Abstract
Sports-related concussions (SRCs) are associated with neuromuscular control deficits in athletes following return to play. However, the connection between SRC and potentially disrupted neural regulation of lower extremity motor control has not been investigated. The purpose of this study was to investigate brain activity and connectivity during a functional magnetic resonance imaging (fMRI) lower extremity motor control task (bilateral leg press) in female adolescent athletes with a history of SRC. Nineteen female adolescent athletes with a history of SRC and nineteen uninjured (without a history of SRC) age- and sport- matched control athletes participated in this study. Athletes with a history of SRC exhibited less neural activity in the left inferior parietal lobule/supramarginal gyrus (IPL) during the bilateral leg press compared to matched controls. Based upon signal change detected in the brain activity analysis, a 6 mm region of interest (seed) was defined to perform secondary connectivity analyses using psychophysiological interaction (PPI) analyses. During the motor control task, the left IPL (seed) was significantly connected to the right posterior cingulate gyrus/precuneus cortex and right IPL for athletes with a history of SRC. The left IPL was significantly connected to the left primary motor cortex (M1) and primary somatosensory cortex (S1), right inferior temporal gyrus, and right S1 for matched controls. Altered neural activity in brain regions important for sensorimotor integration and motor attention, combined with unique connectivity to regions responsible for attentional, cognitive, and proprioceptive processing, indicate compensatory neural mechanisms may underlie the lingering neuromuscular control deficits associated with SRC.
Keywords: fMRI, Bilateral Motor Control, Inferior Parietal Lobule, Motion Analysis
Introduction
An estimated 1.9 million sports-related concussions (SRCs) occur in pediatric and adolescent athletes each year (Bryan, Rowhani-Rahbar, Comstock, Rivara, & Seattle Sports Concussion Research, 2016; Meehan, d'Hemecourt, Collins, & Comstock, 2011), and generally result in acute neural alterations, as well as cognitive, behavioral, and neuromuscular deficits (Bonnette et al., 2020; Hammeke et al., 2013; Howell et al., 2019; Kaushal et al., 2019; Lancaster et al., 2016; Murray et al., 2021; Newsome et al., 2016; Wilde et al., 2019). While resolution of many cognitive and clinical symptoms may occur in the days and weeks following initial injury (Broglio et al., 2022; McCrory et al., 2017), many go unrecognized by athletes and/or are not reported in a timely fashion, thus reducing opportunities for clinical management and positive long term outcomes. Further, athletes who have sustained a concussion exhibit lingering neuromuscular control deficits that persist beyond typical clinical clearance and return to play (Buckley, Munkasy, Tapia-Lovler, & Wikstrom, 2013; De Beaumont et al., 2011; Howell, Beasley, Vopat, & Meehan, 2017; Howell et al., 2020; Howell, Buckley, Lynall, & Meehan, 2018; Howell et al., 2019; Howell, Osternig, & Chou, 2015; Howell, Stracciolini, Geminiani, & Meehan, 2017; Hugentobler et al., 2016; Lapointe et al., 2018; Quatman-Yates et al., 2015). Interestingly, female athletes appear to be more susceptible to SRC injury than males (McGroarty, Brown, & Mulcahey, 2020) and are at a ~2.8 times greater risk for musculoskeletal (MSK) injuries following SRC relative to their uninjured female counterparts (Herman et al., 2017). Persistent neuromuscular control deficits following a SRC are likely related to the 1.6 - 3.9 times increased risk of subsequent lower extremity injury following return to play (Biese et al., 2021; Brooks et al., 2016; Fino et al., 2019; Gilbert, Burdette, Joyner, Llewellyn, & Buckley, 2016; Herman et al., 2017; Lynall, Mauntel, Padua, & Mihalik, 2015; Lynall et al., 2017; Nordstrom, Nordstrom, & Ekstrand, 2014). Risk of subsequent lower extremity injury has been shown to be elevated for up to three years following the initial concussive injury (McPherson, Shirley, Schilaty, Larson, & Hewett, 2020), however, the precise timeframe for increased MSK injury risk and specific relationship between SRC and altered neuromuscular control is not clearly defined.
Athletes with a history of SRC exhibit maladaptive landing biomechanics (Dubose et al., 2017; Lapointe et al., 2018) and conservative gait strategies (e.g., reduced step length and gait velocity) (Buckley et al., 2013; Gagné et al., 2019; Howell, Beasley, et al., 2017; Howell et al., 2015; Murray et al., 2021). Most often, neuromuscular alterations are observed under cognitively demanding conditions (e.g., dual-task) (Howell, Beasley, et al., 2017; Howell et al., 2018; Lapointe et al., 2018), suggesting a potential shift in attentional resources or a breakdown in multisensory (cognitive, visual, attention, and sensorimotor) integration to achieve a previously ‘automated’ task. Despite elevated risk for subsequent MSK injury following SRC, current clinical treatments tend to prioritize the resolution of cognitive and behavioral symptoms rather than lingering neuromuscular control deficits. Residual neuromuscular impairments are hypothesized to be the result of SRC-related insults within the central nervous system (CNS; brain and spinal cord) (Wilkerson, Grooms, & Acocello, 2017) and characterization of brain alterations following SRC may help to better understand the sequela of neuromuscular deficits. Better understanding of the mechanisms involved in SRC-related MSK injury risk could optimize clinical treatment strategies for athletes who sustain an SRC.
Alterations to the CNS in athletes post SRC have been evaluated using numerous techniques including, but not limited to, functional magnetic resonance imaging (fMRI), diffusion weighted imaging (DWI), magnetic resonance spectroscopy (MRS), electroencephalography (EEG), and transcranial magnetic simulation (TMS) (Charney et al., 2020; Churchill, Caverzasi, Graham, Hutchison, & Schweizer, 2017; Conley et al., 2018; De Beaumont, Lassonde, Leclerc, & Theoret, 2007; Kaushal et al., 2019; Keightley et al., 2014; Lancaster et al., 2018; Lancaster et al., 2016; McCuddy et al., 2018; Meier et al., 2020; Narayana et al., 2019; Newsome et al., 2016; Wilde et al., 2019; Yuan et al., 2021; Zhu et al., 2015). Notably, fMRI is considered the gold standard for non-invasively evaluating both cortical and subcortical brain functioning with high spatial resolution, providing mechanistic insight into human behavior (indirect assessment of neural activity via the quantification of the blood oxygen level dependent [BOLD] signal) (Friston, Frith, Turner, & Frackowiak, 1995; Logothetis, Pauls, Augath, Trinath, & Oeltermann, 2001). However, acquiring high quality fMRI data has been historically limited to the evaluation of neural activity while a participant is at rest, performing cognitive tasks, or while completing fine motor control tasks. Thus, potential lingering alterations in neural activity important for the coordination of dynamic lower extremity movements in athletes with a history of SRC is unknown. Recent methodological advances now allow for high quality fMRI data to be acquired while participants perform more dynamic lower extremity motor control tasks including ankle plantarflexion/dorsiflexion, isolated knee flexion/extension movements, simulated gait, and simulated bilateral lower extremity landing (Criss, Onate, & Grooms, 2020; Grooms et al., 2022; Grooms et al., 2019; Jaeger et al., 2014; Newton et al., 2008). Three-dimensional motion analysis methods integrated in the MR environment now further permit concurrent biomechanical quantification of lower extremity movement during fMRI scanning (Anand, Diekfuss, Bonnette, et al., 2020; Anand et al., 2021; Strong et al., 2023). Supported by these technological advancements, we aimed to evaluate brain function (i.e., BOLD signal activity and connectivity) during a motor control task, consisting of bilateral ankle, knee, and hip flexion/extension movement against resistance (i.e., bilateral leg press) (Grooms et al., 2022; Slutsky-Ganesh, Anand, Diekfuss, Myer, & Grooms, 2023), in female adolescent athletes with a history of SRC relative to matched controls. We hypothesized that female adolescent athletes with a history of SRC would exhibit distinct neural strategies during a lower extremity motor control task relative to controls.
Method
Participants
One hundred and six female adolescent athletes from local middle and high schools were recruited to take part in a larger, ongoing research study related to anterior cruciate ligament (ACL) injury risk factors, which included biomechanical and neuroimaging assessments. A questionnaire was used to assess prior history of SRC (described below). After excluding those with a history of neuropsychiatric disorders, non-sports related brain injuries, history of neurotrauma, claustrophobia, orthodontic hardware, and/or contraindications to MRI (n = 61), 45 female adolescent athletes indicated prior head impact exposure that resulted in concussion-related symptoms (symptoms acquired from the Sport Concussion Assessment Tool [SCAT]5) (Echemendia et al., 2017). Fourteen subjects were then excluded due to being left-handed (n = 3), had incomplete fMRI data (n = 1), incidental finding (n = 1), no concurrent biomechanics data (n = 2), unknown claustrophobia requiring scanning to be ceased (n = 1), or no age- and sport- matched controls available (n = 6). During data preprocessing, 12 additional subjects were excluded due to poor quality fMRI data (n = 10 with excessive head motion during task-based motor task [> 1mm] and n = 2 with functional registration errors). The final sample included 19 currently asymptomatic female adolescent athletes designated to the history of SRC group.
The 19 right hand/foot dominant female athletes (15.2 ± 1.2 years; 165.6 ± 8.0 cm; 62.2 ± 5.3 kg; 22.8 ± 2.2 body mass index [BMI]; 6.2 ± 3.8 years of sport participation) with a self-reported history of SRC (time since injury 16.9 ± 15.9 months; range: 1 - 61 months) were age- and sport- matched to nineteen right hand/foot dominant female adolescent athletes with no history of SRC (15.2 ± 1.2 years; 162.0 ± 6.2 cm; 61.4 ± 8.6 kg; 23.3 ± 2.9 BMI; 6.3 ± 3.2 years of sport participation) from the same local middle or high school sports teams (Table 1). Due to the preliminary nature of this study, our analyses included athletes who were presently asymptomatic but reported a history of SRC within any time frame. The study was approved by the institutional review board at Emory University and all data was collected at Emory Sports Performance And Research Center (SPARC). Participants and parents/legal guardians signed a written informed assent and consent prior to MRI screening.
Table 1.
Participant Demographics
| Group | Matched Controls (n=19) (MD ± SD) |
History of SRC (n=19) (MD ± SD) |
t-statistic | df | p-value |
|---|---|---|---|---|---|
| Age (years) | 15.21 ± 1.27 | 15.2 ± 1.27 | .000 | 36 | 1.000 |
| Height (cm) | 162.03 ± 6.18 | 165.56 ± 8.00 | −1.527 | 36 | .135 |
| Weight (kg) | 61.35 ± 8.60 | 62.23 ± 5.32 | −.381 | 36 | .706 |
| Body Mass Index (kg/m2) | 23.35 ± 2.91 | 22.77 ± 2.25 | .673 | 36 | .506 |
| Time Since SRC (months) | -- | 16.9 ± 15.9 | |||
| Sport Participation (years) | 6.32 ± 3.20 | 6.21 ± 3.29 | .100 | 36 | .921 |
|
| |||||
| Head Motion | |||||
| Absolute | 0.31 ± 0.12 | 0.32 ± 0.12 | −.276 | 36 | .784 |
| Relative | 0.10 ± 0.03 | 0.12 ± 0.05 | −1.426 | 36 | .163 |
|
| |||||
| Sport, n (%) | |||||
| Basketball | 3 (7.9) | 3 (7.9) | |||
| Soccer | 6 (15.8) | 6 (15.8) | |||
| Volleyball | 10 (26.3) | 10 (26.3) | |||
Note: All demographics are reported as mean (MD) ± standard deviation (SD), with p-values from independent samples t-tests to quantify if any significant between-group differences.
