Abstract
Objectives:
Technology has substantial potential to transform and extend care for persons with chronic pain, a burdensome and costly condition. To catalyze the development of impactful applications of technology in this space, we developed the Pain Tech Landscape model (PTL), which integrates pain care needs with characteristics of technological solutions.
Methods:
Our interdisciplinary group representing experts in pain and human factors research developed PTL through iterative discussions. To demonstrate one potential use of the model, we apply data generated from a narrative review of selected pain and technology journals (2000–2020) in the form of heat map overlays, to reveal where pain tech research attention has focused to date.
Results:
The PTL comprises three two-dimensional planes, with pain care needs on each x-axis (measurement to management), and technology applications on the y-axes according to: a) user agency (user- to system-driven), b) usage timeframe (temporary to lifelong), and c) collaboration (single-user to collaborative). Heat maps show that existing applications reside primarily in the “user-driven/management” quadrant (e.g., self-care apps). Examples of less developed areas include artificial intelligence and internet of things (i.e., internet-linked household objects), and collaborative/social tools for pain management.
Conclusion:
Collaborative development between the pain and tech fields in early developmental stages using the PTL as a common language could yield impactful solutions for chronic pain management. The PTL could also be used to track developments in the field over time. We encourage periodic re-assessment and refinement of the PTL model, which can also be adapted to other chronic conditions.
Keywords: chronic pain, technology, mHealth, eHealth, pain care
Introduction
Technological developments – including machine learning/artificial intelligence, sensors, and personal technologies such as smartphones—are reshaping the treatment of chronic health conditions (1, 2). Care for individuals with chronic pain may particularly benefit from this transformation. About one in five Americans lives with chronic pain, and nearly 1 in 10 experiences high-impact chronic pain that substantially disrupts their life or work activities (3, 4). Health care costs related to chronic pain exceed $300B per year (5). Much of this cost is driven by care for chronic low back pain, which is the leading cause of disability in the U.S. and worldwide (6). Health care systems struggle to accommodate the needs of individuals with chronic pain, and much pain care falls far short of the multidimensional, biopsychosocial care that is considered optimal (5).
Technology (henceforth we use the blanket term “tech” to encompass mHealth/mobile health, eHealth, digital health, and related terms) has substantial potential to enhance and augment the care of this costly and often debilitating condition. Tech-based strategies can broaden access and extend the capability of health care systems to provide pain care (7), and equip individuals to be more capable self-managers of their conditions (8, 9). Recent meta-analyses and systematic reviews have examined a variety of tech-based tools to assess or treat pain in diverse populations and with varying pain diagnoses (7, 10–16). These reviews conclude similarly that tech has potential to improve pain-related outcomes, although methodological shortcomings such as lack of long-term follow-up are common, and questions about what works best for whom remain largely unanswered.
To date, uptake of tech-based interventions for chronic pain and other chronic illnesses by providers and health systems remains limited (17). Barriers to uptake have been identified, from the perspective of patients (e.g., motivation, access, concerns about trustworthiness and security), providers (e.g., familiarity, time constraints) and systems (e.g., integration, reimbursement) (1, 18). Sustained engagement by users also remains an important challenge. Consumer technologies show a high rate of abandonment in real world settings. Digital health technologies are abandoned by approximately one third of users after three months and by half of users after six months (19–21), and a quarter of mHealth apps are used only once (22). In the context of pain research, tech-based interventions also show suboptimal retention; for example, in one meta-analysis, internet-based cognitive-behavioral therapy (CBT) had almost double the dropout rate of face-to-face CBT (23). Given the need for sometimes lifelong management of chronic pain, it is critical to improve our understanding of how engagement, either continuous or intermittent, in tech use can be maintained over extended periods of time and how tech can adapt to the changing needs of patients living with diverse pain conditions. A related issue, highlighted in a recent systematic review, is that tech tools developed via research for pain management seldom end up being available to real-world users, given barriers to commercialization and other challenges to disseminating the tools (24).
Early tech applications in chronic pain care often consisted of translating existing pain management strategies to digital platforms (e.g., symptom tracking via a device instead of on paper, CBT via mHealth/eHealth platforms). More recently, tech applications in pain care have leveraged new capabilities that include machine learning (25), chatbots (26, 27), gamification, environmental and physiological sensing, virtual reality environments (28) and ongoing assessment-intervention loops that deliver just-in-time assistance. While promising, more research needs to be done on these newer technologies to establish a solid evidence base for their use in pain care. For example, machine learning (ML) has been used most often for classification or diagnosis of patients with pain yet there are still relatively few trials of ML-guided pain treatments, including behavioral or psychosocial (29) Chatbots, or digital conversational agents, have been used fairly extensively in depression and anxiety treatment, but rarely for psychological treatments for pain. (30, 31) While some sensor technologies, such as activity trackers, are now a standard component of many cognitive-behavioral pain interventions, other sensor applications, such as pain assessment, are still in their infancy. (32)
Although the evidence base for any given technology takes time to develop, current trends in technological innovation and consumer use suggest that we are on the cusp of a rapid acceleration of tech options for pain care. For example, passive monitors (wearables) are becoming more ubiquitous and accurate, offering opportunities to measure a broader array of behavioral and physiological states. Low-cost virtual reality (VR) hardware for use at home, including devices that can be used with smartphones, are now available (28, 33–35), enable new opportunities for social distraction and support (36), and at least one VR treatment for pain has been approved as a digital therapeutic by the Food & Drug Administration (37). Artificial intelligence (AI) is increasingly being employed in pain research, diagnosis, and personalized treatment. Examples include applying ML on brain imaging data to predict aspects of pain perception (38), developing classification techniques to accurately distinguish MRI-based brain biomarkers of chronic pain patients (39), and using computer vision to detect pain-related affect (40) or facial expressions (41) trained on datasets like EmoPain (42). AI models have also been trained to generate user-specific pain self-management recommendations delivered through smartphones (43) and to recognize pain-related behaviors in smart homes (i.e., homes with internet-enabled features that can be controlled remotely) (44). Lötsch and Ultsch (45) provide a review of pain research involving ML and point to the continued application of intelligent technology in this area.
