive identification. The organism may not have been a Neisseria species, it may have been a Neisseria species other than N. gonorrhoeae, or it may have been rendered nonviable by the oxidase reagent. Despite this, the specimen was considered falsely negative by the LCx assay.
There were 11 specimens which were LCx positive and culture negative. Eight of these specimens were confirmed as positive by the pilin assay, while the results for three of these specimens were not confirmed. Therefore, eight specimens were considered falsely negative by culture. Reasons for false-negative cultures could include prior antimicrobial therapy, loss of viability of the organisms due to transport, sampling error, or selective inhibition due to vancomycin in the media. Only one of these eight patients reported antibiotic use in the weeks previous to testing. That patient had been on penicillin for 2 weeks. In that patient, the positive LCR could have been due to detection of nonviable organisms. The media were plated at bedside and immediately incubated, thus effectively eliminating any loss of viability due to transport. It is unlikely that the low sensitivity of culture is related to sampling error, as there was no significant difference in the recovery of N. gonorrhoeae from specimens collected before versus those collected after the LCR swab. The media employed did contain vancomycin so that vancomycin-susceptible N. gonorrhoeae would not have been detected. However, when both vancomycin-containing media and media without vancomycin have been employed in our setting, no vancomycin-susceptible N. gonorrhoeae has been detected.
Three specimens were considered falsely positive by the LCx assay. There can be significant psychological and social impact from false-positive N. gonorrhoeae reports. For this reason, we examined the false-positive results more closely. According to the manufacturer, specimens with S/CO ratios of 0.8 to 1.2, which fall within the equivocal zone, should be retested. If the retest S/CO ratio is >1, the specimen is considered positive. All of the specimens which fell within the equivocal zone were negative upon repeat testing. However, all three of the false-positive specimens fell above the gray zone. One specimen had an S/CO ratio of 1.31, and a second specimen had an S/CO ratio of 1.26. According to the package insert, these should be considered positive results. Repeat testing, the pilin assay, and culture confirmed that both of these specimens were, in fact, negative. The third specimen had an original S/CO ratio of 4.02 and an S/CO ratio of 3.06 on repeat testing. The pilin assay was negative. The pilin assay is not as sensitive as the LCx assay (1) and thus may not have been able to detect low numbers of organisms if they were present. Alternatively, the LCx assay result may have been falsely positive or the pilin assay result may have been falsely negative due to some interfering substance. If the specimen was positive because of contamination during specimen processing, the pilin assay would have been expected to be positive also. Negative controls included in each assay or testing of laboratory surfaces and equipment performed routinely to monitor for contamination might have been expected to be positive if contamination had occurred. None of these were positive during any of the testing.
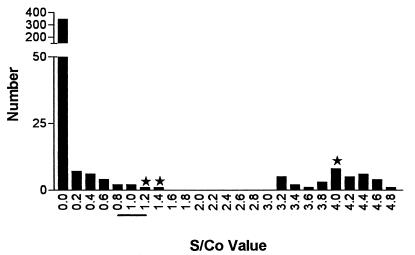
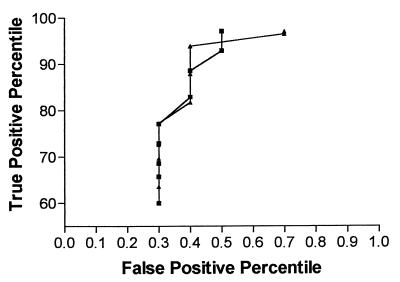
Examination of the frequency histogram of the S/CO results (Fig. 1) demonstrates a clear separation of the true-positive and -negative results. Expansion of the gray zone to S/CO ratios of 0.8 to 2.0 would allow for the detection of low-level false-positive results without significantly increasing the number of repeat tests. Analysis of receiver operating characteristic curves (Fig. 2) by utilizing the manufacturer’s equivalent zone and the expanded equivalent zone suggests that a wider gray zone could decrease the number of false-positive reports and improve the sensitivity and specificity of the assay.
FIG. 1.
Frequency distribution of S/CO ratios. ★, false-positive S/CO ratio; —, manufacturer’s equivocal zone.
FIG. 2.
Receiver operating characteristic curves. Symbols: ▴, manufacturer’s equivocal zone; ■, proposed equivocal zone.
The positive predictive value of the LCx assay in our population, with a prevalence of 8.4%, was 91.7%. In a low-prevalence population, the positive predictive value would decrease. In this setting, expansion of the gray zone could be an important step in improving the predictive value of positive results by decreasing the number of false-positive results.