Abbreviations: SRC, sports-related concussion; df, degrees of freedom
Though the present study was restricted to the data available from participants enrolled within the larger study, prior literature supported our final sample size as adequate for the proposed analyses. Specifically, statistically significant differences in neural activity during the bilateral fMRI leg press were reported when classifying groups by ACL injury risk landing biomechanics with a total n = 9 (high injury risk n = 4, low injury risk n = 5) (Grooms et al., 2022) . Statistically significant relationships in bilateral fMRI leg press neural activity and biomechanics related to ACL injury risk were also reported with a total n = 29 (ACL injury risk landing variables) and n = 17 (in-scanner coordination metrics) (Grooms et al., 2022; Slutsky-Ganesh et al., 2023). Thus, a total n = 38 (history of SRC group n = 19, matched controls n = 19) was considered appropriately powered for this preliminary study.
Procedures
History of Concussion Questionnaire
All participants completed a concussion history questionnaire that was modified from prior literature (Llewellyn, Burdette, Joyner, & Buckley, 2014) (see supplementary appendix). The questionnaire was comprised of two sections. The first section of the questionnaire asked athletes about head injuries they acquired during sport participation in which they did not seek medical attention and continued playing. Provided with a list of 22 concussion-related symptoms (cognitive, behavioral, emotional, and vestibular) from the SCAT5 (Echemendia et al., 2017), athletes were asked to respond if they had suffered a blow to the head during sport which resulted in any of the listed symptoms. Symptom scores for the history of SRC group are reported in Table 2. Upon indication of one or more symptoms, athletes were asked how many times those symptoms had occurred following a hit to the head, and about their most recent incident with a hit or blow that resulted in symptoms. The purpose of this first section was to identify athletes who may have suffered a possible concussion but failed to report or did not know they had suffered a concussion. The second section of the questionnaire asked about the athlete’s history of clinically diagnosed concussions. They were provided with a formal definition of concussion, and then asked who diagnosed it and how many prior concussions they had sustained. The same 22 concussion-related symptoms described in the first section were again provided so athletes could indicate which symptoms they experienced following their most recent clinically diagnosed SRC. To achieve the purpose of this study, all athletes who indicated concussion-like symptoms after a hit or blow to the head (n = 10) or indicated a prior history of clinically diagnosed SRC (n = 9) were included in the SRC history group.
Table 2.
SRC Concussion Symptom Scores
| Symptoms | % Of reported symptoms Diagnosed Concussion (n = 9) |
% Of reported symptoms Concussion Symptom (n = 10) |
|---|---|---|
| Headache | 100 | 100 |
| Dizziness | 88.98 | 80.00 |
| Emotional | 22.22 | 10.00 |
| Didn’t Feel Right | 77.78 | 30.00 |
| Confusion | 55.56 | 20.00 |
| Sensitivity to Noise | 66.67 | 30.00 |
| Drowsiness | 33.33 | 10.00 |
| Blurred Vision | 55.56 | 70.00 |
| Irritable | 11.11 | 20.00 |
| Difficultly Concentrating | 44.44 | 10.00 |
| Pressure in Head | 66.67 | 50.00 |
| Feeling Slowed Down | 66.67 | 30.00 |
| Neck Pain | 22.22 | 20.00 |
| Balance Problems | 22.22 | 20.00 |
| Sadness | 22.22 | 10.00 |
| Difficulty Remembering | 44.44 | 10.00 |
| Trouble Sleeping | 22.22 | 10.00 |
| In a Fog | 44.44 | 20.00 |
| Nausea or Vomiting | 11.11 | 20.00 |
| Sensitivity to Light | 44.44 | 40.00 |
| Nervous or Anxious | 11.11 | 20.00 |
| Fatigue or Low Energy | 44.44 | 20.00 |
| Other | 11.11 | 10.00 |
Note: Symptoms are reported as percentages between those with a clinical diagnosed concussion and those with self-reported concussion symptoms following hit or blow to the head.
Abbreviations: SRC, sports-related concussion
MRI Acquisition Parameters
MRI acquisition was conducted on a 3.0T GE SIGNA Premier Scanner (General Electric; Milwaukee, Wisconsin) equipped with a 48-channel phased array head coil. Scans included T1-weighted Magnetization-Prepared RApid Gradient-Echo (MP-RAGE) sequences to acquire high resolution anatomical images with the following parameters: repetition time (TR) = 2000ms; echo time (TE) = 2.4ms; field of view (FOV) = 256×256 mm; matrix = 256×256 mm; and slice thickness = 1mm. Functional MRI acquisition included multi-band whole-brain gradient-echo echo-planar imaging (EPI) sequences with the following parameters: TR = 1500ms; TE = 30ms; FOV = 240×240 mm; reconstructed matrix = 128×128 mm; slice thickness = 4 mm; voxel size= 3×3×4; axial slices = 44; through-plane acceleration factor = 2.
fMRI Lower Extremity Motor Control Apparatus
We utilized previously published methods to design a lower extremity motor control apparatus that permitted bilateral lower extremity movement during fMRI . The MRI-compatible lower extremity motor control apparatus used in this study was an extension from the previously described unilateral task (Anand et al., 2021; Grooms et al., 2022). In brief, the lower extremity motor control apparatus is comprised of two independent horizontal sliding foot pedals which allow for individual unilateral movement, as well as bilateral and alternating movement (Figure 1). Elastic resistance bands are anchored at three different points, including a fixation point at the center of the board such that the band is wrapped around each individual foot pedal and positioned on the lateral side of each leg (adjacent to each subject’s greater trochanter). The addition of the elastic band provides standardized resistance against the ankle, knee, and hip during various flexion/extension movements (manufacturer peak rated force ~9.1kg).
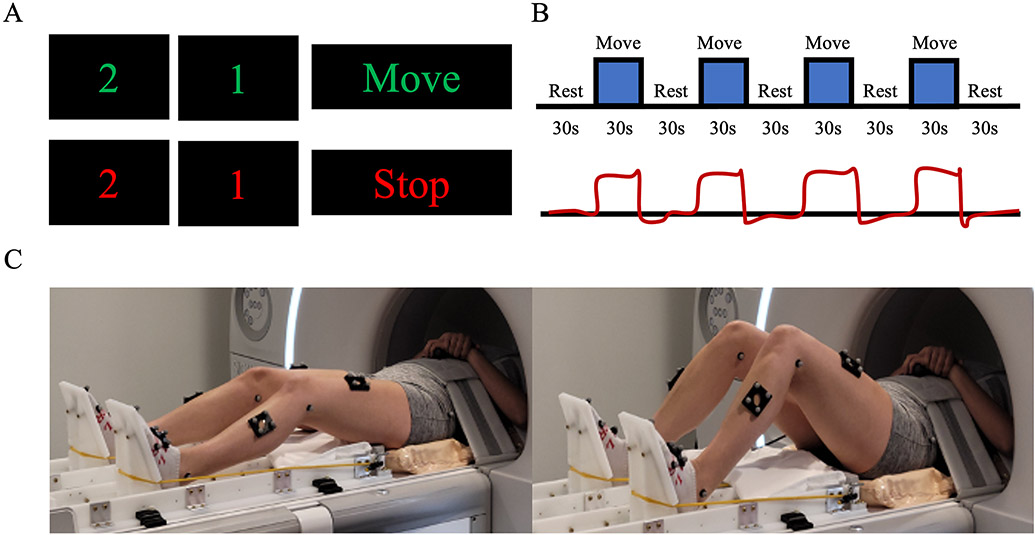
Figure 1:
fMRI lower extremity motor control apparatus and paradigm. A) Visual cue with countdown indicating the start and stop of each 30 second movement block. B) fMRI block design paradigm and modeled hemodynamic response function, which includes 5-30 seconds of rest interleaved with 4-30 seconds of movement. C) Participant completing the bilateral leg press to a 1.27 Hz metronome. Participants moved both legs simultaneously for approximately 18 flexion/extension cycles during each movement block.
Participant Preparation
Prior to fMRI motor control paradigm acquisition, all participants underwent guided MRI movement training with a research scientist. Athletes performed two bilateral leg press practice trials on the same MRI-compatible lower extremity motor control apparatus prior to the scan. The research scientist instructed the participants to maintain limited head movement during the movement trials and guided them through the bilateral movement task. Athletes were told to move both legs simultaneously and match their peak knee extension and flexion in sync with a 1.27 Hz metronome. This standardized approach was designed to provide athletes with a clear understanding of expectations, to ensure robust data collection with limited head motion artifact.
fMRI Lower Extremity Motor Control Paradigm
Participants were placed in the MRI in a supine position with their head in the 48-channel phased array head coil. The head coil was further equipped with a mirror, allowing participants to view a high-definition television monitor (NordicNeuroLab, Bergen Norway) located at the end of the MRI table (outside the bore). Participants were told to maintain their gaze on the monitor throughout the duration of the scan as this is where they would receive visual information about the initiation and termination of the task. All participants wore a set of custom-made headphones for hearing protection, communication during scanning, and for presentation of auditory cues for each movement task. A pair of pneumatic headphones from Scan Sound Inc., (model PAD-HS) (Deerfield, FL, USA) with a slim profile were modified with memory foam padding to conform to the participants head and fit inside the 48-channel head coil. Head padding at the top of the head, around the headphones, and beneath the neck was used to secure the participant’s head and ensure participants were comfortable within the head coil. Additional pads were placed between the shoulder and the head coil to further reduce head movement. Fluidized positioners were placed underneath the participant’s back and head to keep their body from moving in the z plane (superior and/or inferior) during the movement tasks. Two Velcro straps, affixed to the MRI table, were placed across the chest and pelvis also for the purpose of reducing unnecessary movement that could result in motion-related artifacts.
The fMRI lower extremity motor control task was set up in Psychtoolbox 3.0.17 using MATLAB 2022a (Mathworks, Natick, MA) and employed a block design in which participants completed cyclical rest and move blocks. All participants completed bilateral ankle, knee, and hip flexion/extension movements against resistance paced by a 1.27 Hz metronome (audible during move blocks, only). Participants completed a total of four blocks of 30s of flexion/extension movements alternated with 30s of rest. Participants were prompted with a visual countdown at the end of each move/rest block (2-1-Move, 2-1-Stop, respectively) to guide the onset and termination of each movement cycle (Figure 1).
