Abstract
Background
Chronic sensory loss is a common and undertreated consequence of many forms of neurological injury. Emerging evidence indicates that vagus nerve stimulation (VNS) delivered during tactile rehabilitation promotes recovery of somatosensation.
Objective
Here, we characterize the amount, intensity, frequency, and duration of VNS therapy paradigms to determine the optimal dosage for VNS-dependent enhancement of recovery in a model of peripheral nerve injury (PNI).
Methods
Rats underwent transection of the medial and ulnar nerves in the forelimb, resulting in chronic sensory loss in the paw. Eight weeks after injury, rats were implanted with a VNS cuff and received tactile rehabilitation sessions consisting of repeated mechanical stimulation of the previously denervated forepaw paired with short bursts of VNS. Rats received VNS therapy in 1 of 6 systematically varied dosing schedules to identify a paradigm that balanced therapy effectiveness with a shorter regimen.
Results
Delivering 200 VNS pairings a day 4 days a week for 4 weeks produced the greatest percent improvement in somatosensory function compared to any of the 6 other groups (One Way analysis of variance at the end of therapy, F[4 70] P = .005).
Conclusions
Our findings demonstrate that an effective VNS therapy dosage delivers many stimulations per session, with many sessions per week, over many weeks. These results provide a framework to inform the development of VNS-based therapies for sensory restoration.
Keywords: spaced training, massed training, vagal nerve stimulation, nerve injury, neurorehabilitation, dosage
Introduction
Somatosensory loss is a common consequence of many forms of neurological injury, including stroke and peripheral nerve damage.1-3 Vagus nerve stimulation (VNS) therapy has emerged as a non-pharmacological approach to treat impairments arising from neurological injury. VNS exerts its action by engaging neuromodulator networks to enhance synaptic plasticity in combination with rehabilitative training.4-6 Previous preclinical models and a pilot clinical study demonstrate that repeatedly pairing short bursts of VNS with tactile retraining exercises can promote recovery of somatosensory loss compared to rehabilitation alone.7-9 Using a congruent approach, VNS therapy recently received FDA approval for the rehabilitation of upper limb motor deficits in individuals with chronic ischemic stroke. 10 As this implementation is translated to clinical practice and VNS therapy is expanded to target other conditions, it would be valuable to define optimal paradigms of VNS delivery to maximize recovery.7-9
Many studies have investigated the optimal electrical stimulation parameters for VNS, but to date, no study has systematically investigated the impact of different dosing regimens on the efficacy of VNS therapy. The importance of dosing and schedule is well-recognized in neurorehabilitation, and characterizing the most effective approach for VNS therapy has clear potential for clinical impact. Presently, pre-clinical and clinical studies deliver hundreds of pairings per day, several times per week, across several weeks. For instance, in a case study examining sensory recovery with VNS therapy, a patient received 300 VNS-touch pairings 5 days a week over 6 weeks. 9 While evidence suggests VNS therapy enhances recovery of somatosensory function, implementation of a high-volume, long-duration therapy dosage poses a practical challenge with the direct translation to broader clinical use. Therapy schedules that require a regular commute to the clinic and many hours of therapy may not be feasible for patients, may place excessive burden on care providers, and increase the cost of implementation. 11 Therefore, it would be valuable to understand practical dosage requirements to facilitate the translation of VNS therapy to target the restoration of somatosensation.
One aspect of dose in the context of neurorehabilitation is the quantity and distribution of intervention that produces improvements. We sought to characterize the amount, intensity, frequency, and duration of VNS therapy to determine the optimal dosage for VNS-dependent enhancement of recovery in a model of chronic sensory loss. To do so, we explore the number of total VNS-touch pairings received throughout the therapy, the number of VNS-touch pairings in a single therapy session, the number of sessions per week, and the period in days or weeks over which the therapy is delivered on VNS-dependent recovery.12,13 We systematically varied each dosage paradigm to identify a paradigm that balanced effectiveness with a shorter regimen (Figure 1). These experiments provide a framework to guide the development and selection of VNS-based therapies for sensory restoration (Figures 2 and 3).
Figure 1.
Experimental design and therapy schedules. The No VNS group received tactile rehabilitation without VNS stimulation. All other groups received tactile rehabilitation paired with VNS stimulation in various therapy schedules. The Moderate Weekly VNS, Intense Weekly VNS, and Intense Daily VNS explore the effects of reducing session frequency while varying the amount of VNS-touch pairings, and therapy duration. The Short Daily VNS and Rapid Moderate Daily groups explore the effects of reducing individual session time by altering amount of VNS-touch pairings or interstimulus interval between trials.