Of some concern is the low sensitivity of the “gold standard” traditional culture method. The bedside plating and immediate incubation employed during this study certainly allowed for optimum recovery, and yet the resolved sensitivity was only 77.1%. These findings are consistent with the 84% reported by Ching et al. (4) and the 50% reported by Buimer et al. (3) and support the suggestion by Ching et al. (4) that N. gonorrhoeae infection in females is significantly underdiagnosed.
In this study, the LCx assay had a resolved sensitivity of 94.3%. This is similar to that reported by Ching et al. and Buimer et al. (3, 4). Although the sample number was small, these data suggest that the LCx assay is a statistically significantly more sensitive test (McNemar test, P = 0.0265) than culture for detection of N. gonorrhoeae in female endocervical specimens.
ACKNOWLEDGMENT
This study was supported in part by Abbott Laboratories, Inc., Abbott Park, Ill.
REFERENCES
- 1.Abbott Laboratories. Personal communication.
- 1a.Beam C A. Strategies for improving power in diagnostic research. Am J Radiol. 1992;159:631–637. doi: 10.2214/ajr.159.3.1503041. [DOI] [PubMed] [Google Scholar]
- 2.Birkenmeyer L, Blog F, Armstrong A S. Preliminary evaluation of the ligase chain reaction for specific detection of Neisseria gonorrhoeae. J Clin Microbiol. 1992;30:3089–3094. doi: 10.1128/jcm.30.12.3089-3094.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buimer M, van Doornum G J J, Ching S, Peerbooms P G, Plier P K, Ram D, Lee H H. Detection of Chlamydia trachomatis and Neisseria gonorrhoeae by ligase chain reaction-based assays with clinical specimens from various sites: implication for diagnostic testing and screening. J Clin Microbiol. 1996;34:2395–2400. doi: 10.1128/jcm.34.10.2395-2400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ching S, Lee H H, Hook E W, Jacobs M R, Zenilman J. Ligase chain reaction for detection of Neisseria gonorrhoeae in urogenital swabs. J Clin Microbiol. 1995;33:3111–3114. doi: 10.1128/jcm.33.12.3111-3114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donders G G, van Gerven V, de Wet H G, van Straten A M, de Boer F. Rapid antigen tests for Neisseria gonorrhoeae and Chlamydia trachomatis are not accurate for screening women with disturbed vaginal lactobacillary flora. Scand J Infect Dis. 1996;28:559–562. doi: 10.3109/00365549609037960. [DOI] [PubMed] [Google Scholar]
- 6.Hale Y M, Melton M E, Lewis J S, Willis D E. Evaluation of the PACE 2 Neisseria gonorrhoeae assay by three public health laboratories. J Clin Microbiol. 1993;31:451–453. doi: 10.1128/jcm.31.2.451-453.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwen P C, Walker R A, Warren K L, Kelly D M, Hinrichs S H, Linder J. Evaluation of nucleic acid-based test (PACE 2C) for simultaneous detection of Chlamydia trachomatis and Neisseria gonorrhoeae in endocervical specimens. J Clin Microbiol. 1996;33:2587–2591. doi: 10.1128/jcm.33.10.2587-2591.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Kehl S, Georgakas K, Sedmak G, Gradus S, Singh A, Foldy S. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Comparison of Abbott LCx ligase chain reaction with culture for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in endocervical specimens, abstr. C-53; p. 140. [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. Assessment of the clinical accuracy of laboratory tests using receiver operating characteristic (ROC) plots; approved guideline. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Quality assurance for commercially prepared microbiological culture media—second edition; approved standard. NCCLS document M22-A2. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1996. [Google Scholar]
- 10.Stary A, Kopp B, Zahel B, Nerad S, Teodorowicz L, Horting-Muller I. Comparison of DNA-probe test and culture for the detection of Neisseria gonorrhoeae in genital samples. Sex Transm Dis. 1993;20:243–247. doi: 10.1097/00007435-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Thomas E, Scott S D, Grefkees I, Hession G, Pollock R, Martin T, Alvritton W. Validity and cost-effectiveness of the Gonozyme test in the diagnosis of gonorrhea. Can Med Assoc J. 1986;134:121–124. [PMC free article] [PubMed] [Google Scholar]
- 12.Thomason J L, Gelbart S M, Sobieski V J, Anderson R J, Schulien M B, Hamilton P R. Effectiveness of Gonozyme for detection of gonorrhea in low-risk pregnant and gynecologic populations. Sex Transm Dis. 1989;16:28–31. doi: 10.1097/00007435-198901000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Valspolder F, Mutsaers J A, Blog F, Notowicz A. Value of a DNA probe assay (Gen-Probe) compared with that of culture for diagnosis of gonococcal infection. J Clin Microbiol. 1993;31:107–110. doi: 10.1128/jcm.31.1.107-110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]