Despite this rising wave of innovation, we were not able to identify any existing conceptual framework or model linking technology and pain care. A shared framework could provide a common language for pain researchers and tech developers that could spark innovation in pain tech design and applications, ultimately leading to greater use and impact of tech for pain treatment. To this end, we present a novel framework, the Pain Tech Landscape (PTL), which overlays pain care activities with characteristics of tech tools. In order to illustrate one potential application of PTL, we conduct and apply results from a narrative review of leading pain and tech journals in the form of “heat map” overlays to depict in these two dimensional spaces where research attention on tech for pain care has focused to date, highlighting opportunities for future innovation. We conclude by suggesting additional applications for the PTL and briefly summarize key ethical issues to keep in mind when developing new, tech-based methods to relieve the suffering of people living with persistent pain.
Core principles of chronic pain management
New applications of tech should align with current best practices for pain care (46). As these were considered in the development of the PTL and to provide context for non-pain researchers, we briefly summarize them here. Chronic pain care should be individualized and patient-centered, in a manner that fosters a strong therapeutic alliance with a clinician. Thus, mHealth apps and other tech strategies must incorporate input from key user groups throughout the development and implementation process. A multimodal/multidisciplinary approach based on the biopsychosocial model of care is recommended, including medications (when they outweigh risks), restorative therapies (PT/OT), interventional approaches, behavioral approaches, and complementary and integrative health approaches. Treatment outcomes should focus on quality of life, improved functionality, and activities of daily living, and tech applications can standardize and automate quantification and monitoring of these outcomes. As an often-lifelong condition, chronic pain requires management approaches that can be integrated into the lives of affected individuals, highlighting the need to incorporate methods that promote sustained use over time.
Given the importance of integrated, patient-centered, multimodal pain care models (5), it is important to consider how tech can support the incorporation of the key components of such models in the primary care setting, where most people receive pain care. These include (47): enhanced provider education and decision support (e.g., tech can facilitate efficient provision of patient-reported information to providers for use at the point of care); care coordination (e.g., tech can be useful as a surrogate electronic health record carried by the patient); patient education and activation (e.g., tech is particularly suited for “just in time” education and support); and increased access to multimodal care. Emerging research is also establishing the importance of considering and positively shaping the mindset of patients with chronic pain regarding perceptions of physical activity levels and symptom management strategies (48, 49). Finally, because chronic pain is often accompanied by co-occurring problems such as depression, fatigue, and cognitive dysfunction (50), developers should also consider how their solutions can accommodate or target symptoms of these comorbidities.
The Pain Tech Landscape (PTL) model
We propose that pain researchers and technology developers could benefit from using dimensional models of pain tech, wherein key treatment activities and care stages are reflected by spectra that overlap with the functionality and use case(s) that a piece of technology targets. To this end, our interdisciplinary group representing experts in pain and human factors research developed the Pain Tech Landscape (PTL) model through a series of iterative discussions. Prior work has similarly offered design dimensions as a way to organize and navigate the range of capabilities offered by emerging technology (51, 52), though such characterizations have been geared towards general personal health informatics tools and/or dimensions specific to intervention and behavior change. We have also been inspired by recent conceptual frameworks, including lived informatics (53) and practice-based models of eHealth (54, 55) that conceptualize digital technology in terms of functionality that can support health tracking, communication, decision-making, and action. We build on these prior frameworks to offer a conceptual schema that characterizes a life cycle of assessment, treatment, and ongoing care, tailored to the pain context.
Central to the PTL (see Figure 1) is a measurement—management x-axis, given that measurement, in the form of pain and functional assessment, typically precedes and influences management strategies (though we note that in practice, pain measurement/assessment and management are iterative and mutually informing). Measurement refers to approaches and tools used to collect and evaluate health indicators and other information relevant to an individual’s pain status and outcomes. Management refers to treatment-oriented interventions, therapies, and other care activities that aim to promote pain relief and improved functioning.