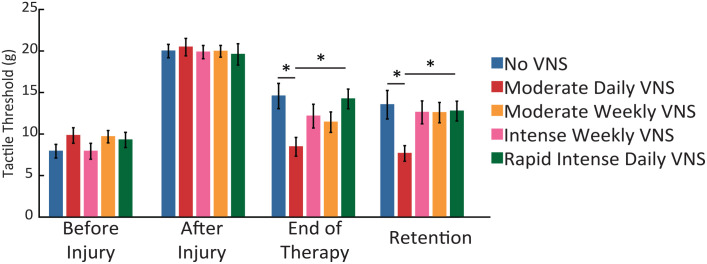
Figure 2.
VNS therapy requires daily sessions over many weeks to drive somatosensory recovery. After injury, withdrawal thresholds are significantly worse in all groups, indicating chronic somatosensory impairments. The Moderate Daily VNS therapy schedule significantly improves somatosensory thresholds compared to equivalent tactile rehabilitation without VNS and all groups receiving 4 sessions of therapy. Error bars indicate mean ± standard error of the mean.
*Denotes P < .05.
Figure 3.
VNS requires many VNS-touch pairings per session to drive somatosensory recovery. After injury, withdrawal thresholds are significantly worse in all groups. The Moderate Daily VNS therapy schedule significantly improves somatosensory thresholds compared to equivalent tactile rehabilitation without VNS, and all groups receiving 50 stimulations per session and 2 seconds ISI. Error bars indicate mean ± standard error of the mean.
*Denotes P < .05.
Methods
Two hundred and ten female Sprague Dawley rats weighing approximately 250 g were used in the course of this study (Charles River Labs). All rats were housed in a reversed 12:12 light-dark cycle. All handling, housing, stimulation, and surgical procedures were approved by the University of Texas at Dallas Institutional Animal Care and Use Committee (Protocol: 14-10).
Peripheral Nerve Injury
All rats underwent complete transection of the right median and ulnar nerves proximal to the elbow, followed by tubular repair, as previously described.7,8,14,15 Rats were deeply anesthetized with ketamine hydrochloride (50 mg/kg, IP), xylazine (20 mg/kg, IP), and acepromazine (5 mg/kg) and supplemented as necessary to maintain areflexia. A small incision was made proximal to the elbow of the right forelimb, and the median and ulnar nerves were carefully isolated and exposed. Both nerves were transected 1 cm proximal to the elbow. Immediately following transection, the proximal and distal stumps of each nerve were sutured 1 mm from the ends of an 8 mm saline-filled polyurethane tube (Micro-Renathane, 0.095 in inner diameter, 0.066 in outer diameter; Braintree Scientific, Braintree, MA), resulting in a 6 mm gap between nerve stumps. The skin incision was sutured and treated with antibiotic ointment. All rats were given sustained-release buprenorphine (1.2 mg/kg) immediately following surgery. Rats were placed in Elizabethan collars for approximately 1 week following injury to limit autophagia.
Vagus Nerve Cuff Implantation
VNS cuff implantation procedures were performed as described in previous studies.7,8,14,15 Approximately 9 weeks after transection of the median and ulnar nerves, rats were anesthetized with ketamine hydrochloride (50 mg/kg i.p.), xylazine (20 mg/kg i.p.), and acepromazine (5 mg/kg i.p.) and placed in a stereotactic apparatus. An incision was made down the midline of the head to expose the skull. Bone screws were inserted into the skull at points surrounding the lambdoid suture and over the cerebellum. A 2-channel connector was mounted to the screws using acrylic. The rat was then removed from the stereotaxic apparatus and placed in a supine position. An incision was made on the left side of the neck, and the overlying musculature was blunt dissected to isolate the vagus nerve. The nerve was placed into a bipolar stimulating cuff electrode, and the electrode leads were tunneled subcutaneously and connected with the 2-channel skull-mounted connector. Incised skin was then sutured closed. All rats received buprenorphine (1.2 mg/kg) following surgery. To confirm cuff electrode functionality and proper placement, VNS-dependent activation of the Hering–Breuer reflex was assessed as in previous studies.16,17 To do so, blood oxygenation saturation was monitored via pulse oximetry during 5 trains of VNS (0.8 mA, 30 Hz, 100 µs pulse width, up to 5 s train duration, with an inter-stimulus duration of 30 s). If stimulation failed to produce a reliable decrease in oxygen saturation, the cuff electrode was replaced.
