Abstract
Objectives:
Nonalcoholic fatty liver disease is the most common chronic liver disease in children. Elafibranor, a dual peroxisome proliferator-activated receptor α/δ agonist, has been proposed as a treatment for NASH. The aims were to: 1. describe pharmacokinetics, safety, and tolerability of oral elafibranor at 2 doses (80 and 120mg) in children 8-17 years and 2. assess changes in aminotransferases.
Methods:
Children with NASH were randomized to open-label elafibranor 80mg or 120mg daily for 12 weeks. The intent-to-treat analysis included all participants who received at least one dose. Standard descriptive statistics and PK analyses were performed.
Results:
Ten males (mean 15.1yrs, SD 2.2) with NASH were randomized to 80mg (n=5) or 120mg (n=5). Baseline mean ALT was 82 U/L (SD 13) and 87 U/L (SD 20) for 80mg and 120mg groups, respectively. Elafibranor was rapidly absorbed and well tolerated. Elafibranor plasma exposure increased between the 80mg and 120mg dose with a 1.9- and 1.3-fold increase in median Cmax and AUC0-24, respectively. End of treatment mean ALT was 52 U/L (SD 20) for the 120mg group, with a relative mean ALT change from baseline of −37.4% (SD 23.8%) at 12 weeks.
Conclusions:
Once daily dosing of elafibranor was well tolerated in children with NASH. There was a 37.4% relative reduction from mean baseline ALT in the 120mg group. Decreasing ALT may be associated with improvement in liver histology, thus could be considered a surrogate for histology in early phase trials. These results may support further exploration of elafibranor in children with NASH.
Keywords: NAFLD, fatty liver, pediatric
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in children affecting an estimated 10% of children in the United States.1 NAFLD has a range of severity including steatosis alone, steatosis with hepatocellular injury or steatohepatitis (NASH), and fibrosis or cirrhosis.3 In children, NASH may have a pattern of inflammation and fibrosis that differs from adults, with predominantly portal histological changes.4 Therefore, children with NASH may not have the same response to a given therapy as adults with NASH. Currently no pharmacologic therapy exists for children with NASH.
Elafibranor (GFT505), is an oral, liver-targeted drug candidate that was being developed for the treatment of NASH with fibrosis in adults.5 Elafibranor is a dual peroxisome proliferator-activated receptor (PPAR) α/δ agonist, with a 5-fold selectivity for activation of PPARα over PPARδ. GFT1007 is the main metabolite of elafibranor and shows similar activation. In preclinical models, Elafibranor improved insulin sensitivity, glucose homeostasis, lipid metabolism and inflammation.6 To date, elafibranor has been well tolerated with few discontinuations due to adverse events.5,7
Considering the need for a treatment for NASH in children, and the potential beneficial effects of elafibranor, elafibranor may be a treatment option for children with NASH. We hypothesized that oral administration of elafibranor once per day for 12 weeks would be safe and result in a decrease in alanine aminotransferase (ALT) in children with NASH. The study aims were to assess: 1) pharmacokinetics (PK) of elafibranor following once daily oral administration of two dose levels (80 and 120mg) in children ages 8 to 17 years; 2) safety and tolerability of two doses of elafibranor in children ages 8 to 17 years and 3) changes in serum ALT, aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT).
METHODS
Study Participants
This was an open-label, multicenter, parallel group study to assess the PK, safety, pharmacodynamics (PD) and the relationship between PK and PD following 12 weeks of once daily oral administration of elafibranor 80mg and 120mg in children and adolescents, 8 to 17 years, with histologically confirmed NASH. Dosing was chosen based on prior adult data.5, 7
Children with NASH were enrolled at pediatric centers at UC San Diego and Columbia University. Participants underwent detailed phenotyping including demographic, clinical, and histological evaluation. Inclusion criteria were children ages 8-17 years and biopsy-proven NASH. Diagnosis of NASH was based on standard clinical, laboratory, and histologic features as previously described.4,8,9 Each participant received standardized counseling for nutrition and exercise based on clinical guidelines for NAFLD at each visit. Written consent for participants was obtained from a parent or guardian, and written assent was obtained from all children prior to participation.