Figure 1:
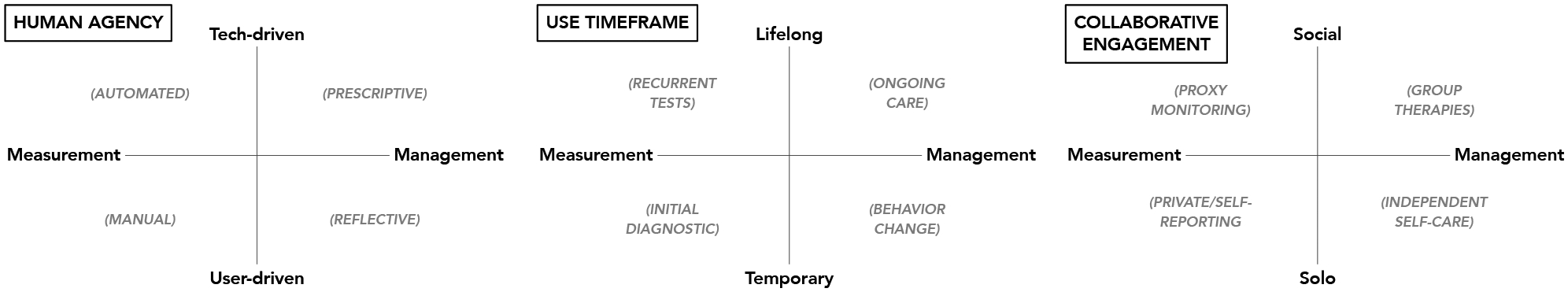
A graphical representation of the Pain Tech Landscape (PTL) model, which aims to bridge pain and tech disciplines by characterizing key activities and stages of pain management in terms of design dimensions, functional requirements, and use cases that tech-based applications can support.
We then offer three intersecting dimensions that we believe are critical factors to consider with respect to tech-based applications in pain care (see Figure 1). These include: 1) the degree of human agency vs. automation-supported; 2) the anticipated timeframe over which the technology will be used; and 3) the degree of independent vs. collaborative use of the tool. We see these as three core aspects of pain care that tech developers must consider; however, they are not intended to be exhaustive, and we encourage other researchers to identify additional relevant dimensions. To highlight specific pain care activities (and corresponding tech use cases), our graphical representation includes labels within each grid quadrant. Next, we describe each of these sectors, providing illustrative examples of recent pain tech applications.
Human Agency:
Tech-augmented measurement and management can vary from agentic, user-driven interactions to largely automated, tech-driven functionality. For example, manual measurement involves subjective self-reports from a patient or proxy using an interactive interface such as an Ecological Momentary Assessment (EMA) tool, while passive monitoring that leverages sensors off, on, or in the body is more tech-driven measurement. Similarly, tech-driven management is largely prescriptive, instructing a user to do something (e.g., “take your pain medication now”) and/or simply intervening on the user’s behalf (e.g., alerting a pain care team if a fall has been detected after initiating a pain medication that has fall risk as a side effect).
Anticipated Timeframe:
Some tech tools are deliberately designed for temporary use. For example, tech might be utilized for one-time assessments that employ ML at the clinic (e.g., to detect pain-related biomarkers in the brain (39) from an MRI scan captured upon patient intake) or leverage inertial sensors in a personal wearable or smartphone device available at home (e.g., to perform a forward flexion test to confirm a diagnosis when a person is unable to travel to visit a trained examiner). Similarly, shorter-term tech applications might be intended for use only until the user reaches a specific goal (e.g., a tool that delivers a rehabilitative treatment course until pain subsides, or that guides self-experimentation activities to help a user discover pain triggers). However, patients with chronic pain may benefit from tools that they can employ, intermittently or continuously, over time to monitor and/or manage their condition over months, years, or even lifelong. In these situations, tech would need to be designed to promote sustained adherence over time. More passive and automated measurement and management may therefore be welcomed in such circumstances, which also highlights one of many ways that the three (agency/time/collaborative) dimensions naturally and effectively overlap.
Collaboration:
Finally, pain management can be more or less collaborative, with more “solo” technology interfacing with only the individual as part of independent self-reporting and self-care, while more social tech could engage informal and formal care teams as part of monitoring an individual’s condition or might connect an individual with social networks such as online forums for support or group-based physical therapy sessions.
To facilitate collaboration between pain and tech researchers using the PTL as a meeting point, we describe classes of potential tech applications for pain in Table 1 and provide brief examples of how tech has already—or might in the near future—be applied to support an individual’s pain care needs. This categorization scheme can aid pain researchers and technology developers in making sense of the evolving landscape of technological development that can be applied to pain care. We note that this summary represents the current state of tech development. In the future, new tools will be developed and new combinations of existing tools will be integrated to support the broader pain measurement and management lifecycle.
Table 1:
Classification of Tech Tools and Applications to Pain Care
| CLASSES/TYPES OF TECHNOLOG | PAIN CARE APPLICATIONS AND PTL-INSPIRED USE CASES |
|---|---|
Artificial intelligence (AI)
|
Automated detection of neural pain markers, inference of acute pain incidents (e.g., bracing/grimace detection), and adaptive therapeutic recommendations |
| Big data/data analytics/data mining/data science | Observational studies that surface trends in wide-scale pain prevalence, effects and complications of pain treatments, and other pain-relevant public health metrics (e.g., burden of disease) |
| Self-monitoring tools/apps (small data, self-experimentation) | Self-tracking and analysis to identify personal factors that may improve or worsen symptoms |
Pervasive/ubiquitous sensing
|
Can be used to track activity or physiological parameters related to pain (e.g., continuous passive monitoring to detect pain attacks, falls, and motion impairments) as well as to trigger just-in-time adaptive interventions (JITAI) or optimally release medication |
| Interactive interfaces Self-(or proxy) report (prompted--EMA, IVR--or episodic reporting) |
Can provide frequent, in-context, real-time data on subjective symptoms (pain, fatigue) and mood states, which can supplement retrospective assessments during clinical visits, as well as offer user-facing alerts (e.g., treatment or appointment reminders) and informatics (e.g., visualizations, reports, dashboards) to aid self-awareness and self-care |
Extended reality (XR)
|
Can implement existing or novel behavioral and rehabilitative interventions such as augmented cognitive-behavioral therapy, VR for management of procedure-related acute pain, and XR gaming for rehabilitation and sensory retraining. |
Computer-mediated human-to-human communication (CMC)
|
Can be integrated into multimodal chronic pain interventions (e.g., use of SMS to prompt adaptive behaviors; intermittent phone or video therapy sessions) to promote increased engagement, participation and outcomes and/or to complement other tech-based interventions (e.g., use of online, phone or video delivered “coaching”; use of social media peer-support groups). |
Speech technologies
|
Can provide automated instruction or feedback to individuals for psychosocial support and education to facilitate effective pain self-management. |
| Games (exergames, serious games) | “Serious”, or therapeutic, games can provide support, motivation, and skills training for pain self-management. Games for entertainment can provide distraction from pain. Exergames can help increase physical activity |
| E-learning (includes web-or mobile-based learning platforms) | Can be used to teach self-management strategies to individuals living with pain, or to providers for their treatment of patients with pain. |
The PTL’s dimensions, together with the classes and examples presented in Table 1, aim to clarify a variety of appropriate and feasible ways to apply specific technologies to specific research and treatment needs. Our hope is that users and developers will find this schema and related examples helpful as they continue to leverage tech in innovative ways to enhance the assessment and management of chronic pain.