Tactile Rehabilitation
Sessions of tactile rehabilitation were performed as previously described.7,8 All rats received tactile rehabilitation, which began approximately 10 weeks after peripheral nerve injury (PNI) and followed 1 of 7 schedules (Table 1, Figure 1). During each session, rats were placed in individual acrylic chambers (14 cm × 15 cm) with a mesh floor. Each session consisted of a predetermined number of individual touches to the ventral surface of the right (injured) forepaw with a paintbrush (Kiss Products, Port Washington, NY). The paintbrush was applied across the paw and digits in varying directions, with an approximate upward force of 10 g.
Table 1.
Therapy Schedule Summary.
| Group | Paw brush touches per session | VNS pairings per session | Average session time (min) | Sessions per week | Therapy duration (wk) | ISI (s) | Total pairings |
|---|---|---|---|---|---|---|---|
| No VNS | 200 | 0 | 34 | 4× | 4 | 10 | 3200 |
| Moderate daily VNS | 200 | 200 | 34 | 4× | 4 | 10 | 3200 |
| Moderate weekly VNS | 200 | 200 | 34 | 1× | 4 | 10 | 800 |
| Intense weekly VNS | 800 | 800 | 134 | 1× | 1 | 10 | 3200 |
| Rapid intense daily VNS | 800 | 800 | 27 | 4× | 4 | 2 | 3200 |
| Short daily VNS | 50 | 50 | 9 | 4× | 4 | 10 | 800 |
| Rapid daily VNS | 200 | 200 | 7 | 4× | 4 | 2 | 3200 |
VNS Delivery
All rats, except those in the No VNS group, received a train of VNS to coincide with the delivery of each mechanical stimulus during tactile rehabilitation sessions. The quantity of, and interval between, each pairing was specific to each group. The total number of pairings, timing between each pairing, and length of each session are interacting factors. These parameters were modified specific to each rat’s group assignment (Table 1, Figure 1). The electrical stimulation parameters of VNS were consistent across groups, with each 0.5-second stimulation train consisting of 0.8 mA, 100-µs biphasic pulses delivered at 30 Hz.
Mechanosensory Withdraw Sensory Testing
Mechanosensory detection thresholds were assessed in all rats according to standard procedures.7,8,14,15 Testing was performed in an acrylic chamber (19.5 cm × 9.6 cm) with a wire mesh floor. During each session, rats were allowed to acclimate to the behavioral chamber for 30 minutes before testing commenced. Mechanical withdrawal thresholds of the left and right forepaws were tested using a dynamic plantar aesthesiometer (Cat. No. 37450; Ugo Basile, Lugano, Switzerland). The actuator filament (0.5 mm diameter) was applied to the plantar surface of the forepaw, and a linearly increasing force was applied (20-second ramp time, 50 g maximal force). The point at which paw withdrawal occurred was automatically recorded for analysis. The left and right forepaws were alternately tested with a minimum of 1 minute between consecutive tests. A complete testing session consisted of 5 recorded withdrawal thresholds for each paw. Trials resulting in paw withdrawal due to spontaneous exploratory activity were excluded from the analysis. Testing was performed before the injury, before therapy, weekly during treatment, and 4 weeks after the conclusion of therapy by experimenters blinded to the experimental group.
Exclusion Criteria
All exclusion criteria were defined a priori. Rats were excluded if their withdrawal thresholds were greater than 15 g before injury or below 15 g at the post-injury timepoint. Fifteen rats were excluded based on pre-injury values, while 51 were excluded based on post-injury values. Twenty-six rats were excluded due to health complications. Thirteen rats were also excluded due to failure of the stimulating cuff. After exclusions, 105 rats were dynamically allocated to 1 of the 7 balanced groups (No VNS (n = 15), Moderate Daily VNS(n = 15), Moderate Weekly VNS(n = 15), Intense Weekly VNS (n = 15), Intense Daily VNS(n = 15), Light Weekly VNS(n = 15), Rapid Weekly VNS(n = 15).
Statistical Analysis
Mechanical withdrawal thresholds were analyzed using a 2-way repeated measures analysis of variance (ANOVA), followed by post hoc Bonferroni-Corrected unpaired t-test where appropriate. A 1-way ANOVA followed by post hoc Bonferroni-Corrected unpaired t-tests were used to compare measurements within a single time point. Paired t-tests were used to compare measurements within subjects from pre-injury to week 8 post-injury time points, where applicable. Statistical tests for each comparison are noted in the tests. Figures depict the mean ± standard error of the mean.
