Abstract
Introduction
The septin 9 blood test is indicated for colorectal cancer screening in individuals who decline first-line tests, but participation in this context is unclear. We conducted a randomized controlled trial to compare re-offering colonoscopy and fecal immunochemical test (FIT) alone vs. also offering the blood test among individuals who declined colonoscopy and FIT.
Methods
Screen-eligible Veterans aged 50–75 years who declined colonoscopy and FIT within the previous six months were randomized to 1) letter and telephone outreach to re-offer screening with colonoscopy/FIT only (control) or 2) additionally offering the blood test as a second-line option (intervention). The primary outcome was completion of any screening test within 6 months. The secondary outcome was completion of a full screening strategy within 6 months, including colonoscopy for those with a positive non-invasive test.
Results
Of 359 participants who completed follow-up, 9.6% in the control group and 17.1% in the intervention group completed any screening (7.5% difference, P=0.035). Uptake of colonoscopy and FIT was similar in the two groups. The full screening strategy was completed in 9.0% and 14.9% in the control and intervention groups, respectively (5.9% difference, P=0.084).
Conclusions
Among individuals who previously declined colonoscopy and FIT, offering a blood test as a secondary option increased screening by 7.5% without decreasing uptake of first-line screening options. However, completion of a full screening strategy did not increase. These findings indicate that a blood test is a promising method to improve colorectal cancer screening, but obtaining a timely colonoscopy after a positive non-invasive test remains a challenge; ClincialTrials.gov number, NCT03598166.
Keywords: liquid biopsy, septin 9, second-line, veteran
INTRODUCTION
High quality evidence demonstrates the efficacy of colorectal cancer screening,1 which receives a Grade A recommendation from the US Preventive Services Task Force (USPSTF).2 Despite the availability of several testing options and widespread insurance coverage, only 71.6% of Americans aged 50 to 75 years were up-to-date with screening in 2020.3 Test-specific barriers to screening include bowel preparation and invasiveness for colonoscopy and fecal aversion for stool-based tests.4 A blood test can circumvent these barriers and could theoretically improve colorectal cancer screening uptake.
The only blood test currently approved by the US Food and Drug Administration (FDA) detects methylated septin 9 DNA, which is a biomarker for colorectal cancer.5 Samples are run in triplicate using real-time polymerase chain reaction and are considered positive if any of the 3 replicates detects methylated septin 9. The FDA-approved version of the test has a sensitivity of 68% and specificity of 79% for colorectal cancer.6
The septin 9 test is indicated for individuals who have declined first-line screening tests,7 but screening uptake in this clinical context is unknown. To assess the effect of offering a blood test on colorectal cancer screening, we conducted a single-center randomized controlled trial among individuals who had previously declined colonoscopy and fecal immunochemical testing (FIT).
METHODS
Study Population
This study was a randomized controlled trial (ClinicalTrials.gov, NCT03598166) that was approved by the Institutional Review Boards at both the VA New York Harbor Health Care System and NYU Langone Health. All participants were recruited from the VA New York Harbor Health Care System. Eligible participants were aged 50 to 75 years, at average risk for colorectal cancer (defined as no personal history of colorectal neoplasia, proximal hyperplastic polyps, hereditary cancer syndrome, or inflammatory bowel disease, and no first-degree relative with colorectal cancer diagnosed before age 60 years), did not have limited life expectancy due to severe comorbidities, eligible for screening, and had declined both colonoscopy and FIT based on electronic health record documentation within the previous 6 months.
Study Procedure
We initially screened 360 individuals who met eligibility criteria based on information in the electronic health record and block randomized them 1:1 to the intervention or control group. All participants received outreach by letter and phone call. The letter informed participants that our records indicated they were overdue for colorectal cancer screening and had declined colonoscopy and FIT within the past 6 months. The letter also stated that the recommended screening options are colonoscopy and FIT and provided a number to call to facilitate scheduling. For the intervention group, the letter reiterated a strong recommendation to screen with colonoscopy or FIT, but also offered an FDA-approved blood test for screening if the participant was unwilling to undergo those two first-line options. The blood test option was not offered to the control group. In addition, all participants were encouraged to complete a survey about colorectal cancer screening attitudes and beliefs, which was included with the letter. We made scripted phone calls starting 1 week after the letter was sent and attempted up to 5 calls per participant. During the calls, 21 participants assigned to the intervention group and 23 assigned to the control group were found to be ineligible—the majority of whom had received screening outside of the VA that was not documented in the electronic health record—and these individuals were replaced by new participants via additional rounds of block randomization in a 1:1 ratio until a predetermined date (Figure 1). For participants who preferred colonoscopy or FIT, we notified the primary care provider of their preference by email but did not order a FIT or a gastroenterology consult for colonoscopy.