Biomechanics Data Acquisition
Three-Dimensional movement biomechanical data were collected using 8 high-speed (120Hz) MRI-compatible motion analysis cameras (Qualisys, Qualisys Corp., Gothenburg, Sweden). Participants were outfitted with 12 mm reflective skin markers placed bilaterally at the following landmarks: the anterior superior iliac spine (ASIS), greater trochanter, medial and lateral femoral condyles, medial and lateral malleoli, head of first and fifth metatarsals, and first distal phalanx. The thigh and shank were fitted with a rigid cluster with four marker clusters, and the dorsal aspect of each foot was fitted with a rigid cluster of three markers, allowing for concurrent measurements of lower extremity biomechanics during fMRI. A static T-pose capture was done prior to fMRI scanning for anatomical skeleton calibration for all biomechanics calculations. A sacral marker that was placed for skeleton calibration was removed before the participants were set up on the MRI table. A second static capture was done in supine position to improve automatic recognition and tracking.
Biomechanics Preprocessing
The biomechanics data were preprocessed in QTM (Qualisys Track Manage, Qualisys Corp., Gothenburg, Sweden) and all misidentified and unidentified marker trajectories were corrected. A lower body skeleton was developed in Visual3D (C-Motion Corp., Germantown, Maryland) to model the pelvis, femur, shank, and foot. The static pose capture was used to define the individualized skeleton from the anatomical markers and the rigid marker clusters were used for tracking segments defined by the anatomical markers. Custom in-house pipelines were developed using MATLAB 2022a (Mathworks, Natick, MA) to process all datasets through Visual3D and extract the joint kinematic data for higher-level analyses. Time series measures of knee sagittal and frontal plane angles were obtained for the entire duration of the tasks and the data was analyzed for each movement block.
Biomechanics Dependent Variables
Biomechanics measurements included mean knee frontal and sagittal plane range of motion across the four fMRI movement blocks. Additionally, the number of cycles (flexion/extension movements) for the left and right leg were recorded to quantify stimulus exposure. The total number of cycles for each leg were then averaged, demeaned, and included as a covariate of no interest in the fMRI general linear model (GLM) analysis.
Demographics
SPSS software (Version 28.0; IBN Corp., Armonk, NY) was used to evaluate between-group (SRC vs controls) demographic data (age, weight, height, BMI, and years of sport participation) and head motion (absolute and relative motion) using independent t-tests. Significance was set a priori at alpha < .05.
fMRI Pre-processing
fMRI data was processed using FMRIB’s Software Library (FSL) (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). Data underwent standardized preprocessing, which included brain extraction, motion correction using MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002), spatial smoothing (Gaussian kernel=6 mm full width at half maximum), slice timing correction, and intensity normalization. Functional images were linearly registered to anatomical T1-weighted MP-RAGE scans, and non-linearly registered to standardized space (Montreal Neurological Institute template: MNI-152). Following initial preprocessing, data underwent independent component analysis for automated removal of motion artifact (ICA-AROMA) (Pruim et al., 2015). Following ICA-AROMA, data was then visually inspected and underwent quality assurance using the interface for batch processing data using ICA-AROMA (INFOBAR) (Anand, Diekfuss, Slutsky-Ganesh, et al., 2020). Usable data was determined based on the BOLD signal model fit, a stable baseline, and < 1 mm absolute head motion. Following visual inspection, all data then underwent a high-pass filter with 100 s cutoff. Individual subject level analyses for the bilateral leg press trial were completed using the FSL FEAT tool (Woolrich, Ripley, Brady, & Smith, 2001). Time series analyses were carried out using FILM prewhitening with local autocorrelation correction, a 30s block design, (30s move/30s rest) and a cluster-wise threshold of z > 3.1 and p < 0.05.
Primary fMRI Analyses: Task-related Activation
Higher-level group analyses included a two-sided independent t-test to assess differences in task-related activation in athletes with a history of SRC compared to matched-controls (contrasts with total n =38: [history of SRC > matched-controls; matched-controls > history of SRC]). Due to a potential variation in gray matter volume within the population sample (athletes age ranged from 12-18 years old), a gray matter voxel-wise covariate was included in the analyses (Oakes et al., 2007). Further, although athletes were instructed to move to the pace of the 1.27 Hz metronome to standardize movement cycles, there was some variation amongst participants in the total flexion/extension cycles completed during fMRI (M = 71.8, SD = 8.4). Therefore, total cycles were included as a covariate of no interest in analyses (see Biomechanics Dependent Variables Subsection). All higher-level analyses were computed across the entire brain with multiple comparison correction using cluster-based thresholding of z > 3.1 and p < 0.05.
History of SRC Classification Secondary Analyses.
Given our decision to combine data for those who reported a clinical diagnosis of concussion and those who only reported symptoms of a concussion, we performed a one way between-subjects ANOVA with group as the independent factor (diagnosed concussion [n = 10], symptoms of concussion [n = 9], matched controls [n = 19]) and % mean BOLD signal change from the left inferior parietal lobule (IPL) as the dependent variable. The % BOLD signal change for each subject was extracted from the resultant cluster (left IPL) from the overall activation analysis using FSL’s featquery tool. Additionally, given the large range of time from the initial SRC incident (diagnosed or self-reported symptoms) in the present study, we examined the relationship between time since SRC and functional brain activity, using time since SRC incident as a covariate of interest in the higher level GLM analysis.
Task-related Connectivity
Psychophysiological interaction (PPI) analysis allows for investigation of task-related connectivity (i.e., temporally correlated neural activity) between a seed region and other brain regions. A seed region was defined based on the results from the group analysis described above. PPI was applied using the seed region of interest (ROI) and task-elicited connectivity was evaluated during the bilateral leg press. Specifically, to identify task specific changes in functional connectivity, we generated an interaction regressor by mean-centering the task time course and demeaning the seed region time course data. The GLM included a regressor variable to capture the physiological measure, which in this case was the BOLD time series from the seed region, and a psychological contrast (block design) along with the interaction term of the task design (O'Reilly, Woolrich, Behrens, Smith, & Johansen-Berg, 2012). Brain regions which had temporally correlated neural activity with the seed region during the bilateral leg press were then identified. Higher-level group analyses included a two-sided independent t-test to assess differences in task-related connectivity in athletes with a history of SRC compared to matched-controls (contrasts with total n =38: [history of SRC > matched controls; matched controls > history of SRC]). As PPI is novel to the fMRI lower extremity motor control literature (not analyzed previously with the fMRI bilateral leg press), we also reported each group’s task-related connectivity, regardless of statistically significant between-group differences (exploratory post hoc analyses). Specifically, two separate independent models for matched controls (n = 19) and history of SRC (n = 19) were performed, using one samples t-tests, to determine significant connectivity for each group, respectively. All task-related connectivity analyses included a multiple comparison correction using cluster-based thresholding of z > 3.1 and p < 0.05.
Biomechanics Data Analyses
Independent sample t-tests were used to compare the matched controls and the athletes with a history of SRC for all biomechanical dependent variables (number of cycles, mean frontal, and sagittal plane range of motion), with an alpha set a priori at p < 0.05.
Results
Demographics
All demographic data can be found in Table 1. Overall, there were no significant differences in age, height, weight, or BMI between the matched controls and athletes with a history of SRC. Additionally, absolute and relative head motion between the groups was not statistically significant (all p > .05). Concussion symptom scores for the history of SRC group (diagnosed concussion and concussion symptoms are detailed in Table 2.
Biomechanics
Overall, there were no significant between-group differences in mean frontal or sagittal plane range of motion measures for both limbs between the matched-controls and athletes with a history of SRC (all p’s > .05). Additionally, there were no significant differences in the number of flexion/extension cycles completed between the groups (p > .05; see Table 3).
Table 3.
fMRI Biomechanical Variables
| Variables | Matched Controls (M ± SD) |
History of SRC (M ± SD) |
t-statistic | df | p value |
|---|---|---|---|---|---|
| Left Mean Sagittal ROM | 27.69 ± 12.62 | 24.83 ± 11.64 | .725 | 36 | 0.473 |
| Right Mean Sagittal ROM | 25.52 ± 10.91 | 23.25 ± 10.13 | .668 | 36 | 0.508 |
| Left Mean Frontal ROM | 2.78 ± 2.06 | 2.77 ± 2.10 | .014 | 36 | 0.989 |
| Right Mean Frontal ROM | 3.12 ± 2.48 | 2.78 ± 1.58 | .511 | 36 | 0.613 |
| Total Cycles | 72.87 ± 5.37 | 70.71 ± 10.64 | .789 | 36 | 0.435 |
Note: Biomechanics measures for each leg were calculated during the bilateral leg press. Range of motion and total number of cycles are reported as mean (MD) ± standard deviation (SD), with p-values from independent samples t tests to quantify if any significant between-group differences.
Abbreviations: SRC, sports-related concussion; ROM, range of motion; df, degrees of freedom
Task-related Activation
fMRI analysis revealed that athletes with a history of SRC displayed less activity relative to matched controls in the left inferior parietal lobule/supramarginal gyrus (‘IPL’ henceforth; MNI coordinates: x = −52, y = −40, z = 50; Voxels: 184; p = 0.025; Z max = 4.34) during the bilateral leg press. See Figure 2 for a visual representation of the significant cluster.
Figure 2:
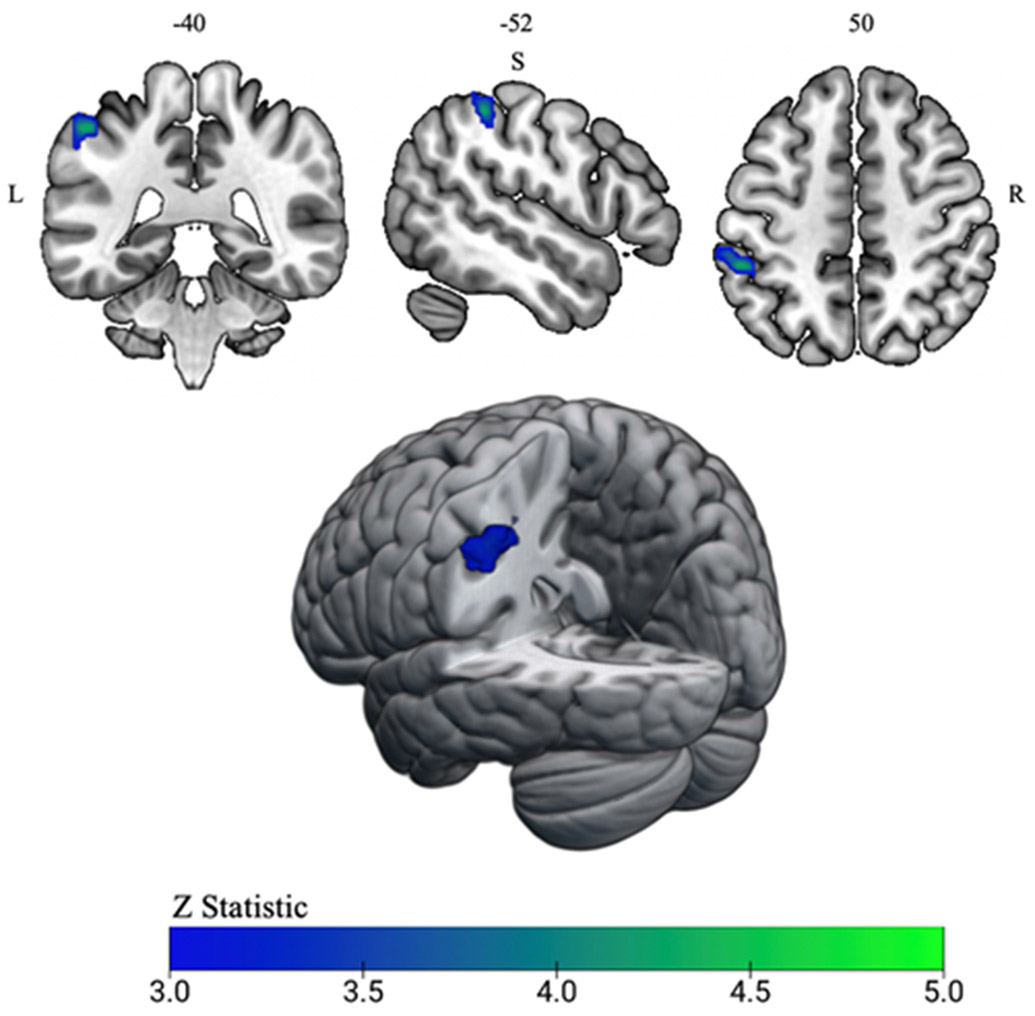
fMRI activity results from bilateral leg press task. A single significant cluster in the left inferior parietal lobule/supramarginal gyrus (IPL) (MNI coordinates: x =−52, y= −40, z = 50; shown in blue) during the bilateral leg press task was identified for the history of sports-related concussion group. Athletes with a history of sports-related concussion demonstrated less activity within the left IPL relative to matched controls. The left IPL cluster surpassed z threshold of 3.1 and a cluster significance of p < 0.05.