Results
Rats underwent transection and repair of the median and ulnar nerves in the forelimb to produce chronic hyposensation in the ventral surface of the right forepaw. As expected, mechanosensory withdrawal thresholds in the injured forelimb were significantly elevated 8 weeks post-injury compared to pre-injury for all groups, indicative of a chronic impairment in somatosensation (Pre-Injury vs Post-Injury, Repeated Measures ANOVA, effect of time, F[130 1], P = 5.5 × 10−20). No differences in withdrawal thresholds were observed across groups before beginning therapy (Pre-Injury vs Post-Injury, Repeated Measures ANOVA, effect of group, F[130 1], P = .27).
To evaluate the effects of VNS therapy dosing, rats received sessions of tactile rehabilitation paired with VNS across different therapy schedules (Figure 1; Table 1). A significant interaction was observed between the time of post-lesion training and end of therapy (Repeated Measures ANOVA Post vs End of Therapy, F[1 100], P = 8.84 × 10−9). Additionally, a significant effect of treatment was observed at the end of the therapy (1 Way ANOVA, at End of Therapy, group effect, F[4 70], P = .01). Consistent with prior studies, Moderate Daily VNS significantly decreased forelimb withdrawal thresholds compared to equivalent tactile rehabilitation without VNS, indicative of restoration of somatosensory function (Moderate Daily VNS vs No VNS, at End of Therapy; Unpaired-test, Bonferroni corrected, P = .01). VNS-dependent improvements were maintained 4 weeks after the cessation of treatment, suggesting lasting improvements (Moderate Daily VNS, End of Therapy vs Retention; Paired t-test, P = .55). These findings confirm previous reports that delivery of many VNS-touch parings per session, for many sessions a week, over many weeks, enhances somatosensory recovery.
Reducing the Number of Sessions
We next sought to identify a shorter therapy regimen that could effectively enhance somatosensory recovery. Therefore, we first attempted to determine whether reducing the frequency of VNS therapy sessions per week from 4 (Moderate Daily VNS) to 1 (Moderate Weekly VNS) would impact the degree of recovery. Rats in the Moderate Weekly VNS group received 200 VNS-touch pairings per session once per week for 4 weeks. Moderate Weekly VNS did not significantly improve recovery compared to equivalent tactile rehabilitation without VNS (Moderate Weekly VNS vs No VNS at End of therapy; Unpaired test; P = .26). Additionally, the Moderate Weekly VNS schedule displayed a strong trend toward a reduced recovery of withdrawal thresholds compared to the Moderate Daily VNS schedule (Moderate Daily VNS vs Moderate Weekly VNS at End of Therapy; Unpaired test; P = .052).
The failure of Moderate Weekly VNS to improve recovery could be attributed to the reduction in the amount of VNS-touch pairings received. Thus, we tested a paradigm that also comprises 3200 total VNS-touch pairings, equivalent to the amount delivered in the effective Moderate Daily VNS condition, while reducing the frequency of sessions per week. This schedule termed Intense Weekly VNS, consisted of 800 VNS-touch pairings once weekly for 4 weeks (Figure 1). Intense Weekly VNS failed to improve recovery compared to those animals receiving Moderate Daily VNS (Intense Weekly VNS stims vs Moderate Daily VNS; Unpaired t-test across groups; P = .51) and did not confer significant benefits compared to rehabilitation without VNS (Intense Weekly VNS vs No VNS at End of therapy; Unpaired test; P = 1.0).
The failure of Intense Weekly VNS to improve recovery indicates that the amount of stimulation is not the determining factor in VNS efficacy. Instead, the lack of effectiveness could be ascribed to an insufficient frequency of sessions per week. Therefore, we tested if a matched number of VNS-touch pairings condensed into a single week would improve recovery. Rats in the Rapid Intense Daily VNS group received 800 VNS-touch pairings per session for 4 consecutive sessions during a single week at an interstimulus interval of 2 seconds instead of 10 seconds (Figure 1). Rapid Intense Weekly VNS failed to improve recovery and withdrawal thresholds were significantly impaired compared to Moderate Daily VNS (Rapid Intense Daily VNS vs Moderate Daily VNS, at End of Therapy; Unpaired t-test across groups; P = .02), even though both groups received the same total number of VNS-touch pairings. Additionally, Intense Daily VNS was not significantly different from rehabilitation without VNS (Rapid Intense Daily VNS vs No VNS at End of therapy; Unpaired test; P = 1.0). These findings indicate that the distribution of VNS paired with tactile rehabilitation must be delivered for multiple days per week, for multiple weeks, to enhance recovery.