Figure 1.
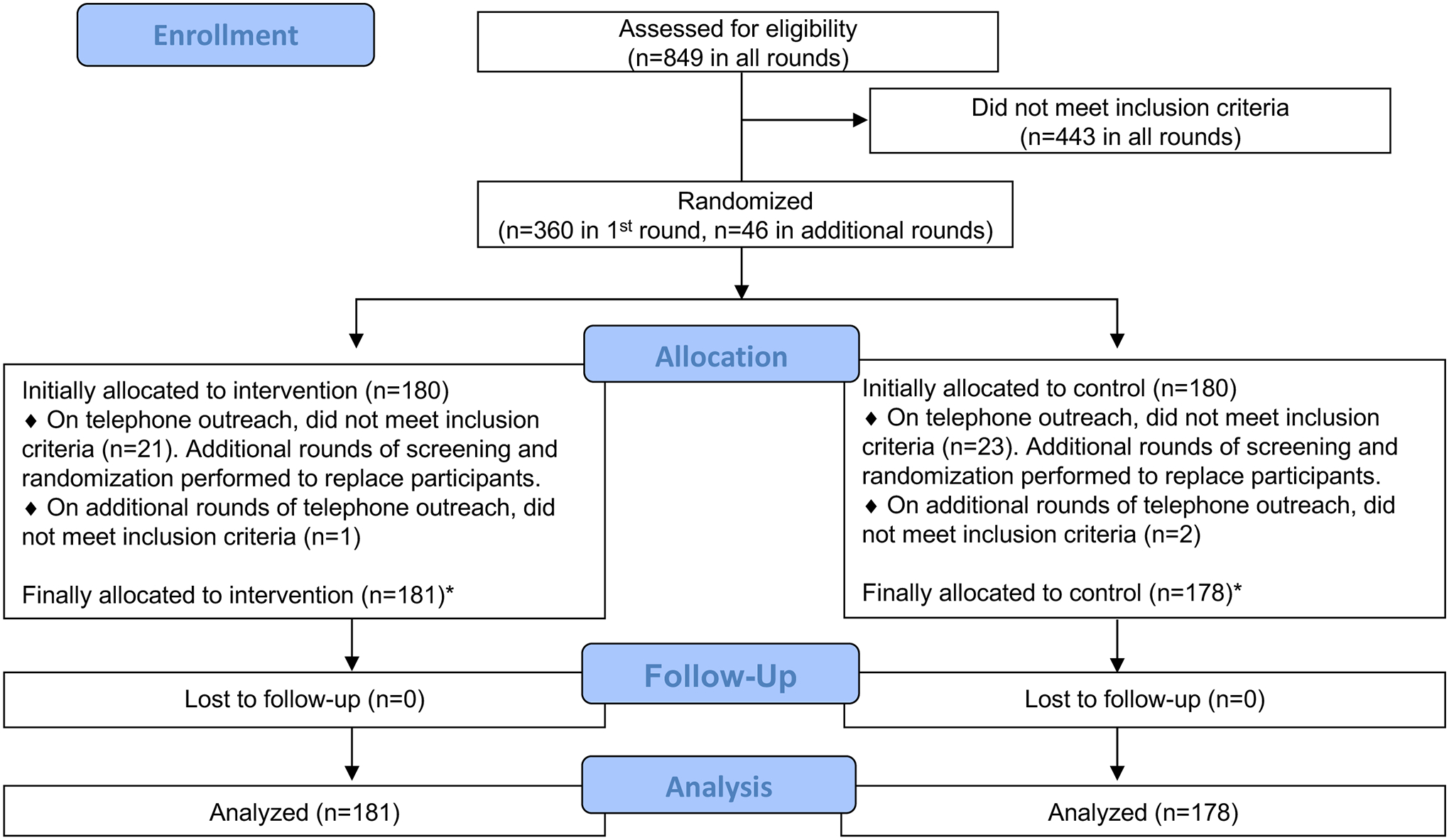
Study Flow Diagram
*Imbalance in groups is explained by additional rounds of block randomization and exclusion. Randomization was always 1:1, regardless of the number of participants already allocated to either group.