History of SRC Classification Secondary Analyses
There was a significant group effect on % mean BOLD signal change, F (2, 35) = 10.80, p < .001. Post hoc comparisons using the Tukey honest significant difference (HSD) test indicated that the % BOLD signal change for both the diagnosed concussion and symptom of concussion groups were lesser than the matched controls (p = .01 and p < .001, respectively). However, there was no significant difference in % BOLD signal change between the diagnosed concussion and symptom of concussion groups (p = .59). These data indicate that the lesser left IPL task-related activation observed for those with a history of SRC was not uniquely driven by whether athletes had reported a diagnosis of concussion or only reported symptoms of a concussion (see Figure 3 for a visual representation). Additionally, given that time from concussion may be a factor in functional activity during the bilateral leg press task, we evaluated the brain activity for the history of SRC group with the addition of time since concussion as a covariate of interest. Higher-level GLM analysis revealed that time since concussion was not directly related to brain activity during the bilateral leg press task (p > .05 and z < 3.1), indicating that more or less time since the concussive incident to the present study did not uniquely affect neural activity.
Figure 3:
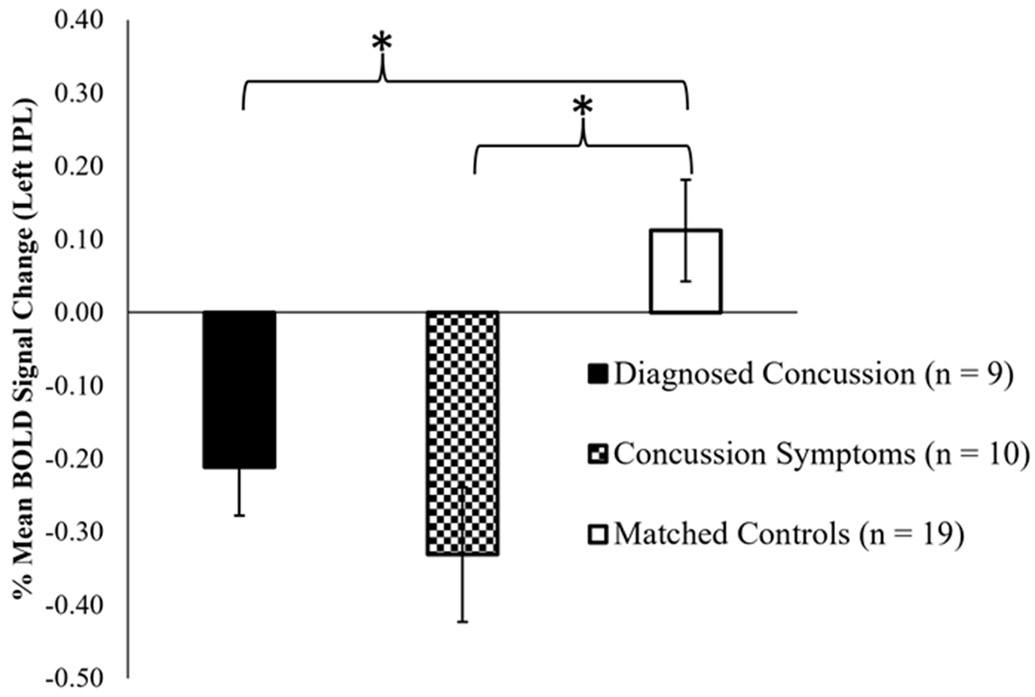
fMRI secondary brain activity analysis for history of SRC subgroups and matched controls. The % Blood Oxygen Level Dependent (BOLD) signal change from the resultant left inferior parietal lobule (IPL) cluster from the overall activation analysis was calculated to visualize activation in the region across groups (Diagnosed Concussion, Concussion Symptoms, and Matched controls). There were no significant differences in left IPL deactivation between the diagnosed and concussion symptoms groups (p = .59). * However, the diagnosed concussion and concussion symptom group each exhibited more deactivation in the left IPL relative to matched control subjects (p = .01 and p < .001), respectively. Error bars represent +/− 1 standard error of the mean.
Seed Creation
The significant between-group differences for task-related activation were used to motivate a seed region for PPI analyses reported below. Specifically, we created a 6 mm diameter spherical seed at the peak coordinates from the ‘group comparison of task-related activation’ cluster (left IPL; MNI coordinates: x = −52, y = −40, z = 50).
Task-Related Connectivity
No statistically significant differences in task-related connectivity between the two groups was observed (p’s > .05, z’s >3.1).
Exploratory Post Hoc Analyses
Matched controls had significant task-related connectivity between the left IPL (i.e., seed region) and the left primary motor cortex (M1) and primary somatosensory cortex (S1), the right inferior temporal gyrus, and the right S1 (Figure 4; Table 4). The history of SRC group displayed significant task-related connectivity between the left IPL and the bilateral posterior cingulate gyrus/precuneus and the right IPL (Figure 5; Table 4).
Figure 4:
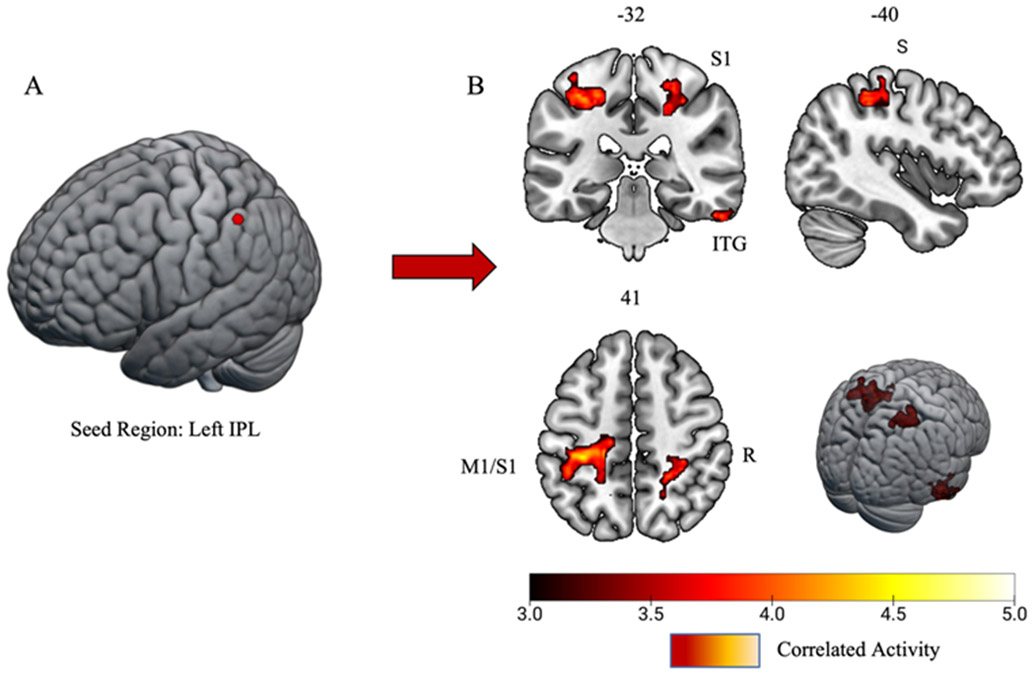
Matched controls task-related connectivity results. A) 6mm spherical seed region placed in the left inferior parietal lobule/supramarginal gyrus (IPL). B) Task-related connectivity results yielded significant connections with the left primary motor cortex/primary somatosensory cortex (M1/SI), right inferior temporal gyrus (ITG), and right somatosensory cortex (S1). Significant clusters surpassed z threshold of 3.1 and a cluster significance of p < 0.05.
Table 4.
Task-Related Connectivity Using Psychophysiological Interaction Analysis (PPI)
| Matched-Controls | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Clusters | Brain Regions | Side | Voxels (#) | p value | Z max | MNI Coordinates (xyz) |
| 1 | Primary Motor/Primary Somatosensory Cortex | Left | 921 | p<0.0001 | 4.86 | −28, −26, 52 |
| 2 | Inferior Temporal Gyrus | Right | 429 | p<0.001 | 4.29 | 56, −30, −26 |
| 3 | Primary Somatosensory Cortex | Right | 428 | p<0.001 | 4.4 | 30, −34, 54 |
|
| ||||||
| History of SRC | ||||||
|
| ||||||
| Clusters | Brain Regions | Side | Voxels (#) | p value | Z max | MNI Coordinates (xyz) |
| 1 | Posterior Cingulate / Precuneous Cortex | Bilateral | 405 | p=0.001 | 4.85 | 4, −38, 46 |
| 2 | Right Inferior Parietal Lobule/Supramarginal Gyrus | Right | 393 | p=0.002 | 4.65 | 62, −38, 34 |
Note: A 6mm spherical seed was place at the Left Inferior Parietal Lobule/Supramarginal Gyrus (MNI coordinates: x =−52, y= −40, z = 50).