Reducing the Duration of Sessions
The failure to reduce overall therapy time by reducing the frequency and duration suggests that daily repetition is required to enhance somatosensory recovery. One plausible alternative to reduce the burden of therapy that maintains the daily and weekly exposure to VNS is to shorten individual sessions. Therefore, we evaluated whether 2 modifications to shorten each session, namely reducing the amount of VNS-touch pairings per session or reducing the time between individual VNS-touch pairings, would impact the degree of somatosensory recovery. We observed a significant effect of session length on somatosensory thresholds (1 Way ANOVA, End of Therapy, group effect, F[2 94], P = 3.4 × 10−15). To design a shorter session while maintaining frequency and duration, we first reduced the amount of daily VNS-touch pairings. In the Short Daily VNS schedule, rats received 50 VNS-touch pairings per session 4 times a week for 4 weeks (Figure 1). Short Daily VNS failed to improve recovery and was significantly not different compared to Moderate Daily VNS (Short Daily VNS vs Moderate Daily VNS; Unpaired t-test across groups; P = .4.) Additionally, Short Daily VNS was not significantly different from animals receiving tactile rehabilitation without VNS (Short Daily VNS vs No VNS; Unpaired t-test across groups; P = 1.0).
The failure of the prior conditions indicates that it is critical to deliver many VNS-touch pairings multiple times a week for multiple weeks. We next sought to shorten the amount of therapy time while maintaining the Moderate Daily VNS schedule. To do so, we tested a Rapid Daily VNS schedule with a shortened interval between VNS-touch pairings. In the Rapid Daily VNS schedule, rats received 200 VNS-touch pairings, 4 times a week for 4 weeks at an interstimulus interval of 2 seconds instead of 10 seconds (Figure 1). Rapid Daily VNS failed to improve recovery compared to those animals receiving Moderate Daily VNS and was not significantly different from animals that did not receive VNS (Rapid Daily VNS vs Moderate Daily VNS; Unpaired t-test across groups; P = .12; Rapid Daily VNS vs No VNS; Unpaired t-test across groups; P = 1.0). These findings suggest that the distribution of VNS-touch pairings in time is critical in determining the magnitude of recovery.
Moderate Daily VNS Produces the Greatest Degree of Somatosensory Recovery
Our findings demonstrate that Moderate Daily VNS produces the greatest percent improvement in somatosensory function compared to any of the 6 other groups (Figure 4A, 1 Way ANOVA at the end of therapy, F[4 70] P = .005). Moderate Daily VNS achieved significantly greater percent recovery than those animals that did not receive VNS (No VNS vs Moderate Daily VNS at End of Therapy; Unpaired-test; P = .007; Intense Daily VNS vs Moderate Daily VNS at End of Therapy; Unpaired-test; P = .02). Moderate VNS therapy produced a significantly higher percent recovery than a matched amount of VNS delivered once a week or over a single week (Intense Weekly VNS vs Moderate Daily VNS stims at End of Therapy Unpaired-test; P = .036). These findings indicate that timing and dosage of VNS therapy are key determinants in enhancing somatosensory function after PNI.
Figure 4.
VNS efficacy cannot be linked to any single dosing factor. (A) VNS delivered in the Moderate Daily VNS schedule results in a robust improvement in recovery, whereas other VNS therapy schedules do not facilitate recovery compared to tactile therapy without stimulation. (B-H) No single factor characterized in this study can account for the effects of VNS therapy.
No single factor characterized in this study can account for the effects of VNS therapy. The interactions between amount, intensity, frequency, and duration of VNS play a significant role in rehabilitative outcomes (Figure 4, B-H). Moderate Daily VNS consistently outperformed all groups as the most effective therapy protocol, which suggests that the interaction between these factors is responsible for the enhanced recovery observed in the Moderate Daily VNS group.
Discussion
Emerging evidence from preclinical and clinical studies demonstrates that combining tactile rehabilitation with VNS enhances recovery of somatosensation after neurological injury.7-9 Optimizing the efficacy of this potential therapy is critical to facilitating clinical translation. An effective and minimally burdensome therapy would benefit both patients and providers. Here, we sought to comprehensively characterize the minimum amount, intensity, frequency, and duration of VNS therapy required for VNS-dependent enhancement of recovery in a model of chronic sensory loss.