Phenotyping of cohort
Demographic data were obtained via a structured interview. Weight, height, and waist measurements were performed in duplicate and BMI, BMI percentile and BMI z-score were calculated using Centers for Disease Control and Prevention instructions. Participants fasted overnight for 10 hours before phlebotomy for laboratories including: complete blood count with differential, PT/INR, comprehensive metabolic panel, creatinine clearance, bilirubin direct, GGT, creatine phosphokinase, C-reactive protein, thyroid stimulating hormone, fasting insulin, c-peptide, and lipid panel. Study timeline is available in supplemental material
Statistical Analysis
The primary objective of this study was to characterize individual PK parameters in children 8 to 17 years of age, by collecting rich PK samples to support non-compartmental analysis. Inter-participant variability of PK parameters in adults was low to moderate (coefficient of variation [CV]% ranged from 23.3% to 68.3%). Assuming similar variability in children, a sample size of 20 participants was selected to target a 95% CI [confidence interval] within 60% and 140% of the geometric mean estimates of clearance and volume of distribution for elafibranor with at least 80% power. Additionally, 20 participants was sufficient to evaluate the safety and tolerability and explore the responsiveness (efficacy) of once daily oral dosing of 80mg and 120mg of elafibranor for up to 12 weeks. Per protocol, enrollment of participants 11-20 was based upon a review of the first 10 participants by the data safety monitoring board (DSMB).
Analysis Populations
Three populations were used for analysis: safety, PK and intent-to-treat (ITT). The safety population was defined as all participants who received at least one dose of study drug and have at least one post-baseline safety assessment. The pharmacokinetic population was defined as all participants who received at least one dose of study drug, did not have protocol deviations or adverse events that may significantly affect PK, and have at least one post-dose PK sample. All randomized participants who received at least one dose of study drug were the ITT population. PK and safety data were analyzed based on the PK population and the safety population, respectively. Efficacy data were analyzed based on the ITT population.
Pharmacokinetics
Individual plasma concentrations for elafibranor and its active metabolite GFT1007, were summarized descriptively by planned time points for each dose group. Individual plasma concentration-time profiles of elafibranor were plotted on semi-logarithmic scales.
Pharmacokinetic parameters of elafibranor and GFT1007 were estimated using noncompartmental analysis methods in Phoenix WinNonlin 8.0, using actual elapsed time from dosing. Plasma concentrations ≥ the qualified lower limit of the assay were used in the pharmacokinetic analysis. Calculated PK parameters included: maximum concentration (Cmax), time to maximum concentration (Tmax), area under the plasma concentration time curve over 24 hours (AUC0-24), and trough concentrations at 24 hours post-dose in plasma (Ctrough).
Safety and Efficacy
Safety evaluations consisted of adverse events, vital signs, ECG, and laboratory measurements (hematology, coagulation, biochemistry, and urinalysis) and were summarized by dose group and overall. Efficacy evaluations were summarized by dose group and by time-point using descriptive statistics and were based on the change from baseline. Based on adult data, gastrointestinal symptoms and fatigue are considered common non-serious adverse reactions.5,7
RESULTS
Ten males with NASH were randomized to either 80mg (n=5) or 120mg (n=5) (Supplemental Figure 1). Mean age was 15.1 years (SD 2.2) and 90% reported Hispanic ethnicity (Table 1). Baseline mean BMI z-score was 2.9 (SD 0.35). Of the ten participants, four had stage 0-1 fibrosis, six had stage 2-3 fibrosis, five had zone 1 NASH, and five had zone 3 NASH using NASH-CRN criteria.4 At baseline, mean ALT was 82 U/L (SD 13) in the 80mg group and 87 U/L (SD 20) in the 120mg group. After these ten children completed their PK studies, the DSMB reviewed all data and recommended continued enrollment including children in the younger age group. However, further enrollment was interrupted by the COVID-19 pandemic and was not re-initiated by the sponsor following discontinuation of evaluation of elafibranor in NASH in adults after failure to meet the primary endpoint in a phase 3 trial.