Abbreviations: SRC, sports-related concussion; MNI, Montreal Neurological Institute
Figure 5:
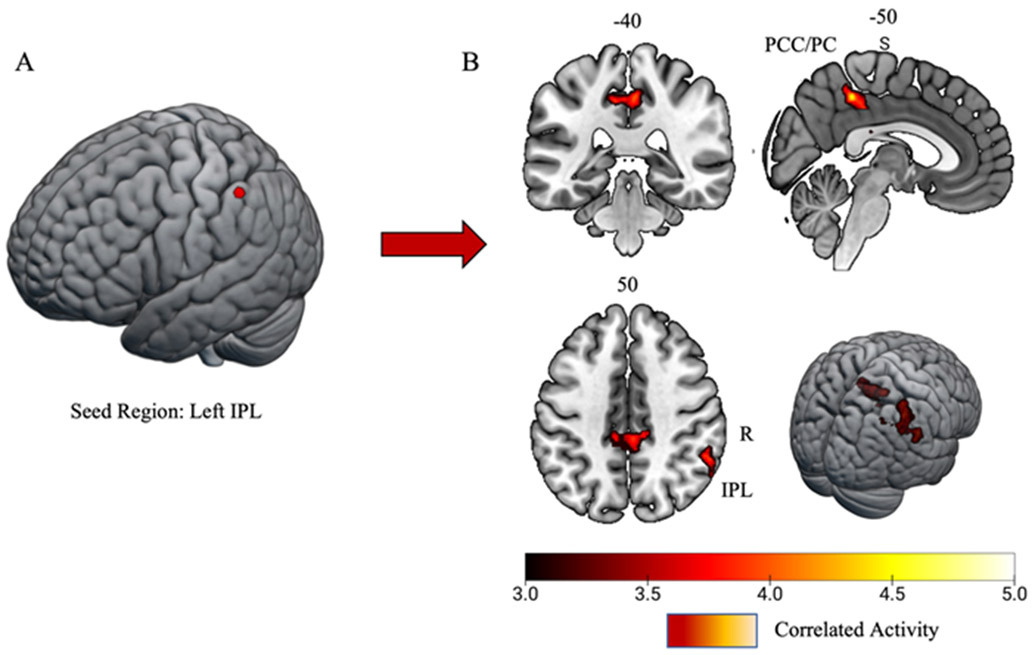
History of sports-related concussion task-related connectivity results. A) 6mm spherical seed region placed in the left inferior parietal lobule/supramarginal gyrus (IPL). B) Task-related connectivity results yielded significant connectivity with the bilateral posterior cingulate gyrus/precuneus cortex (PCC/PC) and the right IPL. Both clusters surpassed z threshold of 3.1 and a cluster significance of p < 0.05.
Discussion
The purpose of this study was to utilize an fMRI lower extremity motor control paradigm (bilateral leg press) to investigate task-evoked neural activity and connectivity in female adolescent athletes with a history of SRC. By examining the effects of concussion history on brain function during a bilateral leg press, we aimed to advance prior work that has evaluated motor system impairments resulting from SRCs (Charney et al., 2020; De Beaumont et al., 2007). Overall, the findings from this study revealed that female adolescent athletes with a history of SRC exhibited less activation within the left IPL relative to matched controls. Using the left IPL as a seed ROI, task-related connectivity for matched controls and for those with a history of SRC were identified to further characterize each group’s neural strategy. Despite no significant between-group differences in task-related connectivity, exploratory post hoc analyses revealed distinct neural strategies employed during the motor task (via independent statistical models). Matched controls displayed significant sensorimotor task-related neural connectivity, whereas those with a history of SRC displayed significant task-related neural connectivity within brain regions related to attention and cognition (Leech & Sharp, 2014), spatial processing, and proprioception (Ben-Shabat, Matyas, Pell, Brodtmann, & Carey, 2015; Iandolo et al., 2018). The present fMRI results in athletes with a history of SRC indicated disruptions in sensorimotor integration that may impair motor attention, potentially serving as the mechanism for the lingering deficits in neuromuscular function following SRC. Moreover, task-related connectivity within brain regions responsible for attention and proprioception for those with a history of SRC may indicate maladaptive allocation of neural resources to achieve the bilateral leg press constraints.
Task-related Activation
Female adolescent athletes with a history of SRC had less brain activity in the left IPL during the bilateral leg press relative to matched controls, specifically within the region of the supramarginal gyrus that borders the intraparietal sulcus. The inferior parietal cortex is important for balance control and postural stability (Surgent, Dadalko, Pickett, & Travers, 2019), with the left IPL playing a critical role in spatial and temporal timing judgements (Assmus et al., 2003), as well as overt and covert attentional tasks (Silk, Bellgrove, Wrafter, Mattingley, & Cunnington, 2010). Original investigations of the left IPL suggested that individuals with parietal lesions exhibited difficulty with disengaging attention from one task and reorienting their focus towards another (Posner, Walker, Friedrich, & Rafal, 1984). Further work has described the juxtaposition of orienting attention and overt eye movement, allowing for identification of parietal attention systems specifically related to regulating limb movement response, termed ‘motor attention’ (Rushworth, Nixon, Renowden, Wade, & Passingham, 1997). Early TMS studies further demonstrated the left IPLs role in motor attention, as temporary disruption of the left IPL affects a participant’s ability to orient attention away from one task to another (Rushworth, Ellison, & Walsh, 2001). Specifically, when individuals receive an orienting cue for a movement, motor preparation task switching is disrupted and the participant struggles to disengage motor attention from one movement to another (Rushworth, Ellison, et al., 2001).
This role of the left IPL for the integration of visuospatial attention and motor control (Rushworth, Johansen-Berg, Gobel, & Devlin, 2003; Rushworth, Paus, & Sipila, 2001; Villiger et al., 2013) highlights a distinct mechanism whereby a breakdown of attentional resources may facilitate a loss of motor stability after SRC (Avedesian, Singh, Diekfuss, Myer, & Grooms, 2021). While this study did not specifically evaluate the effects of dual-task conditions on lower extremity motor control (e.g., by adding a secondary cognitive task), previous findings have shown that athletes with a concussive injury exhibit greater functional activity in the supramarginal gyrus during balance assessment under dual-task conditions (Urban et al., 2021). While divergent from the results reported here, activation differences in the left IPL may highlight a potential brain biomarker related to altered motor control and attention following SRC. Specifically, during basic motor control (balance), dual-task conditions may evoke a neural compensatory response (hyperactivation) to achieve desired motor output following initial concussion injury, whereas the reduction in activation found in athletes with a history of SRC during a dynamic motor task (bilateral leg press) may indicate the neuromotor system is less prepared for incoming dual-task demands for athletes with a history of SRC. Thus, requiring a greater redistribution of attentional resources to initiate movement. Alternatively, relatively greater versus lesser left IPL activity may be task-specific, with each uniquely interacting with dual-task demands for athletes with a history of SRC. Considering the mounting evidence for gait alterations seen under dual-task conditions when motor attention is taxed (Howell, Beasley, et al., 2017; Howell et al., 2018; Howell et al., 2015; Howell, Stracciolini, et al., 2017), our findings may provide a neurologic link between SRC history and dual-task cost for neuromuscular control (i.e., the performance difference resulting from a shift between undivided and divided attention). Moreover, considering that dual-task cost examinations have revealed a breakdown in motor coordination and postural stability (Hugentobler et al., 2016; Quatman-Yates et al., 2015) in both acute and asymptomatic concussion stages (Gagné et al., 2019; Howell, Beasley, et al., 2017; Howell et al., 2018; Howell et al., 2015; Howell, Stracciolini, et al., 2017), the degradation of motor coordination when attentional resources are utilized for a cognitive task may be due to altered motor attention associated activity and connectivity.
Task-related Connectivity
The left IPL was used to motivate a seed region to further characterize the neural strategies employed during the fMRI leg press. Despite no significant between-group differences in task-related connectivity between athletes with a history of SRC and matched controls, exploratory analyses provided further, preliminary insight into the neural strategies each group utilized.
Matched Controls
PPI analyses revealed a primarily sensorimotor task-related connectivity strategy in the matched control group. Specifically, results showed task-related connectivity of the left IPL with the left primary motor and primary somatosensory cortex, the right primary somatosensory cortex, and the right inferior temporal gyrus. Previous functional connectivity investigations of the human parietal lobule revealed that the left supramarginal gyrus is connected to the left primary motor cortex (Rushworth, Behrens, & Johansen-Berg, 2006; Zhang & Li, 2014). Moreover, effective communication between brain regions responsible for sensorimotor integration is critical for motor performance, with movement output relying on sensory information to help shape optimal motor responses (Gale, Flanagan, & Gallivan, 2021). These connectivity patterns between the left and right somatosensory regions along with the left primary motor cortex suggest that matched controls maintain a connectivity pattern in which sensory, motor, and sensorimotor integration brain regions are working in concert for lower extremity motor performance (i.e., regions important for motor control are operating ‘in sync’ to move both limbs). While we hypothesize the sensorimotor connectivity strategy employed for matched controls is an appropriate and expected neural strategy in matched female adolescent athletes, future research is warranted given the limited prior literature utilizing fMRI, bilateral lower extremity motor control, and/or PPI analyses.
Athletes with a History of SRC
PPI analyses revealed that athletes with a history of SRC exhibited a task-related connectivity neural strategy that relied on regions important for attention and cognition. Specifically, results showed task-related connectivity of the left IPL with the bilateral posterior cingulate gyrus (PCG)/precuneus cortex, and the right IPL. The PCG is part of the posterior cingulate cortex (PCC), an area that has been noted for its central role in the default mode network (DMN). The DMN is known to exhibit highly correlated activity at rest, but also shows deactivation during externally directed attentional tasks (Raichle et al., 2001). Disruptions in the DMN, specifically within the PCC, is evidenced in individuals following brain injuries (Bonnelle et al., 2011; Yount et al., 2002). Further, alterations within resting state PCC and precuneus connectivity are associated with impaired sustained attention (Bonnelle et al., 2011). Considering that the PCG plays a critical role in attention, and the right IPL is responsible for additional visuospatial attention processing, proprioception, and attention orienting (Corbetta & Shulman, 2002; Numssen, Bzdok, & Hartwigsen, 2021), connectivity among the left IPL, the PCG and right IPL, during the bilateral leg press task, may be suggestive of an attentional-based compensatory connectivity strategy to successfully complete the motor task. In summary, these task-related connectivity results shed light into the underlying mechanism for neural dysregulation, specifically distinct neural compensatory strategies for the reduced activity in the left IPL, which may be impairing dual-task motor behavior in athletes with a history of SRC.
Clinical Implications
Findings from this study may inform future neurotherapeutic targets for lower extremity injury risk reduction strategies in adolescent female athletes with a history of SRC. Athletes with a history of SRC exhibit altered gait (Howell, Beasley, et al., 2017; Howell et al., 2020; Howell et al., 2018; Howell et al., 2015; Howell, Stracciolini, et al., 2017), jump landing biomechanics (Lapointe et al., 2018; Lynall et al., 2018), postural stability (Hugentobler et al., 2016; Quatman-Yates et al., 2015), as well as an increased risk of lower-extremity MSK injury (Howell et al., 2020; Lynall et al., 2015; Lynall et al., 2017; McPherson, Nagai, Webster, & Hewett, 2020) despite being cleared for return to sport. Thus, interventional techniques that seek to address lingering neuromuscular impairments are crucial to the overall health of adolescent athletes. Considering that our findings revealed those with a history of SRC exhibited alterations within motor attention regions and subsequent recruitment of additional neurocognitive resources, a breakdown of motor coordination under cognitively demanding scenarios that lead to lower extremity MSK injury may be due to attentional deficits. Moreover, our findings provide a first step towards isolating a potential CNS disruption following SRC that may contribute to future lower extremity injury. Restoration of IPL activity and connectivity to motor and somatosensory regions as seen in the matched controls may improve motor coordination recovery under dual-task conditions in athletes with a history of SRC. Thus, our data indicate that clinical management of SRC should continue to aim to address motor control and motor attention alterations through targeted sensorimotor rehabilitation that optimize cognitive and motor performance. For instance, current clinical approaches that utilize dual-task interventions may be particularly beneficial to correct motor and cognitive dysfunction in athletes post-SRC (Fritz & Basso, 2013; Fritz, Cheek, & Nichols-Larsen, 2015; Ingriselli et al., 2014).