Consistent with previous reports, Moderate Daily VNS generates robust, significant improvements in recovery of somatosensory function compared to equivalent tactile rehabilitation without VNS. The effectiveness of this paradigm is consistent with improvements observed with a similar strategy in previous preclinical models and a pilot clinical.7-9 VNS-dependent restoration of somatosensory function was maintained for at least a month after cessation of therapy, suggesting long-lasting benefits. This durable recovery is consistent with the principle that VNS paired with tactile rehabilitation mediates recovery by enhancing synaptic plasticity in sensory networks. 15 Together, these findings confirm that VNS paired with tactile rehabilitation represents a viable means to improve tactile function recovery in a chronic sensory loss model.
The paradigms of effective VNS therapy currently employed in preclinical and clinical studies, including Moderate Daily VNS, deliver hundreds of pairings per day, several times per week, across several weeks. While VNS therapy improves recovery of somatosensory function, there are practical challenges with directly translating this approach to broader clinical use. Travel to the clinic for daily sessions and the long duration of each session increase the inconvenience and burden on patients. Therefore, the primary aim of the present study was to identify a paradigm that balances effectiveness and brevity. One way to shorten the therapy duration is to deliver fewer sessions per week. Moderate Weekly VNS, Intense Weekly VNS, and Rapid Intense Daily VNS all received 2-quarter the number of sessions than Moderate Daily VNS (Figure 1). However, these paradigms all failed to replicate the robust improvements in somatosensory function observed with Moderate Daily VNS. Moreover, these paradigms were unable to enhance recovery compared to tactile rehabilitation without VNS. Together, the failure of these shorter protocols to improve sensory function indicates that a minimum number of therapy sessions is necessary for VNS to enhance recovery. There is no clear consensus on the optimal number of therapy sessions, yet our results show that more than 4 sessions are needed for there to be a significant VNS-driven improvement.14,18,19 In these shortened VNS therapy schedules, the delivery of VNS itself does not seem to compensate for the reduced repetition of rehabilitation.
It is reasonable to expect that the total number of VNS-tactile pairings would be the primary determinant of the degree of recovery, and consequent reductions in the total number of pairings would reduce efficacy. However, the observation that Intense Weekly VNS and Rapid Intense Daily VNS, which deliver an equivalent number of pairings as Moderate Daily VNS, failed to improve recovery reveals that the total amount of VNS pairings alone is not the sole determinant in the degree of recovery. Various VNS studies indicate that there is a ceiling of number of effective VNS pairings per session, and that exceeding this ceiling produces no further effects.20-22 This is predicated on the notion that VNS engages neuromodulatory networks to promote plasticity, and these systems are prone to desensitization. 23 It is conceivable that this desensitization threshold was surpassed in both the Intense Weekly VNS and Rapid Intense Daily VNS groups. Therefore, despite both Intense groups receiving an equivalent number of pairings as the effective Moderate Daily paradigm, if only a certain number of those pairings each session meaningfully contributes to recovery, the overall consequence would be indistinguishable from the VNS paradigm that delivers fewer daily pairings. The result of this observation is that an optimal paradigm requires VNS pairings to be spread across many sessions, thus minimizing presumptive within-session desensitization, to drive enhanced recovery.
The observation that schedules with a low frequency of sessions fail to improve recovery indicates that daily repetition is a necessary component of an effective VNS therapy. The week-long interval between therapy sessions likely contributed to the failure of Moderate Weekly VNS and Intense Weekly VNS. There is no agreement on the appropriate amount of time between sessions, although some evidence indicates that the amount of therapy in a session interacts with session frequency to influence rehabilitative outcomes. The greater the amount of therapy in a session, the longer the period between sessions can occur without negatively affecting the rehabilitative outcome, and vice versa.19,24 Although the Intense Weekly VNS group received 4-fold more VNS-touch pairings during a session than Moderate VNS, the Intense Weekly VNS group failed to demonstrate enhanced recovery suggesting the interval between sessions was too long. These findings support using VNS paradigms in which multiple sessions are delivered each week.