Table 1:
Study population and clinical characteristics with change from baseline to end of treatment and end of trial* mean (SD)
| Characteristic | 80mg (n = 5) | 120mg (n = 5) | ||||
|---|---|---|---|---|---|---|
| Age, mean (SD) | 14.5 (2.2) | 15.7 (2.3) | ||||
| Sex, N | ||||||
| Male | 5 | 5 | ||||
| Ethnicity, N (%) | ||||||
| Hispanic | 4 | 5 | ||||
| Non-Hispanic | 1 | 0 | ||||
| Change in Parameters |
Baseline | End of Treatment |
End of Trial (n=4) |
Baseline | End of Treatment |
End of Trial (n=4) |
| Weight (SD), kg | 86.0 (18.9) | 87.6 (21.8) | 90.0 (23.2) | 96.8 (25.6) | 97.0 (25.5) | 97.5 (25.7) |
| BMI Z score (SD) | 2.7 (0.2) | 2.7 (0.4) | 2.9 (0.1) | 3.0 (0.5) | 2.9 (0.5) | 2.9 (0.5) |
| ALT (SD), U/L | 82 (13) | 100 (51) | 114 (52) | 87 (20) | 52 (20) | 58 (15) |
| AST (SD), U/L | 38 (6) | 46 (17) | 48 (13) | 38 (9) | 29 (6) | 30 (8) |
| GGT (SD), U/L | 74 (31) | 85 (64) | 125 (74) | 44 (21) | 28 (16) | 30 (17) |
| Fasting Glucose (SD), mg/dL | 106 (11) | 112 (18) | 118 (18) | 94 (7) | 95 (2) | 100 (4) |
| Fasting Insulin (SD), mIU/L | 61 (36) | 71 (40) | 101 (80) | 54 (21) | 46 (10) | 39 (9) |
| LDL (SD), mg/dL | 89 (19) | 93 (39) | 107 (40) | 70 (27) | 62 (27) | 69 (26) |
| HDL (SD), mg/dL | 38 (9) | 43 (12) | 41 (12) | 43 (4) | 50 (8) | 49 (5) |
| Triglycerides (SD), mg/dL | 113 (72) | 128 (51) | 170 (29) | 127 (68) | 81 (35) | 113 (53) |
SD: Standard deviation
BMI : body mass index
ALT : alanine aminotransferase
AST : aspartate aminotransferase
GGT : gamma-glutamyl transferase
LDL: low density lipoprotein
HDL: high density lipoprotein
there was no significant difference in age, anthropometric parameters or laboratory values between the two groups at baseline
Pharmacokinetics
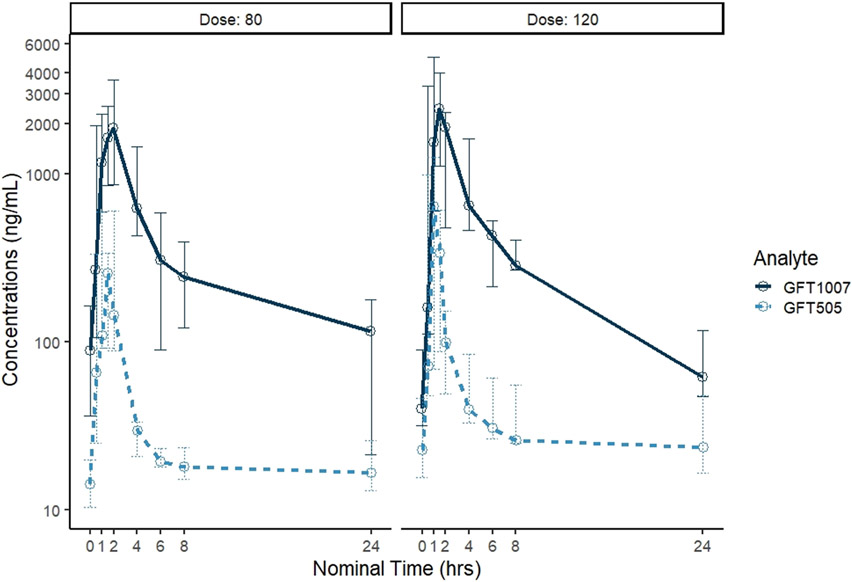
The median (min-max) Cmax values for elafibranor for the 80 and 120mg groups were 336 (93-607) and 639 (221-1258) ng/mL, respectively (Table 2, Figure 1). Median Cmax values for GFT1007 for the 80 and 120mg groups were 2545 (863-3666) and 2458 (1122-5001) ng/mL, respectively. At steady state, both doses of elafibranor were rapidly absorbed and transformed to its active metabolite, GFT1007. Elafibranor plasma exposure increased between the 80mg and 120mg dose levels with a 1.9 and 1.3-fold increase in median Cmax and AUC0-24, respectively, suggesting relative linearity. No differences in GFT1007 exposure were observed between the 2 groups.