Participants in the intervention group who preferred the blood test were scheduled for a research visit, and only these individuals were asked to provide written informed consent. Following the manufacturer’s instructions,7 20 mL of blood was collected in two 10 mL lavender top K2EDTA tubes. Within 4 hours of collection, the samples were centrifuged for 12 minutes at 1350 +/− 150 relative centrifugal force, the plasma was transferred to a 15 mL conical tube, and this was centrifuged for an additional 12 minutes. A minimum of 3.5 mL of plasma was then transferred into a cryovial and frozen. The sample was then delivered to a commercial laboratory (Lenco Diagnostic Laboratory, Brooklyn, NY), where the assay was performed within 14 days. The research team obtained the test results within 1 week from an online portal, and this information was shared with participants via phone call. The test result and recommended action was also entered in the electronic health record. Participants with a negative blood test were recommended to undergo repeat screening with any method in 1 year. For those with a positive test, the research team placed a referral to gastroenterology.
Study Outcomes
The primary outcome was receipt of any colorectal cancer screening test within 6 months of outreach. We performed a sensitivity analysis to exclude individuals whose 6 month follow up period overlapped with the suspension of all non-urgent procedures due to COVID-19, which lasted from March 13 to June 15, 2020. The secondary outcome was completion of a full screening strategy—defined as a colonoscopy with adequate bowel preparation, a negative FIT or blood test, or a positive FIT or blood test followed by a colonoscopy—within 6 months of outreach. To account for additional time needed to schedule a diagnostic colonoscopy following a positive FIT or blood test, we conducted a sensitivity analysis that extended the follow up period to 6 months after a positive non-invasive test result. In an exploratory analysis, we assessed whether completing any screening test within 6 months was associated with age, sex, or race and ethnicity. We also calculated the overall test positivity for FIT and the blood test.
Sample Size Calculation and Statistical Analysis
Two previous studies reported that uptake of the septin 9 blood test was 84–94%.8,9 However, since our study population consisted of individuals who had previously declined both colonoscopy and FIT, we estimated a substantially lower uptake of 50%. To detect a 15% absolute difference in screening uptake with an alpha of 0.05, 80% power, and drop-out of 10 patients per group, the required sample size was 180 participants per group or 360 participants overall.
Analyses were performed using Stata version 16 (StataCorp, College Station, TX). The chisquared and Fisher’s exact tests, depending on expected cell counts, were used to compare proportions in the two groups. Univariable and multivariable logistic regression was used to assess the association between demographic characteristics and the primary outcome. Results were considered statistically significant if the two-sided P value was < 0.05.
RESULTS
Participant Demographics
A total of 359 eligible participants—178 in the control group and 181 in the intervention group—were recruited and all completed 6 months of follow up (Figure 2). The slight imbalance in the two groups was caused by additional rounds of randomization to replace ineligible participants. Overall, 75.8% of participants were aged 60–75 years and 95.5% were male (Table 1). In terms of race and ethnicity, the study population was 40.9% non-Hispanic Black, 36.2% non-Hispanic White, 15.3% Hispanic, 1.4% Asian, 0.8% Native Hawaiian/Pacific Islander, and 5.3% other/unknown. The control and intervention groups did not differ in these demographic characteristics.
Figure 2.
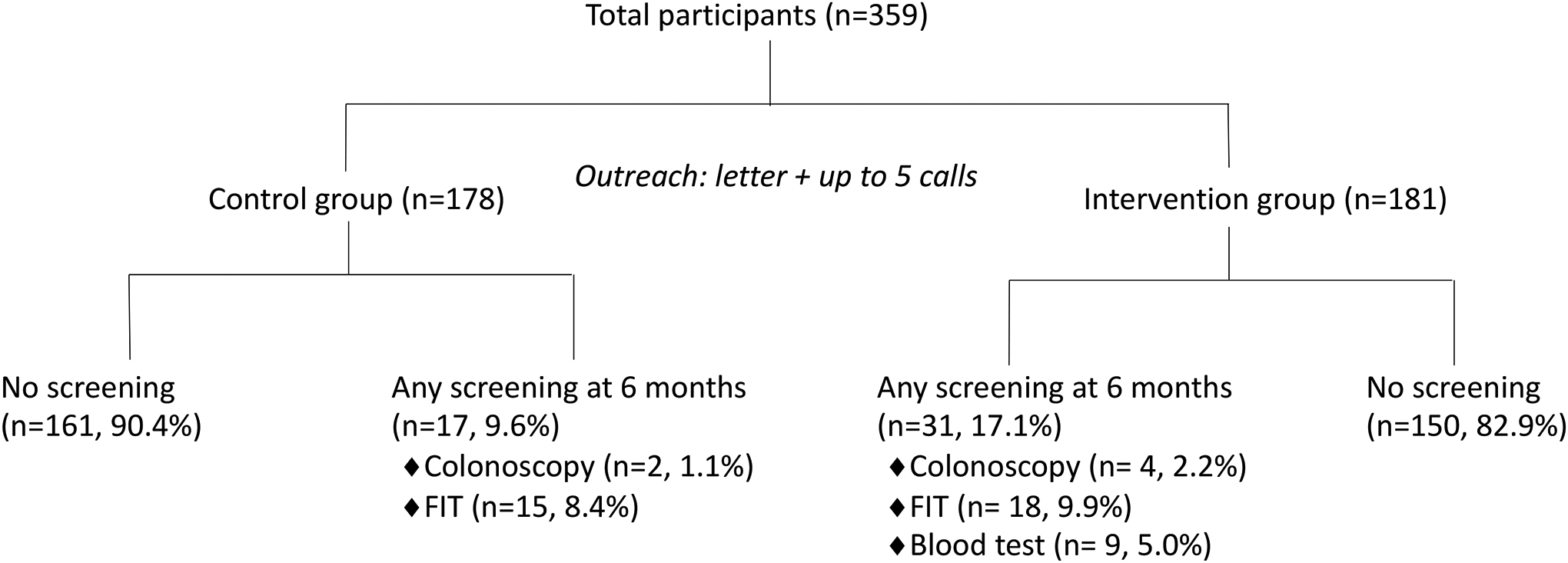
Study Procedures and Outcomes
Table 1.