Adjunctive motor learning strategies to current clinical care may support or accelerate the long-term restoration of motor function for female athletes with a history of SRC (Avedesian et al., 2021). For instance, the OPTIMAL (Optimizing Performance Through Intrinsic Motivation and Attention for Learning) theory of motor learning which has robustly shown to improve the acquisition, retention, and transfer of motor skills (Wulf & Lewthwaite, 2016) is theorized to promote adaptive neuroplasticity related to injury prevention, rehabilitation, exercise, and play (OPTIMAL PREP) (Diekfuss, Bonnette, et al., 2020; Diekfuss et al., 2021; Diekfuss, Hogg, et al., 2020). Specifically, athletes with a history of SRC motor behavior and associated neural functioning may be uniquely supported when adopting an external focus of attention, are provided autonomy support, and/or are provided enhanced expectancies for future performance (Avedesian et al., 2021). For instance, recent work demonstrated that a single session of visual biofeedback double-leg squat training with real-time positive feedback (i.e., enhanced expectancies) reduced dual-task cost for single-leg postural control in healthy controls (Williams et al., 2022). However, we emphasize that sensorimotor-based interventions such as OPTIMAL PREP, remain largely theoretical and necessitate future research before clinical implementation. Specifically, future randomized controlled trials are needed to a) evaluate the relative effectiveness of sensorimotor-based interventions on restoring motor and cognitive function and b) characterize underlying mechanisms to support more targeted, future application of neurotherapeutics aiming to restore neuromuscular control in athletes with a history of SRC (i.e., the degree of brain activity/connectivity changes in response to a given sensorimotor intervention).
Limitations
The current findings reported in this study are limited to a small sample of female adolescent athletes and thus cannot be generalizable to male populations or older athletes. Additionally, self-reporting for SRC is inherently limited as athletes may fail to accurately recall prior concussion history. Moreover, a lack of consistent medical reporting/diagnostic criteria may not capture athletes with a prior concussion. This investigation only evaluated coarse kinematic movement parameters (mean range of motion), no kinetic measures were taken (force applied) and the task was a relatively slow and controlled movement. We recognize that athletes with differing anthropometrics could also have affected the amount of force exerted and neural activity during the bilateral leg press, but our matching procedure likely minimized any potential confounds of between group differences in force applied on the study outcomes. Thus, the present paradigm may only partially reflect the cognitive and motor demands that athletes experience during dynamic sport activities. Future prospective research should consider larger sample sizes with a cohort of males for comparison, incorporate more objective measures of head impact exposure (head impact accelerometry), and consider the use of additional physiology-related assessments, such as eye tracking, respiration, and/or heart rate variability, to control for potential confounding variables that may be unique to those with a history of SRC (e.g., acute oculomotor deficits). Additionally, refined diagnostic criteria to classify SRC magnitude (e.g., probe at loss of consciousness [mild to moderate TBI/SRC]) and integrated MRI-compatible technologies that permit measurement of kinetic data (MR-safe loadcells to evaluate joint torque) concurrent with neural activity is warranted.
Conclusion
Overall, the findings from this study revealed that SRC was associated with less neural activity in a brain region responsible for sensorimotor integration and motor attention. Athletes without history of SRC utilized functional connectivity with regions within the sensorimotor network, whereas those with a history of SRC exhibited connectivity between sensorimotor and attentional brain regions, during the bilateral leg press. The current findings support our initial hypothesis that female adolescent athletes with a history of SRC would display distinct brain activity and task-related connectivity during the bilateral leg press task. Further functional activity in the left IPL may be indicative of a sensorimotor integration disruption and potential impairment of the motor attention system during the bilateral leg press in athletes with a history of SRC. The elevated functional connectivity between the left IPL and attentional regions for the history of SRC group suggest alterations of the motor attention system. The study results point toward opportunities for future research to apply targeted therapies that remediate both neural and motor dysfunction before athletes return to play.
Supplementary Material
Acknowledgements:
The authors would like to thank the athletes, parents, and coaching staff from the Georgia high schools for their participation and overall support for this study. We also thank Philip K. Wong for evaluating all neuroimaging datasets for potential incidental findings.
Funding:
This research was directly supported by NIH/NIAMS/NINDS research grants U01AR067997, R01AR077248, R01AR076153, and T32NS7453-20. This work was also partially funded by internal support from the Department of Orthopaedics at Emory University.
Conflict of Interest Statement:
Gregory D. Myer has consulted with Commercial entities to support application to the US Food and Drug Administration but has no financial interest in the commercialization of the products. Dr. Myer’s institution receives current and ongoing grant funding from National Institutes of Health/NIAMS Grants U01AR067997, R01 AR070474, R01AR055563, R01AR076153, R01 AR077248 and has received industry sponsored research funding related to brain injury prevention and assessment with Q30 Innovations, LLC, and ElMinda, Ltd. Dr. Myer receives author royalties from Human Kinetics and Wolters Kluwer. Dr. Myer is an inventor of biofeedback technologies (2017 Non-Provisional Patent Pending-Augmented and Virtual reality for Sport Performance and Injury Prevention Application filed 11/10/2016 (62/420,119), Software Copyrighted.) designed to enhance rehabilitation and prevent injuries and receives licensing royalties. Dr. Myer and Dr. Diekfuss receive inventor-related royalties resultant from biofeedback technologies (Include Health: LIC1907082014-0706). Dr. Grooms has current and ongoing funding support from the National Institutes of Health/National Center for Complementary and Integrative Health (R21 AT009339-02) and National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR076153, R01AR077248) and the US Department of Defense Congressionally Directed Medical Research Program Peer Reviewed Orthopaedic Research Program (OR170266), research award (81XWH-18-1-0707). Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. Dr. Diekfuss also receives author royalties from Kendall Hunt Publishing Company.
Data Availability Statement
The data that supports the findings of the current manuscript are available from the corresponding author upon reasonable request.
References
- Anand M, Diekfuss JA, Bonnette S, Short I, Hurn M, Grooms DR, & Myer GD (2020). Validity of an MRI-compatible motion capture system for use with lower extermity neuroimaging paradigms. Int J Sports Phys Ther, 15(6), 936–946. doi: 10.26603/ijspt20200936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand M, Diekfuss JA, Slutsky-Ganesh AB, Bonnette S, Grooms DR, & Myer GD (2020). Graphical interface for automated management of motion artifact within fMRI acquisitions: INFOBAR. SoftwareX, 12, 100598. doi: 10.1016/j.softx.2020.100598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand M, Diekfuss JA, Slutsky-Ganesh AB, Grooms DR, Bonnette S, Barber Foss KD, … Myer GD (2021). Integrated 3D motion analysis with functional magnetic resonance neuroimaging to identify neural correlates of lower extremity movement. Journal of Neuroscience Methods, 355, 109108. doi: 10.1016/j.jneumeth.2021.109108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmus A, Marshall JC, Ritzl A, Noth J, Zilles K, & Fink GR (2003). Left inferior parietal cortex integrates time and space during collision judgments. NeuroImage, 20 Suppl 1, S82–88. doi: 10.1016/j.neuroimage.2003.09.025 [DOI] [PubMed] [Google Scholar]
- Avedesian JM, Singh H, Diekfuss JA, Myer GD, & Grooms DR (2021). Loss of motor stability after sports-related concussion: Opportunities for motor learning strategies to reduce musculoskeletal injury risk. Sports Medicine, 51(11), 2299–2309. doi: 10.1007/s40279-021-01527-5 [DOI] [PubMed] [Google Scholar]
- Ben-Shabat E, Matyas TA, Pell GS, Brodtmann A, & Carey LM (2015). The right supramarginal gyrus is important for proprioception in healthy and stroke-affected participants: A functional MRI study. Frontiers of Neurology, 6, 248. doi: 10.3389/fneur.2015.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biese KM, Stamm JM, Wichman DM, Hetzel SJ, Post EG, & Bell DR (2021). Association of lower extremity injuries and injury mechanism with previous concussion history in adolescent athletes. Physical therapy in sport: official journal of the Association of Chartered Physiotherapists in Sports Medicine, 48, 76–82. doi: 10.1016/j.ptsp.2020.12.018 [DOI] [PubMed] [Google Scholar]
- Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, … Sharp DJ (2011). Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. Journal of Neuroscience, 31(38), 13442–13451. doi: 10.1523/JNEUROSCI.1163-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnette S, Diekfuss JA, Grooms D, Myer GD, Meehan WP 3rd, & Howell DR (2020). Integrated linear and nonlinear trunk dynamics identify residual concussion deficits. Neuroscience letters, 729, 134975. doi: 10.1016/j.neulet.2020.134975 [DOI] [PubMed] [Google Scholar]
- Broglio SP, McAllister T, Katz BP, LaPradd M, Zhou W, & McCrea MA (2022). The natural history of sport-related concussion in collegiate athletes: Findings from the NCAA-DoD CARE Consortium. Sports Medicine, 52(2), 403–415. doi: 10.1007/s40279-021-01541-7 [DOI] [PubMed] [Google Scholar]
- Brooks MA, Peterson K, Biese K, Sanfilippo J, Heiderscheit BC, & Bell DR (2016). Concussion increases odds of sustaining a lower extremity musculoskeletal injury after return to play among collegiate athletes. American Journal of Sports Medicine, 44(3), 742–747. doi: 10.1177/0363546515622387 [DOI] [PubMed] [Google Scholar]
- Bryan MA, Rowhani-Rahbar A, Comstock RD, Rivara F, & Seattle Sports Concussion Research, C. (2016). Sports- and recreation-related concussions in US youth. Pediatrics, 138(1). doi: 10.1542/peds.2015-4635 [DOI] [PubMed] [Google Scholar]