Table 2:
Pharmacokinetic parameters for two dose levels of elafibranor
| Analyte | Dose (mg) | Statistics | Cmax (ng/mL) | Tmax (hr) | AUC0-24 (hr*ng/mL) |
Ctrough Day 29 (ng/mL) |
|---|---|---|---|---|---|---|
| N | 5 | 5 | 5 | 5 | ||
| GFT505 | 80 | Median | 336 | 1.5 | 1033 | 14.2 |
| Min; Max | 93; 607 | 0.52; 2.0 | 598; 1389 | 10.4; 20.0 | ||
| 120 | Median | 639 | 1.0 | 1322 | 22.7 | |
| Min; Max | 221; 1258 | 0.98; 1.5 | 799; 2643 | 15.7; 46.5 | ||
| GFT1007 | 80 | Median | 2545 | 2.0 | 10244 | 88.5 |
| Min; Max | 863; 3666 | 1.5; 2.0 | 5272; 14972 | 36.2; 165 | ||
| 120 | Median | 2458 | 1.5 | 9008 | 40.1 | |
| Min; Max | 1122; 5001 | 1.0; 1.5 | 7541; 15305 | 31.1; 90.2 |
Cmax: maximum serum concentration
Tmax: time to peak drug concentration
AUC0-24: area under the plasma concentration-time curve over the last 24-h dosing interval
Ctrough: trough concentration
Figure 1.
Elafibranor (GFT 505) and GFT 1007 concentrations over time for each dose level: median (min/max).
Pharmacodynamics
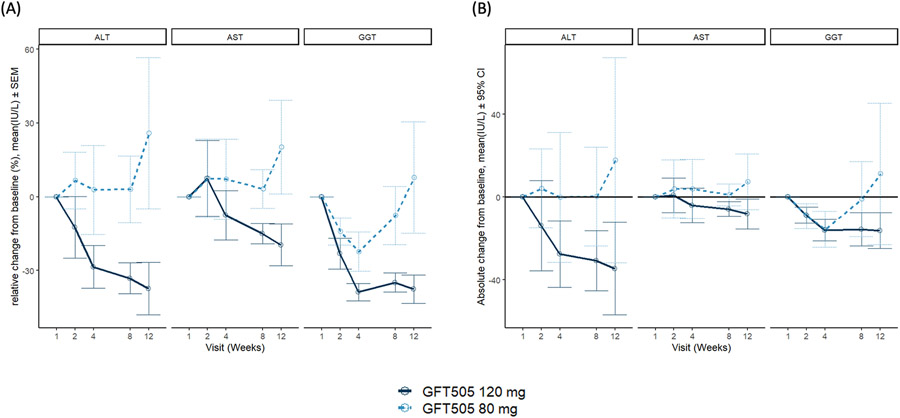
Mean ALT at end of treatment (day 85) was 100 U/L (SD 51) for the 80mg group and 52 U/L (SD 20) for the 120mg group, with a mean change in ALT of +18 U/L (SD 57) and −35 U/L (SD 26), respectively (Table 1, Figure 2). ALT in the 120mg group decreased through each visit with a relative mean ALT change from baseline of −37.4% (SD 23.8%) at 12 weeks (Figure 2). Four weeks after the end of treatment, ALT was still decreased in the 120mg group with a mean relative change of −29.3% (SD 21.6%) from baseline but not in the 80mg group (45.9% [SD 56.2%]). Table 1 depicts change in key metabolic parameters from baseline to end of trial.
Figure 2.
Change from baseline in liver transaminases (A) Absolute change from baseline in ALT, AST, and GGT in the two dose levels over a 12-week treatment course. (B) Relative change from baseline in ALT, AST, and GGT in the two dose levels over a 12-week treatment course
Safety
All participants completed the full study duration of 85 days. Elafibranor was generally well tolerated, and there were no serious adverse events or events that led to drug or participant withdrawal (supplemental table 1).
DISCUSSION
In this open-label, multicenter, parallel group study, we evaluated the PK, PD, safety and tolerability of two dose levels of elafibranor in ten male adolescents with NASH. Additionally, we assessed changes in serum transaminases over 12 weeks. Elafibranor was well tolerated, and all children completed the full study. Elafibranor was absorbed rapidly with median Tmax of the active metabolite in the 120mg group of 1.5 hours. Additionally, plasma exposure increased between the 80mg and 120mg in an approximately linear fashion. In the secondary aim, we noted a decrease in relative mean ALT by 37% at 12 weeks in the 120mg cohort.