Using the PTL to map existing applications of pain tech and identify gaps
To demonstrate one potential “use case” for this new model—i.e., illustrating areas on the two-dimensional grids with prior research attention as well as gaps that may represent opportunities for new design and implementation --we conducted an informal narrative review of selected journals and proceedings, using the PTL to visually represent our findings. Our goal was not to conduct a formal or exhaustive review, as there are a number of high-quality existing systematic reviews and meta-analyses on tech for pain, as noted above. Rather, we sought to characterize general directions in research on pain tech as reflected in articles published in several specific high-profile pain and tech journals/conference proceedings over the last 20 years. We chose this strategy because publication in one of these prestigious venues indicates a certain degree of rigor and acceptance into the mainstream.
Methods: Review of leading pain journals, tech journals and flagship conference proceedings
We manually reviewed the Tables of Contents of all issues of the pain-focused journals Pain and the Journal of Pain published between 2000 and 2020, labeling in a spreadsheet tech-focused articles (whether an original research article or review) with the class of technology (see Table 1) that was the topic of the article. Because of our manual search approach for these two pain-focused journals, we did not employ search terms or use a publication database. We then reviewed the list, looking at the relative prevalence of each category overall and over each five year period. To characterize work from technology-focused research communities, we similarly retrieved and labeled pain-related papers published between 2000 and 2020 in the prominent publishing outlets on human-centered systems. Specific outlets included the conference on Human Factors in Computing Systems (CHI), Journal of Medical Internet Research (JMIR), and Interactive, Mobile, Wearable and Ubiquitous Technologies (IMWUT/UbiComp), and other technical venues (e.g., Institute of Electrical and Electronics Engineers (IEEE), Pervasive Health, and Tangible Embedded and Embodied Interaction (TEI)) that emphasize applications in health including pain. Because hundreds of papers appear in these conferences every year, the Association for Computing Machinery (ACM) Digital Library and the online database for non-ACM venue were searched using the keyword “pain”. Titles and abstracts of returned papers were manually reviewed to verify relevance to pain tech, and then labeling was conducted similar to the process performed for the pain-focused journal articles.
These steps were conducted independently by two reviewers (MJ, EM) and any discrepancies in classification were discussed and resolved. Our final step was to collaboratively review the findings from both pain and tech publications, to use as a springboard for discussion of observed trends and concentrations, agreed on by the interdisciplinary group of authors. These recognized concentrations were then applied to creating the heat maps, described below, using a tool called Heatmapper (56). This approach provides an at-a-glance impression of emphasized vs. underserved areas in pain tech over the last two decades.
Results
Figure 2 illustrates the heatmap representation of the research trends we observed overlaid on the three PTL grids, with deeper, red coloring indicating a larger number of published papers and lighter, purple coloring indicating fewer publications related to that particular topic. Generally, the Human Agency PTL grid shows that user-driven applications are considerably more prominent in the literature than tech-driven, on both measurement (e.g., Ecological Momentary Assessment or EMA) and management (e.g., e-learning) sides of pain care. The Use Timeframe PTL grid shows that most research attention has been in longer-term measurement of pain-related phenomena (vs. temporary or one-off); a common example is wearables/sensors. Finally the Collaborative Engagement PTL grid shows that there has been relatively little development in the social/collaborative space; for tech that involves only one user, approximately an equal amount of attention has been given to the management and measurement aspects of pain care.