The failure to reduce overall therapy time by reducing the schedule of daily and weekly sessions suggests that daily repetition is required to enhance somatosensory recovery. One plausible alternative to reduce the burden of therapy that maintains the daily and weekly schedule is to shorten individual sessions. There are 2 simple means to reduce the duration of a session: deliver fewer VNS pairings or deliver the same number of VNS pairings faster. We evaluated these approaches individually by either reducing the total number of VNS-touch pairings per session or reducing the time between individual VNS-touch pairings. The Short Daily VNS groups received 1-quarter of the daily amount of VNS-touch pairings than the Moderate Daily VNS group. Although this group-maintained frequency and duration compared to the Moderate Daily VNS group, it failed to match the extent of recovery. Given that this group matched Moderate Daily VNS in all other aspects except the daily and overall number of VNS-touch pairings, these findings lead to the conclusion that there is a minimum threshold of daily VNS pairings required to drive enhancement of recovery. These findings are consistent with prior studies, 25 and indicate that an effective VNS therapy should deliver sufficient stimulation pairing in each session.
Finally, we attempted to shorten each session by reducing the interval between VNS pairings. The Rapid Moderate Daily VNS group matched the features Moderate Daily VNS group, except that VNS-touch pairings occurred 5 times faster than Moderate Daily VNS, substantially reducing the duration of daily sessions. The Rapid Moderate Daily VNS paradigm failed to produce the robust recovery observed with Moderate Daily VNS and did not yield significant improvements compared with tactile rehabilitation without VNS. This reduction in the efficacy of VNS at shorter intervals is consistent with prior preclinical studies evaluating VNS-dependent plasticity. 25 This raises the prospect that longer intervals between pairings could produce larger effects, an avenue we did not explore in this study. Although this could improve the efficacy of VNS therapy, it would lengthen the duration of each session, which is contrary to the central goal of this study. Regardless, these findings highlight the need to consider the duration between VNS pairings as a determining factor in the efficacy of VNS therapy.
Moderate Daily VNS was the only group able to drive significantly enhanced recovery compared to the No VNS group. The inability to replicate the extent of recovery in Moderate Daily VNS by all other groups suggests that a single dimension cannot define the critical dose of VNS therapy required to produce a significant improvement. Thus, it is essential to consider multiple factors in optimizing the therapy. The findings from this study demonstrate that an effective VNS therapy involves delivering multiple stimulations per session, conducting several sessions per week, and continuing therapy over several weeks. These results offer empirical evidence to guide the design of VNS paradigms that enhance neurological recovery. Therapists can utilize the principles observed in this study to design a VNS therapy schedule that is effective and tailored to the patient’s ability to attend clinic sessions.
Acknowledgments
We would like to thank Alfonso Reyes for the construction of VNS stimulator cuffs. We also thank, Mamoon Aslam-Syed Mian, Nikit Valmiki, Immaculate Wambugu, Sakina Husain, Afaf Nabeeha, and Abishek Sule for their help in behavioral training and testing. Finally, thank you to David Pruitt and Michael Borland for their guidance in constructing this manuscript.
Footnotes
Abbreviations: VNS: vagus nerve stimulation
PNI: peripheral nerve injury
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MPK has a financial interest in MicroTransponder, Inc., which is developing closed-loop VNS for stroke. RLR is the co-founder and CEO of XNerve, which is developing nerve stimulation therapies. The other authors declare that no competing interests exist.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Congressionally Directed Medical Research Program DM190663
ORCID iD: Andrea D. Ruiz  https://orcid.org/0000-0002-3918-3882
https://orcid.org/0000-0002-3918-3882
References
- 1. Broeks JG, Lankhorst GJ, Rumping K, Prevo AJH. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil. 1999;21(8):357-364. doi: 10.1080/096382899297459 [DOI] [PubMed] [Google Scholar]
- 2. Hundepool CA, Ultee J, Nijhuis THJ, Houpt P, Group “zero” R, Hovius SER. Prognostic factors for outcome after median, ulnar, and combined medianeulnar nerve injuries: a prospective study. J Plast Reconstr Aesthet Surg. 2015;68:1-8. doi: 10.1016/j.bjps.2014.09.043 [DOI] [PubMed] [Google Scholar]
- 3. Lundborg G, Rosén B. Hand function after nerve repair. Acta Physiol. 2007;189(2):207-217. doi: 10.1111/j.1748-1716.2006.01653.x [DOI] [PubMed] [Google Scholar]