Participant Demographics
| Variable | Overall (n=359) | Control (n=178) | Intervention (n=181) |
|---|---|---|---|
| Age (years) | |||
| 50–59 | 87 (24.2%) | 38 (21.3%) | 49 (27.1%) |
| 60–69 | 148 (41.2%) | 78 (43.8%) | 70 (38.7%) |
| 70–75 | 124 (34.5%) | 62 (34.8%) | 62 (34.3%) |
| Male sex | 343 (95.5%) | 171 (96.1%) | 172 (95.0%) |
| Race/Ethnicity | |||
| Non-Hispanic White | 130 (36.2%) | 68 (38.2%) | 62 (34.3%) |
| Non-Hispanic Black | 147 (40.9%) | 70 (39.3) | 77 (42.5%) |
| Hispanic | 55 (15.3%) | 26 (14.6%) | 29 (16.0%) |
| Asian | 5 (1.4%) | 3 (1.7%) | 2 (1.1%) |
| Native Hawaiian/Pacific Islander | 3 (0.8%) | 1 (0.6%) | 2 (1.1%) |
| Other/Unknown | 19 (5.3%) | 10 (5.6%) | 9 (5.0%) |
Findings
The first invitation letter was mailed on Jan 11, 2019 and the last phone call occurred on June 10, 2020. The contact rate, defined as the percentage of participants who either spoke to the research team by phone or returned a survey, was 253/359 (70.5%). The mean number of calls made was 3.4 in the control group and 3.5 in the intervention group. Of the 359 participants, 76 (21.2%) expressed an interest in colorectal cancer screening during phone conversations (Table 2): 19 (5.3%) preferred colonoscopy, 33 (9.2%) preferred FIT, 22 (12.2% of the intervention group) preferred the blood test, 1 (0.3%) preferred either colonoscopy or FIT, and 1 (0.6% of the intervention group) preferred either FIT or the blood test.
Table 2.
Stated Preference for Screening Tests
| Preferred test, n (%) | Overall (n=359) | Control (n=178) | Intervention (n=181) |
|---|---|---|---|
| Preferred no screening | 283 (78.8) | 148 (83.2) | 135 (74.6) |
| Colonoscopy | 19 (5.3) | 7 (3.9) | 12 (6.6) |
| FIT | 33 (9.2) | 23 (12.9) | 10 (5.5) |
| Blood test | - | - | 22 (12.2) |
| Colonoscopy or FIT | 1 (0.3) | - | 1 (0.6) |
| FIT or blood test | - | - | 1 (0.6) |
For the primary outcome, 17/178 (9.6%) participants in the control group and 31/181 (17.1%) of those in the intervention group completed any form of colorectal cancer screening within 6 months of outreach (P = 0.035, Figure 3). This represented a 7.5% or 1.8 fold increase in screening in the intervention group. Of the 17 individuals in the control group who underwent screening, 2 (1.1%) received colonoscopy and 15 (8.4%) received FIT. Of the 31 individuals in the intervention group who underwent screening, 4 colonoscopies (2.2%), 18 FITs (9.9%), and 9 blood tests (5.0%) were completed (Figure 2). There was no difference in the proportion of colonoscopy or FIT completed in the two groups. Of the 6 individuals in both groups who underwent primary screening colonoscopy, 2 had nonadvanced adenoma and 1 had advanced adenoma. In the sensitivity analysis that excluded participants whose 6 month follow up period overlapped with the COVID-19 pause in elective procedures, 13/157 (8.3%) in the control group and 30/161 (18.6%) in the intervention group completed any screening test (P = 0.007). The intervention group had a 10.4% or 2.3 fold higher screening uptake.