There are three PPAR isotypes α, β/δ, and γ. PPARs are regulators of fatty acid metabolism, glucose metabolism, inflammation, and fibrogenesis9 with their main ligands being fatty acids and metabolites.11 PPARα is expressed in organs with high rates of fatty acid metabolism, such as hepatocytes, and the result of PPARα activation is a reduction in triglyceride accumulation in the liver.12 Historically, PPAR agonists have been used in treatment of metabolic syndrome, hypertriglyceridemia, and mixed hyperlipidemia, and type 2 diabetes.13 Given their physiologic properties, PPARs may be implicated in NASH pathogenesis. Furthermore, human liver PPARα gene expression is inversely correlated with NASH severity14 and PPARα agonists are seen as potential therapeutic options for NASH.15
Elafibranor is a PPAR α/δ agonist and was evaluated in pre-clinical models and clinical trials for NAFLD. In pre-clinical rodent models of NASH with fibrosis, elafibranor demonstrated improvement in liver inflammatory markers as well as hepatic steatosis and fibrosis.6 In an 8-week trial of 80mg daily dosing in 22 males with obesity and insulin resistance, elafibranor improved insulin sensitivity, triglycerides, and aminotransferases without notable side effects.16 In a double-blind, placebo-controlled study of adults with obesity and either pre-diabetes or dyslipidemia, 80mg daily dose elafibranor significantly improved triglycerides, insulin resistance, and fasting plasma glucose.17 Subsequently, in a phase 2, multi-center, randomized, placebo-controlled trial, adults with NASH were given 80mg or 120mg of daily elafibranor for one year.5 Although the phase 2 trial failed to meet its pre-specified primary endpoint, in a post-hoc analysis, using an updated endpoint, NASH resolved without fibrosis worsening in a higher proportion in those taking the 120mg dose (n = 91) compared to the 80mg dose (n = 93) (19% vs 12%, p = 0.045). A phase 3 placebo-controlled trial (RESOLVE-IT; NCT02704403) evaluating the effect of elafibranor in over 1000 adults with NASH and fibrosis receiving 120mg for 72 weeks was discontinued after interim results showed no significant difference compared to placebo in the histologic endpoints with 19.2% in the treatment group achieving the primary endpoint of NASH resolution compared to 14.7% in the placebo group.7,18 As a result, additional development of elafibranor in NASH was discontinued due to lack of efficacy, but not due to safety reasons; a larger trial in children was therefore not conducted and the present trial was discontinued. These data on a dual PPAR α/δ agonist are still encouraging and may be a potential option for trials in the future.19
The current paradigm for drug development is to first assess efficacy in adults, then consider trials in children, as children represent a vulnerable subgroup per FDA guidelines. The assurance of safety prior to testing a drug in children is important. However, the reliance of pediatric drug development on adult drug development does not consider many aspects of NAFLD in children that are different than what is observed in adults and that NAFLD in children may be a distinct disease with differences in the pathophysiology and histology that hinder extrapolation of results from adults to children. Rather than the adult histologic characteristics of ballooning hepatocytes and zone 3 predominant pattern, children can have borderline zone 1 NASH with portal predominance, and this has been described as the pediatric pattern of disease.5 Therapeutic targets in children may differ compared to adults as well. For example, in a clinical trial with cysteamine bitartrate for pediatric NAFLD, children with the prototypical pediatric zone 1 NASH pattern were much more likely to have a histological response than children with other NASH patterns.20 NAFLD can often be a “silent” phenomenon, and in the absence of therapy, NAFLD in children can have high morbidity. At diagnosis, nearly 15% of children present with advanced fibrosis.21 Furthermore, NASH is now the leading indication for liver transplantation in young adults. Additionally, in a recent population-based study of children and young adults, those with NAFLD had higher rates of mortality compared to healthy controls over a 20-year period, with the causes of mortality being cancer, liver disease, and cardiovascular disease.22 The FDA, in a guidance statement to industry, acknowledged these challenges in therapeutic development in NASH, and stated that extrapolation of efficacy from adults to children based solely on PK/PD is not appropriate. Additionally, more