Figure 2:
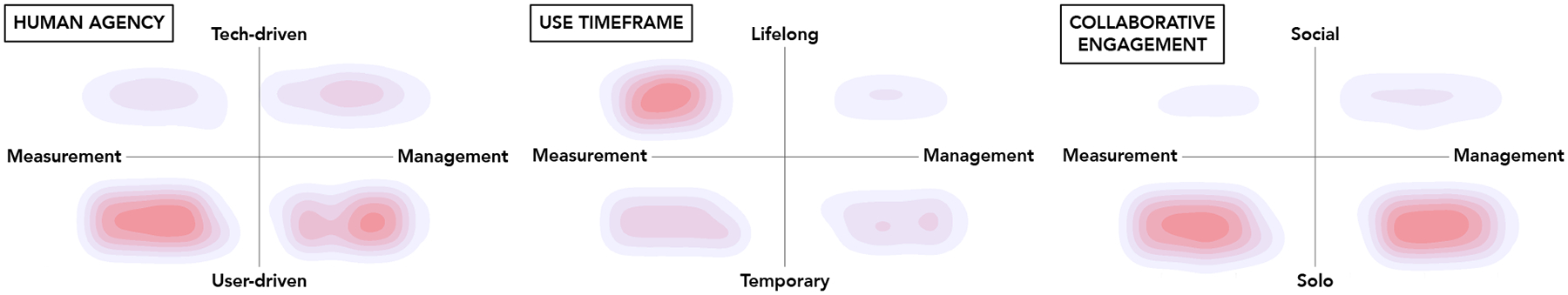
Heatmap depictions of the areas where the literature on pain tech has focused more (red) or less (purple) attention over the last 20 years.
Put in terms of specific tools, some research waves over this time period are particularly notable. First, in tandem with the growing ubiquity of personal mobile devices/smartphones, our analysis of the data documents a heavy focus in tech journals and proceedings from approximately 2011 to 2015 on transitioning traditional paper-based instruments to a smartphone/wireless device interface, together with some effort to digitally connect users with therapists, skill-building information, or other basic resources to aid self-management. From 2016 to 2020, our review highlights more recent explorations into the design of: emerging self-reporting modalities (e.g., tangible interfaces — i.e., devices a user interacts with via touch, or VR-based body maps to express pain in more immersive and embodied ways, the investigation of patients’ use of social media as part of coping, and the application of ML to diagnose pain as well as enhance fundamental understanding of pain mechanisms (though these pain-centered efforts tend to reflect and leverage the mainstream appearance and adoption of such technologies). In the pain journals, the majority of tech-focused articles over the last 20 years have addressed e-learning (i.e., educational or support strategies delivered by electronic means; usually internet or app-based); and ecological momentary assessments/self-reporting (for tracking pain and other symptoms). The next most common tech focus has been VR, and, in the last 5 years of the period under review, ML/AI.
Discussion
Our review highlights how prior work has largely focused on the measurement (vs. management) side of pain care, as well as more reflective vs. prescriptive forms of management, which we believe is due to researchers erring on the side of do-no-harm applications, as the pain tech field continues to emerge. Based on projections regarding technology trends that will define the next decade, we expect to see an emphasis on pain-centered technologies that support more artificially intelligent functionality, robotics, and augmented reality experiences. We also anticipate applications that fit into Internet of Things (IoT) (i.e., internet-enabled household objects) and other ecosystems of devices (e.g., especially wearables and speech interfaces). We foresee the integration of multiple classes of technology into closed-loop systems for monitoring and intervention, which can adaptively deliver treatments tailored in response to a user’s captured data. An example is smart environmental spaces that can leverage a variety of sensors and deliver multimodal, personal, and/or social oriented care as needed (with the latter being a notable underdeveloped area according to our Collaborative Engagement heatmap).
Finally, novel tech applications may benefit not only care for pain, but also the science around pain. For example, the development of digital biomarkers (i.e., behavioral or physiological data from wearable or other sensors) for subjectively experienced symptoms such as pain intensity, could lead to a deeper basic understanding of pain as a complex physiological, neurological, and psychosocial phenomenon. Further, though our focus is on characterizing pain tech from a functionality standpoint (e.g., capable of assisting with the measurement and/or management of pain), the fact that our review surfaced less than 5 papers involving randomized controlled trials (RCTs) also highlights a need for pain tech researchers to engage in more rigorous evaluations of these tools.
Conclusions and Future Directions
Effective management of chronic pain remains a major challenge for patients, providers, and health systems. Tech brings the possibility that pain care (including supporting self-management) can be provided in new, better, and more accessible ways. We maintain that more deliberate collaborations between pain researchers, technologists, and other stakeholders could enhance the efficacy and uptake of tech applications to the pain care space. Our novel framework, the Pain Tech Landscape (PTL), represents a potentially valuable tool for stakeholders from diverse disciplines to use as a common reference point to understand needs and functionality from both the technology and pain care perspectives, and as such may facilitate cross-disciplinary discussions and planning.
We suggest that this framework could also be used to track developments in the field over time, and we encourage periodic re-assessment and refinement of the PTL model. Other potential uses of the model include tracking where evidence of efficacy is strongest, or where adoption/uptake greatest. We look forward to expansion of the model from the pain research and technology development communities, as it is intended as a foundation for a shared, standardized conceptualization that can be used to both situate and guide future work in the pain tech space.
While not directly addressed in the PTL framework, we would be remiss not to mention that attention to ethics, access, and potential bias is paramount when developing new tech applications and when planning studies. First, tech applications for health are a potential threat to privacy and autonomy, particularly with respect to continuous sensing technologies and connection to the internet of things. Second, while tech has the potential to broaden access to high-quality pain care among minoritized and marginalized populations, it also poses potential dangers to these groups. One of these is that groups that historically have been made vulnerable will be excluded from advances in pain tech where a digital divide still exists or where costly new tech is needed for a given pain care application. Another major concern is that pain tech devices and decision-making algorithms will have “built in” biases such that they do not perform as well in historically excluded groups, and may place these groups at greater risk of harm (in an example from outside of pain care, pulse oximeters yield biased results when used on darker skin, leading to poorer clinical care; 57). AI models in health care tend to be trained using data from dominant groups that do not reflect the diversity of the populations in which these models may ultimately be used, limiting their utility (58). External validation of new AI tools with data from diverse populations is therefore an essential step (58). Additionally, pain researchers and technologists who are training AI algorithms should, where possible, use data sets that are either publicly available, or provide a detailed report on the characteristics of the population from whom the training data was collected, so that any potential limitations and biases are made evident to future users (59).