Figure 3.
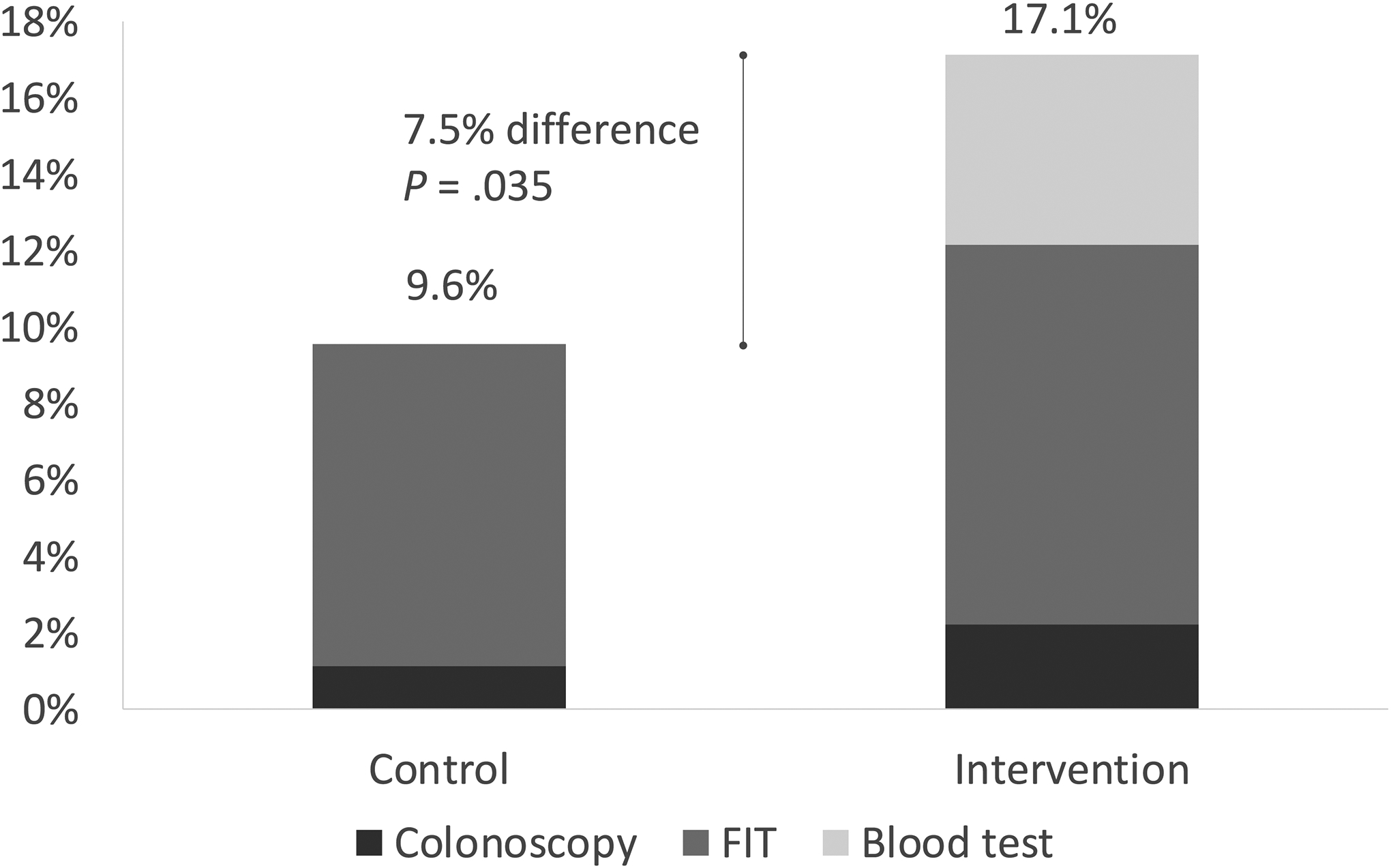
Screening Uptake 6 months after Outreach
For the secondary outcome, the proportion of participants who completed a full screening strategy within 6 months was 16/178 (9.0%) in the control group and 27/181 (14.9%) in the intervention group (P = 0.084). Of the 5 individuals who had a positive FIT (n=3) or blood test (n=2) within 6 months of outreach, only 1 participant with a positive blood test underwent a colonoscopy during the same period, which revealed an advanced adenoma. No other individuals completed diagnostic colonoscopy when the follow up period was extended to 6 months after the date of the FIT or blood test result.
