Abstract
Accumulating evidence suggests that disrupted brain insulin signaling promotes the development and progression of Alzheimer’s disease (AD), driving clinicians to target this circuitry. While both traditional and more modern antidiabetics show promise in combating insulin resistance, intranasal insulin appears to be the most efficient method of boosting brain insulin. Furthermore, intranasal delivery elegantly avoids adverse effects from peripheral insulin administration. However, there remain significant open questions regarding intranasal insulin’s efficacy, safety, and potential as an adjunct or mono-therapy. Thus, this review aims to critically evaluate the present evidence and future potential of intranasal insulin as a meaningful treatment for AD.
This article is part of the Special Issue entitled ‘Metabolic Impairment as Risk Factors for Neurodegenerative Disorders.’
Keywords: Alzheimer’s disease, Brain insulin resistance, Intranasal insulin, Cognition, Neurodegeneration
1. The Alzheimer’s disease insulin connection
Alzheimer’s disease (AD) is hallmarked by amyloid beta (Aβ) plaques, neurofibrillary tangles, widespread cortical neuronal loss, and cognitive impairment (Selkoe, 2001; Hardy and Selkoe, 2002; Iqbal et al., 2016). Several lines of evidence converge to suggest that central insulin resistance plays a causal role in the development and progression of AD (Ma et al., 2009; De Felice et al., 2009). For instance, several landmark studies have revealed reduced brain insulin receptor sensitivity and insulin receptor expression in post-mortem AD brains (Steen et al., 2005; Moloney et al., 2010; Talbot et al., 2012). Additionally, diabetes, a condition inextricably linked to insulin resistance, has also been identified as a significant risk factor for developing AD. For instance, a recent meta-analysis of longitudinal population-based studies (involving 1,746,777 individuals) revealed that the risk of AD is increased by roughly 50% in diabetics as compared to the general population (Zhang et al., 2017). Further supporting this conclusion, extensive work has been done to elucidate the specific mechanistic pathways underlying the connection (reviewed in Ferreira et al., 2014; Biessels and Reagan, 2015; Vieira et al., 2017; Bloom et al., 2017). Thus, the above evidence combines to advocate for central insulin resistance as a primary feature of AD pathology.
With this in mind, ongoing clinical trials targeting AD primarily utilize therapeutics designed to reduce brain insulin resistance (Bomfim et al., 2012; Talbot and Wang, 2014). However, addressing brain insulin levels directly may also represent a viable complimentary strategy for curbing AD pathology. Supporting this, reduced central insulin has been observed in AD patients (Craft et al., 1998). In this context, great hopes have been attached to the use of the intranasal delivery route to increase central nervous system (CNS) insulin. With this in mind, the present review briefly describes the intranasal delivery method. Thereafter, it critically evaluates the efficacy, safety, and future potential of intranasal insulin in the treatment of AD symptomology.
2. Mechanisms of delivery
Animal studies have shown that insulin can be transferred, without compromising its biological properties, along olfactory and trigeminal pathways to the brain via the nasal route (Thorne et al., 1995, 2004, 2008; Liu et al., 2001; Ross et al., 2004; Ma et al., 2007; Lochhead and Thorne, 2012; Renner et al., 2012a,b; Dhuria et al., 2016). The olfactory nerve terminates in the olfactory bulb, while the trigeminal nerve enters the brain through both the pons and the cribriform plate, allowing for drug delivery to both the anterior and posterior regions of the brain (Lochhead and Thorne, 2012; Renner et al., 2012b). Transport of substances along the olfactory and trigeminal nerve pathways is thought to occur through both intracellular and extracellular mechanisms (Chapman et al., 2013).
As in animals (Salameh et al., 2015; Ramos-Rodriguez et al., 2017), intranasal delivery appears to raise brain insulin levels in humans. In a study involving 36 healthy humans (9 female, 27 male, 25–41 years of age) (Born et al., 2002), cerebrospinal fluid (CSF) and serum concentrations of insulin were measured dynamically within 80 min of intranasal administration (40 international units (IU) regular human insulin) in samples of CSF and venous blood. Predictably, CSF concentrations began to rise within 10 min of administration and peaked after 30 min; however, serum concentrations remained unaltered (Born et al., 2002). Interestingly, there are several studies in which acute intranasal insulin administration was followed by a transient increase in serum insulin, as well as a reduction in plasma glucose within the physiological range (Benedict et al., 2011; Heni et al., 2012; Brünner et al., 2013; Thienel et al., 2017). However, these short-lived increases in serum insulin due to intranasal insulin are substantially less problematic than the larger, more sustained increases resulting from peripheral administration.
Several studies support the hypothesis that intranasal insulin reaches the brain in physiologically relevant levels in humans. For instance, a single intranasal dose of 60 IU significantly changed brain activity 30 min post-administration in healthy men (Hallschmid et al., 2004a). Of note, intranasal insulin increases resting-state functional connectivity between the hippocampus and other regions of the brain, which may explain some of its cognitive benefits (Zhang et al., 2015; Kullmann et al., 2017). Finally, in line with findings in mice that brain insulin is anorexigenic and regulates whole-body energy metabolism (Brüning et al., 2000; Morton et al., 2006), intranasal insulin reduces body fat, alters CNS processing of food cues, results in lower ad-libitum food intake, and improves peripheral insulin signaling in humans (Hallschmid et al., 2004b; Benedict et al., 2008, 2011; Guthoff et al., 2010; Hallschmid et al., 2012; Jauch-Chara et al., 2012; Iwen et al., 2014). While these measures are not directly related to AD, combined with the above evidence they indicate that intranasal insulin indeed reaches the CNS, and does so at therapeutically useful concentrations.