- 4. Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol. 2017;289:21-30. doi: 10.1016/J.EXPNEUROL.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hulsey DR, Hays SA, Khodaparast N, et al. Reorganization of motor cortex by Vagus nerve stimulation requires cholinergic innervation. Brain Stimul. 2016;9(2):174-181. doi: 10.1016/j.brs.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hulsey DR, Shedd CM, Sarker SF, Kilgard MP, Hays SA. Norepinephrine and serotonin are required for vagus nerve stimulation directed cortical plasticity. Exp Neurol. 2019;320:112975. doi: 10.1016/j.expneurol.2019.112975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Darrow MJ, Mian TM, Torres M, et al. Restoration of somatosensory function by pairing Vagus nerve stimulation with tactile rehabilitation. Ann Neurol. 2020;87(2):194-205. doi: 10.1002/ana.25664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Darrow MJ, Mian TM, Torres M, et al. The tactile experience paired with Vagus nerve stimulation determines the degree of sensory recovery after chronic nerve damage. Behav Brain Res. 2021;396:112910. doi: 10.1016/J.BBR.2020.112910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kilgard MP, Rennaker RL, Alexander J, Dawson J. Vagus nerve stimulation paired with tactile training improved sensory function in a chronic stroke patient. NeuroRehabilitation. 2018;42(2):159-165. Accessed March 28, 2022. http://eprints.gla.ac.uk/159189/http://eprints.gla.ac.uk [DOI] [PubMed] [Google Scholar]
- 10. Dawson J, Liu CY, Francisco GE, et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet. 2021;397:1545-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silva MA. Predictors of Retention in Physical Therapy: client-, Disease-, and Treatment-Related Factors. Marquette University; 2010. [Google Scholar]
- 12. Keith RA. Treatment strength in rehabilitation. Arch Phys Med Rehabil. 1997;78(12):1298-1304. doi: 10.1016/S0003-9993(97)90300-2 [DOI] [PubMed] [Google Scholar]
- 13. Lohse KR, Lang CE, Boyd LA. Is more better? Using metadata to explore dose-response relationships in stroke rehabilitation. Stroke. 2014;45(7):2053-2058. doi: 10.1161/STROKEAHA.114.004695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meyers EC. Enhancing Plasticity Using Vagus Nerve Stimulation Improves Recovery Following Neurological Injury. University of Texas at Dallas; 2017. [Google Scholar]
- 15. Meyers EC, Kasliwal N, Solorzano BR, et al. Enhancing plasticity in central networks improves motor and sensory recovery after nerve damage. Nat Commun. 2019;10(1):1-14. doi: 10.1038/s41467-019-13695-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bucksot JE, Morales Castelan K, Skipton SK, Hays SA. Parametric characterization of the rat Hering-Breuer reflex evoked with implanted and non-invasive vagus nerve stimulation. Exp Neurol. 2020;327:113220. doi: 10.1016/J.EXPNEUROL.2020.113220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rios MU, Bucksot JE, Rahebi KC, Engineer CT, Kilgard MP, Hays SA. Protocol for construction of rat nerve stimulation cuff electrodes. Methods Protoc. 2019;2(1):19. doi: 10.3390/MPS2010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Forrest G, Reppel A, Kodsi M, Smith J, Enix D. Inpatient rehabilitation facilities: the 3-hour rule. Medicine. 2019;98(37):e17096. doi: 10.1097/MD.0000000000017096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamada C, Itaguchi Y, Fukuzawa K. Effects of the amount of practice and time interval between practice sessions on the retention of internal models. PLoS One. 2019;14(4):e0215331. doi: 10.1371/journal.pone.0215331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morrison RA, Danaphongse TT, Abe ST, et al. High intensity VNS disrupts VNS-mediated plasticity in motor cortex. Brain Res. 2021;1756:147332. doi: 10.1016/j.brainres.2021.147332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hays SA, Khodaparast N, Hulsey DR, et al. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke. 2014;45(10):3097-3100. doi: 10.1161/STROKEAHA.114.006654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ganzer PD, Darrow MJ, Meyers EC, et al. Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury. eLife. 2018;7:e32058. doi: 10.7554/eLife.32058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hays SA, Ruiz A, Bethea T, et al. Vagus nerve stimulation during rehabilitative training enhances recovery of forelimb function after ischemic stroke in aged rats. Neurobiol Aging. 2016;43:111-118. doi: 10.1016/J.NEUROBIOLAGING.2016.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Donovan JJ, Radosevich DJ. A meta-analytic review of the distribution of practice effect: now you see it, now you don’t. J Appl Psych. 1999;84(5):795-805. [Google Scholar]
- 25. Borland MS, Engineer CT, Vrana WA, et al. The interval between VNS-tone pairings determines the extent of cortical map plasticity. Neuroscience. 2018;369:76-86. doi: 10.1016/j.neuroscience.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]