Further, many existing tech applications for pain are not optimized for use by vulnerable populations; e.g., older adults have barriers to using some tech tools (60), although design guidelines are emerging on how to address these challenges (60) and creative solutions have been implemented for offering tech support to patients in the clinical setting (2). It is also imperative that individuals living with chronic pain and other community-based partners, particularly those from historically underrepresented groups, are engaged in the tech development process. A growing literature has addressed issues of ethics and equity in tech for health care; see Bauer & Lizotte (61) for an in-depth discussion of an intersectional approach to fairness, accountability, transparency and ethnics (FATE) in AI; Owens & Walker (62) for discussion of making algorithms anti-racist; and Sim (1) for a comprehensive examination of ethical issues and potential harms in mobile health.
As a final note, while this paper has discussed chronic pain in a disease-agnostic way, it is possible that specific technologies may be more or less helpful for different pain conditions (e.g., sickle cell, fibromyalgia), and that future tech solutions can leverage the growing understanding of the mechanisms underlying specific pain conditions. Similarly, the PTL could be applied, with some modifications, to other chronic health conditions for which digital solutions can promote patient engagement and better clinical care in an accessible way, furthering tech’s potential to substantially benefit public health.
Conflicts of Interest and Source of Funding:
We do not have any financial, consultant, institutional, or other relationships that might lead to bias or a conflict of interest to disclose. This work was supported in part by funding from the National Institutes of Health [K01 AG050706-01A1 to M.R.J.; P30AG059297 to R.B.F.; U24AT009769 to R.D.K.; and P30AG022845, R01AG070055, R01DK131050 and K24AG053462 to M.C.R.]
Abbreviations:
- ML
Machine learning
- AI
Artificial intelligence
- PTL
Pain Tech Landscape
- EMA
Ecological Momentary Assessment
- MRI
Magnetic Resonance Imaging
Contributor Information
Mary R. Janevic, University of Michigan School of Public Health, 1415 Washington Heights, Ann Arbor, Michigan 48109-2029.
Elizabeth Murnane, Dartmouth College Thayer School of Engineering.
Roger B. Fillingim, University of Florida College of Dentistry.
Robert D. Kerns, Yale University.
M. Cary Reid, Weill Cornell Medicine.
References
- 1.Sim I Mobile devices and health. New England Journal of Medicine. 2019;381(10):956–68. [DOI] [PubMed] [Google Scholar]
- 2.Milani RV, Bober RM, Lavie CJ. The role of technology in chronic disease care. Progress in Cardiovascular Diseases. 2016;58(6):579–83. [DOI] [PubMed] [Google Scholar]
- 3.Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. Morbidity and Mortality Weekly Report. 2018;67(36):1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janevic MR, McLaughlin SJ, Heapy AA, Thacker C, Piette JD. Racial and Socioeconomic Disparities in Disabling Chronic Pain: Findings From the Health and Retirement Study. The Journal of Pain. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Institute of Medicine. Relieving pain in America a blueprint for transforming prevention, care, education, and research. Washington, D.C.: National Academies Press; 2011. xvii, 364 p. p. [PubMed] [Google Scholar]
- 6.Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, et al. What low back pain is and why we need to pay attention. The Lancet. 2018;391(10137):2356–67. [DOI] [PubMed] [Google Scholar]
- 7.Hewitt S, Sephton R, Yeowell G. The effectiveness of digital health interventions in the management of musculoskeletal conditions: systematic literature review. Journal of medical Internet research. 2020;22(6):e15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Education and Counseling. 2002;48(2):177–87. [DOI] [PubMed] [Google Scholar]
- 9.Nunes F, Verdezoto N, Fitzpatrick G, Kyng M, Grönvall E, Storni C. Self-care technologies in HCI: Trends, tensions, and opportunities. ACM Transactions on Computer-Human Interaction (TOCHI). 2015;22(6):1–45. [Google Scholar]
- 10.Bhattarai P, Newton-John T, Phillips JL. Quality and usability of arthritic pain self-management apps for older adults: a systematic review. Pain Medicine. 2018;19(3):471–84. [DOI] [PubMed] [Google Scholar]
- 11.Du S, Liu W, Cai S, Hu Y, Dong J. The efficacy of e-health in the self-management of chronic low back pain: A meta analysis. International Journal of Nursing Studies. 2020;106:103507. [DOI] [PubMed] [Google Scholar]
- 12.Moman RN, Dvorkin J, Pollard EM, Wanderman R, Murad MH, Warner DO, et al. A systematic review and meta-analysis of unguided electronic and mobile health technologies for chronic pain—is it time to start prescribing electronic health applications? Pain Medicine. 2019;20(11):2238–55. [DOI] [PubMed] [Google Scholar]
- 13.Dario AB, Cabral AM, Almeida L, Ferreira ML, Refshauge K, Simic M, et al. Effectiveness of telehealth-based interventions in the management of non-specific low back pain: a systematic review with meta-analysis. The Spine Journal. 2017;17(9):1342–51. [DOI] [PubMed] [Google Scholar]
- 14.Martorella G, Boitor M, Berube M, Fredericks S, Le May S, Gelinas C. Tailored web-based interventions for pain: systematic review and meta-analysis. Journal of Medical Internet Research. 2017;19(11):e8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundararaman LV, Edwards RR, Ross EL, Jamison RN. Integration of mobile health technology in the treatment of chronic pain: a critical review. Regional Anesthesia and Pain Medicine. 2017;42(4):488–98. [DOI] [PubMed] [Google Scholar]