3. Evaluation of efficacy
3.1. Animal studies
This section covers the effects of intranasal insulin on the core mechanisms involved in AD (briefly summarized in Fig. 1). Several studies involving both cognitively healthy and AD animal models have investigated the effects of intranasal insulin on CNS parameters affected by AD. With respect to cognition, the vast majority of animal studies have produced encouraging results. Both acute and repeated intranasal treatment with insulin, spanning from days to several weeks, improve cognitive functions that typically deteriorate in AD. Specifically, they produce significant improvements in spatial memory, working memory, decision making, motor memory, and recognition of novel objects. Studies in both wildtype and 3xTg-AD mice - a transgenic model of AD - demonstrate improved spatial memory functions following treatment with intranasal insulin for ≥1 month (Apostolatos et al., 2012; Mao et al., 2016). Similar improving effects on spatial memory are found after acute intranasal delivery of insulin to another transgenic mouse model of AD, the SAMP8 mice (Salameh et al., 2015). Of note, administration of insulin when given 5 min post training, but not 24 h post training, boosted spatial memory in SAMP8 mice (Salameh et al., 2015). This indicates that the timing of intranasal insulin administration may be critical in facilitating its memory-improving effects. In separate experiments involving wildtype and 3xTg-AD mice, repeated intranasal treatment with insulin mitigated acute and long-term impairments in learning and retention of spatial memory that typically occur following anesthesia (Zhang et al., 2016; Chen et al., 2017). Memory-improving effects have also been demonstrated for other species, including cats and rats. For instance, in cats infected with post intracranial feline immunodeficiency virus, treatment 5 days per week with intranasal insulin for six weeks improved motor speed, gait variance, decision making, and motor memory (Mamik et al., 2016). Thus, the weight of the evidence supports the efficacy of intranasal insulin to improve cognition in animals.
Fig. 1.
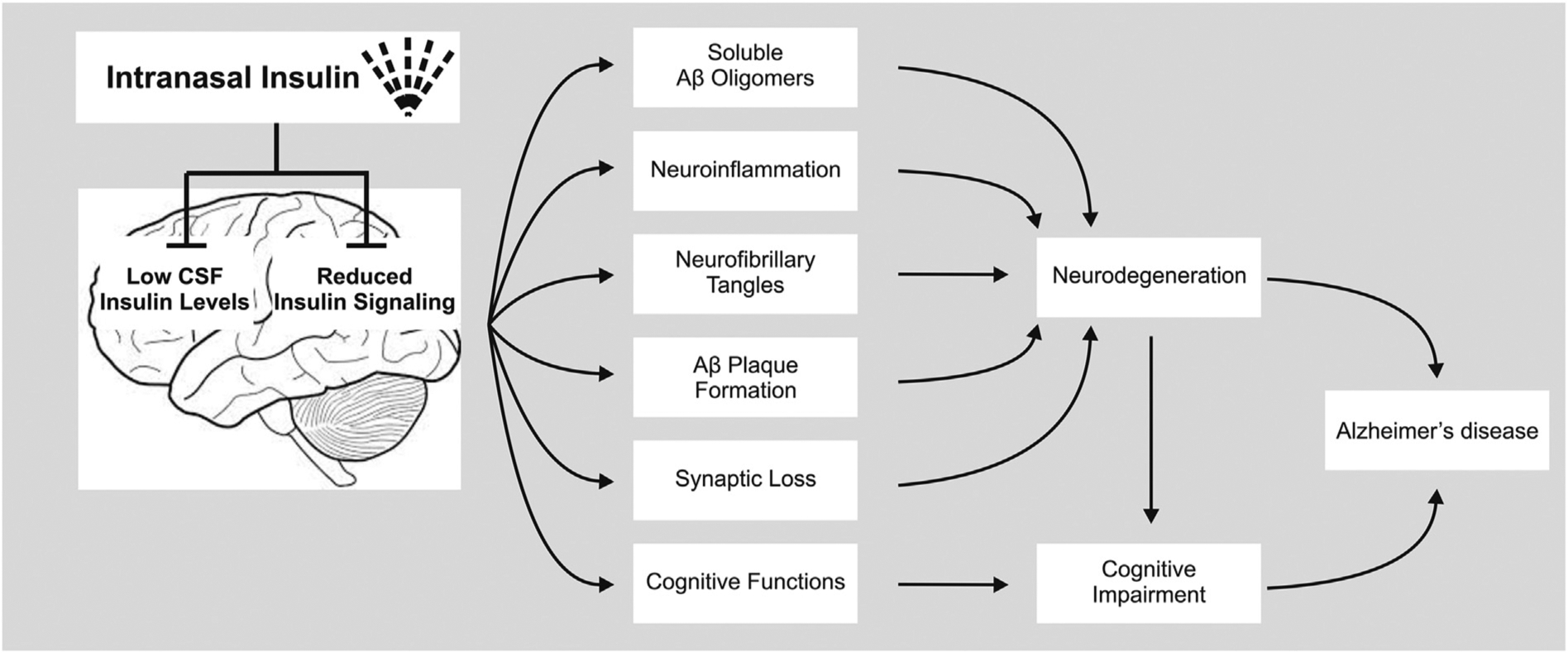
Mechanisms involved in Alzheimer’s disease that are likely promoted by brain insulin resistance and a lack of brain insulin and counteracted by intranasal insulin therapy. Abbreviations: Aβ, amyloid beta; AD, Alzheimer disease.