We did not observe any association between age, sex, and race and ethnicity with the primary outcome (Table 3). Overall test positivity, including tests that were completed after 6 months, was 4/49 (8.2%) for FIT and 2/11 (18.2%) for the blood test.
Table 3.
Association of Demographic Characteristics with Any Screening at 6 Months
| Variable | Univariable OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|
| Age, years | ||
| 50–59 | Ref | Ref |
| 60–69 | 1.09 (0.51–2.33) | 1.01 (0.47–2.17) |
| 70–75 | 0.80 (0.35–1.82) | 0.72 (0.31–1.66) |
| Sex | ||
| Male | Ref | Ref |
| Female | 0.42 (0.05–3.25) | 0.64 (0.12–3.49) |
| Race/Ethnicity | ||
| Non-Hispanic White | Ref | Ref |
| Non-Hispanic Black | 0.82 (0.40–1.64) | 0.77 (0.38–1.58) |
| Hispanic | 1.24 (0.53–2.92) | 1.14 (0.48–2.72) |
| Asian | 4.34 (0.80–23.68) | 4.13 (0.75–22.67) |
| Native Hawaiian/Pacific Islander | 0.87 (0.04–17.51) | 0.97 (0.05–20.38) |
| Other/Unknown | 0.87 (0.21–3.57) | 0.83 (0.20–3.54) |
Multivariable model includes age, sex, and race/ethnicity
DISCUSSION
In a randomized controlled trial of individuals who had declined colonoscopy and FIT for colorectal cancer screening, offering a blood test as a secondary option increased screening by 7.5% (1.8 fold) compared to re-offering colonoscopy and FIT alone. Excluding participants whose follow up may have been disrupted by the 3-month suspension of non-urgent procedures caused by COVID-19 resulted in a 10.4% (2.3 fold) higher uptake in the intervention group, which suggests the effect of the intervention may have been larger under normal circumstances. There was no difference in the proportion of participants who completed a full screening strategy in the two groups, and the number of individuals who completed a diagnostic colonoscopy after a positive FIT or blood test was low. Finally, we did not observe a decrease in colonoscopy or FIT use among participants who were offered the blood test as a secondary option.
This is the first trial to show the effect of offering a blood test for colorectal cancer screening in individuals who have declined both colonoscopy and FIT, which is important because the septin 9 test is indicated for individuals who have declined first-line screening tests. Although several other screening modalities are listed in the USPSTF recommendations2—including stool DNA-FIT, CT colonography, and flexible sigmoidoscopy—colonoscopy and FIT are by far the most widely used tests in the US and worldwide.10,11 In addition, the main concerns regarding convenience and tolerability for colonoscopy (bowel preparation, invasiveness) and FIT (need for stool sample) also apply to the other tests recommended by the USPSTF. Therefore, individuals who have declined both colonoscopy and FIT represent the de facto target population for the septin 9 test in most clinical scenarios.
Two previous studies showed high acceptance of the septin 9 blood test among average-risk individuals, but these did not offer the blood test both under its current FDA-approved indication and in a real-world setting. In a German study, 109 individuals who declined screening colonoscopy during routine clinic visits were immediately offered a choice between FIT and the blood test.8 The vast majority of participants (97.2%) completed a non-invasive screening test, with 82.6% undergoing the blood test during the same visit and 14.7% choosing FIT. FIT and the blood test were presented as equal options in the study, which is not consistent with the current indication for the blood test. In a US-based randomized controlled trial of persons who were overdue for screening, 99.5% of those in the blood test arm completed screening within 6 weeks, compared to 88.1% in the FIT arm and 20.2% in the usual care arm.9 It is unclear whether all participants had been offered both colonoscopy and FIT, but more importantly, the combined 93.7% screening uptake in the two intervention arms indicates a strong selection bias toward screening among those who enrolled in the trial. We re-offered all participants both colonoscopy and FIT as first-line tests and only required consent for individuals who underwent the blood test, which approximated the actual conditions under which the test would be used and minimized selection bias. Our finding that offering a blood test can increase screening in individuals who had declined FIT is also novel, and it indicates that participants perceive blood and stool tests as distinct non-invasive modalities.