- 16.Slattery BW, Haugh S, O’Connor L, Francis K, Dwyer CP, O’Higgins S, et al. An evaluation of the effectiveness of the modalities used to deliver electronic health interventions for chronic pain: systematic review with network meta-analysis. Journal of Medical Internet Research. 2019;21(7):e11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuire BE, Henderson EM, McGrath PJ. Translating e-pain research into patient care. Pain. 2017;158(2):190–3. [DOI] [PubMed] [Google Scholar]
- 18.Vo V, Auroy L, Sarradon-Eck A. Patients’ perceptions of mHealth apps: meta-ethnographic review of qualitative studies. JMIR mHealth and uHealth. 2019;7(7):e13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clawson J, Pater JA, Miller AD, Mynatt ED, Mamykina L, editors. No longer wearing: investigating the abandonment of personal health-tracking technologies on craigslist. Proceedings of the 2015 ACM international joint conference on pervasive and ubiquitous computing; 2015. [Google Scholar]
- 20.Lazar A, Koehler C, Tanenbaum TJ, Nguyen DH, editors. Why we use and abandon smart devices. Proceedings of the 2015 ACM international joint conference on pervasive and ubiquitous computing; 2015. [Google Scholar]
- 21.Attig C, Franke T. Abandonment of personal quantification: A review and empirical study investigating reasons for wearable activity tracking attrition. Computers in Human Behavior. 2020;102:223–37. [Google Scholar]
- 22.Vaghefi I, Tulu B. The continued use of mobile health apps: insights from a longitudinal study. JMIR mHealth and uHealth. 2019;7(8):e12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macea DD, Gajos K, Daglia Calil YA, Fregni F. The efficacy of Web-based cognitive behavioral interventions for chronic pain: a systematic review and meta-analysis. The Journal of Pain. 2010;11(10):917–29. [DOI] [PubMed] [Google Scholar]
- 24.Higgins KS, Tutelman PR, Chambers CT, Witteman HO, Barwick M, Corkum P, et al. Availability of researcher-led eHealth tools for pain assessment and management: barriers, facilitators, costs, and design. Pain Reports. 2018;3(Suppl 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piette JD, Newman S, Krein SL, Marinec N, Chen J, Williams DA, et al. Artificial Intelligence (AI) to improve chronic pain care: Evidence of AI learning. Intelligence-Based Medicine. 2022:100064. [Google Scholar]
- 26.Hauser-Ulrich S, Künzli H, Meier-Peterhans D, Kowatsch T. A smartphone-based health care chatbot to promote self-management of chronic pain (SELMA): pilot randomized controlled trial. JMIR mHealth and uHealth. 2020;8(4):e15806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson B, Black CM, Corey D. The Evolving Role of Medical Chatbots.Vein Magazine. 2018. Available from: https://www.veindirectory.org/magazine/article/practice-management/medical-chatbots
- 28.Trost Z, France C, Anam M, Shum C. Virtual reality approaches to pain: toward a state of the science. Pain. 2021;162(2):325–31. [DOI] [PubMed] [Google Scholar]
- 29.Jenssen MDK, Bakkevoll PA, Ngo PD, Budrionis A, Fagerlund AJ, Tayefi M, et al. Machine Learning in Chronic Pain Research: A Scoping Review. Applied Sciences. 2021;11(7):3205. [Google Scholar]
- 30.Abd-Alrazaq AA, Rababeh A, Alajlani M, Bewick BM, Househ M. Effectiveness and Safety of Using Chatbots to Improve Mental Health: Systematic Review and Meta-Analysis. J Med Internet Res. 2020;22(7):e16021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartlett J, Fisher E, Likkanen S, Turunen J, Skog M, Eccleston C. The design and development of an embodied Semi-Autonomous Mentoring Intelligence (SAMI) for use in virtual reality interventions, operationalized for the self-management of chronic pain. Frontiers in Virtual Reality. 2022. [Google Scholar]
- 32.Naranjo-Hernández D, Reina-Tosina J, Roa LM. Sensor Technologies to Manage the Physiological Traits of Chronic Pain: A Review. Sensors. 2020;20(2):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang H, Shen J, Wheeler KK, Patterson J, Lever K, Armstrong M, et al. Efficacy of smartphone active and passive virtual reality distraction vs standard care on burn pain among pediatric patients: A randomized clinical trial. JAMA Network Open. 2021;4(6):e2112082–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darnall BD, Krishnamurthy P, Tsuei J, Minor JD. Self-administered skills-based virtual reality intervention for chronic pain: randomized controlled pilot study. JMIR Formative Research. 2020;4(7):e17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Logan DE, Simons LE, Caruso TJ, Gold JI, Greenleaf W, Griffin A, et al. Leveraging virtual reality and augmented reality to combat chronic pain in youth: position paper from the interdisciplinary network on virtual and augmented technologies for pain management. Journal of Medical Internet Research. 2021;23(4):e25916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang A, Sun Y, Tamir N, Won AS, editors. Pain Experience in Social VR: The Competing Effect on Objective Pain Tolerance and Subjective Pain Perception. 2020 IEEE Conference on Virtual Reality and 3D User Interfaces Abstracts and Workshops (VRW); 2020: IEEE. [Google Scholar]
- 37.Wetsman N VR treatment for chronic pain gets FDA authorization. The Verge. 2021. [Google Scholar]