There are, however, a minority of studies that do not support this conclusion. For instance, the longest animal trial to date investigating intranasal insulin’s impact on memory produced no significant effect on memory, as measured by odorant and object recognition (C57BL6/J mice treated with daily intranasal insulin for 2 months) (Bell and Fadool, 2017). Additionally, in a rat model, intranasal insulin actually reduced performance in the Morris water maze task (Anderson et al., 2017). However, such seemingly contradicting findings are sparse.
The above-mentioned findings, with some exception, favor intranasal insulin for treating cognitive deficits in patients suffering from AD. It is important, though, to additionally examine whether intranasal insulin alters the turnover of brain metabolites that are involved in the etiology of AD. In a study in 9-month old 3xTg-AD female mice treated acutely with propofol, one-week treatment with daily intranasal insulin attenuated hyperphosphorylation of tau, promoted brain insulin signaling, and led to up-regulation of protein phosphatase 2A, a major tau phosphatase in the brain. Intranasal insulin also resulted in down-regulation of several tau protein kinases, including cyclin-dependent protein kinase 5, calcium/calmodulin-dependent protein kinase II, and c-Jun N-terminal kinase (Chen et al., 2014a). These results were confirmed in older female wildtype mice treated with intranasal insulin prior to anesthesia (Zhang et al., 2016), as well as in a type 2 diabetes rat model in which intranasal therapy for 4 weeks markedly reduced tau hyperphosphorylation in the rat brain (Yang et al., 2013). However, as with cognition, there is conflicting evidence regarding its impact on brain metabolites. For example, one-week of intranasal insulin therapy in 3xTg-AD mice had no effect on CNS levels of total tau, tau phosphorylation, tau kinases and tau phosphatase (Chen et al., 2014b). Additional studies in transgenic mouse models of tauopathy, with longer duration of intranasal insulin therapy and follow-up, are therefore needed to evaluate the therapeutic capacity of intranasal insulin to alter AD taupatholgy.
One week of daily intranasal insulin led to a 50% reduction of the Aβ 40 levels in the forebrains of 9-month-old female 3xTg-AD mice (Chen et al., 2014b). Further reinforcing the idea that intranasal insulin may slow amyloidogenesis in AD, treating female 4.5-month-old APP/PS1 mice for 6 weeks with daily intranasal insulin reduced amyloid plaques in both the hippocampus and cortex, and lowered concentrations of soluble Aβ oligomers in the brain (Mao et al., 2016). The latter finding is intriguing from a therapeutic point of view, as soluble Aβ oligomers are considered the most neurotoxic form of Aβ peptides in the brain (Ferreira et al., 2007). Finally, treating six-month-old female Sprague-Dawley rats with daily intranasal insulin for six days reduced hippocampal Aβ concentration (Subramanian and John, 2012).
As indicated by animal studies, intranasal insulin may possibly exert positive effects on additional neuropathological processes, thereby eventually curbing the development and progression of AD. For instance, 9-month-old 3xTg-AD mice treated with a daily dose of intranasal insulin for one week exhibited signs of reduced microglia activation and increased levels of synaptic proteins (Chen et al., 2014b). A chronic activation of microglial cells is hypothesized to result in neuroinflammation, and has been identified as contributor to AD pathogenesis (Tejera and Heneka, 2016). On the other hand, a downregulation of presynaptic and postsynaptic proteins has been proposed to be related to cognitive impairments in AD (Reddy et al., 2005). These findings could thus be seen as an additional support for the therapeutic potential of intranasal insulin. However, somewhat dampening enthusiasm, repeated doses of intranasal insulin in 21-month-old male rats resulted in glial overactivation (Anderson et al., 2017). Moreover, in contrast to robust effects seen after seven days of intranasal insulin (Marks et al., 2009), only modest effects on the activation of proteins along the insulin downstream signaling pathway in the olfactory bulb of C57BL6/J mice could be observed after 2-month of intranasal insulin therapy (Bell and Fadool, 2017). If confirmed by future studies, the latter could suggest that neurons in the olfactory bulb become less responsive to brain insulin under conditions of chronic intranasal insulin therapy. Whether this is true for neuronal responses to insulin in other brain regions is unclear.