- Buckley TA, Munkasy BA, Tapia-Lovler TG, & Wikstrom EA (2013). Altered gait termination strategies following a concussion. Gait Posture, 38(3), 549–551. doi: 10.1016/j.gaitpost.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney MF, Howell DR, Lanois C, Starr TC, Liao H, Coello E, … Lin AP (2020). Associations between neurochemistry and gait performance following concussion in collegiate athletes. Journal of Head Trauma Rehabilitation, 35(5), 342–353. doi: 10.1097/HTR.0000000000000616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill NW, Caverzasi E, Graham SJ, Hutchison MG, & Schweizer TA (2017). White matter microstructure in athletes with a history of concussion: Comparing diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI). Human Brain Mapping, 38(8), 4201–4211. doi: 10.1002/hbm.23658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley AC, Cooper PS, Karayanidis F, Gardner AJ, Levi CR, Stanwell P, … Iverson GL (2018). Resting state electroencephalography and sports-related concussion: A systematic review. Journal of Neurotrauma. doi: 10.1089/neu.2018.5761 [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. doi: 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Criss CR, Onate JA, & Grooms DR (2020). Neural activity for hip-knee control in those with anterior cruciate ligament reconstruction: A task-based functional connectivity analysis. Neuroscience letters, 730, 134985. doi: 10.1016/j.neulet.2020.134985 [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Lassonde M, Leclerc S, & Theoret H (2007). Long-term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery, 61(2), 329–336; discussion 336-327. doi: 10.1227/01.NEU.0000280000.03578.B6 [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Mongeon D, Tremblay S, Messier J, Prince F, Leclerc S, … Theoret H (2011). Persistent motor system abnormalities in formerly concussed athletes. Journal of Athletic Training, 46(3), 234–240. doi: 10.4085/1062-6050-46.3.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekfuss JA, Bonnette S, Hogg JA, Riehm C, Grooms DR, Singh H, … Myer GD (2020). Practical training strategies to apply neuro-mechanistic motor learning principles to facilitate adaptations towards injury-resistant movement in youth. Journal of Science in Sport and Exercise, 3(1), 3–16. doi: 10.1007/s42978-020-00083-0 [DOI] [Google Scholar]
- Diekfuss JA, Grooms DR, Hogg JA, Singh H, Slutsky-Ganesh AB, Bonnette S, … Myer GD (2021). Targeted application of motor learning theory to leverage youth neuroplasticity for enhanced injury-resistance and exercise performance: OPTIMAL PREP. Journal of Science in Sport and Exercise, 3(1), 17–36. doi: 10.1007/s42978-020-00085-y [DOI] [Google Scholar]
- Diekfuss JA, Hogg JA, Grooms DR, Slutsky-Ganesh AB, Singh H, Bonnette S, … Myer GD (2020). Can we capitalize on central nervous system plasticity in young athletes to inoculate against injury? Journal of Science in Sport and Exercise, 2(4), 305–318. doi: 10.1007/s42978-020-00080-3 [DOI] [Google Scholar]
- Dubose DF, Herman DC, Jones DL, Tillman SM, Clugston JR, Pass A, … Chmielewski TL (2017). Lower extremity stiffness changes after concussion in collegiate football players. Medicine and Science in Sports Exercise, 49(1), 167–172. doi: 10.1249/MSS.0000000000001067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echemendia RJ, Meeuwisse W, McCrory P, Davis GA, Putukian M, Leddy J, … Herring S (2017). The sport concussion assessment tool 5th edition (SCAT5): Background and rationale. British Journal of Sports Medicine, 51(11), 848–850. doi: 10.1136/bjsports-2017-097506 [DOI] [PubMed] [Google Scholar]
- Fino PC, Becker LN, Fino NF, Griesemer B, Goforth M, & Brolinson PG (2019). Effects of recent concussion and injury history on instantaneous relative risk of lower extremity injury in division I collegiate athletes. Clinical Journal of Sport Medicine, 29(3), 218–223. doi: 10.1097/JSM.0000000000000502 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, & Frackowiak RS (1995). Characterizing evoked hemodynamics with fMRI. NeuroImage, 2(2), 157–165. doi: 10.1006/nimg.1995.1018 [DOI] [PubMed] [Google Scholar]
- Fritz NE, & Basso DM (2013). Dual-task training for balance and mobility in a person with severe traumatic brain injury: A case study. Journal of Neurolgic Physical Therapy, 37(1), 37–43. doi: 10.1097/NPT.0b013e318282a20d [DOI] [PubMed] [Google Scholar]
- Fritz NE, Cheek FM, & Nichols-Larsen DS (2015). Motor-cognitive dual-task training in persons with neurologic disorders: A systematic review. Journal Neurolgic Physical Therapy, 39(3), 142–153. doi: 10.1097/NPT.0000000000000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagné M-È, McFadyen BJ, Cossette I, Fait P, Gagnon I, Sirois K, … Ouellet M-C (2019). Alterations in dual-task walking persist two months after mild traumatic brain injury in young adults. Journal of Concussion, 3, 2059700219878291. doi: 10.1177/2059700219878291 [DOI] [Google Scholar]
- Gale DJ, Flanagan JR, & Gallivan JP (2021). Human somatosensory cortex is modulated during motor planning. Journal of Neuroscience, 41(27), 5909–5922. doi: 10.1523/JNEUROSCI.0342-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert FC, Burdette GT, Joyner AB, Llewellyn TA, & Buckley TA (2016). Association between concussion and lower extremity injuries in collegiate athletes. Sports Health, 8(6), 561–567. doi: 10.1177/1941738116666509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grooms DR, Diekfuss JA, Criss CR, Anand M, Slutsky-Ganesh AB, DiCesare CA, & Myer GD (2022). Preliminary brain-behavioral neural correlates of anterior cruciate ligament injury risk landing biomechanics using a novel bilateral leg press neuroimaging paradigm. PloS one, 17(8), e0272578. doi: 10.1371/journal.pone.0272578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grooms DR, Diekfuss JA, Ellis JD, Yuan W, Dudley J, Foss KDB, … Myer GD (2019). A novel approach to evaluate brain activation for lower extremity motor control. Journal of Neuroimaging, 29(5), 580–588. doi: 10.1111/jon.12645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammeke TA, McCrea M, Coats SM, Verber MD, Durgerian S, Flora K, … Rao SM (2013). Acute and subacute changes in neural activation during the recovery from sport-related concussion. Journal of International Neuropsycholgical Society: JINS, 19(8), 863–872. doi: 10.1017/S1355617713000702 [DOI] [PubMed] [Google Scholar]
- Herman DC, Jones D, Harrison A, Moser M, Tillman S, Farmer K, … Chmielewski TL (2017). Concussion may increase the risk of subsequent lower extremity musculoskeletal injury in collegiate athletes. Sports Medicine, 47(5), 1003–1010. doi: 10.1007/s40279-016-0607-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DR, Beasley M, Vopat L, & Meehan WP 3rd. (2017). The effect of prior concussion history on dual-task gait following a aoncussion. Journal of Neurotrauma, 34(4), 838–844. doi: 10.1089/neu.2016.4609 [DOI] [PubMed] [Google Scholar]
- Howell DR, Bonnette S, Diekfuss JA, Grooms DR, Myer GD, Wilson JC, & Meehan WP 3rd. (2020). Dual-task gait stability after concussion and subsequent injury: An exploratory investigation. Sensors (Basel, Switzerland), 20(21). doi: 10.3390/s20216297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DR, Buckley TA, Lynall RC, & Meehan WP 3rd. (2018). Worsening dual-task gait costs after concussion and their association with subsequent sport-related injury. Journal of Neurotrauma, 35(14), 1630–1636. doi: 10.1089/neu.2017.5570 [DOI] [PubMed] [Google Scholar]
- Howell DR, Myer GD, Grooms D, Diekfuss J, Yuan W, & Meehan WP 3rd. (2019). Examining motor tasks of differing complexity after concussion in adolescents. Archives of Physical Medicine and Rehabilitation, 100(4), 613–619. doi: 10.1016/j.apmr.2018.07.441 [DOI] [PubMed] [Google Scholar]
- Howell DR, Osternig LR, & Chou LS (2015). Return to activity after concussion affects dual-task gait balance control recovery. Medicine and Science in Sports Exercise, 47(4), 673–680. doi: 10.1249/MSS.0000000000000462 [DOI] [PubMed] [Google Scholar]
- Howell DR, Stracciolini A, Geminiani E, & Meehan WP 3rd. (2017). Dual-task gait differences in female and male adolescents following sport-related concussion. Gait Posture, 54, 284–289. doi: 10.1016/j.gaitpost.2017.03.034 [DOI] [PubMed] [Google Scholar]
- Hugentobler JA, Gupta R, Slater R, Paterno MV, Riley MA, & Quatman-Yates C (2016). Influence of age on postconcussive postural control measures and future implications for assessment. Clinical Journal of Sport Medicine, 26(6), 510–517. doi: 10.1097/JSM.0000000000000286 [DOI] [PubMed] [Google Scholar]
- Iandolo R, Bellini A, Saiote C, Marre I, Bommarito G, Oesingmann N, … Inglese M (2018). Neural correlates of lower limbs proprioception: An fMRI study of foot position matching. Human Brain Mapping, 39(5), 1929–1944. doi: 10.1002/hbm.23972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingriselli JM, Register-Mihalik JK, Schmidt JD, Mihalik JP, Goerger BM, & Guskiewicz KM (2014). Outcomes, utility, and feasibility of single task and dual task intervention programs: preliminary implications for post-concussion rehabilitation. Journal of Science and Medicine in Sport, 17(6), 580–585. doi: 10.1016/j.jsams.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger L, Marchal-Crespo L, Wolf P, Riener R, Michels L, & Kollias S (2014). Brain activation associated with active and passive lower limb stepping. Frontiers in human neuroscience, 8, 828. doi: 10.3389/fnhum.2014.00828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, & Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. doi: 10.1016/s1053-8119(02)91132-8 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, & Smith SM (2012). Fsl. NeuroImage, 62(2), 782–790. doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Kaushal M, Espana LY, Nencka AS, Wang Y, Nelson LD, McCrea MA, & Meier TB (2019). Resting-state functional connectivity after concussion is associated with clinical recovery. Human Brain Mapping, 40(4), 1211–1220. doi: 10.1002/hbm.24440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley ML, Saluja RS, Chen JK, Gagnon I, Leonard G, Petrides M, & Ptito A (2014). A functional magnetic resonance imaging study of working memory in youth after sports-related concussion: is it still working? Journal of Neurotrauma, 31(5), 437–451. doi: 10.1089/neu.2013.3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Meier TB, Olson DV, McCrea MA, Nelson LD, & Muftuler LT (2018). Chronic differences in white matter integrity following sport-related concussion as measured by diffusion MRI: 6-Month follow-up. Human Brain Mapping, 39(11), 4276–4289. doi: 10.1002/hbm.24245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Olson DV, McCrea MA, Nelson LD, LaRoche AA, & Muftuler LT (2016). Acute white matter changes following sport-related concussion: A serial diffusion tensor and diffusion kurtosis tensor imaging study. Human Brain Mapping, 37(11), 3821–3834. doi: 10.1002/hbm.23278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe AP, Nolasco LA, Sosnowski A, Andrews E, Martini DN, Palmieri-Smith RM, … Broglio SP (2018). Kinematic differences during a jump cut maneuver between individuals with and without a concussion history. International Journal of Psychophysiology, 132(Pt A), 93–98. doi: 10.1016/j.ijpsycho.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Leech R, & Sharp DJ (2014). The role of the posterior cingulate cortex in cognition and disease. Brain, 137(Pt 1), 12–32. doi: 10.1093/brain/awt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn T, Burdette GT, Joyner AB, & Buckley TA (2014). Concussion reporting rates at the conclusion of an intercollegiate athletic career. Clinical Journal of Sport Medicine, 24(1), 76–79. doi: 10.1097/01.jsm.0000432853.77520.3d [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, & Oeltermann A (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature, 412(6843), 150–157. doi: 10.1038/35084005 [DOI] [PubMed] [Google Scholar]
- Lynall RC, Blackburn JT, Guskiewicz KM, Marshall SW, Plummer P, & Mihalik JP (2018). Reaction time and joint kinematics during functional movement in recently concussed individuals. Archives of Physical Medicine and Rehabilitation, 99(5), 880–886. doi: 10.1016/j.apmr.2017.12.011 [DOI] [PubMed] [Google Scholar]