An absolute increase of 7.5% in screening uptake in the intervention group—which rose to 10.4% in the sensitivity analysis that accounted for disruptions caused by COVID-19—is a clinically significant improvement given that outreach to re-offer the first-line tests alone resulted in only 9.6% uptake. An Australian trial that randomized individuals who had previously declined screening with FIT (including those who had also declined a different blood test) to FIT, the blood test, or a choice between the two options found no difference in uptake in the three groups at 3 months.12 In that study, re-offering FIT—the primary method of screening in Australia—resulted in 12.0% uptake, which is comparable to the 9.6% uptake in our control group. Screening in our intervention group may have been higher because the septin 9 blood test is FDA-approved, whereas the blood test used in the Australian trial was investigational.
Although individuals in the intervention group had higher participation for any screening, the difference between the two groups in completing a full screening strategy was not statistically significant. This highlights the importance of performing diagnostic colonoscopy after a positive noninvasive test, which is challenging in many settings13,14 and may be especially difficult in individuals who had previously declined participation in screening. Our study was not powered to compare follow up colonoscopy rates for FIT and the blood test, which were similar in the two previous septin 9 studies.8,9 However, assessing both the follow up colonoscopy rate for positive blood tests and potential interventions to improve it, as has been done for FIT,15–17 are important areas for future investigation.
The proportion of participants who screened with first-line colonoscopy or FIT was similar in the two groups. This offers reassurance that a blood test, when presented appropriately as a second-line option, does not appear to divert individuals away from screening modalities with more robust evidence for colorectal cancer prevention. Providing individuals with second-line screening options may actually enhance adherence with first-line strategies by emphasizing the importance of screening and the recommendation to use first-line options. Future studies should explore this hypothesis. Furthermore, based on our findings and those from the aforementioned US and Australian trials,9,12 re-offering first-line tests to individuals who have previously declined screening appears to be a sensible approach with reasonable yield. Data on the participation rate of individuals who declined and are re-offered screening is sparse, but a Dutch population-based mailed FIT screening program found that 19–23% of nonparticipants completed screening on a subsequent round of invitation.18 Our results also support previous data that showed presenting patients with a choice in screening modality improves overall uptake.19
The septin 9 test is the only FDA-approved blood test for colorectal cancer screening, but currently it is not recommended by national organizations that issue screening guidelines in the US, including the USPSTF. Several other blood-based screening tests have been developed, but it remains to be seen whether any of these new tests will be considered first-line options. Even if new blood tests are recommended as first-line options in national guidelines, individual institutions may still offer them as secondary options after colonoscopy and FIT because of the lack of long-term data. Therefore, our results may be generalizable to blood tests other than septin 9.
As expected, the positivity rate of the septin 9 test (18%) was more double that of FIT (8%), which has important implications for programmatic screening and the required colonoscopy capacity. Additionally, whether the positivity rate for septin 9 changes on subsequent rounds of testing is unknown and currently under investigation.
Several limitations of the study warrant acknowledgment. First, because the blood test was not clinically available at our institution, we obtained blood samples during a separate research appointment rather than as part of a clinical visit. Therefore, our results likely underestimate blood test uptake in the clinical scenario where patients can provide the sample at an on-site laboratory immediately after a medical appointment without the need for documented informed consent. On the other hand, the additional engagement required for the blood test—a research visit and a gastroenterology referral for positive tests—may have increased participation for this screening modality. Second, this was a single-center trial in a VA population, which was older and predominantly male. These results may need replication in a non-VA population. Third, we made up to 5 phone calls to contact participants, which is a higher level of outreach than is typically feasible in a clinical setting. However, since the outreach was identical for both the intervention and control groups, this most likely would not have affected the primary outcome.
In summary, among individuals who had previously declined screening with colonoscopy and FIT, re-offering colonoscopy and FIT with a second-line blood test option increased screening by 7.5% more than only re-offering colonoscopy and FIT. There was no decrease in uptake of first-line screening tests. However, there was no difference in the proportion of individuals who completed a full screening strategy. These results demonstrate that a subset of those who have declined first-line screening options are receptive to a blood test. Ensuring diagnostic evaluation after a positive non-invasive test remains a challenge and priority.
“What You Need to Know”.
Background
The septin 9 blood test, the only FDA-approved blood test for colorectal cancer screening, is indicated for individuals who have declined first-line tests. However, the participation rate in this context is unclear.
Findings
In a randomized controlled trial, 17.1% of individuals who had previously declined first-line tests and were reoffered the first-line tests and the blood test completed screening within 6 months, compared to 9.6% of individuals who were only reoffered the first-line tests (7.5% difference, P=0.035).