3.2. Cognitively healthy humans
Studies in cognitively healthy humans have led to insights into the effects of acute and long-term intranasal insulin administration on cognition. In first randomized controlled proof-of-concept intervention trials involving either normal-weight or obese young adults (<40 yrs old), 8 weeks of daily intranasal doses of regular insulin improved delayed recall of word lists (Benedict et al., 2004; Hallschmid et al., 2008). This long-term effect was even stronger when insulin aspart, a fast-acting insulin analog, was delivered via the nasal route for 8 weeks (Benedict et al., 2007). More recent studies have even presented findings suggesting that a single dose of intranasal insulin is already sufficient to improve cognitive performance in cognitively healthy humans (Benedict et al., 2008; Novak et al., 2014; Brünner et al., 2015). For instance, a single intranasal dose of insulin improved visuospatial memory and verbal fluency functions in a sample of elderly non-demented type 2 diabetes patients and aged-matched subjects (Novak et al., 2014), possibly as a result of increased resting-state functional connectivity between the hippocampal regions and multiple regions within the default mode network (Zhang et al., 2015). Whether intranasal insulin influences tau metabolism and turnover of Aβ peptides in the circulation or in CSF has, however, not thoroughly been investigated in cognitively healthy humans. The latter is quite important given the long window of preclinical AD (Jack et al., 2013). To our best knowledge, there is just one study in the literature in which 10 and 20 IU of insulin were effective in reducing plasma concentrations of Aβ42 levels in cognitively healthy elderly subjects (mean age >70 years). This effect was only seen in those who did not carry copies of the apolipoprotein E epsilon4 (APOE4) AD risk allele (Reger et al., 2008a). Whereas some studies observed that plasma concentrations of Aβ42 concentrations were higher in subjects at risk for future AD (Lue et al., 2017), others have shown that lower plasma concentrations of Aβ42 concentrations were inversely correlated with brain Aβ load (Lui et al., 2010). With these conflicting data in mind, caution is warranted when concluding that reduced plasma concentrations of Aβ42 levels upon intranasal insulin administration provide supportive evidence for neuroprotective properties of nasal insulin.
To summarize, findings in cognitively healthy humans are encouraging in that they suggest that intranasal insulin may, in a dose- and memory task-dependent manner, improve cognition. It should be noted though that some studies report null-findings. For instance, in cognitively healthy subjects aged >70 years used as controls for patients with mild cognitive impairment (MCI, the prodromal state of AD) and AD, a single dose of intranasal insulin (ranging from 10 to 60 IU) failed to improve attention, working memory, and story recall (Reger et al., 2006, 2008a). Maybe even more alarming, a recent study in young adults (<30 years old) found that intranasal insulin, administered prior to sleep, impaired the acquisition of new contents in both the declarative and procedural memory systems on the next day (Feld et al., 2016). Further complicating the story, the latter finding could suggest that the direction in which intranasal insulin alters memory functions in humans may be subject to circadian regulation. Finally, the vast majority of studies dealing with effects of intranasal insulin on memory in cognitively healthy humans did not report adverse effects, except for some reporting transient drops in plasma glucose (Benedict et al., 2008) and an acute elevation of blood pressure (Benedict et al., 2005). At first glance, this speaks for a favorable side effect profile of intranasal insulin in humans, at least in the long-term. On the other hand, caution is warranted, as it cannot be ruled out that these studies did not thoroughly monitor possible side effects during and after cessation of intranasal insulin intervention.
3.3. AD patients
The last decade has seen a surge in clinical trials for intranasal insulin in patients with amnestic MCI and AD. The weight of the evidence, with some exceptions (Stein et al., 2011; Rosenbloom et al., 2014), demonstrates that both acute and chronic administration of various insulin formulations improves several aspects of cognition (Reger et al., 2006, 2008a,b; Craft et al., 2012, 2017; Claxton et al., 2013, 2015). This includes - but is not limited to - verbal memory, memory savings, and selective attention. Moreover, cognitively impaired patients treated with intranasal insulin exhibit signs of functional improvement (Reger et al., 2008b; Craft et al., 2012). Noteworthy, while cognition of APOE4-negative patients generally benefitted from nasal insulin (e.g. Reger et al., 2006; Reger et al., 2008a; Craft et al., 2012), in APOE4-positive patients, mixed results have been obtained. For instance, while intranasal administration of regular insulin did not alter or even impair cognitive functions (Rosenbloom et al., 2014), three weeks (but not 4 months) of daily intranasal doses of insulin detemir improved cognitive functions in APOE4-positive patients (Claxton et al., 2015; Craft et al., 2017). Adding further complexity, APOE4-negative women and men exhibit different dose-response patterns. Cognition was generally improved in men treated for 4 months with intranasal doses of 20 and 40 IU regular insulin daily, respectively. In contrast, APOE4-negative females’ cognitive performance declined over time on the high but not low dose of insulin (Claxton et al., 2013). The mechanistic basis of APOE-related treatment differences remains unknown.
In the largest study to date (Craft et al., 2012) involving 104 cognitively impaired patients with either MCI or AD in which subjects were assigned to three study arms (20 IU regular insulin daily; 40 IU regular insulin daily; placebo daily for 4 months), intranasal insulin therapy improved delayed memory (in the 20 IU group) and preserved caregiver-rated functional ability (both groups). Intriguingly, over the 4 month trial, the parietotemporal, frontal, precuneus, and cuneus brain regions showed reduced progression of hypometabolism in those treated with insulin (both doses) (Craft et al., 2012). Some of these results were later confirmed in a smaller trial showing that intranasal administration of regular insulin (40 IU daily for four months) improved memory and preserved brain volume (Craft et al., 2017).
Studies performed in MCI and AD patients have shown that intranasal insulin not only alters cognitive functions but also levels of AD biomarkers in both the circulation and CSF. For instance, cognitively impaired patients treated with 20 IU of regular insulin (twice daily) for 21 days exhibited an increased Aβ40/42 ratio in blood, both under fasting and postprandial conditions (Reger et al., 2008b). The increased ratio could indicate that less Aβ42 was produced in the brain (and/or periphery). Alternatively, it could suggest that less Aβ42 reached the circulation due to increased deposition in the brain. In a separate clinical trial testing the acute effects of four intranasal doses of regular insulin (10, 20, 40, or 60 IU), higher doses were linked with increased plasma concentrations of Aβ42 in APOE4 negative patients. No such dose-response relationship was seen in patients with APOE4 (Reger et al., 2008a). Finally, with respect to CSF AD biomarker profiles, daily intranasal doses of regular insulin for 4 months affected neither tau nor Aβ peptides in cognitively impaired patients (Craft et al., 2012). These null findings could be confirmed in a smaller study (n = 36) in that treating MCI and AD patients with daily doses of regular insulin did not alter CSF concentration of Aβ42, tau, and tau-P181 (). The same study, however, found a lower ratio of tau-P181 to CSF Aβ42 following regular insulin treatment relative to placebo (Craft et al., 2017). The ratio of tau-P181 to CSF Aβ42 has recently been identified as a sensitive marker of AD pathology (Harari et al., 2014).