- Lynall RC, Mauntel TC, Padua DA, & Mihalik JP (2015). Acute lower extremity injury rates increase after concussion in college athletes. Medicine and Science in Sports Exercise, 47(12), 2487–2492. doi: 10.1249/MSS.0000000000000716 [DOI] [PubMed] [Google Scholar]
- Lynall RC, Mauntel TC, Pohlig RT, Kerr ZY, Dompier TP, Hall EE, & Buckley TA (2017). Lower extremity musculoskeletal injury risk after concussion recovery in high school athletes. Journal of Athletic Training, 52(11), 1028–1034. doi: 10.4085/1062-6050-52.11.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse W, Dvorak J, Aubry M, Bailes J, Broglio S, … Vos PE (2017). Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. British Journal of Sports Medicine, 51(11), 838–847. doi: 10.1136/bjsports-2017-097699 [DOI] [PubMed] [Google Scholar]
- McCuddy WT, Espana LY, Nelson LD, Birn RM, Mayer AR, & Meier TB (2018). Association of acute depressive symptoms and functional connectivity of emotional processing regions following sport-related concussion. Neuroimage Clinical, 19, 434–442. doi: 10.1016/j.nicl.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGroarty NK, Brown SM, & Mulcahey MK (2020). Sport-related concussion in female athletes: A systematic review. Orthopaedic Journal of Sports Medicine, 8(7), 2325967120932306. doi: 10.1177/2325967120932306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson AL, Nagai T, Webster KE, & Hewett TE (2020). Musculoskeletal injury risk after sport-related concussion: Response. American Journal of Sports Medicine, 48(2), NP17–NP18. doi: 10.1177/0363546519894290 [DOI] [PubMed] [Google Scholar]
- McPherson AL, Shirley MB, Schilaty ND, Larson DR, & Hewett TE (2020). Effect of a concussion on anterior cruciate ligament injury risk in a general population. Sports Medicine, 50(6), 1203–1210. doi: 10.1007/s40279-020-01262-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan WP 3rd, d'Hemecourt P, Collins CL, & Comstock RD (2011). Assessment and management of sport-related concussions in United States high schools. American Journal of Sports Medicine, 39(11), 2304–2310. doi: 10.1177/0363546511423503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier TB, Giraldo-Chica M, Espana LY, Mayer AR, Harezlak J, Nencka AS, … McCrea MA (2020). Resting-state fMRI metrics in acute sport-related concussion and their association with clinical recovery: A study from the NCAA-DOD CARE Consortium. Journal of Neurotrauma, 37(1), 152–162. doi: 10.1089/neu.2019.6471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray NG, Moran R, Islas A, Pavilionis P, Szekely B, Alphonsa S, … Cipriani D (2021). Sport-related concussion adopt a more conservative approach to straight path walking and turning during tandem gait. Journal of Clinical Translational Research, 7(4), 443–449. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/34667890 [PMC free article] [PubMed] [Google Scholar]
- Narayana S, Charles C, Collins K, Tsao JW, Stanfill AG, & Baughman B (2019). Neuroimaging and Neuropsychological Studies in Sports-Related Concussions in Adolescents: Current State and Future Directions. Front Neurol, 10, 538. doi: 10.3389/fneur.2019.00538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome MR, Li X, Lin X, Wilde EA, Ott S, Biekman B, … Levin HS (2016). Functional connectivity is altered in concussed adolescent athletes despite medical clearance to return to play: A preliminary report. Frontiers of Neurology, 7(116), 116. doi: 10.3389/fneur.2016.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton JM, Dong Y, Hidler J, Plummer-D'Amato P, Marehbian J, Albistegui-Dubois RM, … Dobkin BH (2008). Reliable assessment of lower limb motor representations with fMRI: use of a novel MR compatible device for real-time monitoring of ankle, knee and hip torques. NeuroImage, 43(1), 136–146. doi: 10.1016/j.neuroimage.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom A, Nordstrom P, & Ekstrand J (2014). Sports-related concussion increases the risk of subsequent injury by about 50% in elite male football players. British Journal of Sports Medicine, 48(19), 1447–1450. doi: 10.1136/bjsports-2013-093406 [DOI] [PubMed] [Google Scholar]
- Numssen O, Bzdok D, & Hartwigsen G (2021). Functional specialization within the inferior parietal lobes across cognitive domains. eLife, 10, e63591. doi: 10.7554/eLife.63591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly JX, Woolrich MW, Behrens TE, Smith SM, & Johansen-Berg H (2012). Tools of the trade: psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience, 7(5), 604–609. doi: 10.1093/scan/nss055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes TR, Fox AS, Johnstone T, Chung MK, Kalin N, & Davidson RJ (2007). Integrating VBM into the general linear model with voxelwise anatomical covariates. NeuroImage, 34(2), 500–508. doi: 10.1016/j.neuroimage.2006.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, & Rafal RD (1984). Effects of parietal injury on covert orienting of attention. Journal of Neuroscience, 4(7), 1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, & Beckmann CF (2015). ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage, 112, 267–277. doi: 10.1016/j.neuroimage.2015.02.064 [DOI] [PubMed] [Google Scholar]
- Quatman-Yates CC, Bonnette S, Hugentobler JA, Mede B, Kiefer AW, Kurowski BG, & Riley MA (2015). Postconcussion postural sway variability changes in youth: The benefit of structural variability analyses. Pediatric Physical Therapy: the official publication of the Section on Pediatrics of the American Physical Therapy Association, 27(4), 316–327. doi: 10.1097/PEP.0000000000000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL (2001). A default mode of brain function. PloS one, 98(2), 676–682. doi: 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, & Johansen-Berg H (2006). Connection patterns distinguish 3 regions of human parietal cortex. Cerebral Cortex, 16(10), 1418–1430. doi: 10.1093/cercor/bhj079 [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Ellison A, & Walsh V (2001). Complementary localization and lateralization of orienting and motor attention. Nature Neuroscience, 4(6), 656–661. doi: 10.1038/88492 [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Johansen-Berg H, Gobel SM, & Devlin JT (2003). The left parietal and premotor cortices: Motor attention and selection. NeuroImage, 20 Suppl 1, S89–100. doi: 10.1016/j.neuroimage.2003.09.011 [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Nixon PD, Renowden S, Wade DT, & Passingham RE (1997). The left parietal cortex and motor attention. Neuropsychologia, 35(9), 1261–1273. doi: 10.1016/s0028-3932(97)00050-x [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Paus T, & Sipila PK (2001). Attention systems and the organization of the human parietal cortex. Journal of Neuroscience, 21(14), 5262–5271. doi: 10.1523/JNEUROSCI.21-14-05262.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk TJ, Bellgrove MA, Wrafter P, Mattingley JB, & Cunnington R (2010). Spatial working memory and spatial attention rely on common neural processes in the intraparietal sulcus. NeuroImage, 53(2), 718–724. doi: 10.1016/j.neuroimage.2010.06.068 [DOI] [PubMed] [Google Scholar]
- Slutsky-Ganesh AB, Anand M, Diekfuss JA, Myer GD, & Grooms DR (2023). Lower extremity Interlimb coordination associated brain activity in young female athletes: A biomechanically instrumented neuroimaging study. Psychophysiology, 60(4), e14221. doi: 10.1111/psyp.14221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong A, Grip H, Arumugam A, Boraxbekk CJ, Selling J, & Häger CK (2023). Right hemisphere brain lateralization for knee proprioception among right-limb dominant individuals. Frontiers of Human Neuroscience, 17, 969101. doi: 10.3389/fnhum.2023.969101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgent OJ, Dadalko OI, Pickett KA, & Travers BG (2019). Balance and the brain: A review of structural brain correlates of postural balance and balance training in humans. Gait Posture, 71, 245–252. doi: 10.1016/j.gaitpost.2019.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban K, Schudlo L, Keightley M, Alain S, Reed N, & Chau T (2021). Altered brain activation in youth following concussion: Using a dual-task paradigm. Developmental Neurorehabilitation, 24(3), 187–198. doi: 10.1080/17518423.2020.1825539 [DOI] [PubMed] [Google Scholar]
- Villiger M, Estevez N, Hepp-Reymond MC, Kiper D, Kollias SS, Eng K, & Hotz-Boendermaker S (2013). Enhanced activation of motor execution networks using action observation combined with imagination of lower limb movements. PloS one, 8(8), e72403. doi: 10.1371/journal.pone.0072403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, Newsome MR, Ott SD, Hunter JV, Dash P, Redell J, … Levin H (2019). Persistent disruption of brain connectivity after sports-related concussion in a female athlete. Journal of Neurotrauma, 36(22), 3164–3171. doi: 10.1089/neu.2019.6377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson GB, Grooms DR, & Acocello SN (2017). Neuromechanical considerations for postconcussion musculoskeletal injury risk management. Current Sports Medicine Reports, 16(6), 419–427. doi: 10.1249/JSR.0000000000000430 [DOI] [PubMed] [Google Scholar]
- Williams AM, Hogg JA, Diekfuss JA, Kendall SB, Jenkins CT, Acocello SN, … Wilkerson GB (2022). Immersive real-time biofeedback optimized with enhanced expectancies improves motor learning: A feasibility study. Journal of Sport Rehabilitation, 31(8), 1023–1030. doi: 10.1123/jsr.2021-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, & Smith SM (2001). Temporal autocorrelation in univariate linear modeling of fMRI data. NeuroImage, 14(6), 1370–1386. doi: 10.1006/nimg.2001.0931 [DOI] [PubMed] [Google Scholar]
- Wulf G, & Lewthwaite R (2016). Optimizing performance through intrinsic motivation and attention for learning: The OPTIMAL theory of motor learning. Psychonomic Bulletin & Review, 23(5), 1382–1414. doi: 10.3758/s13423-015-0999-9 [DOI] [PubMed] [Google Scholar]
- Yount R, Raschke KA, Biru M, Tate DF, Miller MJ, Abildskov T, … Bigler ED (2002). Traumatic brain injury and atrophy of the cingulate gyrus. Journal of Neuropsychiatry and Clinical Neurosciences, 14(4), 416–423. doi: 10.1176/jnp.14.4.416 [DOI] [PubMed] [Google Scholar]
- Yuan W, Diekfuss JA, Barber Foss KD, Dudley JA, Leach JL, Narad ME, … Myer GD (2021). High school sports-related concussion and the effect of a jugular vein compression collar: A prospective longitudinal investigation of neuroimaging and neurofunctional outcomes. Journal of Neurotrauma, 38(20), 2811–2821. doi: 10.1089/neu.2021.0141 [DOI] [PubMed] [Google Scholar]
- Zhang S, & Li CS (2014). Functional clustering of the human inferior parietal lobule by whole-brain connectivity mapping of resting-state functional magnetic resonance imaging signals. Brain Connectivity, 4(1), 53–69. doi: 10.1089/brain.2013.0191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu DC, Covassin T, Nogle S, Doyle S, Russell D, Pearson RL, … Kaufman DI (2015). A potential biomarker in sports-related concussion: brain functional connectivity alteration of the default-mode network measured with longitudinal resting-state fMRI over thirty days. Journal of Neurotrauma, 32(5), 327–341. doi: 10.1089/neu.2014.3413 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of the current manuscript are available from the corresponding author upon reasonable request.