Implications for patient care
A blood test can improve colorectal cancer screening uptake in individuals who have previously declined colonoscopy and fecal immunochemical testing.
Funding:
This study was funded by Epigenomics and a grant from the New York Society for Gastrointestinal Endoscopy’s Florence Lefcourt Endoscopy Research Award to PSL. PSL is also supported by grant K08 CA230162 from the National Cancer Institute. The funding sources had no role in the analysis of data, preparation of the manuscript, or decision to submit the manuscript for publication.
COI/Financial Disclosures:
PSL has received research support from Epigenomics and Freenome and is on the advisory board for Guardant Health. The remaining authors have no disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: This material is the result of work supported in part by resources from the Veterans Health Administration. The content is solely the responsibility of the authors and does not represent the views of the U.S. Department of Veterans Affairs or the U.S. government.
The authors had access to the study data and have reviewed and approved the final manuscript.
REFERENCES
- 1.Lin JS, Perdue LA, Henrikson NB, et al. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021;325:1978–1998. [DOI] [PubMed] [Google Scholar]
- 2.US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:Influence of family size and birth order on risk of cancer: a population-based study. [Google Scholar]
- 3.Richardson LC, King JB, Thomas CC, et al. Adults Who Have Never Been Screened for Colorectal Cancer, Behavioral Risk Factor Surveillance System, 2012 and 2020. Prev Chronic Dis 2022;19:E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones RM, Woolf SH, Cunningham TD, et al. The relative importance of patient-reported barriers to colorectal cancer screening. Am J Prev Med 2010;38:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.deVos T, Tetzner R, Model F, et al. Circulating methylated SEPT9 DNA in plasma is a biomarker for colorectal cancer. Clin Chem 2009;55:1337–1346. [DOI] [PubMed] [Google Scholar]
- 6.Potter NT, Hurban P, White MN, et al. Validation of a real-time PCR-based qualitative assay for the detection of methylated SEPT9 DNA in human plasma. Clin Chem 2014;60:1183–1191. [DOI] [PubMed] [Google Scholar]
- 7.Epigenomics. Epi proColon Instructions for Use. 2016. Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf13/p130001c.pdf [Accessed June 7, 2022].
- 8.Adler A, Geiger S, Keil A, et al. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol 2014;14:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liles EG, Coronado GD, Perrin N, et al. Uptake of a colorectal cancer screening blood test is higher than of a fecal test offered in clinic: A randomized trial. Cancer Treat Res Commun 2017;10:27–31. [Google Scholar]
- 10.Centers for Disease Control and Prevention. QuickStats: Percentage of Adults Aged 50–75 Years Who Met Colorectal Cancer (CRC) Screening Recommendations* — National Health Interview Survey, United States, 2018. MMWR Morb Mortal Wkly Rep 2020;69:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navarro M, Nicolas A, Ferrandez A, et al. Colorectal cancer population screening programs worldwide in 2016: An update. World J Gastroenterol 2017;23:3632–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young GP, Chen G, Wilson CJ, et al. “Rescue” of Nonparticipants in Colorectal Cancer Screening: A Randomized Controlled Trial of Three Noninvasive Test Options. Cancer Prev Res Phila Pa 2021;14:803–810. [DOI] [PubMed] [Google Scholar]
- 13.Selby K, Senore C, Wong M, et al. Interventions to ensure follow-up of positive fecal immunochemical tests: An international survey of screening programs. J Med Screen 2021;28:51–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Issaka RB, Singh MH, Oshima SM, et al. Inadequate Utilization of Diagnostic Colonoscopy Following Abnormal FIT Results in an Integrated Safety-Net System. Am J Gastroenterol 2017;112:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selby K, Baumgartner C, Levin TR, et al. Interventions to Improve Follow-up of Positive Results on Fecal Blood Tests: A Systematic Review. Ann Intern Med 2017;167:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Issaka RB, Bell-Brown A, Snyder C, et al. Perceptions on Barriers and Facilitators to Colonoscopy Completion After Abnormal Fecal Immunochemical Test Results in a Safety Net System. JAMA Netw Open 2021;4:e2120159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mog AC, Liang PS, Donovan LM, et al. Timely Colonoscopy After Positive Fecal Immunochemical Tests in the Veterans Health Administration: A Qualitative Assessment of Current Practice and Perceived Barriers. Clin Transl Gastroenterol 2022;13:e00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapidzic A, Grobbee EJ, Hol L, et al. Attendance and yield over three rounds of population-based fecal immunochemical test screening. Am J Gastroenterol 2014;109:1257–1264. [DOI] [PubMed] [Google Scholar]
- 19.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012;172:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]