4. Future potential
Cumulatively, the above findings support the use of intranasal insulin as a novel therapeutic in the treatment of AD. However, by virtue of being favorable, these results also highlight the need for follow-up research. As discussed, to date, the effects of intranasal insulin have only been investigated in relatively smaller samples (treatment arms with less than 50 patients) for no more than 4 months. Additionally, gender, APOE genotype, and the type of insulin formulation appear to considerably modify patient response. With this in mind, more studies with longer durations and specific sample populations are needed to paint a clearer picture of the connection between insulin and AD pathology.
One major point of exploration must be the impact of intranasal insulin on central insulin receptor expression and sensitivity in AD. Chronic elevation of systemic insulin has been linked to impaired insulin response in peripheral tissues (Shimomura et al., 2000). By analogy, it is reasonable to speculate that in the long term, chronic intranasal administration may produce similar adverse effects centrally. Long-duration trials that include a late-stage test of acute brain insulin resistance could address this concern. Even such trials may not go far enough: the extent to which dose timing and frequency impact central insulin resistance is also a mystery. This despite the fact that, for instance, insulin sensitivity of peripheral tissues exhibits a clear circadian pattern in healthy humans (Boden et al., 1996). Additionally, the impact of dose frequency is poorly understood. With these thoughts in mind, future trials investigating both timing and frequency may help to develop an optimal dosing “schedule” that effectively balances efficacy and safety.
Finally, intranasal insulin’s potential as a combination treatment is obvious. Antidiabetics such as GLP-1 agonists are designed to offset brain insulin resistance (Bomfim et al., 2012; Talbot and Wang, 2014). Furthermore, antidiabetics facilitate an increased response to insulin treatment (Ebinger et al., 2000). As one major concern with chronic intranasal insulin is its possible impact on brain insulin resistance, utilizing it in conjunction with antidiabetics may help elucidate its full potential in the treatment of AD.
Funding
The work of CB is supported by the Novo Nordisk Foundation (NNF14OC0009349), the Swedish Brain Research Foundation (FO2016-0092), and the Swedish Research Council (2015-03100). The work of CAG is supported by the National Science Foundation, NSF IOS-1656626 (USA), and the National Institutes of Health CTT COBRE, P20 GM109091-03 (USA). The funders did not have any role in design of the review, interpretation of the discussed literature, or in the writing process. We apologize to the many researchers who have contributed to the field and who because of space constraints have not been cited herein.
Disclosure statement
The authors are unaware of any affiliation, funding, or financial holdings that might be perceived as affecting the objectivity of this review article.
Abbreviations:
- AD
Alzheimer’s disease
- Aβ
Amyloid beta
- APOE4
Apolipoprotein E epsilon4
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- IU
International units
- MCI
Mild cognitive impairment
Footnotes
Conflicts of interest
The authors have nothing to disclose and no conflicts of interest to report.
References
- Anderson KL, Frazier HN, Maimaiti S, Bakshi VV, Majeed ZR, Brewer LD, Porter NM, Lin AL, Thibault O, 2017. Impact of single or repeated dose intranasal zinc-free insulin in young and aged F344 rats on cognition, signaling, and brain metabolism. J. Gerontol. A Biol. Sci. Med. Sci 72 (2), 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolatos A, Song S, Acosta S, Peart M, Watson JE, Bickford P, Cooper DR, Patel NA, 2012. Insulin promotes neuronal survival via the alternatively spliced protein kinase CδII isoform. J. Biol. Chem 287 (12), 9299–9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell GA, Fadool DA, 2017. Awake, long-term intranasal insulin treatment does not affect object memory, odor discrimination, or reversal learning in mice. Physiol. Behav 174, 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W, 2004. Intranasal insulin improves memory in humans. Psychoneur-oendocrinology 29 (10), 1326–1334. [DOI] [PubMed] [Google Scholar]
- Benedict C, Dodt C, Hallschmid M, Lepiorz M, Fehm HL, Born J, Kern W, 2005. Immediate but not long-term intranasal administration of insulin raises blood pressure in human beings. Metabolism 54 (10), 1356–1361. [DOI] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Schmitz K, Schultes B, Ratter F, Fehm HL, Born J, Kern W, 2007. Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology 32 (1), 239–243. [DOI] [PubMed] [Google Scholar]
- Benedict C, Kern W, Schultes B, Born J, Hallschmid M, 2008. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J. Clin. Endocrinol. Metab 93 (4), 1339–1344. [DOI] [PubMed] [Google Scholar]
- Benedict C, Brede S, Schiöth HB, Lehnert H, Schultes B, Born J, Hallschmid M, 2011. Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men. Diabetes 60 (1), 114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, Reagan LP, 2015. Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurosci 16 (11), 660–671. [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, Urbain JL, 1996. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes 45 (8), 1044–1050. [DOI] [PubMed] [Google Scholar]
- Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG, 2012. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Aβ oligomers. J. Clin. Invest 122 (4), 1339–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL, 2002. Sniffing neuropeptides: a transnasal approach to the human brain. Nat. Neurosci 5 (6), 514–516. [DOI] [PubMed] [Google Scholar]
- Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR, 2000. Role of brain insulin receptor in control of body weight and reproduction. Science 289 (5487), 2122–2125. [DOI] [PubMed] [Google Scholar]
- Brünner YF, Benedict C, Freiherr J, 2013. Intranasal insulin reduces olfactory sensitivity in normosmic humans. J. Clin. Endocrinol. Metab 98 (10), E1626–E1630. [DOI] [PubMed] [Google Scholar]
- Brünner YF, Kofoet A, Benedict C, Freiherr J, 2015. Central insulin administration improves odor-cued reactivation of spatial memory in young men. J. Clin. Endocrinol. Metab 100 (1), 212–219. [DOI] [PubMed] [Google Scholar]
- Bloom GS, Lazo JS, Norambuena A, 2017. Reduced brain insulin signaling: a seminal process in Alzheimer’s disease pathogenesis. Neuropharmacology. 10.1016/j.neuropharm.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CD, Frey WH 2nd, Craft S, Danielyan L, Hallschmid M, Schiöth HB, Benedict C, 2013. Intranasal treatment of central nervous system dysfunction in humans. Pharm. Res 30 (10), 2475–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Run X, Liang Z, Zhao Y, Dai CL, Iqbal K, Liu F, Gong CX, 2014a. Intranasal insulin prevents anesthesia-induced hyperphosphorylation of tau in 3xTg-AD mice. Front. Aging Neurosci 6, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhao Y, Dai CL, Liang Z, Run X, Iqbal K, Liu F, Gong CX, 2014b. Intranasal insulin restores insulin signaling, increases synaptic proteins, and reduces Aβ level and microglia activation in the brains of 3xTg-AD mice. Exp. Neurol 261, 610–619. [DOI] [PubMed] [Google Scholar]
- Chen Y, Dai CL, Wu Z, Iqbal K, Liu F, Zhang B, Gong CX, 2017. Intranasal insulin prevents anesthesia-induced cognitive impairment and chronic neurobehavioral changes. Front. Aging Neurosci 9, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton A, Baker LD, Wilkinson CW, Trittschuh EH, Chapman D, Watson GS, Cholerton B, Plymate SR, Arbuckle M, Craft S, 2013. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer’s disease. J. Alzheimers Dis 35(4), 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S, 2015. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J. Alzheimers Dis 44 (3), 897–906. [DOI] [PubMed] [Google Scholar]
- Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D Jr., 1998. Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology 50 (1), 164–168. [DOI] [PubMed] [Google Scholar]
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B, 2012. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch. Neurol 69 (1), 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, Claxton A, Baker LD, Hanson AJ, Cholerton B, Trittschuh EH, Dahl D, Caulder E, Neth B, Montine TJ, Jung Y, Maldjian J, Whitlow C, Friedman S, 2017. Effects of regular and long-acting insulin on cognition and Alzheimer’s disease biomarkers: a pilot clinical trial. J. Alzheimers Dis 57 (4), 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL, 2009. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc. Natl. Acad. Sci. U. S. A 106 (6), 1971–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhuria SV, Fine JM, Bingham D, Svitak AL, Burns RB, Baillargeon AM, Panter SS, Kazi AN, Frey WH 2nd, Hanson LR, 2016. Food consumption and activity levels increase in rats following intranasal Hypocretin-1. Neurosci. Lett 627, 155–159. [DOI] [PubMed] [Google Scholar]
- Ebinger M, Jehle DR, Fussgaenger RD, Fehmann HC, Jehle PM, 2000. Glucagon-like peptide-1 improves insulin and proinsulin binding on RINm5F cells and human monocytes. Am. J. Physiol. Endocrinol. Metab 279 (1), E88–E94. [DOI] [PubMed] [Google Scholar]
- Feld GB, Wilhem I, Benedict C, Rüdel B, Klameth C, Born J, Hallschmid M, 2016. Central nervous insulin signaling in sleep-associated memory formation and neuroendocrine regulation. Neuropsychopharmacology 41 (6), 1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira ST, Vieira MN, De Felice FG, 2007. Soluble protein oligomers as emerging toxins in Alzheimer’s and other amyloid diseases. IUBMB Life 59 (4–5), 332–345. [DOI] [PubMed] [Google Scholar]
- Ferreira ST, Clarke JR, Bomfim TR, De Felice FG, 2014. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimers Dement. 10 (1 Suppl), S76–S83. [DOI] [PubMed] [Google Scholar]
- Guthoff M, Grichisch Y, Canova C, Tschritter O, Veit R, Hallschmid M, Häring HU, Preissl H, Hennige AM, Fritsche A, 2010. Insulin modulates food-related activity in the central nervous. J. Clin. Endocrinol. Metab 95 (2), 748–755. [DOI] [PubMed] [Google Scholar]
- Hallschmid M, Schultes B, Marshall L, Mölle M, Kern W, Bredthauer J, Fehm HL, Born J, 2004a. Transcortical direct current potential shift reflects immediate signaling of systemic insulin to the human brain. Diabetes 53 (9), 2202–2208. [DOI] [PubMed] [Google Scholar]
- Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W, 2004b. Intranasal insulin reduces body fat in men but not in women. Diabetes 53 (11), 3024–3029. [DOI] [PubMed] [Google Scholar]