- 38.Rosa MJ, Seymour B. Decoding the matrix: benefits and limitations of applying machine learning algorithms to pain neuroimaging. Pain. 2014;155(5):864–7. [DOI] [PubMed] [Google Scholar]
- 39.Boissoneault J, Sevel L, Letzen J, Robinson M, Staud R. Biomarkers for musculoskeletal pain conditions: use of brain imaging and machine learning. Current Rheumatology Reports. 2017;19(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olugbade TA. Automatic Monitoring of Physical Activity Related Affective States for Chronic Pain Rehabilitation: UCL (University College London; ); 2018. [Google Scholar]
- 41.Prkachin KM, Hammal Z. Computer mediated automatic detection of pain-related behavior: prospect, progress, perils. Frontiers in Pain Research (Lausanne, Switzerland: ). 2021;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aung MS, Kaltwang S, Romera-Paredes B, Martinez B, Singh A, Cella M, et al. The automatic detection of chronic pain-related expression: requirements, challenges and the multimodal EmoPain dataset. IEEE transactions on affective computing. 2015;7(4):435–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabbi M, Aung MS, Gay G, Reid MC, Choudhury T. Feasibility and acceptability of mobile phone–based auto-personalized physical activity recommendations for chronic pain self-management: Pilot study on adults. Journal of Medical Internet Research. 2018;20(10):e10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fritz RL, Wilson M, Dermody G, Schmitter-Edgecombe M, Cook DJ. Automated smart home assessment to support pain management: multiple methods analysis. Journal of Medical Internet Research. 2020;22(11):e23943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lötsch J, Ultsch A. Machine learning in pain research. Pain. 2018;159(4):623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Health UDo, Services H. Pain management best practices inter-agency task force report: Updates, gaps, inconsistencies, and recommendations. Washington, DC: US Department of Health and Human Services. 2019. [Google Scholar]
- 47.Peterson K, Anderson J, Bourne D, Mackey K, Helfand M. Effectiveness of models used to deliver multimodal care for chronic musculoskeletal pain: A rapid evidence review. Journal of General Internal Medicine. 2018;33(1):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boswell MA, Evans KM, Zion SR, Boles DZ, Hicks JL, Delp SL, et al. Mindsets Predict Physical Activity and Relate to Chosen Management Strategies in Individuals with Knee Osteoarthritis. medRxiv. 2021. [DOI] [PubMed] [Google Scholar]
- 49.Boswell MA, Evans KM, Zion SR, Boles DZ, Hicks JL, Delp SL, et al. Mindset is associated with future physical activity and management strategies in individuals with knee osteoarthritis. Annals of Physical and Rehabilitation Medicine. 2022:101634-. [DOI] [PubMed] [Google Scholar]
- 50.Phillips K, Clauw DJ. Central pain mechanisms in the rheumatic diseases: future directions. Arthritis Rheum. 2013;65(2):291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murnane E, Choudhury T. Mobile and sensor technology as a tool for health measurement, management, and research with aging populations. 2020. [Google Scholar]
- 52.Murnane EL. A Framework for Domain-Driven Development of Personal Health Informatics Technologies. 2017. [Google Scholar]
- 53.Epstein DA, Ping A, Fogarty J, Munson SA, editors. A lived informatics model of personal informatics. Proceedings of the 2015 ACM international joint conference on pervasive and ubiquitous computing; 2015. [Google Scholar]
- 54.Black AD, Car J, Pagliari C, Anandan C, Cresswell K, Bokun T, et al. The impact of eHealth on the quality and safety of health care: a systematic overview. PLoS medicine. 2011;8(1):e1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shaw T, McGregor D, Brunner M, Keep M, Janssen A, Barnet S. What is eHealth (6)? Development of a conceptual model for eHealth: qualitative study with key informants. Journal of Medical Internet Research. 2017;19(10):e8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, Maciejewski A, et al. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Research. 2016;44(W1):W147–W53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moran-Thomas A How a popular medical device encodes racial bias. Boston Review. 2020. August 5, 2020. Available at: https://www.bostonreview.net/articles/amy-moran-thomas-pulse-oximeter/
- 58.Celi LA, Cellini J, Charpignon M-L, Dee EC, Dernoncourt F, Eber R, et al. Sources of bias in artificial intelligence that perpetuate healthcare disparities—A global review. PLOS Digital Health. 2022;1(3):e0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daneshjou R, Smith MP, Sun MD, Rotemberg V, Zou J. Lack of transparency and potential bias in artificial intelligence data sets and algorithms: a scoping review. JAMA dermatology. 2021;157(11):1362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Czaja SJ. Usability of technology for older adults: Where are we and where do we need to be. Journal of Usability Studies. 2019;14(2):61–4. [Google Scholar]
- 61.Bauer GR, Lizotte DJ. Artificial intelligence, intersectionality, and the future of public health. American Public Health Association; 2021. p. 98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Owens K, Walker A. Those designing healthcare algorithms must become actively anti-racist. Nature Medicine. 2020;26(9):1327–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