longitudinal data are needed to identify appropriate inclusion/exclusion criteria in children as well as endpoints that consider the histologic differences seen in children.23 Non-invasive imaging endpoints, such as MRI proton density fat fraction (MRI-PDFF) for steatosis and MR Elastography (MRE) for fibrosis, should also be considered as outcome measures. MRI-PDFF has been successfully used in trials in children,24 and MRE can accurately detect presence of fibrosis in children.25 To equitably include children in clinical trials, once safety is established, more proof-of-concept trials with these imaging markers could be utilized to establish potential efficacy leading to late phase trials with histologic endpoints.26,27
This was the first study of elafibranor in children and therefore utilized an open-label design. The study was designed to assess safety, PK, and changes in biochemical markers. The study did not have histologic outcomes, but the improvement in ALT was clinically meaningful for the 120mg dose and ALT is a potential indicator of histologic improvement in children.28 A clear limitation was the premature discontinuation of this trial due to adult phase 3 data of non-efficacy in histologic endpoints in the RESOLVE-IT trial. Only males were studied, but there is no known effect of gender on elafibranor efficacy, safety or PK in adults. Also, this study did not include a placebo comparator to evaluate safety, tolerability, or efficacy. Ultimately, additional studies with a more inclusive population of children with well-defined clinical endpoints are needed to assess if PPAR α/δ agonists may be useful in treating NAFLD in children.
Elafibranor was generally safe and well-tolerated in children 12-17 years with NASH and resulted in a decrease in ALT in the 120mg dosing group. These data support pursuing larger drug trials with this same mechanism of targeting PPAR α/δ agonists in children, as we have yet to find effective medication treatments for NASH in children. Given that children with NASH have high morbidity and potential for early mortality, urgent action is needed to find efficacious therapies. Although assessment of safety and tolerability of new therapeutic options in children is paramount, limiting NASH clinical trials in pediatrics to drugs that are efficacious in adults may limit progress in children.
Supplementary Material
What is Known:
Nonalcoholic steatohepatitis (NASH) is common in children and the leading cause of liver transplantation in young adults.
No pharmacologic therapy exists for children with NASH and mainstay of treatment is lifestyle changes.
Elafibranor is a dual peroxisome proliferator-activated receptor α/δ agonist that was being developed as treatment in adults for NASH with fibrosis.
What is New:
Elafibranor was generally safe and well tolerated in children 8–17 years with NASH.
Elafibranor was rapidly absorbed.
Elafibranor 120mg daily for 12 weeks decreased relative mean ALT by 37.4%.
Funding:
The study was funded in part through clinical research contracts from Genfit Corporation to UC San Diego and Columbia University. The study was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001442 and UL1TR001873. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Clinical Trial Number: NCT03883607
https://clinicaltrials.gov/ct2/show/NCT03883607?term=elafibranor&draw=2&rank=2
Conflicts of Interest:
Nidhi P. Goyal: none declared
Ali Mencin: none declared
Kimberly P. Newton: none declared
Janis Durelle: none declared
Carissa Carrier: none declared
Patricia Ugalde-Nicalo: none declared
Benoit Noel: shareholder of GENFIT
Julie Mouton: none declared
Dawn Vargas: none declared
David Magrez: shareholder of GENFIT
Bachirou Tadde: none declared
Pascal Birman: none declared
Brookie M. Best: none declared
Carol Addy: none declared
Jeffrey B. Schwimmer: reports grants to UCSD from Intercept and Seraphina outside the submitted work
REFERENCES
- 1.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–1393. [DOI] [PubMed] [Google Scholar]
- 2.Alkhouri N. Liver Transplantation for Nonalcoholic Steatohepatitis (NASH) in Children and Young Adults: The True Burden of Pediatric Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;148(4):S–1046. [Google Scholar]
- 3.Carter-Kent C, Yerian LM, Brunt EM, et al. Nonalcoholic steatohepatitis in children: A multicenter clinicopathological study. Hepatology. 2009;50(4):1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005. Sep;42(3):641–9. [DOI] [PubMed] [Google Scholar]
- 5.Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150(5):1147–1159.e5. [DOI] [PubMed] [Google Scholar]