- Hallschmid M, Benedict C, Schultes B, Born J, Kern W, 2008. Obese men respond to cognitive but not to catabolic brain insulin signaling. Int. J. Obes. (Lond) 32 (2), 275–282. [DOI] [PubMed] [Google Scholar]
- Hallschmid M, Higgs S, Thienel M, Ott V, Lehnert H, 2012. Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes 61 (4), 782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari O, Cruchaga C, Kauwe JS, Ainscough BJ, Bales K, Pickering EH, Bertelsen S, Fagan AM, Holtzman DM, Morris JC, Goate AM, Alzheimer’s disease neuroimaging initiative, 2014. Phosphorylated tau-Aβ42 ratio as a continuous trait for biomarker discovery for early-stage Alzheimer’s disease in multiplex immunoassay panels of cerebrospinal fluid. Biol. Psychiatry 75 (9), 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ, 2002. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297 (5580), 353–356. [DOI] [PubMed] [Google Scholar]
- Heni M, Kullmann S, Ketterer C, Guthoff M, Linder K, Wagner R, Stingl KT, Veit R, Staiger H, Häring HU, Preissl H, Fritsche A, 2012. Nasal insulin changes peripheral insulin sensitivity simultaneously with altered activity in homeostatic and reward-related human brain regions. Diabetologia 55 (6), 1773–1782. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX, 2016. Tau and neurodegenerative disease: the story so far. Nat. Rev. Neurol 12 (1), 15–27. [DOI] [PubMed] [Google Scholar]
- Iwen KA, Scherer T, Heni M, Sayk F, Wellnitz T, Machleidt F, Preissl H, Häring HU, Fritsche A, Lehnert H, Buettner C, Hallschmid M, 2014. Intranasal insulin suppresses systemic but not subcutaneous lipolysis in healthy humans. J. Clin. Endocrinol. Metab 99 (2), E246–E251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr1., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ, 2013. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12 (2), 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauch-Chara K, Friedrich A, Rezmer M, Melchert UH, Scholand-Engler GH, Hallschmid M, Oltmanns KM, 2012. Intranasal insulin suppresses food intake via enhancement of brain energy levels in humans. Diabetes 61 (9), 2261–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Veit R, Scheffler K, Machann J, Häring HU, Fritsche A, Preissl H, 2017. Intranasal insulin enhances brain functional connectivity mediating the relationship between adiposity and subjective feeling of hunger. Sci. Rep 7 (1), 1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XF, Fawcett JR, Thorne RG, DeFor TA, Frey WH 2nd, 2001. Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J. Neurol. Sci 187 (1–2), 91–97. [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, Thorne RG, 2012. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev 64 (7), 614–628. [DOI] [PubMed] [Google Scholar]
- Lue LF, Sabbagh MN, Chiu MJ, Jing N, Snyder NL, Schmitz C, Guerra A, Belden CM, Chen TF, Yang CC, Yang SY, Walker DG, Chen K, Reiman EM, 2017. Plasma levels of Aβ42 and tau identified probable Alzheimer’s dementia: findings in two cohorts. Front. Aging Neurosci 9, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JK, Laws SM, Li QX, Villemagne VL, Ames D, Brown B, Bush AI, De Ruyck K, Dromey J, Ellis KA, Faux NG, Foster J, Fowler C, Gupta V, Hudson P, Laughton K, Masters CL, Pertile K, Rembach A, Rimajova M, Rodrigues M, Rowe CC, Rumble R, Szoeke C, Taddei K, Taddei T, Trounson B, Ward V, Martins RN, AIBL Research group, 2010. Plasma amyloid-beta as a biomarker in Alzheimer’s disease: the AIBL study of aging. J. Alzheimers Dis 20 (4), 1233–1242. [DOI] [PubMed] [Google Scholar]
- Ma YP, Ma MM, Ge S, Guo RB, Zhang HJ, Frey WH 2nd, Xu GL, Liu XF, 2007. Intranasally delivered TGF-beta1 enters brain and regulates gene expressions of its receptors in rats. Brain Res. Bull 74 (4), 271–277. [DOI] [PubMed] [Google Scholar]
- Mamik MK, Asahchop EL, Chan WF, Zhu Y, Branton WG, McKenzie BA, Cohen EA, Power C, 2016. Insulin treatment prevents neuroinflammation and neuronal injury with restored neurobehavioral function in models of HIV/AIDS neurodegeneration. J. Neurosci 36 (41), 10683–10695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech W, Gant DJ, Chen PP, Hudspeth B, Chen C, Zhao Y, Vinters HV, Frautschy SA, Cole GM, 2009. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J. Neurosci 29 (28), 9078–9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao YF, Guo Z, Zheng T, Jiang Y, Yan Y, Yin X, Chen Y, Zhang B, 2016. Intranasal insulin alleviates cognitive deficits and amyloid pathology in young adult APPswe/PS1dE9 mice. Aging Cell. 15 (5), 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA, 2009. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J. Neurosci 29 (20), 6734–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C, 2010. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol. Aging 31(2), 224–243. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW, 2006. Central nervous system control of food intake and body weight. Nature 443 (7109), 289–295. [DOI] [PubMed] [Google Scholar]
- Novak V, Milberg W, Hao Y, Munshi M, Novak P, Galica A, Manor B, Roberson P, Craft S, Abduljalil A, 2014. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care 37 (3), 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Rodriguez JJ, Sanchez-Sotano D, Doblas-Marquez A, Infante-Garcia C, Lubian-Lopez S, Garcia-Alloza M, 2017. Intranasal insulin reverts central pathology and cognitive impairment in diabetic mother offspring. Mol. Neurodegener 12 (1), 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W Jr., Kaye J, Manczak M, 2005. Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J. Alzheimers Dis 7 (2), 103–117. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Frey WH 2nd, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, Craft S, 2006. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol. Aging 27 (3), 451–458. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, Plymate SR, Cherrier MM, Schellenberg GD, Frey WH 2nd, Craft S, 2008a. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J. Alzheimers Dis 13 (3), 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S, 2008b. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 70 (6), 440–448. [DOI] [PubMed] [Google Scholar]
- Renner DB, Frey WH 2nd, Hanson LR, 2012a. Intranasal delivery of siRNA to the olfactory bulbs of mice via the olfactory nerve pathway. Neurosci. Lett 513 (2), 193–197. [DOI] [PubMed] [Google Scholar]
- Renner DB, Svitak AL, Gallus NJ, Ericson ME, Frey WH 2nd, Hanson LR, 2012b. Intranasal delivery of insulin via the olfactory nerve pathway. J. Pharm. Pharmacol 64 (12), 1709–1714. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MH, Barclay TR, Pyle M, Owens BL, Cagan AB, Anderson CP, Frey WH 2nd, Hanson LR, 2014. A single-dose pilot trial of intranasal rapid-acting insulin in apolipoprotein E4 carriers with mild-moderate Alzheimer’s disease. CNS Drugs 28 (12), 1185–1189. [DOI] [PubMed] [Google Scholar]
- Ross TM, Martinez PM, Renner JC, Thorne RG, Hanson LR, Frey WH 2nd, 2004. Intranasal administration of interferon beta bypasses the blood-brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J. Neuroimmunol 151 (1–2), 66–77. [DOI] [PubMed] [Google Scholar]
- Salameh TS, Bullock KM, Hujoel IA, Niehoff ML, Wolden-Hanson T, Kim J, Morley JE, Farr SA, Banks WA, 2015. Central nervous system delivery of intranasal insulin: mechanisms of uptake and effects on cognition. J. Alzheimers Dis 47 (3), 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, 2001. Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev 81(2), 741–766. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL, 2000. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol. Cell 6 (1), 77–86. [PubMed] [Google Scholar]
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM, 2005. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J. Alzheimers Dis 7 (1), 63–80. [DOI] [PubMed] [Google Scholar]
- Stein MS, Scherer SC, Ladd KS, Harrison LC, 2011. A randomized controlled trial of high-dose vitamin D2 followed by intranasal insulin in Alzheimer’s disease. J. Alzheimers Dis 26 (3), 477–484. [DOI] [PubMed] [Google Scholar]
- Subramanian S, John M, 2012. Intranasal administration of insulin lowers amyloid-beta levels in rat model of diabetes. Indian J. Exp. Biol 50 (1), 41–44. [PubMed] [Google Scholar]
- Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE, 2012. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Invest 122 (4), 1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Wang HY, 2014. The nature, significance, and glucagon-like peptide-1 analog treatment of brain insulin resistance in Alzheimer’s disease. Alzheimers Dement. 10 (1 Suppl), S12–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejera D, Heneka MT, 2016. Microglia in Alzheimer’s disease: the good, the bad and the ugly. Curr. Alzheimer Res 13 (4), 370–380. [DOI] [PubMed] [Google Scholar]
- Thienel M, Wilhelm I, Benedict C, Born J, Hallschmid M, 2017. Intranasal insulin decreases circulating cortisol concentrations during early sleep in elderly humans. Neurobiol. Aging 54, 170–174. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Emory CR, Ala TA, Frey WH 2nd, 1995. Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 692 (1–2), 278–282. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Pronk GJ, Padmanabhan V, Frey WH 2nd, 2004. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 127 (2), 481–496. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Hanson LR, Ross TM, Tung D, Frey WH 2nd, 2008. Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience 152 (3), 785–797. [DOI] [PubMed] [Google Scholar]
- Vieira MNN, Lima-Filhoa RAS, De Felice FG, 2017. Connecting Alzheimer’s disease to diabetes: underlying mechanisms and potential therapeutic targets. Neuropharmacology. 10.1016/j.neuropharm.2017.11.014. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ma D, Wang Y, Jiang T, Hu S, Zhang M, Yu X, Gong CX, 2013. Intranasal insulin ameliorates tau hyperphosphorylation in a rat model of type 2 diabetes. J. Alzheimers Dis 33 (2), 329–338. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hao Y, Manor B, Novak P, Milberg W, Zhang J, Fang J, Novak V, 2015. Intranasal insulin enhanced resting-state functional connectivity of hippocampal regions in type 2 diabetes. Diabetes 64 (3), 1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen C, Hua S, Liao H, Wang M, Xiong Y, Cao F, 2017. An updated meta-analysis of cohort studies: diabetes and risk of Alzheimer’s disease. Diabetes Res. Clin. Pract 124, 41–47. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dai CL, Chen Y, Iqbal K, Liu F, Gong CX, 2016. Intranasal insulin prevents anesthesia-induced spatial learning and memory deficit in mice. Sci. Rep 6, 21186. [DOI] [PMC free article] [PubMed] [Google Scholar]