- 6.Staels B, Rubenstrunk A, Noel B, et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58(6):1941–1952. [DOI] [PubMed] [Google Scholar]
- 7.Harrison SA, Ratzui V, Bedossa P, et al. RESOLVE-IT Phase 3 of Elafibranor in NASH: Final Results of the Week 72 Interim Surrogate Efficacy Analysis. In: The Liver Meeting, AASLD. Nov 2020. Poster #LP23. [Google Scholar]
- 8.Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017. Feb;64(2):319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newton KP, Hou J, Crimmins NA, et al. Nonalcoholic Steatohepatitis Clinical Research Network. Prevalence of Prediabetes and Type 2 Diabetes in Children With Nonalcoholic Fatty Liver Disease. JAMA Pediatr. 2016. Oct 3;170(10):e161971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68(5):879–887. [DOI] [PubMed] [Google Scholar]
- 11.Francque S, Szabo G, Abdelmalek MF, et al. Nonalcoholic steatohepatitis: the role of peroxisome proliferator-activated receptors. Nat Rev Gastroenterol Hepatol. 2021;18(1):24–39. [DOI] [PubMed] [Google Scholar]
- 12.Bougarne N, Weyers B, Desmet SJ, et al. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr Rev. 2018;39(5):760–802. [DOI] [PubMed] [Google Scholar]
- 13.Botta M, Audano M, Sahebkar A, Sirtori CR, Mitro N, Ruscica M. PPAR Agonists and Metabolic Syndrome: An Established Role? Int J Mol Sci. 2018;19(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francque S, Verrijken A, Caron S, et al. PPARα gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J Hepatol. 2015;63(1):164–173. [DOI] [PubMed] [Google Scholar]
- 15.Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62(3):720–733. [DOI] [PubMed] [Google Scholar]
- 16.Cariou B, Hanf R, Lambert-Porcheron S, et al. Dual peroxisome proliferator-activated receptor α/δ agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care. 2013;36(10):2923–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cariou B, Zaïr Y, Staels B, Bruckert E. Effects of the new dual PPAR α/δ agonist GFT505 on lipid and glucose homeostasis in abdominally obese patients with combined dyslipidemia or impaired glucose metabolism. Diabetes Care. 2011;34(9):2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. https://ir.genfit.com/news-releases/news-release-details/genfit-announces-results-interim-analysis-resolve-it-phase-3 . [Google Scholar]
- 19.Feng Z, Xiang J, Liu H, et al. Design, Synthesis, and Biological Evaluation of Triazolone Derivatives as Potent PPARα/δ Dual Agonists for the Treatment of Nonalcoholic Steatohepatitis. J Med Chem. 2022;65(3):2571–2592. [DOI] [PubMed] [Google Scholar]
- 20.Schwimmer JB, Lavine JE, Wilson LA, et al. In Children with Nonalcoholic Fatty Liver Disease, Cysteamine Bitartrate Delayed Release Improves Liver Enzymes but does not Reduce Disease Activity Scores. Gastroenterology. Published online 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwimmer JB, Newton KP, Awai HI, et al. Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38(10):1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon TG, Roelstraete B, Hartjes K, et al. Non-alcoholic fatty liver disease in children and young adults is associated with increased long-term mortality. J Hepatol. 2021;75(5):1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.FDA. No Title. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/noncirrhotic-nonalcoholic-steatohepatitis-liver-fibrosis-developing-drugs-treatment
- 24.Schwimmer JB, Ugalde-Nicalo P, Welsh JA, et al. Effect of a Low Free Sugar Diet vs Usual Diet on Nonalcoholic Fatty Liver Disease in Adolescent Boys: A Randomized Clinical Trial. JAMA. 2019;321(3):256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwimmer JB, Behling C, Angeles JE, et al. Magnetic resonance elastography measured shear stiffness as a biomarker of fibrosis in pediatric nonalcoholic fatty liver disease. Hepatology. 2017;66(5):1474–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alkhouri N, Kohli R, Feldstein AE. Designing Clinical Trials in Pediatric Nonalcoholic Steatohepatitis: Tips for Patient Selection and Appropriate Endpoints. Hepatol Commun. 2019;3(12):1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vos MB, Dimick-Santos L, Mehta R, et al. Factors to Consider in Development of Drugs for Pediatric Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;157(6):1448–1456.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newton KP, Lavine JE, Wilson L, et al. Alanine Aminotransferase and Gamma-Glutamyl Transpeptidase Predict Histologic Improvement in Pediatric Nonalcoholic Steatohepatitis. Hepatology. 2021. Mar;73(3):937–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.